Abstract
In recent years there has been a significant advancement in the role of gut microbiota in regulating gastrointestinal motility. The bidirectional cross talk between the host and gut microbiota has been implicated in the regulation of both physiological and pathophysiological conditions. Intestinal dysbiosis or alteration in the composition of intestinal microbiota can result in impaired host intestinal permeability, immune response, and metabolism, leading to a proinflammatory state. In this review, we focus on the role of the gut microbiome in regulating gastrointestinal motility and shaping the enteric nervous system. We highlight the mechanisms of microbial metabolites in regulating intestinal motility. Several host factors such as diet and genetic predisposition can influence the gut microbial diversity and ultimately contribute to dysbiosis. Intestinal dysbiosis can contribute to the pathophysiology of disorders such as irritable bowel syndrome and chronic intestinal pseudo-obstruction. Manipulation of the gut microbiome is a promising therapeutic target for the treatment of motility disorders. Modification of gut microbiota through diet, antibiotics, probiotics, prebiotics, and fecal microbiota transplantation are all promising strategies for the treatment of gastrointestinal motility disorders that are currently under investigation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Motility disorders
- Transplantation
- Fecal microbiota
- Enteric neurons
- Irritable bowel syndrome
- Amyotrophic lateral sclerosis
6.1 Introduction
There is evolving evidence that interplay exists between the gut microbiota and the enteric nervous system (ENS), resulting in altered gastrointestinal motility. The conventional wisdom was that motor patterns influence the size, location, and diversity of the microbiota (Quigley 2011). Recent literature and studies have challenged this view, and several studies have demonstrated that the gut microbiota may exert significant control over the enteral nervous system of the host. The bidirectional cross talk between the gut microbiota and the host has been established; yet, the exact dynamics of the relationship remain an active field of investigation.
Several gastrointestinal disorders have been linked to alterations in gut microbiota, including irritable bowel syndrome (IBS), acute diarrhea, inflammatory bowel disease (IBD), and small intestinal bacterial overgrowth syndrome (SIBO) (Aziz et al. 2013). Alterations in gastrointestinal flora with the use of antibiotics can lead to Clostridium difficile-infected diarrhea (Kelly and LaMont 2008). Alterations in intestinal bacteria, and the subsequent production of carcinogens may contribute to the pathogenesis of colon cancer (Rowland 2009).
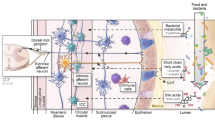
In a newborn, the gastrointestinal tract is sterile at birth. It is colonized within the first few days to weeks after birth by the microbiota. This microbiota is essential for survival as it confers a symbiotic and commensal advantage to the host. Antibiotics use in the early stages of life can predispose to gastrointestinal disorders later in life. The human microbiome contains nearly 100 trillion cells, about tenfold the number of human cells, and encodes about 150 times more genes than human cells (Ley et al. 2006). Metagenomic sequencing of fecal samples of 124 Europeans found that 99% of the genes in the human intestinal microbiome are bacterial. There are 1000–1150 prevalent bacterial species and each individual has at least 160 such species, which are largely shared (Qin et al. 2010). Most of the bacteria found in the human GI tract belong to the Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria phyla (Gill et al. 2006). The gut microbiota performs three broad functions, which are well described and include metabolic, immunological, and trophic functions . The metabolic function of bacteria is to extract calories from complex oligosaccharides and promote the absorptive capacity of the intestinal epithelium (Sekirov et al. 2010). The concentration of bacteria varies with age and location in the gastrointestinal tract. The distal colon has the maximum concentration of bacteria, approaching almost 1011 bacteria per gram. The colon is populated mainly by anaerobic bacteria owing to the low oxygen concentration in the colon, including Bacteroides, Bifidobacterium, Clostridium, Lactobacillus, and Porphyromonas. The stomach and proximal intestine have relatively small numbers of bacteria because of the acidic environment. Gram-negative and anaerobic bacteria populate the terminal ileum (Mackie et al. 1999). In this chapter, we first describe the influence of microbiota in shaping the ENS. Next, we discuss how microbial metabolites can regulate intestinal motility. Host factors can affect gut microbial diversity, and we demonstrate how dysbiosis can lead to motility disorders such as IBS and chronic intestinal pseudo-obstruction (CIPO) .
