Abstract
The molecular biology of pancreatic neuroendocrine tumors (pNETs) carcinogenesis is poorly understood and is generally different from that of exocrine pancreatic neoplasms. pNETs represent a rare group of neoplasms with heterogeneous clinicopathological features. They are generally sporadic but can also arise within very rare hereditary syndromes, such as multiple endocrine neoplasia type I (MEN-I), von Hippel-Lindau disease (VHL), neurofibromatosis type 1 (NF1), and tuberous sclerosis complex (TSC). In these syndromes although a specific genotype/phenotype association with pNETs has been described, exact mechanisms leading to tumors development are still debated. Some clinical and biological features of pNETs associated with hereditary syndromes are similar in sporadic cases.
The presence of germline mutations has been indeed recently proved also in a high proportion of sporadic pNETs (17%) by whole genoming sequencing. These mutations include (beyond the well-known MEN1 and VHL) also other genes (such as BRCA2, or other of the mTOR pathway). Overall, main genomic changes involve gain of 17q, 7q, 20q, 9p, 7p, 9q and loss of 11q, 6q, 11p, 3p, 1p, 10q, 1q that identify the region of putative candidate oncogenes or tumor suppressor genes (TSGs) respectively. For some of them a possible relevant prognostic role has been described. “Classical” oncogenes involved in exocrine neoplasms (k-Ras, c-Jun, c-Fos) are of limited relevance in pNETs; on the contrary, overexpression of Src-like kinases and cyclin DI oncogene (CCNDI) has been described. As for TSGs, p53, DPC4/Smad, and Rb are not implicated in pNETs tumorigenesis, while for p16INK4a, TIMP-3, RASSF1A, and hMLH1 more data are available, with data suggesting a role for methylation as silencing mechanism. Different molecular pathways and the role of tyrosine kinase receptors have also been investigated in pNETs (EGF, c-KIT) with interesting findings especially for VEGF and m-TOR, which encourage clinical development. Microarray analysis of expression profiles has recently been employed to investigate pNETs, with a number of different strategies, even if these studies suffer from a number of limitations, mainly related with the poor repeatability and the poor concordance between different studies. However, apart from methodological limits, molecular biology studies are needed to better know this group of neoplasms, aiming at identifying novel markers and targets for therapy also highlighting relations with clinical outcome. Besides biomarkers recent studies are currently focusing on the role of the immune system in tumor pathogenesis of pNETs, paving the way to a new therapeutic approach also in these rare tumors: the immunotherapy.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
The molecular biology of pancreatic neuroendocrine tumors (pNETs) is poorly understood, and overall oncogenes and tumor suppressor genes (TSGs) more frequently involved in exocrine neoplasms, and particularly in pancreatic cancer, are not relevant. pNETs are generally sporadic, as their carcinogenesis is based on somatic mutations [1]. However, oncosuppressors responsible for pNETs can be involved by germline mutations, which are present also in a significant rate of sporadic pNETs [2]. This process may be spontaneous, without a previous family history, or more frequently inherited, as a part of well-described syndromes. The present paragraph will review in depth existing evidences for the molecular pathogenesis of pNETs, with a summary of data from studies of familial syndromes, genetic instability, as well as those examining the role of oncogenes, TSGs, and an insight into more recent microarray studies. A brief overview of the expression of growth factors and their receptors as possible therapeutic targets will also be presented.
Inherited Pancreatic Endocrine Tumors
The following hereditary syndromes have been associated with pNETs: multiple endocrine neoplasia type I (MEN-I), von Hippel-Lindau disease (VHL), von Recklinghausen’s disease (neurofibromatosis 1 or NF1), and tuberous sclerosis complex (TSC) [3]. The latter three are phakomatoses, rare neurocutaneous syndromes characterized by uncontrolled growth of ectodermal tissues from which endocrine tumors arise.
The pNETs occurring in these hereditary forms are primarily nonfunctioning tumors or insulinomas, with different incidence, and do not differ from those detected as sporadic [3] (Table 1).
Multiple Endocrine Neoplasia Type I (MEN-I)
The most frequent inherited syndrome causing pNETs is MEN-I , a rare autosomal dominant disorder (incidence 1:20,000–40,000) clinically defined by the presence of two or more of the following neoplasms: gastroenteropancreatic neuroendocrine tumors, parathyroid gland adenomas, pituitary adenomas, with other neoplastic lesions (i.e., thyroid adenomas, multiple lipomas, bronchial or thymic carcinoids) occurring occasionally [4]. About 10% of pNETs occur as a part of MEN-I.
The MEN-I syndrome is the result of an inactivating mutation of the Menin gene, an oncosuppressor gene located on chromosome 11q13 [4].
This gene, consisting in 10 exons, encodes for a 68 KDa nuclear protein of 610 amino acids, named Menin. Menin functions include binding and inactivation of many nuclear transcription factors (especially JunD but also SMAD3, mSin3a, and trithorax family histone methyltransferase complex), upregulation of cell cycle inhibitors expression (p27KIPI and p18Ink4c), and influence on DNA repair process, all of which result in inhibition of cellular proliferation [5,6,7,8].
The spectrum of possible mutations is greatly various. In the last decade, more than 1,300 germline variants (the half of which with pathological effect) have been identified, and 10–12% of them occur without a positive family history. Some 23% are nonsense mutations, 9% splicing-site mutations, 41% frameshift deletions or insertions, 6% in-frame deletions or insertions, 20% missense mutations, and 1% whole or partial gene deletions [4].
Even though any genotype/phenotype association with pNETs have been described, the exact mechanism leading to the neoplasia is still debated and the role of Menin on cell cycle negative control and DNA stability is somehow controversial.
Gene mapping in MEN-I patients have shown loss of heterozygosis (LOH) in half of the cases, confirming the oncosuppressor function of Menin and the tumorigenesis Knudson’s two-hit hypothesis. LOH of the Menin gene and other somatic mutations on wild-type allele behave as a second hit after a first hit germline, inherited mutation. LOH on Menin allele, as described in sporadic pNETs, can also involve other terminal region of 11q, suggesting implications of additional genes in neoplastic development and progression. A heterogeneity among tumors even in the same patient, suggesting that different tumor-specific tumorigenic mechanisms may contribute to the pathogenesis of MEN1 tumors. The present study supports the clinical applicability of the WES strategy to research on multiple tumor samples and blood [9, 10].
pNETs patients with pathological Menin gene mutation do not differ from sporadic forms in terms of clinical features (age of onset, hormone and/or neoplasia-related symptoms), but only 10% develop metastases, especially in the case of tumors larger than 3–5 cm (irrespectively to its histotype) [1, 3].
In up to 80–90% of cases, endocrine pancreatic involvement consists in endocrine islet cell hyperplasia, without somatic LOH on Menin, and microadenomatosis (multiple indolent tumors <5 mm). These latter kind of lesions are characterized by trabecular structure and distinctive stroma, and, in spite of being asymptomatic and without metastases, in about 50% of the cases LOH of Menin gene is detectable [11,12,13].
In a variable percentage of MEN-I patients (20–60%), microadenomatosis is associated to one or multiple pancreatic “macro-tumors,” which are larger than 5 mm but less than 3–5 cm. These neoplasms are NF pNETs in about 80% of cases, 15–20% insulinomas, 3% glucagonomas, and rarely VIPomas or gastrinomas [1,2,3].
These tumors are often clinically silent and just 10% of cases lead to metastases, but they are often associated with other symptomatic more aggressive gastrointestinal neuroendocrine tumors, especially duodenal gastrinomas and somatostatinomas [3, 14, 15].
In fact, although 20–60% of MEN-I patients have Zollinger-Ellison syndrome (20–40% associated with gastric carcinoid type II), gastrinomas arise far more frequently in the duodenum as single or multiple small tumors (not unfrequently undetectable) rather than as pNETs [3, 16, 17].