6.2 Microbiota Effects on the Enteric Nervous System
6.2.1 Early Development of the ENS
The effects of the microbiota on the development of the ENS are best illustrated in studies performed by colonizing germ-free animals with microbiota. Structural and functional differences have been found in the jejunal and ileal myenteric plexus of postnatal germ-free mice compared with specific pathogen-free mice. Postnatal germ-free mice also have decreased nerve density, decreased neurons per ganglion, and an increased ratio of nitrergic neurons compared with specific pathogen-free mice. In addition, decreased jejunal and ileal contractility has been observed in postnatal germ-free mice compared with specific pathogen-free mice (Collins et al. 2014). A recent animal experiment performed by McVey Neufeld et al. (2013) showed that the microbiome is essential for normal gut intrinsic afferent neuron excitability in the mouse. The investigators examined the electrophysiological properties of myenteric plexus neurons in germ-free mice, specific pathogen-free mice, and germ-free mice conventionalized with intestinal bacteria. Significant findings from this study included decreased neuronal excitability in the myenteric afterhyperpolarization neurons and prolonged post-action potential slow afterhyperpolarization in germ-free mice compared with the other two groups of mice. Another animal study examined small bowel myoelectrical activity in germ-free rats colonized with specific bacterial strains. Interestingly, colonization with anaerobic strains including Clostridium tabificum alone, and Lactobacillus acidophilus and Bifidobacterium bifidum in combination stimulated small intestinal transit by stimulating phase III of the migrating motor complex (MMC) sooner, whereas aerobic strains such as Micrococcus luteus and Escherichia coli suppressed or had no significant effect on the initiation of phase III of the MMC respectively (Husebye et al. 2001). The mechanism by which the microbiota modulates the MMC is unclear; however, the results of this study suggest that changes that arise in the local environment as a result of anaerobic metabolism may influence small intestinal motility. These landmark animal experiments provide evidence that the microbiota are necessary for the normal development and physiological function of the ENS.
6.2.2 Neurohormones
Serotonin (5-HT) functions as both a neurotransmitter and a local hormone, which is present in the gastrointestinal tract and in the central nervous system. It plays diverse roles in digestion, including initiation of intestinal secretion and peristalsis. Serotonin activates both intrinsic excitatory and inhibitory enteric motor neurons. It can stimulate cholinergic neurons to release acetylcholine, which results in smooth muscle contraction, or it can stimulate inhibitory nitrergic neurons to release nitric oxide, which results in smooth muscle relaxation (Sikander et al. 2009). Several small studies suggest that gut microbiota might modulate the biosynthesis and release of 5-HT in the gastrointestinal tract. The gut microbiota produces short chain fatty acids (SCFAs) via fermentation of carbohydrates, and SCFAs modulate the release of 5-HT from enterochromaffin cells in vivo (Fukumoto et al. 2003). The presence of the cholera toxin induces release of 5-HT in the rat jejunum (Bearcroft et al. 1996). In-vitro studies suggest that infection with enteropathogenic Escherichia coli might decrease the activity of the serotonin transporter in intestinal epithelial cells, resulting in decreased concentrations and uptake of 5-HT (Esmaili et al. 2009).
6.2.3 Microbial Metabolism
Short chain fatty acids such as butyrate, propionate, and acetate are produced as by-products of enteric bacterial fermentation of resistant starches. The proportions of each SCFA produced vary based on the presence of a particular microbiota and dietary fiber intake. Gram-positive anaerobic bacteria from the Firmicutes phylum, such as Faecalibacterium prausnitzii and Eubacterium rectale/Roseburia spp., are significant producers of SCFAs. The most widely studied SCFA is butyrate, which is essential for enterocyte function. Butyrate provides a source of energy for enterocytes, mediates intestinal epithelial cell turnover, modulates production of inflammatory cytokines, and alters gastrointestinal motility (Canani et al. 2011). The motor effects of SCFAs differ based on chain length. In-vitro and in-vivo rat studies have shown that butyrate increases the proportion of ascending excitatory cholinergic neurons and increases colonic contractile activity; however, these findings were not demonstrated for propionate or acetate (Soret et al. 2010). In a study performed on guinea pigs, butyrate increased the frequency of full-length propagations in the proximal colon and increased the velocity of propagation in the distal colon. In contrast, propionate blocked full and short propagations and had a biphasic effect on nonpropagating contractions, and acetate decreased short and total propagations (Hurst et al. 2014). The presence of SCFAs may also increase the proportion of ascending excitatory cholinergic neurons and colonic contractile activity (Soret et al. 2010). The mechanisms by which SCFAs affect gastrointestinal motility are an active area of study.