Von Hippel-Lindau Disease (VHL)
pNETs also occur in a significant percentage of individuals affected by Von Hippel-Lindau disease (VHL). It is a very rare (1:30,000–1:50,000) autosomal dominant phakomatosis with a variable phenotype characterized by the presence of at least one of these major manifestations: single retinal or cerebellar hemangioblastoma (HB), renal cell carcinoma (RCC) or pheochromocytoma and other more rare multiorgan lesions such as pancreatic cysts or pNETs, renal cysts, endolymphatic sac tumors, epididymal papillary cystoadenomas, paragangliomas, polycythemia, and other rare tumors [18].
The gene responsible for this disease is VHL gene, an oncosuppressor of three exons located on 3p25-26 that by alternative splicing can encode for two proteins (pVHL), respectively of 213 and 160 amino acids [18].
The two VHL products accomplish to similar activities in the cytoplasm; in particular, they make an ubiquitin complex with cullin-2, Rbx1, and elongins B named VBC, which in case of normoxia binds and inactivates hypoxia-inducible factor (HIF) [14].
Inactivating mutation of VHL gene causes an overexpression of HIF, especially of vascular endothelial growth factor which lines to tumorigenesis [15].
Until now, more than 300 germline mutations have been found, 60% of which are truncating or missense mutations while 40% are deletions. These mutations are associated with different phenotypical expressions: only patients with missense mutations develop pheochromocytoma (VHL type 2) associated (2b) or not (2a) to RCC, whereas patients affected by other mutations will develop the remaining related disease manifestations (VHL type 1) [15, 18].
Disease penetrance grows by age (90% at 65 years), as germline mutations have to be followed by another somatic event in the wild-type allele.
As far as pNETs, LOH in the VHL allele or, less frequently, methylation or neomutation are frequent findings [15, 19]. Indeed, pancreatic involvement by multiple indolent cysts is typical of VHL (50–75%), but pNETs are also frequent (5–17%) [20].
Strict associations between specific mutations and phenotypic expression of pNETs have been reported, but tumor cells show a typical LOH in chromosome 3p which is not limited to the VHL gene, but also involves other adjacent genes (such as not papillary renal carcinoma-1) possibly implicated with tumorigenesis and progression [20].
Biological and clinical features of VHL-associated pNETs are similar to sporadic forms: they are typically nonfunctioning and asymptomatic, generally expressing somatostatin receptors and in 30–50% of cases are multifocal in the pancreas [3, 20, 21].
However, pNETs arising in VHL disease are usually small (<2–3 cm) and without liver metastases in about 80–90%, with a consequent better prognosis compared to sporadic ones. This difference is most likely due to earlier detection (at a mean of 35 vs. 58 years) thanks to investigations due to other malignancies’ symptoms [2, 3, 21].
Von Recklinghausen’s Disease or Neurofibromatosis Type 1 (NF-1)
Occurrence of gastroenteropancreatic NETs in NF-1 is less frequent than in MEN-I and VHL disease, and in particular the rate of pNET is very low [22].
NF-1 is an autosomal dominant phakomatoses (1:3,000–1:4,000) with high penetrance, defined by multiple café-au-lait skin spots, neurofibromas of any type and localization (10% malignant), and characterized by predisposition to various other malignancies development (3–30%) such as gliomas, myeloid leukemia, and pheochromocytoma [23].
NF-1 arises from mutation of the NF-1 gene, a large oncosuppressor of 50 exons located on the 17q11.2 chromosome. Its product, called neurofibromin, is a GTPase acting as a negative regulator of mitonegic Ras pathway, especially of the mTOR signaling [24].
Many NF-1 gene mutations have been identified, of which up to 50% arising “de novo”; however, all the significant genotype/phenotype association have been demonstrated [23].
Rate of associated pNETs is undeterminable [3, 25,26,27]. They arise from germline NF1 mutation and deletion; insulinomas and somatostatinomas are similar to sporadic forms as in the tumor cells there is low expression of NF-1. The risk of pNETs development is often increased in this disease, probably because of mTOR pathway upregulation; however, more cases are needed to study the genotype/phenotype relation.
Tuberous Sclerosis Complex (TSC)
The rarest inherited disease associated with gastroenteropancreatic NETs is TSC . This phakomatosis (1:10,000) is a hereditary multiorgan disease transmitted by autosomal dominant inheritance. TSC has a 100% penetrance and a highly variable expression; clinical manifestations are typical skin alterations, renal angiomyolipomas, multiple and diffuse hamartomas, mental retardation, and neurological alterations. pNETs are occasionally associated [28].
Two genes are responsible for this disease: TSC1 (9q34) and TSC2 (16p13.3) that respectively encode for hamartin and tuberin. These two proteins make a dimer that multi-modulates cell growth, interacting with phosphoinositide 3-kinase pathway-mTOR activity and insulin receptor signaling.
Several genotype/phenotype associations have been described and related to many different mutations (50% occurring de novo); somatic tumor cells show a secondary mutation or a large deletion, up to a complete LOH on the two alleles often involving large chromosomal region.
The described cases of pNETs associated with this disease are mainly nonfunctional, and few cases of insulinoma and somatostinoma, with a behavior similar to sporadic forms [5]. In particular, one case of pNETs described in literature, a nonfunctional tumor identified in a child, exhibited a TSC2 gene LOH; this confirms its oncosuppressor role, such as in other TSC-related neoplasm [29, 30].
Genetic Instability in Sporadic Pancreatic Endocrine Tumors
Genetic instability represents the necessary condition for tumor development, through the clonal expansion of cancerous cells that have acquired a selective advantage. Among the different events (point mutations, chromosomal rearrangements, gene amplifications, microsatellite sequences alterations, and epigenetic changes) occurring during the multistep process of somatic cells transformation, alterations in DNA copy number are the commonest events.
Allelic imbalances, that result from incorrect mitotic division and consequent abnormal chromosomal separation, may be revealed by a variety of methods including karyotyping, comparative genomic hybridization (CGH), microsatellite analysis, or, more recently, single nucleotide polymorphisms (SNPs) allelotyping.
Conventional CGH is a molecular cytogenetic genome-wide technique for the analysis of copy number changes in DNA of tumor cells. Through this method, differentially labeled test DNA and normal reference DNA are hybridized simultaneously to normal chromosome spreads and the hybridization is detected with two different fluorochromes. Regions of gain or loss of DNA sequences, such as deletions, duplications, or amplifications, are seen as changes in the ratio of the intensities of the two fluorochromes along the target chromosomes. In brief, the regions frequently identified with decreased copy number are likely to harbor tumor suppressor genes (TSGs), whereas regions with increased copy number may contain dominant oncogenes.
Furthermore, allelotyping, that is the systematic analysis of the allelic losses in single chromosomes thus exploring loss of heterozygosity (LOH), is another strategy to determine the most probable locus of a TSG: it can be based on polymorphic microsatellite DNA or on SNPs, assaying the frequency and extent of lost regions on all chromosomal arms. SNPs allelotyping is more sensitive than microsatellite analysis and is also useful to detect DNA copy number.
Genome-Wide Studies in Sporadic pNETs
During the last decade, several studies with different approaches have addressed to look for specific genomic defects in sporadic pNETs [31,32,33,34,35,36,37,38,39,40,41,42]. As shown in Table 2, CGH has been largely used to explore genetic aberrations. Most of the available data refer to small, heterogeneous tumor series and essentially regard well-differentiated pNETs. In addition, several different tumor classifications have been used by investigators in their studies during time making difficult a possible analysis of pNETs subtypes. In this paragraph, data are presented separating nonfunctioning (NF-) from functioning pNETs (F-pNETs), and among these, further taking account of benign insulinomas, malignant insulinoma, and gastrinomas to possibly identify specific genomic patterns.
In the ten published studies [31, 32, 35,36,37,38, 40,41,42,43] of CGH /genomic wide-allelotyping, 101 NF pNETs have been studied (Tables 3 and 4). The most frequent findings were losses of 11q (38.6%), 6q (37.6%), 11p (33.7%), 3p (26.7%), 1p (27.7%), and 10q (25.7%), while the most frequent gains involved 17q (41%), 7q (35.9%), 12q (34.6%), 14q (34.6%), 4p (32%), and 20q (30.7%).
As for the 31 gastrinomas investigated in seven studies, loss of 3p (19%) and gain of 9p (29%) represented the most common chromosomal aberrations [31, 32, 34,35,36,37, 40].