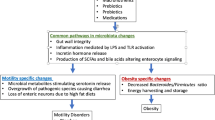
Low-grade inflammation has been observed in patients with IBS. Immunohistochemical studies of intestinal biopsy specimens from IBS patients have shown increased lymphocyte and mast cell infiltration in addition to myenteric neurodegeneration in patients with IBS compared with controls (Chadwick et al. 2002; Tornblom et al. 2002; Barbara et al. 2004). Increased cytokines such as IL-1β, IL-6, IL-8, LPS-induced IL-6, and TNF-α have also been observed in IBS patients (Liebregts et al. 2007). The etiology of the inflammation is thought to be multifactorial, and disruption of the intestinal epithelial barrier resulting in increased permeability has been proposed as a possible mechanism. Lipopolysaccharide (LPS ), a component of cell walls of Gram-negative bacteria, does not permeate the intestinal epithelial barrier under normal conditions. However, LPS crosses the epithelial barrier in settings of increased permeability, such as inflammation and infection, and activates toll-like receptors (TLRs) found on macrophages and dendritic cells. Activation of TLRs serves as protection against further injury. The mechanism by which specific TLRs distinguish between virulent and commensal bacteria is under study (Rakoff-Nahoum et al. 2004).
The host innate immune system is also able to detect the presence of microbial metabolites through pattern recognition receptors and immune-sensing complexes known as inflammasomes. Specifically, the NLRP-6 inflammasome plays a role in colonic homeostasis and can be influenced by microbiota-associated metabolites, including taurine, histamine, and spermine in a mouse model. These metabolites alter the secretion of IL-8 from colonic epithelial cells, which subsequently affects the downstream production of antimicrobial peptides. Disruption of the NLRP-6/IL-8/anti-microbial peptide axis results in increased susceptibility to inflammation in the host (Levy et al. 2015, 2017).
Bile acids serve many functions in the gastrointestinal tract. They are best known for their role in facilitating the absorption of lipids and fat-soluble vitamins; however, they also have antimicrobial properties, increase mucosal permeability, enhance intestinal secretion of water and electrolytes, and participate in signaling pathways in the ENS that modulate motility and sensation. The gut microbiota metabolically alters bile acids through the process of deconjugation. Bile acid malabsorption results in diarrhea, and is commonly seen in patients with ileal resection, Crohn’s disease, and diarrhea-predominant IBS. Several causative mechanisms for bile acid malabsorption have been suggested, including a deficiency of fibroblast growth factor 19 and genetic alterations of receptors involved in bile acid synthesis and transport. Established modalities for treating bile acid diarrhea include bile acid sequestrants such as cholestyramine, colestipol, and colesevelam. Bile acid receptor agonists that bind Farnesoid X receptor, such as obeticholic acid, are under development (Camilleri 2015).
Gut bacteria ferment carbohydrates and produce various gaseous by-products, such as carbon dioxide, hydrogen, methane, and hydrogen sulfide. The microbes generate energy via this process. Interestingly, there is an association between excessive gas production and clinical disorders. Excess hydrogen production has been implicated in SIBO and IBS. SIBO is defined by the presence of excessive bacteria in the small intestine and can be diagnosed using a glucose or lactulose hydrogen breath test or by culturing small intestinal aspirate. During a lactulose hydrogen breath test, lactulose is ingested, which is subsequently digested by the gut bacteria. Excess production of hydrogen is observed as a result of poor transit and the resulting overgrowth of bacteria in the gut (Pimentel et al. 2013).
Methane has been linked to constipation, constipation-predominant IBS, and obesity. Methane gas is a common by-product of fermentation produced by intestinal bacteria and results in slowed small intestinal transit. In animal models, infusion of methane has resulted in 59% slowing of small intestinal transit (Pimentel et al. 2006). Hydrogen sulfide is a toxic, pungent gas that is produced by both microbes and mammalian tissues, and studies have shown that it may play a role in gastrointestinal motility by indirectly mediating secretion, nociception, and smooth muscle relaxation. It has been linked to ulcerative colitis, although the mechanism of action remains unclear (Medani et al. 2011).
6.2.4 Host Factors
The effect of diet and genetics on shaping the gut microbiota has been demonstrated by a comparative study in children from Europe and rural Africa. This study compared the fecal microbiota of European children with the that of African children from a village in Burkina Faso. The African diet was high-fiber, low-fat, low-protein, and vegetarian, whereas the European diet consisted of mostly animal protein, sugars, starch, and low-fiber foods. Analysis of microbiota from the African children showed increased predominance of Bacteroidetes and decreased concentrations of Firmicutes. Specifically, Bacteroidetes such as Prevotella- and Xylanibacter-containing bacterial genes for cellulose and xylan digestion were found in the microbiota of the African children, but not in European children. In contrast, European children were found to have higher concentrations of Firmicutes and Enterobacteriaceae than African children. The concentration of fecal SCFAs also differed between the two groups, with the African cohort having higher concentrations of SCFAs noted in their stool samples. The findings of this study suggest that exposure to diets high in polysaccharides may influence the evolution of the gut microbiota that can best utilize this diet to produce energy (De Filippo et al. 2010).
Obesity has significantly increased in the last few decades, especially in the USA. The gut microbiota has been shown to be different in obese individuals; a decreased concentration of Bacteroidetes has been noted. The pathophysiology is poorly understood, but it is hypothesized that changes in gut flora might lead to altered proinflammatory molecules and changes in host–gene expression, which affect the gut epithelial and endocrine function and have an impact on insulin resistance and adiposity (Ley 2010).
6.3 Microbiota in Neurological Diseases
The bidirectional interaction between the nervous system and gastrointestinal tract is further illustrated by the role of the gut microbiota in the pathogenesis of neurological conditions , particularly Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS). Altered microbial composition has also been observed in multiple sclerosis, autism spectrum disorder, and Alzheimer’s disease (Scheperjans et al. 2015; Zhang et al. 2017; Chen et al. 2016; Luna et al. 2017; Shen et al. 2017).
6.3.1 Parkinson’s Disease
Parkinson’s disease is a neurodegenerative condition resulting from the accumulation of a specific amyloid protein, α-synuclein (ASO), within neurons. Motor deficits seen clinically in PD arise from the deposition of ASO in the dopaminergic neurons of the substantia nigra pars compacta in the midbrain. Gastrointestinal symptoms such as constipation may precede neurological symptoms by many years and arise from ASO deposition in enteric neurons.
Intestinal inflammation is present in patients with PD. Increased serum markers of endotoxins such as LPS binding protein, increased intestinal permeability, and increased mucosal staining of Gram-negative bacteria, ASO, and oxidative stress markers such as nitrotyrosine have been observed in a small human study (Forsyth et al. 2011). Differences in fecal microbiota composition have been identified in PD patients compared with healthy controls. A relative increase in Enterobacteriaceae and a decrease in Prevotellaceae have been observed in PD (Scheperjans et al. 2015; Unger et al. 2016). Decreased concentrations of stool SCFAs have been observed in PD patients compared with healthy controls, and may contribute to the development of gastrointestinal dysmotility (Unger et al. 2016).
Gene–environment interactions are thought to play a role in the pathogenesis of PD. An elegant experiment performed by Sampson et al. (2016) demonstrated that germ-free transgenic mice overexpressing ASO have reduced motor deficits, reduced microglial activation, and fewer ASO inclusions compared with ASO mice colonized with complex microbiota. These findings suggest that the presence of a microbiota might be required for the development of PD. Further experiments by this group have shown that treating germ-free ASO mice with SFCAs induces motor deficits, suggesting a possible mechanism by which microbiota modulate microglia. Colonization of germ-free ASO mice with microbiota obtained from patients with PD also induces clinical symptoms when compared with colonization with healthy donors. Therefore, it is plausible that the microbiota might influence the development of PD in genetically predisposed hosts.
6.3.2 Amyotrophic Lateral Sclerosis
Dysbiosis has been implicated in the development of ALS. ALS is a rapidly progressive, fatal neuromuscular condition affecting motor neurons associated with mutations in the Cu/Zn superoxide dismutase gene (SOD1). The G93A transgenic mouse model, which contains the human SOD1 gene, has been developed to study ALS.
Analysis of the fecal microbiota composition of G93A mice has shown decreased concentrations of butyrate-producing bacteria such as Butyrivibrio fibrisolvens, Escherichia coli, and Fermicus compared with wild-type mice. A loss of integrity of tight junctions and increased permeability of the intestinal epithelium has been observed in G93A mice, suggesting that a disruption of intestinal homeostasis might be involved in the pathogenesis of ALS (Wu et al. 2015). A recent landmark study from Zhang et al. demonstrated that oral butyrate treatment delayed progression of ALS symptoms, and significantly prolonged life span in the G93A mouse. Interestingly, treatment with butyrate also increased the proportion of butyrate-producing bacteria. Improvements in the structural integrity of the murine gut with restoration of tight junction proteins, decreased intestinal permeability, and decreased aggregation of SOD1 mutant proteins were also observed following butyrate treatment. Manipulation of the microbiome and its metabolites may emerge as a new therapeutic target for ALS (Zhang et al. 2017).