In benign insulinomas (116 overall tumor samples in seven studies), most frequent losses were found on 11q (19%), Xq (18%), and 1p (17%), while most frequent gains regarded 9q (41%), 7p (20%), and 7q e 5q (both 19%). Malignant insulinomas (30 tumor samples), defined by the presence of loco-regional advanced or metastatic disease, harbored more genomic alterations than benign counterpart [32, 33, 35,36,37, 39, 40]. In particular, most frequent losses were found on 6q (70%), Y (43%), 2q (33%), 3q (30%), 6p (30%), 10q, 11p, 11q, and Xq (all 23%), while main gains involved 17q (57%), 17p (53%), 12q (53%), 14q (50%), 7q (47%), 20q, and 9q (43%).
The identification of gains and losses on chromosomal regions helps to highlight loci potentially containing putative oncogenes and TSGs. Tables 5 and 6 summarize main losses and gains, together with candidate TSGs and oncogenes, the associated disorders for which a pathogenetic link has been already described and, finally, the prognostic significance of the particular genetic change.
On the whole, NF-pNETs seem to present more genomic aberrations, then malignant insulinomas, with benign insulinomas and gastrinomas presenting the lowest amount of changes. This tendency is consistent with the finding by Speel and colleagues that pNETs larger than 2 cm exhibited significantly more aberrations than lesions smaller than 2 cm given that NF-pNETs are often larger than 2 cm at diagnosis [32].
All these observations strongly suggest that pNETs subtypes may evolve along different molecular pathways: deciphering their specific signatures would help to implement pNETs classification system, with obvious implications for a better understanding of this complex nosological entity.
Prognostic Relevance
Accumulated evidences showing that pNETs from patients with advanced disease harbored significantly higher numbers of genetic aberrations than tumors from patients with localized disease suggest that malignant progression of pNETs progression is driven by the progressive accumulation of multiple genetic changes [32, 36, 39, 50], as is also known to occur in other types of human carcinomas [46].
Another interesting issue is the possible relationship between molecular genetic defects (number and type of genomic changes) and tumor progression or malignancy in pNETs.
Several LOH studies [45, 47,48,49], using microsatellite markers, demonstrated that LOH at chromosome 1, and in particular of its long arm, is a common event among pNETs subtypes (12/27 gastrinomas, 35/40 insulinomas, 10/29 different pNETs subtypes) and was significantly associated with the presence of hepatic metastases regardless of tumor type. Moreover, Chen and colleagues (2003) found in their series of gastrinomas that allelic loss at 1q31-32 as well as 1q21-23 significantly correlated with tumor aggressive growth and postoperative development of liver metastases [48]. Likewise, Yang and colleagues (2005) reported high frequency of LOH at 1q 21.3-23.2 and 1q31.3, significantly associated with malignancy of insulinomas suggesting in these two regions the presence of putative tumor suppressor genes important for aggressive growth of these tumors [49]. Although these two studies narrowed region of potential candidate genes, to date actual genes involved remain undefined (Table 5).
As for chromosome 3, LOH was demonstrated to be a common event (frequency ranged from 33% to 83%) in pNETs regardless of tumor subtypes and its frequency was significantly higher in malignant than in benign neoplasms, on the whole finding a correlation with clinically metastatic disease in several studies [44, 51,52,53]. As common deleted regions were different (3p14.2-21; 3p25.3-p23; 3q27-qter, all outside of the VHL locus) in the same studies, different putative tumor suppressor genes other than VHL on chromosome 3 may play a role in the latest steps of tumorigenesis of sporadic pNETs.
Only one LOH study reported by Barghorn and colleagues (2001) described allelic loss at chromosome 6 in 62.2% of cases in a heterogeneous cohort of pNETs, the majority of which were insulinomas and NF-pNETs (with common deleted regions mapped at 6q22.1 and 6q23-q24), and it was significantly more common in tumors larger than 2 cm in diameter than below this threshold as well as in malignant than in benign tumors [43]. Previously, Speel and colleagues (1999) had reported an overall loss at 6q in 39% of pNETs (with a common deleted region at 6q21-22) and in all of six insulinomas, again indicating a locus harboring a potential TSG involved in tumor development [32]. To further support this hypothesis, combined data from abovementioned genome-wide studies show that 6q loss occurs in 70% of malignant insulinomas and in 37.6% of NF-pNETs, as shown in Table 2 .
Chromosome 17. In a study of 20 mixed functioning and nonfunctioning pancreatic endocrine tumors, Beghelli and colleagues (1998) found allelic losses on 17p13 in ~24% of the chromosomal loci analyzed with a higher frequency of allelic losses significantly associated with a high proliferation index and malignancy of the tumors [54]. Moreover, the absence of p53 gene mutations in nearly all these tumors suggests the existence of another tumor suppressor gene in the same chromosomal area. However, according to genomic-wide studies, loss of 17p is a rare event (<10%) and probably does not play a central role in the majority of endocrine tumors development. On the opposite, gain of 17q is a frequent event, especially in malignant insulinomas (>50%). The oncogene Her-2/Neu, frequently overexpressed in breast and esophageal cancer which is identified as more aggressive phenotype, is located on chromosome 17q21. Her-2/Neu gene amplifications were identified in 40% of 11 gastrinomas [55], the majority of which were locally advanced or metastatic, while in another study by [56] the same gene was amplificated in 14% of 43 gastrinomas and this time higher mRNA levels in tumor cells were correlated with liver metastases [56].
LOH on chromosome 22q was detected in 14 of 15 insulinomas (93%) by Wild and colleagues (2001). The shortest region of overlap implicated a deletion at 22q12.1-q12.2 where hSNF5/INI1 gene is located but no alteration was identified by single strand conformational polymorphism analysis, direct DNA sequencing, or RNA expression analysis [57]. The same group [58] described LOH on chromosome 22q in 22 of 23 pNETs (including nonfunctioning tumors, gastrinomas, and vipomas) showing a LOH rate of 85% at locus 22q12.1, with LOH strongly correlated with the presence or the development of distant metastases [58]. Moreover, LOH on 22q12.3 was significantly associated with distant metastases, an area where two putative candidate gene are located, that is, synapsin3 (SYN3) and tissue inhibitor of metalloproteinase-3 (TIMP-3). Also in this instance, genome-wide studies tend to underestimate genetic changes: in particular, loss of 22q was found in ~20% of NF-pNETs and in less than 10% of other pNETs subtypes.
Sex Chromosomes. According to combined data from genome-wide studies reported, Xq loss mainly occurs in insulinomas (~20% of cases) and one CGH -study also noted an association between Xq loss metastatic disease, raising the hypothesis that X chromosome changes plays a role in defining the more aggressive nature of endocrine lesions [32, 43].
Aberration of X chromosome has been described mainly in gastric carcinoids and pNETs, and in malignant compared with benign endocrine tumors. Pizzi et al. [59] comparing pNETs and endocrine tumors of the ileum and appendix noted that LOH on chromosome X was evident in 60% of malignant gastric and pancreatic tumors but in only 4.5% of benign tumors. Similarly, none of the benign midgut tumors exhibited X chromosome LOH, whereas 15% of malignant tumors contained this aberration [59]. On the whole, an association between X chromosome LOH and malignancy clearly has been found. In LOH analysis, allelic losses on X chromosome were revealed in 50% of type III gastric carcinoids, but not in type I tumors. Again, tumors that exhibited LOH were associated with metastasis [60]. Also in a series of 16 female patients with gastrinomas reported by Chen et al. 56% presented X chromosome LOH, was significantly associated with aggressive postoperative tumor growth and with increased primary tumor size [61]. Missiaglia and colleagues (2002), in their microsatellite and FISH analysis extended to chromosome Y, described that pNETs from females had loss of chromosome X in 40% of cases whereas pNETs from males showed loss of chromosome Y in 36% of case but never had loss of the X chromosome [62]. A significant association of sex chromosome loss with metastases, local invasion Ki-67 > 5% was also described. Sex chromosome loss was found to be an independent variable associated with a shorter survival period and an increased risk of death of approximately fourfold.