6.4 Dysbiosis in Motility Disorders
6.4.1 Small Intestinal Bacterial Overgrowth
Small intestinal bacterial overgrowth is defined by the presence of greater than 105 colony-forming units per ml of bacteria in jejunal aspirate. Clinical manifestations of SIBO include bloating, distention, diarrhea, abdominal pain, and weight loss. Host mechanisms to prevent SIBO include secretion of gastric acid, bile acid and pancreatic fluids, normal gastrointestinal motility, a competent ileocecal valve, and IgA production on the mucosal surface of the intestines (Miazga et al. 2015). The acidity of the stomach prevents bacterial growth in the small intestine. Reduced gastric acid production may occur as a result of increased aging or from exposure to Helicobacter pylori and may lead to SIBO (Dukowicz et al. 2007). The use of proton pump inhibitors has been considered a risk factor for SIBO; however, previous studies have yielded inconsistent results. A large study from the Mayo Clinic including 1,191 patients showed that the results of glucose hydrogen breath testing did not differ between PPI users and non-users . This study also showed that risk factors for a positive breath test included older age and the presence of diarrhea (Ratuapli et al. 2012).
Dysmotility likely plays a role in the pathogenesis of bacterial overgrowth. A small study by Vantrappen et al. (1977) compared the motor complexes seen on manometry tracings from normal subjects and those with SIBO, as defined by a positive bile acid breath test and found that the patients with SIBO had absent or disordered motor complexes. The function of the intestinal motor complexes is thought to be clearance of secretions, desquamated cells and nutrients. Impairment of this mechanism creates an environment that promotes bacterial overgrowth. Another study performed by Stotzer et al. (1996) also showed differences in antroduodenojejunal pressure tracings in healthy subjects compared with patients with SIBO, confirmed by duodenal aspirate and glucose hydrogen breath testing. The most significant finding in this study was the loss of phase III activity in the antrum and small intestine of SIBO patients compared with healthy subjects. Physiologically, phase III of the motor complex functions to clear stomach and intestinal contents during the fasting state. A recent Norwegian study examined intestinal motor patterns in patients with late radiation enteropathy by analyzing small intestinal manometry tracings, gastric pH, and bacterial counts. Interestingly, abnormalities in the MMC were found to be predictors of increased gram-negative bacilli concentrations in patients with late radiation enteropathy (Husebye et al. 1995).
Delayed small intestinal transit time has been demonstrated in patients with SIBO. A recent retrospective study examined 72 patients who underwent wireless motility capsule testing and lactulose hydrogen breath testing. Subjects with positive lactulose hydrogen breath tests were found to have longer small bowel and whole gut transit times than those with normal lactulose hydrogen breath tests. Based on these results, the authors concluded that delayed small intestinal transit contributes to the pathogenesis of SIBO (Roland et al. 2015).
6.4.2 Chronic Intestinal Pseudo-Obstruction
Chronic intestinal pseudo-obstruction clinically presents with the symptoms of obstruction in the absence of a mechanical blockage. CIPO is challenging to identify, and unfortunately many patients may experience recurrent episodes before diagnosis . In CIPO, the intestinal smooth muscle does not effectively contract and propagate luminal contents (De Giorgio et al. 2011).
The pathogenesis of CIPO is not very well understood. Inflammatory and neurodegenerative processes can damage the myenteric ganglia and the interstitial cells of Cajal. Viruses that have been implicated in CIPO include Herpesviridae and John Cunningham virus. Secondary causes of CIPO include neurological disorders such as PD, collagen vascular disease, and endocrine diseases. Chronic alcohol abuse has also been linked to CIPO. Ongoing studies are being performed to identify genetic causes of CIPO. Derangements in gut microbiota occur as a result of altered intestinal motility, and this population frequently presents with symptoms of bacterial overgrowth (Gabbard and Lacy 2013).
6.4.3 Irritable Bowel Syndrome
Dysbiosis has been observed in patients with functional bowel disorders. Functional bowel disorders include IBS, functional diarrhea, chronic idiopathic constipation, and functional bloating. IBS is the most common functional bowel disorder with an estimated 10–20% prevalence in the Western world (Saito et al. 2002). Diagnosis is symptom-based as described by the Rome Criteria. The pathogenesis of functional bowel disorders is multifactorial, with both biological and psychosocial circumstances influencing the course of the disease. Physiological studies suggest that the fecal microbiota might alter the brain–gut axis in functional bowel disorders, which results in aberrant sensorimotor and enteroendocrine function, loss of integrity of the intestinal epithelial barrier, and increased intestinal inflammation (Ringel 2017).
The composition of the fecal microbiota in patients with IBS differs from healthy controls and may also vary by IBS subtype. In a study conducted Rajilic-Stojanovic et al. (2011), phylogenetic microarray analysis of fecal samples show that Bacteroidetes are decreased and Firmicutes are increased in patients with IBS compared with healthy controls. Interestingly, Faecalibacterium species were the only Firmicutes found in lower concentrations in IBS patients compared with healthy controls; Faecalibacterium prausnitzii is known to have anti-inflammatory properties based on previous studies performed in animal models of colitis. Pathogenic species in the Firmicutes phylum including Streptococcus species were found in higher concentrations in IBS patients.
It has been argued that the development of post-infectious IBS (PI-IBS ) provides compelling evidence that the microbiota is involved in the pathogenesis of IBS. PI-IBS is a condition presenting with classic symptoms of IBS following acute gastrointestinal infection. The odds ratios of developing PI-IBS after acute gastroenteritis are six- to sevenfold based on previous studies (Thabane et al. 2007; Halvorson et al. 2006). Risk factors for PI-IBS include younger age, female gender, co-existing psychiatric comorbidities such as anxiety and depression, and prolonged infection and fever. Jalanka-Tuovinen et al. (2014) performed phylogenetic microarray analysis of fecal samples from patients with IBS-D, PI-IBS, and healthy subjects and found that the composition of the microbiota of patients with IBS-D and PI-IBS was similar, and that both differed from healthy controls. However, it should be noted that this study performed by Jalanka-Tuovinen et al. (2014) found increased Bacteroidetes and decreased Firmicutes in the IBS groups, which conflicts with the study discussed earlier by Rajilic-Stojanovic et al. (2011). The lack of consistency between studies suggests that although dysbiosis is observed in IBS, the specific microbiota involved in the pathogenesis is still unclear.
6.5 Conclusion
Manipulation of the gut microbiome has emerged as a therapeutic target for the treatment of motility disorders. Modification of the gut microbiota through diet, antibiotics, probiotics, prebiotics, and fecal microbiota transplantation (FMT) is currently under investigation. Recently, the efficacy of the low fermentable oligo-, di-, monosaccharides, and polyols (FODMAP) diet has been demonstrated in the treatment of IBS. FODMAPs are osmotically active, poorly absorbed carbohydrates that are highly susceptible to bacterial fermentation. A recent meta-analysis shows that IBS symptom severity is reduced on a low-FODMAP diet (Marsh et al. 2016). Although the low-FODMAP diet appears promising, its long-term safety has not been assessed.
Studies examining the use of prebiotics and probiotics are currently underway. Prebiotics are foods ingested by the host that cannot be digested in the small intestine and are subsequently fermented in the colon. Prebiotics selectively promote the growth and proliferation of species of commensal bacteria such as Lactobacillus and Bifidobacterium with known health benefits. Studies addressing the efficacy of prebiotics are limited, but there are data to suggest that ingestion of inulin-type fructans might be beneficial (Quigley and Quera 2006). In contrast to prebiotics, probiotics are live microorganisms ingested by the host. There are many varieties of probiotics available commercially, and clinical efficacy appears to be strain-specific (Whelan 2011). A systematic review of 16 randomized controlled trials demonstrates improvement in IBS symptoms after treatment with Bifidobacterium infantis 35624 (Brenner et al. 2009).
Non-absorbable antibiotics such as rifaximin are the mainstay of treatment for SIBO, and have been recently approved for the treatment of diarrhea-predominant IBS (Pimentel et al. 2011). Early antibiotics exposure, specifically to macrolides and tetracyclines, has been linked to the subsequent development of functional bowel disorders, and treatment with antibiotics such as clindamycin and cephalosporins has been connected to the development of C. difficile infection (Villarreal et al. 2012). These seemingly conflicting findings of antibiotics being both causative and therapeutic suggests that the microbiome might be selectively targeted by antibiotics that may be beneficial or harmful to commensal bacteria.