Recently, in a comparative LOH analysis on X chromosome by Azzoni et al. [63] higher rate of allelic loss was found in poorly differentiated endocrine carcinomas than in well-differentiated endocrine carcinomas with two chromosomal regions, Xq25 and Xq26 showing LOH with a relatively high frequency [63]. Candidate tumor suppressor genes mapping at Xq25 are ODZ1, encoding Tenascin, a glycoprotein of the extracellular matrix involved in morphogenetic movements, tissue repair and tumor spreading and SH2D1A, whose mutation was described in X-linked lymphoproliferative disease and in non-Hodgkin Lymphomas [64]; while potential tumor suppressor genes for Xq26 are MEF, a transcription factor capable to suppress the transcription of the genes encoding for the matrix metalloproteinases, MMP-2 and MMP-9, and interleukin-8 as demonstrated in cell lines of human nonsmall cell lung carcinoma [65]; and GPC-3, a heparan sulfate proteoglycan linked to the cell membrane, involved in the progression of several types of malignant tumors, including mesotheliomas, ovarian, and lung carcinomas [66].
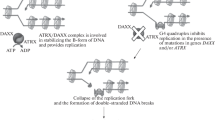
Loss of DAXX or ATRX protein and alternative lengthening of telomeres have also been proved to show a prognostic meaning in pNET cases. They were indeed associated to tumor stage, relapse-free survival, and decreased time of tumor-associated survival in 243 patients affected by pNETs [43].
Final Considerations
The limited resolution of the conventional CGH method, its low reliability (emerged from the observation that some regions – 1p32- pter, 16p, 19, and 22 – showed gains in negative control experiments), and its feature to be a laborious method remain the principal limits. On the other hand, LOH analysis, depending on number and type of microsatellite markers used, often offers contradictory results. For this reason, caution is needed in interpreting their results, awaiting further studies to confirm available data.
Array-CGH technology can improve the resolution of conventional CGH on metaphase chromosomes from 5 to 10 Mb to ≤1 Mb on arrayed DNA. In a series of 27 insulinomas, Jonkers and colleagues (2006) performed a genome-wide array-based CGH analysis detecting in >50% of cases loss of chromosomes 11q and 22q and gains of chromosome 9q with the first two alterations only partially identified before by conventional CGH (11q loss and 22q loss were found in ~20% and ~10% of benign and malignant insulinomas, respectively) [67].
The chromosomal regions of interest included 11q24.1 (56%), 22q13.1 (67%), 22q13.31 (56%), and 9q32 (63%). Comparing their alteration frequencies in tumors with benign, uncertain, and malignant behavior according the most recent WHO classification, the authors suggest that gain of 9q32 and loss of 22q13.1 are early genetic events in insulinomas, occurring independently of the other alterations. Finally, in this study further evidence was found for the accumulation of chromosomal alterations which run parallel with increasing malignant potential.
Genetic Alterations of Oncogenes and Tumor Suppressor Genes, and Expression of Growth Factors and Their Receptors
Oncogenes
The role of k-Ras has been investigated by a number of authors, with findings suggesting limited relevance if any, thus differentiating pancreatic endocrine neoplasms from the exocrine counterpart. K-ras mutations were found in a risible proportion of cases [50, 54, 55, 68,69,70,71,72], without any significant clinical association. Not surprisingly, the BRAF gene, one of the human isoforms of RAF, which is activated by ras, does not seem to have a role in tumorigenesis of pNETs [73]. However, a possible role for the ras signaling pathway in pNETs may depend on inactivation of the TSG RASSF1 (see below).
Similarly, there is limited evidence for a role of either c-Jun or c-Fos [71, 74]. On the other hand, c-Myc is overexpressed in most studies either at the RNA or protein level [50, 68, 75, 76]. The proto-oncogene Bcl-2, which acts as an antiapoptotic factor, has been detected in up to 45% of examined pNETs samples [75]; however, there are no data examining the overall balance of the pro/antiapoptotic machinery in pNETs.
Src is a family of proto-oncogenic nonreceptor tyrosine kinase including nine members. Src-like kinases act downstream of growth factor receptors and integrins transmitting messages that are crucial for several aspects of cell growth and metabolism, as for example cell cycle regulation, cell adhesion, and motility. Overexpression of Lck, a member of Src family, has been recently demonstrated in metastatic progressive pNETs in a microarray study [74]. The expression and activity of Src have been also described in pNETs cell lines and tissues, and inhibition of Src activity has been shown to interfere with adhesion, spreading, and migration of cells [77].
As far as cell cycle, although animals with constitutive activation of CDK4 develop pNETs [78], mutations have not been found in insulinomas [79]. A more relevant role for the cyclin DI oncogene (CCNDI) is suggested by findings of its overexpression and relation with disease stage [80, 81].
The Wnt signaling pathway is relevant for a number of neoplasms, and β-catenin activation is frequently detected in such cancers. However, no mutations of the β-catenin gene have been detected in a study including 108 pNETs, and nuclear accumulation of the β-catenin protein seems a rare and late event [82].
In a further study, 52% of pNETs showed abnormal β-catenin staining, which was related with loss of normal E-cadherin staining and more aggressive behavior [83].
Tumor Suppressor Genes
The role of MEN-I and VHL mutations, either in genetic or sporadic forms, has been summarized in the previous paragraphs.
The role of the p53 TSG has been investigated in a wide number of studies. A rationale for such investigations comes from studies of mice with p53 mutations and pNETs development. However, most studies found no mutations of p53 and/or no overexpression of the mutated protein in human pNETs [50, 54, 55, 68, 69, 72, 73, 84,85,86,87]. These data suggest that findings of LOH at 17q13 may be related with other unknown TSGs.
Similarly, although LOH at 18q is fairly frequent in pNETs, the DPC4/Smad gene has not been found to be mutated in the majority of published papers [50, 68, 88], and the retinoblastoma TSG (Rb) is also not implicated [89].
On the other hand, the p16 INK4a TSG, which encodes for an inhibitor of CDK4, seems relevant for at least a portion of pNETs. Particularly, inactivation of p16, either by mutations or by methylation is common in gastrinomas, but less frequent in NF-pNETs and insulinomas [55, 68, 90, 91].
The expression of the putative tumor suppressor gene tissue inhibitor of metalloproteinase-3 (TIMP-3) has been found to be altered by either promoter hypermethylation or homozygous deletion. The predominant TIMP-3 was described in 44% of examined pNETs, with as significant relation with the metastatic process [92].
The Ras-association domain family 1A (RASSF1A) is a TSG, interacting with ras. It is inactivated in a variety of solid tumors, usually by epigenetic silencing of the promoter or by loss at 3p21.3. RASSF1A induces cell cycle arrest through inhibition of cyclin D1 accumulation. RASSF1A hypermethylation was detected in 10 out of 12 (83%) endocrine tumors [93], and in a further publication RASSF1A silencing by methylation and 3p21.3 deletion was associated with tumors from foregut only, and with malignant behavior [94].
Loss of expression of the p27 protein has instead been paradoxically related with well-differentiated pNETs, with most indolent features, while its expression was associated with metastatic disease [95].
The aberrant promoter methylation of the mismatch repair gene, hMLH1, is associated with microsatellite instability (MSI). Hypermethylation of the hMLH1 promoter has been found in 23% of pNETs. Some 50% of hMLH1-methylated pNETs were found to be microsatellite unstable, and MSI was restricted to pNETs with hMLH1 hypermethylation. Tumors with MSI-positive had a better survival compared with MSI-negative [96].
Growth Factors and Their Receptors (Receptor Tyrosine Kinases)
The expression of growth factors, and their receptors, generally tyrosine kinases, is an interesting issue and offers the opportunity for targeted therapy. Angiogenesis has been studied in depth in transgenic mouse model (Rip1-Tag2) in which mice develop pNETs [97]. Although pNETs are highly vascular, some studies have suggested that they express VEGF, which correlates with a more aggressive tumor [98], while others detailed how pNETs present a wide range of microvascular density (MVD) according to the malignant potential, with malignant tumors showing lower MVD and VEGF expression than benign ones [99].
The surface of pNETs cells presents several other growth factor receptors, including receptor tyrosine kinases such as the epidermal growth factor receptor (EGFR), the stem cell factor (SCF) receptor c-KIT, and the platelet derived growth factor receptors (PDGFR) [100,101,102,103].
The EGFR (ErbB-1) is a member of a receptor tyrosine kinase family also including HER2/Neu (ErbB-2), HER-3 (ErbB-3), and HER-4 (ErbB-4), whose activation after interaction with their ligands leads to a number of downstream cascade molecular events involving cell proliferation and transformation. Although the expression of the EGFR and its phosphorylation seems more relevant in carcinoids than pNETs, phosphorylated-EGFR expression was found to be an unfavorable prognostic marker only in pNETs [104]. As far as other members of the Erb family, the expression and amplification of HER-2/Neu were explored in patients with gastrinoma, with relevant data presented above [55, 56].
c-KIT (CD117) is a type III tyrosine kinase receptor which, once activated by its ligand, stem cell factor (SCF), induces dimerization and autophosphorylation of the receptor at specific tyrosine regions, which acts as docking sites for other intracitosolic proteins important for intracellular signal transduction. Abnormal expression of c-KIT and/or SCF has been described in a variety of solid tumors, and activating mutations of c-KIT are a typical feature of gastrointestinal stromal tumors (GIST). Several studies have investigated the expression of c-KIT, together with other receptor tyrosine kinases in gastroenteropancreatic endocrine tumors, by immunohistochemistry [102, 105]. The results are inconsistent and, as hypothesized for other cancer types, inter-studies disagreement may be explained by different antibodies employed or different immunohistochemistry protocols.
A recent study including 98 pNET samples [2] has proved that sporadic pNETs contain germline mutations in about 17% of patients. These mostly interest genes involved in four main pathways: chromatin remodeling, DNA damage repair, activation of mTOR signaling (including previously undescribed EWSR1 gene fusions), and telomere maintenance, hypoxia, and HIF signaling. Also further mutations involving MUTYH, APOBEC, und BRCA have been described, paving the way to further molecular targets for therapeutic approach.
The (PI3K)/Protein Kinase B/AKT/mTOR Pathway
The mammalian target of rapamycin (mTOR) is a serine-threonine kinase involved in the mechanisms of regulation of cell growth and death through apoptosis. It plays a critical role in transducing a number of different proliferative signals mediated through the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) pathway , principally by activating downstream protein kinases that are required for both ribosomal biosynthesis and translation of key mRNAs of proteins required for cell cycle progression.
The signaling pathways upstream of mTOR include several tumor suppressors, such as PTEN, NF1, the kinase LKB1, and oncogenes such as Ras and Raf. mTOR also mediates signaling downstream of a number of growth factors such as IGF-1 and VEGF (Fig. 1). These signaling pathways converge on the tuberous sclerosis complex (TSC1/TSC2), which inhibits the mTOR activator Rheb, a small GTPase. In turn, activation of the mTOR pathway enhances the activity of HIF1α and of VEGF itself [106, 107].
Schematic representation of the PI3K/AKT/mTOR pathway. Green color indicates overexpression or activation, and red color indicates reduced expression or deactivating mutations. Overall, the balance of such events suggests an important role for this signaling pathway in pNETs. Notably, mutations of TSC1/TSC2 and PTEN may reduce the negative effect of hypoxia on the mTOR pathway
Tumors exhibiting constitutively activated PI3K/AKT/mTOR signaling due to mutations or loss of the abovementioned tumor suppressor genes (PTEN or TSC), or overexpression of upstream genes, are potentially susceptible to mTOR inhibitors, therefore making the investigation of this pathway particularly interesting for pNETs.
Microarray Studies
Global expression profiling has been often employed in the past decade to better understand molecular changes occurring in a number of tumors. This approach has been proved useful to identify novel markers and targets for therapy or to highlight relations with clinical outcome.
However, microarray studies suffer a number of limitations, mainly related with the poor repeatability, and the poor concordance between different studies [108].
Microarray analysis of expression profiles has recently been employed to investigate pNETs, with a number of different strategies. These studies are summarized in Table 7 [74, 109,110,111,112,113,114].
Overall, the studies differ significantly in terms of different samples and design, different platforms and statistical/bioinformatics methods. Two of the studies [74, 113] employed a wider platform. Two main different design subgroups can be identified: (1) comparison of pNETs samples versus purified pancreatic islets [74, 109, 110] and (2) comparison of metastatic versus nonmetastatic pNETs [112,113,114]. One other study compared expression profiles of pooled biopsy material of pNETs with that obtained from other pancreatic pathologies and normal pancreata [111], making its comparison with the other studies of poor sense. However, some of these studies did not provide clinical or histopathologic data sufficient to determine the clinical behavior of the investigated patients, and only one of the studies also compared primary lesions versus liver metastases [74], with findings suggesting a striking similarity between matched primaries and metastases.
Overall, none of the studies could identify novel dysregulated genes associated with a certain clinical behavior or with prognosis or response to treatment. The overlap between the different gene lists is very poor, as previously reported for pancreatic adenocarcinoma [115]. However, some interesting candidates for further evaluation as prognostic factors or therapeutic factors may have been identified.
A single paper examined the expression of microRNAs in pNETs [116]. MicroRNAs are small noncoding RNAs able to regulate gene expression by targeting specific mRNAs for degradation or translation inhibition. A role for microRNAs in tumor development and progression has been ascertained for many human cancers including pancreatic adenocarcinoma. Using a specific custom microarray, Roldo et al. explored the global microRNA expression of 40 pNETs (12 insulinomas, 28 non functioning tumors) compared to normal pancreas, and showed that a common pattern of microRNA distinguishes pNETs from normal pancreas. Specific microRNAs were identified, such as miR-204, primarily expressed in insulinomas and miR-21 which was strongly associated with both high Ki67 and liver metastases.
Conclusion
Research has made significant progresses in the knowledge of pNETs’ molecular biology but still the carcinogenesis involves mechanisms that need to be clarified. This multistep process may involve mutations of oncosuppressors genes , as well as germline mutations, which have been identified also in sporadic tumors. Further studies have to focus on immunotherapy and on the development of new target therapies to offer new treatment options to these patients.
Key Research Points
-
The molecular pathology of pancreatic endocrine tumors has been further investigated in the last 5 years, mostly thanks to whole genomic sequencing.
-
CGH studies suggest a plausible role for a number of TSGs, which is partially confirmed by specific studies. The role of epigenetics changes, especially of methylation deserves more attention.
-
A number of alterations of tyrosine kinase receptors (VEGFR), and molecular pathways (mTOR) expression and activity have been described.
-
Data of microarray studies suffer of the poor heterogeneity of the samples and have not described a specific relation between expression profiles and prognosis or response to therapy.
Future Scientific Directions
-
Future studies should always classify pNETs samples according to clinical and pathological standards, including WHO and TNM classification. Moreover, the tumor behavior (stable or progressive) is an issue in such an “indolent” tumor type.
-
CGH array studies may help identifying putative oncogenes or TSGs.
-
Microarray studies conducted in wide series of well-investigated pNETs with a relation with clinical behavior and follow-up are needed.
-
More in vitro models (animal models and cell lines) are sorely needed to better understand the process of tumor growth and progression, and possibly the role of novel therapies with targeted agents.
-
The relation between pNETs cells and the surrounding stroma has not been investigated and may be important, similarly to pancreatic adenocarcinoma.
-
The main future focus will be the role of the immune system in pNET tumorigenesis and proliferation control, starting from PD1/PDL-1 evaluation, paving the way to immunotherapy application also in these tumors (first trials ongoing).
Clinical Implications
-
Clinicians dealing with pNETs should keep in mind the possibility of inherited disorders, as the diagnostic and therapeutic strategy is different from that of sporadic cases.
-
Molecular alterations may somehow predict the clinical course and possibly suggest the use of certain novel targeted therapies, such as VEGF and mTOR inhibitors.
-
In this view, referral of patients to centers with more experience in clinical and molecular aspects of neuroendocrine tumors should be recommended.
-
Further knowledge about molecular pathways and mutations could pave the way for new tailored target therapies.
References
Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumours: pancreatic endocrine tumours. Gastroenterology. 2008;135(5):1469–92.
Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65–71.
Anlauf M, Garbrecht N, Bauersfeld J, Schmitt A, Henopp T, Komminoth P, Heitz PU, Perren A, Klöppel G. Hereditary neuroendocrine tumours of the gastroenteropancreatic system. Virchows Arch. 2007;451(Suppl 1):S29–38.
Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29(1):22–32.
Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102(3):749–54.
Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005;102(41):14659–64.
Bai F, Pei XH, Nishikawa T, Smith MD, Xiong Y. p18Ink4c, but not p27Kip1, collaborates with Men1 to suppress neuroendocrine organ tumours. Mol Cell Biol. 2007;27(4):1495–504.
Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13(4):587–97.
Bazzi W, Renon M, Vercherat C, Hamze Z, Lacheretz-Bernigaud A, Wang H, Blanc M, Roche C, Calender A, Chayvialle JA, Scoazec JY, Cordier-Bussat M. MEN1 missense mutations impair sensitization to apoptosis induced by wild-type menin in endocrine pancreatic tumour cells. Gastroenterology. 2008;135(5):1698–709.
Kim BY, Park MH, Woo HM, Jo HY, Kim JH, Choi HJ, et al. Genetic analysis of parathyroid and pancreatic tumors in a patient with multiple endocrine neoplasia type 1 using whole-exome sequencing. BMC Med Genet. 2017;18(1):106. https://doi.org/10.1186/s12881-017-0465-9.
Machens A, Schaaf L, Karges W, Frank-Raue K, Bartsch DK, Rothmund M, Schneyer U, Goretzki P, Raue F, Dralle H. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol. 2007;67(4):613–22.
Ballian N, Hu M, Liu SH, Brunicardi FC. Proliferation, hyperplasia, neogenesis, and neoplasia in the islets of Langerhans. Pancreas. 2007;35(3):199–206.
Anlauf M, Perren A, Klöppel G. Endocrine precursor lesions and microadenomas of the duodenum and pancreas with and without MEN1: criteria, molecular concepts and clinical significance. Clin Endocrinol. 2007;67:613–22.
Pereira T, Zheng X, Ruas JL, Tanimoto K, Poellinger L. Identification of residues critical for regulation of protein stability and the transactivation function of the hypoxia-inducible factor-1alpha by the von Hippel-Lindau tumour suppressor gene product. J Biol Chem. 2003;278(9):6816–23.
Woodward ER, Maher ER. Von Hippel-Lindau disease and endocrine tumour susceptibility. Endocr Relat Cancer. 2006;13:415–25.
Berna MJ, Annibale B, Marignani M, Luong TV, Corleto V, Pace A, Ito T, Liewehr D, Venzon DJ, Delle Fave G, Bordi C, Jensen RT. A prospective study of gastric carcinoids and enterochromaffin-like cell changes in multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: identification of risk factors. J Clin Endocrinol Metab. 2008;93(5):1582–91.
Anlauf M, Garbrecht N, Henopp T, Schmitt A, Schlenger R, Raffel A, Krausch M, Gimm O, Eisenberger CF, Knoefel WT, Dralle H, Komminoth P, Heitz PU, Perren A, Klöppel G. Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological feature. World J Gastroenterol. 2006;12(34):5440–6.
Corcos O, Couvelard A, Giraud S, Vullierme MP, O’Toole D, Rebours V, Stievenart JL, Penfornis A, Niccoli-Sire P, Baudin E, Sauvanet A, Levy P, Ruszniewski P, Richard S, Hammel P. Endocrine pancreatic tumours in von Hippel-Lindau disease: clinical, histological, and genetic features. Pancreas. 2008;37(1):85–93.
Lott ST, Chandler DS, Curley SA, Foster CJ, El-Naggar A, Frazier M, Strong LC, Lovel M, Killary AM. High frequency loss of Heterozygosity in von Hippel-Lindau (VHL)-associated and sporadic pancreatic islet cell tumours: evidence for a stepwise mechanism for malignant conversion in VHL tumourigenesis. Cancer Res. 2002;62:1952–5.
Mukhopadhyay B, Sahdev A, Monson JP, Besser GM, Reznek RH, Chew SL. Pancreatic lesions in von Hippel-Lindau disease. Clin Endocrinol. 2002;57:603–8.
Chetty R, Kennedy M, Ezzat S, Asa SL. Pancreatic endocrine pathology in von Hippel-Lindau disease: an expanding spectrum of lesions. Endocr Pathol. 2004;15:141–8.
Perren A, Wiesli P, Schmid S, Montani M, Schmitt A, Schmid C, Moch H, Komminoth P. Pancreatic endocrine tumours are a rare manifestation of the neurofibromatosis type 1 phenotype: molecular analysis of a malignant insulinoma in a NF-1 patient. Am J Surg Pathol. 2006;30(8):1047–51.
McClatchey AI. Neurofibromatosis. Annu Rev Pathol. 2007;2:191–216.
Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschläger M. The mTOR pathway and its role in human genetic diseases. Mutat Res. 2008;659(3):284–92.
Garbrecht N, Anlauf M, Schmitt A, Henopp T, Sipos B, Raffel A, Eisenberger CF, Knoefel WT, Pavel M, Fottner C, Musholt TJ, Rinke A, Arnold R, Berndt U, Plöckinger U, Wiedenmann B, Moch H, Heitz PU, Komminoth P, Perren A, Klöppel G. Somatostatin-producing neuroendocrine tumours of the duodenum and pancreas: incidence, types, biological behavior, association with inherited syndromes, and functional activity. Endocr Relat Cancer. 2008;15(1):229–41.
Nesi G, Marcucci T, Rubio CA, Brandi ML, Tonelli F. Somatostatinoma: clinico-pathological features of three cases and literature reviewed. Gastroenterol Hepatol. 2008;23(4):521–6.
Fujisawa T, Osuga T, Maeda M, Sakamoto N, Maeda T, Sakaguchi K, Onishi Y, Toyoda M, Maeda H, Miyamoto K, Kawaraya N, Kusumoto C, Nishigami T. Malignant endocrine tumour of the pancreas associated with von Recklinghausen’s disease. J Gastroenterol. 2002;37(1):59–67.
Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–68.
Rosner M, Hanneder M, Siegel N, Valli A, Hengstschläger M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat Res. 2008;658(3):234–46.
Francalanci P, Diomedi-Camassei F, Purificato C, Santorelli FM, Giannotti A, Dominici C, Inserra A, Boldrini R. Malignant pancreatic endocrine tumour in a child with tuberous sclerosis. Am J Surg Pathol. 2003;27(10):1386–9.
Terris B, Meddeb M, Marchio A, Danglot G, Fléjou JF, Belghiti J, Ruszniewski P, Bernheim A. Comparative genomic hybridization analysis of sporadic neuroendocrine tumours of the digestive system. Genes Chromosom Cancer. 1998;22(1):50–6.
Speel EJ, Richter J, Moch H, Egenter C, Saremaslani P, Rütimann K, Zhao J, Barghorn A, Roth J, Heitz PU, Komminoth P. Genetic differences in endocrine pancreatic tumour subtypes detected by comparative genomic hybridization. Am J Pathol. 1999;155(6):1787–94.
Stumpf E, Aalto Y, Höög A, Kjellman M, Otonkoski T, Knuutila S, Andersson LC. Chromosomal alterations in human pancreatic endocrine tumours. Genes Chromosom Cancer. 2000;29(1):83–7.
Yu F, Jensen RT, Lubensky IA, Mahlamaki EH, Zheng YL, Herr AM, Ferrin LJ. Survey of genetic alterations in gastrinomas. Cancer Res. 2000;60(19):5536–42.
Speel EJ, Scheidweiler AF, Zhao J, Matter C, Saremaslani P, Roth J, Heitz PU, Komminoth P. Genetic evidence for early divergence of small functioning and nonfunctioning endocrine pancreatic tumours: gain of 9Q34 is an early event in insulinomas. Cancer Res. 2001;61(13):5186–92.
Zhao J, Moch H, Scheidweiler AF, Baer A, Schäffer AA, Speel EJ, Roth J, Heitz PU, Komminoth P. Genomic imbalances in the progression of endocrine pancreatic tumours. Genes Chromosom Cancer. 2001;32(4):364–72.