Fecal microbiota transplantation is a process in which stool from healthy donors is transplanted to patients with the hope of curing an underlying gastrointestinal disorder. Restoration of a healthy gut microbiome to a diseased host underlines the importance of the microbiota in modulating normal gastrointestinal function. The efficacy of FMT has been established for recurrent C. difficile infection, and is currently FDA-approved for this indication (Rossen et al. 2015). Ongoing studies of FMT in the treatment of IBD and motility disorders are limited but promising. A preliminary Chinese study examining 9 patients with CIPO demonstrated an improvement in symptoms of bloating and abdominal pain in addition to the ability to tolerate enteral feeds following FMT via a nasojejunal tube (Gu et al. 2017). A randomized controlled trial examining the efficacy of FMT compared with laxative therapy in patients with slow-transit constipation showed 30% improvement in patients with FMT, although the rate of treatment-related adverse events was higher in the FMT group (Tian et al. 2017). A recently published open label study on FMT in 10 Japanese patients with IBS (8 with IBS-D, 1 with IBS-C, and 1 with IBS-M) demonstrated clinical improvement in symptoms in 6 patients at 4 weeks after FMT. Analysis of stool microbiota composition showed increased diversity following FMT. Higher concentrations of Bifidobacterium species were noted in donor stools from the patients who responded to FMT (Mizuno et al. 2017). Although these early studies are encouraging, FMT for the treatment of motility disorders remains experimental.
References
Aziz Q, Dore J, Emmanuel A, Guarner F, Quigley EM (2013) Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil 25(1):4–15
Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D et al (2004) Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126(3):693–702
Bearcroft CP, Perrett D, Farthing MJ (1996) 5-hydroxytryptamine release into human jejunum by cholera toxin. Gut 39(4):528–531
Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS (2009) The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol 104(4):1033–1049
Camilleri M (2015) Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver 9(3):332–339
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17(12):1519–1528
Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A et al (2002) Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 122(7):1778–1783
Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MM et al (2016) Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 6:28484
Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM (2014) Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil 26(1):98–107
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S et al (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107(33):14691–14696
De Giorgio R, Cogliandro RF, Barbara G, Corinaldesi R, Stanghellini V (2011) Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin North Am 40(4):787–807
Dukowicz AC, Lacy BE, Levine GM (2007) Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (NY) 3(2):112–122
Esmaili A, Nazir SF, Borthakur A, Yu D, Turner JR, Saksena S et al (2009) Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 137(6):2074–2083
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA et al (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6(12):e28032
Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M et al (2003) Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 284(5):R1269–R1276
Gabbard SL, Lacy BE (2013) Chronic intestinal pseudo-obstruction. Nutr Clin Pract 28(3):307–316
Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS et al (2006) Metagenomic analysis of the human distal gut microbiome. Science 312(5778):1355–1359
Gu L, Ding C, Tian H, Yang B, Zhang X, Hua Y et al (2017) Serial frozen fecal microbiota transplantation in the treatment of chronic intestinal pseudo-obstruction: a preliminary study. J Neurogastroenterol Motil 23(2):289–297
Halvorson HA, Schlett CD, Riddle MS (2006) Postinfectious irritable bowel syndrome—a meta-analysis. Am J Gastroenterol 101(8):1894–1899
Hurst NR, Kendig DM, Murthy KS, Grider JR (2014) The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil 26(11):1586–1596
Husebye E, Skar V, Hoverstad T, Iversen T, Melby K (1995) Abnormal intestinal motor patterns explain enteric colonization with gram-negative bacilli in late radiation enteropathy. Gastroenterology 109(4):1078–1089
Husebye E, Hellstrom PM, Sundler F, Chen J, Midtvedt T (2001) Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol 280(3):G368–G380
Jalanka-Tuovinen J, Salojarvi J, Salonen A, Immonen O, Garsed K, Kelly FM et al (2014) Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 63(11):1737–1745
Kelly CP, LaMont JT (2008) Clostridium difficile—more difficult than ever. N Engl J Med 359(18):1932–1940
Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA et al (2015) Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163(6):1428–1443
Levy M, Blacher E, Elinav E (2017) Microbiome, metabolites and host immunity. Curr Opin Microbiol 35:8–15
Ley RE (2010) Obesity and the human microbiome. Curr Opin Gastroenterol 26(1):5–11
Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124(4):837–848
Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S et al (2007) Immune activation in patients with irritable bowel syndrome. Gastroenterology 132(3):913–920
Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J et al (2017) Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell Mol Gastroenterol Hepatol 3(2):218–230
Mackie RI, Sghir A, Gaskins HR (1999) Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr 69(5):1035S–1045S
Marsh A, Eslick EM, Eslick GD (2016) Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr 55(3):897–906
McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA (2013) The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil 25(2):183–e88
Medani M, Collins D, Docherty NG, Baird AW, O’Connell PR, Winter DC (2011) Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis 17(7):1620–1625
Miazga A, Osinski M, Cichy W, Zaba R (2015) Current views on the etiopathogenesis, clinical manifestation, diagnostics, treatment and correlation with other nosological entities of SIBO. Adv Med Sci 60(1):118–124
Mizuno S, Masaoka T, Naganuma M, Kishimoto T, Kitazawa M, Kurokawa S et al (2017) Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 96(1):29–38
Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH et al (2006) Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol 290(6):G1089–G1095
Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J et al (2011) Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 364(1):22–32
Pimentel M, Mathur R, Chang C (2013) Gas and the microbiome. Curr Gastroenterol Rep 15(12):356
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59–65
Quigley EM (2011) Microflora modulation of motility. J Neurogastroenterol Motil 17(2):140–147
Quigley EM, Quera R (2006) Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology 130(2 Suppl 1):S78–S90
Rajilic-Stojanovic M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S et al (2011) Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141(5):1792–1801
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118(2):229–241
Ratuapli SK, Ellington TG, O’Neill MT, Umar SB, Harris LA, Foxx-Orenstein AE et al (2012) Proton pump inhibitor therapy use does not predispose to small intestinal bacterial overgrowth. Am J Gastroenterol 107(5):730–735
Ringel Y (2017) The gut microbiome in irritable bowel syndrome and other functional bowel disorders. Gastroenterol Clin North Am 46(1):91–101
Roland BC, Ciarleglio MM, Clarke JO, Semler JR, Tomakin E, Mullin GE et al (2015) Small intestinal transit time is delayed in small intestinal bacterial overgrowth. J Clin Gastroenterol 49(7):571–576
Rossen NG, MacDonald JK, de Vries EM, D’Haens GR, de Vos WM, Zoetendal EG et al (2015) Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. World J Gastroenterol 21(17):5359–5371
Rowland IR (2009) The role of the gastrointestinal microbiota in colorectal cancer. Curr Pharm Des 15(13):1524–1527
Saito YA, Schoenfeld P, Locke GR 3rd (2002) The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 97(8):1910–1915
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(6):1469–1480.e12
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30(3):350–358
Sekirov I, Russell SL, Antunes LC, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90(3):859–904
Shen L, Liu L, Ji HF (2017) Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis 56(1):385–390
Sikander A, Rana SV, Prasad KK (2009) Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta 403(1–2):47–55
Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP et al (2010) Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138(5):1772–1782
Stotzer PO, Bjornsson ES, Abrahamsson H (1996) Interdigestive and postprandial motility in small-intestinal bacterial overgrowth. Scand J Gastroenterol 31(9):875–880
Thabane M, Kottachchi DT, Marshall JK (2007) Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 26(4):535–544
Tian H, Ge X, Nie Y, Yang L, Ding C, McFarland LV et al (2017) Fecal microbiota transplantation in patients with slow-transit constipation: a randomized, clinical trial. PLoS One 12(2):e0171308
Tornblom H, Lindberg G, Nyberg B, Veress B (2002) Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 123(6):1972–1979
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72
Vantrappen G, Janssens J, Hellemans J, Ghoos Y (1977) The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest 59(6):1158–1166
Villarreal AA, Aberger FJ, Benrud R, Gundrum JD (2012) Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ 111(1):17–20
Whelan K (2011) Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care 14(6):581–587
Wu S, Yi J, Zhang YG, Zhou J, Sun J (2015) Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep 3(4) pii: e12356
Zhang YG, Wu S, Yi J, Xia Y, Jin D, Zhou J et al (2017) Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther 39(2):322–336
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The American Physiological Society
About this chapter
Cite this chapter
Raja, S., Batra, V., Srinivasan, S. (2018). The Influence of Microbiota on Gastrointestinal Motility. In: Sun, J., Dudeja, P. (eds) Mechanisms Underlying Host-Microbiome Interactions in Pathophysiology of Human Diseases. Physiology in Health and Disease. Springer, Boston, MA. https://doi.org/10.1007/978-1-4939-7534-1_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7534-1_6
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4939-7533-4
Online ISBN: 978-1-4939-7534-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)