Tönnies H, Toliat MR, Ramel C, Pape UF, Neitzel H, Berger W, Wiedenmann B. Analysis of sporadic neuroendocrine tumours of the enteropancreatic system by comparative genomic hybridisation. Gut. 2001;48(4):536–41.
Floridia G, Grilli G, Salvatore M, Pescucci C, Moore PS, Scarpa A, Taruscio D. Chromosomal alterations detected by comparative genomic hybridization in nonfunctioning endocrine pancreatic tumours. Cancer Genet Cytogenet. 2005;156(1):23–30.
Jonkers YM, Claessen SM, Perren A, Schmid S, Komminoth P, Verhofstad AA, Hofland LJ, de Krijger RR, Slootweg PJ, Ramaekers FC, Speel EJ. Chromosomal instability predicts metastatic disease in patients with insulinomas. Endocr Relat Cancer. 2005;12(2):435–47.
Chung DC, Brown SB, Graeme-Cook F, Tillotson LG, Warshaw AL, Jensen RT, Arnold A. Localization of putative tumour suppressor loci by genome-wide allelotyping in human pancreatic endocrine tumours. Cancer Res. 1998;58(16):3706–11.
Rigaud G, Missiaglia E, Moore PS, Zamboni G, Falconi M, Talamini G, Pesci A, Baron A, Lissandrini D, Rindi G, Grigolato P, Pederzoli P, Scarpa A. High resolution allelotype of nonfunctional pancreatic endocrine tumours: identification of two molecular subgroups with clinical implications. Cancer Res. 2001;61(1):285–92.
Nagano Y, Kim DH, Zhang L, White JA, Yao JC, Hamilton SR, Rashid A. Allelic alterations in pancreatic endocrine tumours identified by genome-wide single nucleotide polymorphism analysis. Endocr Relat Cancer. 2007;14(2):483–92.
Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146(2):453–60.e5.
Barghorn A, Komminoth P, Bachmann D, Rütimann K, Saremaslani P, Muletta-Feurer S, Perren A, Roth J, Heitz PU, Speel EJ. Deletion at 3p25.3-p23 is frequently encountered in endocrine pancreatic tumours and is associated with metastatic progression. J Pathol. 2001;194(4):451–8.
Ebrahimi SA, Wang EH, Wu A, Schreck RR, Passaro E Jr, Sawicki MP. Deletion of chromosome 1 predicts prognosis in pancreatic endocrine tumours. Cancer Res. 1999;59(2):311–5.
Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–99.
Guo SS, Wu AY, Sawicki MP. Deletion of chromosome 1, but not mutation of MEN-1, predicts prognosis in sporadic pancreatic endocrine tumours. World J Surg. 2002;26(7):843–7.
Chen YJ, Vortmeyer A, Zhuang Z, Huang S, Jensen RT. Loss of heterozygosity of chromosome 1q in gastrinomas: occurrence and prognostic significance. Cancer Res. 2003;63(4):817–23.
Yang YM, Liu TH, Chen YJ, Jiang WJ, Qian JM, Lu X, Gao J, Wu SF, Sang XT, Chen J. Chromosome 1q loss of heterozygosity frequently occurs in sporadic insulinomas and is associated with tumour malignancy. Int J Cancer. 2005;117(2):234–40.
Pavelic K, Hrascan R, Kapitanovic S, Vranes Z, Cabrijan T, Spaventi S, Korsic M, Krizanac S, Li YQ, Stambrook P, Gluckman JL, Pavelic ZP. Molecular genetics of malignant insulinoma. Anticancer Res. 1996;16(4):1707–17.
Chung DC, Smith AP, Louis DN, Graeme-Cook F, Warshaw AL, Arnold A. A novel pancreatic endocrine tumour suppressor gene locus on chromosome 3p with clinical prognostic implications. J Clin Invest. 1997;100(2):404–10.
Nikiforova MN, Nikiforov YE, Biddinger P, Gnepp DR, Grosembacher LA, Wajchenberg BL, Fagin JA, Cohen RM. Frequent loss of heterozygosity at chromosome 3p14.2-3p21 in human pancreatic islet cell tumours. Clin Endocrinol. 1999;51(1):27–33.
Guo SS, Arora C, Shimoide AT, Sawicki MP. Frequent deletion of chromosome 3 in malignant sporadic pancreatic endocrine tumours. Mol Cell Endocrinol. 2002;190(1–2):109–14.
Beghelli S, Pelosi G, Zamboni G, Falconi M, Iacono C, Bordi C, Scarpa A. Pancreatic endocrine tumours: evidence for a tumour suppressor pathogenesis and for a tumour suppressor gene on chromosome 17p. J Pathol. 1998;186(1):41–50.
Evers BM, Rady PL, Sandoval K, Arany I, Tyring SK, Sanchez RL, Nealon WH, Townsend CM Jr, Thompson JC. Gastrinomas demonstrate amplification of the HER-2/neu proto-oncogene. Ann Surg. 1994;219(6):596–601. discussion 602–604.
Goebel SU, Iwamoto M, Raffeld M, Gibril F, Hou W, Serrano J, Jensen RT. Her-2/neu expression and gene amplification in gastrinomas: correlations with tumour biology, growth, and aggressiveness. Cancer Res. 2002;62(13):3702–10.
Wild A, Langer P, Ramaswamy A, Chaloupka B, Bartsch DK. A novel insulinoma tumour suppressor gene locus on chromosome 22q with potential prognostic implications. J Clin Endocrinol Metab. 2001;86:5782–7.
Wild A, Langer P, Celik I, Chaloupka B, Bartsch DK. Chromosome 22q in pancreatic endocrine tumours: identification of a homozygous deletion and potential prognostic associations of allelic deletions. Eur J Endocrinol. 2002;147(4):507–13.
Pizzi S, D’Adda T, Azzoni C, Rindi G, Grigolato P, Pasquali C, Corleto VD, Delle Fave G, Bordi C. Malignancy-associated allelic losses on the X-chromosome in foregut but not in midgut endocrine tumours. J Pathol. 2002;196(4):401–7.
D’Adda T, Candidus S, Denk H, Bordi C, Höfler H. Gastric neuroendocrine neoplasms: tumour clonality and malignancy-associate large X-chromosomal deletions. J Pathol. 1999;189:394–401.
Chen YJ, Vortmeyer A, Zhuang Z, Gibril F, Jensen RT. X-chromosome loss of heterozygosity frequently occurs in gastrinomas and is correlated with aggressive tumour growth. Cancer. 2004;100(7):1379–87.
Missiaglia E, Moore PS, Williamson J, Lemoine NR, Falconi M, Zamboni G, Scarpa A. Sex chromosome anomalies in pancreatic endocrine tumours. Int J Cancer. 2002;98(4):532–8.
Azzoni C, Bottarelli L, Pizzi S, D’Adda T, Rindi G, Bordi C. Xq25 and Xq26 identify the common minimal deletion region in malignant gastroenteropancreatic endocrine carcinomas. Virchows Arch. 2006;448:119–26.
Brandau O, Schuster V, Weiss M, Hellebrand H, Fink FM, Kreczy A, Friedrich W, Strahm B, Niemeyer C, Belohradsky BH, Meindl A. Epstein-Barr virus-negative boys with non-Hodgkin lymphoma are mutated in the SH2D1A gene, as are patients with X-linked lymphoproliferative disease (XLP). Hum Mol Genet. 1999;8:2407–13.
Seki Y, Suico MA, Uto A, Hisatsune A, Shuto T, Isohama Y, Kai H. The ETS transcription factor MEF is a candidate tumour suppressor gene on the X chromosome. Cancer Res. 2002;62:6579–86.
Kim H, Xu GL, Borczuk AC, Busch S, Filmus J, Capurro M, Brody JS, Lange J, D’Armiento JM, Rothman PB, Powell CA. The heparan sulfate proteoglycan GPC3 is a potential lung tumour suppressor. Am J Respir Cell Mol Biol. 2003;29:694–701.
Jonkers YM, Claessen SM, Feuth T, van Kessel AG, Ramaekers FC, Veltman JA, Speel EJ. Novel candidate tumour suppressor gene loci on chromosomes 11q23-24 and 22q13 involved in human insulinoma tumourigenesis. J Pathol. 2006;210(4):450–8.
Moore PS, Orlandini S, Zamboni G, et al. Pancreatic tumours: molecular pathways implicated in ductal cancer are involved in ampullary but in exocrine nonductal or endocrine tumourigenesis. Br J Cancer. 2001;84:253–62.
Pellegata NS, Sessa F, Renault B, et al. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumours progress through different genetic lesions. Cancer Res. 1994;54:1556–60.
Yashiro T, Flton N, Hara H, et al. Comparison of mutations of ras oncogene in human pancreatic exocrine and endoxrine tumours. Surgery. 1993;114:758–64.
Hoffer H, Ruhri C, Putz B, et al. Oncogene expression in endocrine pancreatic tumours. Virchows Arch B Cell Pathol Incl Mol Pathol. 1998;55:355–61.
Sato T, Konishi K, Kimura H, et al. Evaluation of PCNA, p53, K-ras and LOH in endocrine pancreas tumours. Hepato-Gastroenterology. 2000;47:875–9.
Tannapfel A, Vomschloss S, Karhoff D, Markwarth A, Hengge UR, Wittekind C, Arnold R, Hörsch D. BRAF gene mutations are rare events in gastroenteropancreatic neuroendocrine tumours. Am J Clin Pathol. 2005;123(2):256–60.
Capurso G, Lattimore S, Crnogorac-Jurcevic T, Panzuto F, Milione M, Bhakta V, Campanini N, Swift SM, Bordi C, Delle Fave G, Lemoine NR. Gene expression profiles of progressive pancreatic endocrine tumours and their liver metastases reveal potential novel markers and therapeutic targets. Endocr Relat Cancer. 2006;13:541–58.
Wang DG, Johnston CF, Buchanan KD. Oncogene expression in gastroenteropancreatic neuroendocrine tumours: implications for pathogenesis. Cancer. 1997;80:668–75.
Roncalli M, Springall DR, Varndell IM, et al. Oncoprotein immunoreactivity in human endocrine tumours. J Pathol. 1991;163:117–27.
Di Florio A, Capurso G, Milione M, Panzuto F, Geremia R, Delle Fave G, Sette C. Src family kinase activity regulates adhesion, spreading and migration of pancreatic endocrine tumour cells. Endocr Relat Cancer. 2007;14(1):111–24.
Rane SG, Cosenza SC, Mettus RV, Reddy EP. Germ line transmission of the Cdk4(R24C) mutation facilitates tumourigenesis and escare from cellular senescence. Mol Cell Biol. 2002;22:644–56.
Vax VV, Bibi R, Diaz-Cano S, et al. Activating point mutations in cyclin-dependent kinase 4 are not seen in sporadic pituitary adenomas, insulinomas or Leydig cell tumours. J Endocrinol. 2003;178:301–10.
Guo SS, Wu X, Shimoide AT, Wong J, Moatamed F, Sawicki MP. Frequent overexpression of cyclin D1 in sporadic pancreatic endocrine tumours. J Endocrinol. 2003;179:73–9.
Chung DC, Brown SB, Graeme-Cook F, et al. Overexpression of cyclin D1 in sporadic pancreatic endocrine tumours. J Clin Endocrinol Metab. 2000;85:4373–8.
Hervieu V, Lepinasse F, Gouysse G, et al. J Clin Pathol. 2006;59:1300–4.
Chetty R, Serra S, Asa SL. Am J Surg Pathol. 2008;32:413–9.
Wang DG, Johnston CF, Anderson N, et al. Overexpression of the tumour suppressor p53 is not implicated in neuroendocrine tumour carcinogenesis. J Pathol. 1995;175:397–401.
Yoshimoto K, Iwahana H, Fukuda A, et al. Role of p53 mutations in endocrine tumourigenesis: mutation detection by polymerase chain reaction-single strand conformation polymorphism. Cancer Res. 1992;52:5061–4.
Lam KY, Lo CY. Role of p53 tumour suppressor gene in pancreatic endocrine tumours of Chinese patients. Am J Gastroenterol. 1998;93:1232–5.
Bartz C, Ziske C, Wiedenmann B, et al. p53 tumour suppressor gene expression in pancreatic neuroendocrine tumour cells. Gut. 1996;38:403–9.
Bartsch D, Hahn SA, Danichevski KD, et al. Mutations of the DPC4/Smad4 gene in neuroendocrine pancreatic tumours. Oncogene. 1999;18:2367–71.
Chung DC, Smith AP, Louis DN, et al. Analysis of the retinoblastoma tumour suppressor gene in pancreatic endocrine tumours. Clin Endocrinol. 1997;47:423–8.
Bartsch D, Kersting M, Wild A. Low frequency of p16(INK4a) alterations in insulinomas. Digestion. 2000;52:171–7.
Serrano J, Goebel SU, Peghini PL, et al. Alterations in the p16 INK4a/CDKN2A tumour suppressor gene in gastrinomas. J Clin Endocrinol Metab. 2000;85:4146–56.
Wild A, Ramaswamy A, Langer P, Celik I, Fendrich V, Chaloupka B, Simon B, Bartsch DK. Frequent methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene in pancreatic endocrine tumours. J Clin Endocrinol Metab. 2003;88(3):1367–73.
Dammann R, Schagdarsurengin U, Liu L, Otto N, Gimm O, Dralle H, Boehm BO, Pfeifer GP, Hoang-Vu C. Frequent RASSF1A promoter hypermethylation and K-ras mutations in pancreatic carcinoma. Oncogene. 2003;22(24):3806–12.
Pizzi S, Azzoni C, Bottarelli L, Campanini N, D’Adda T, Pasquali C, Rossi G, Rindi G, Bordi C. RASSF1A promoter methylation and 3p21.3 loss of heterozygosity are features of foregut, but not midgut and hindgut, malignant endocrine tumours. J Pathol. 2005;206(4):409–16.
Rahman A, Maitra A, Ashfaq R, Yeo CJ, Cameron JL, Hansel DE. Loss of p27 nuclear expression in a prognostically favorable subset of well-differentiated pancreatic endocrine neoplasms. Am J Clin Pathol. 2003;120(5):685–90.
House MG, Herman JG, Guo MZ, Hooker CM, Schulick RD, Cameron JL, Hruban RH, Maitra A, Yeo CJ. Prognostic value of hMLH1 methylation and microsatellite instability in pancreatic endocrine neoplasms. Surgery. 2003;134(6):902–8. discussion 909.
Parangi S, O’Reilly M, Christofori G, Holmgren L, Grosfeld J, Folkman J, et al. Antiangiogenic therapy of transgenic mice impairs de novo tumour growth. Proc Natl Acad Sci U S A. 1996;93(5):2002–7.
Zhang J, Jia Z, Li Q, Wang L, Rashid A, Zhu Z, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumours. Cancer. 2007;109(8):1478–86.
Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94–101.
Wulbrand U, Wied M, Zofel P, Goke B, Arnold R, Fehmann H. Growth factor receptor expression in human gastroenteropancreatic neuroendocrine tumours. Eur J Clin Investig. 1998;28(12):1038–49.
Srivastava A, Alexander J, Lomakin I, Dayal Y. Immunohistochemical expression of transforming growth factor alpha and epidermal growth factor receptor in pancreatic endocrine tumours. Hum Pathol. 2001;32(11):1184–9.
Fjallskog ML, Lejonklou MH, Oberg KE, Eriksson BK, Janson ET. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumours. Clin Cancer Res. 2003;9(4):1469–73.
Welin S, Fjallskog ML, Saras J, Eriksson B, Janson ET. Expression of tyrosine kinase receptors in malignant midgut carcinoid tumours. Neuroendocrinology. 2006;84(1):42–8.
Papouchado B, Erickson LA, Rohlinger AL, Hobday TJ, Erlichman C, Ames MM, et al. Epidermal growth factor receptor and activated epidermal growth factor receptor expression in gastrointestinal carcinoids and pancreatic endocrine carcinomas. Mod Pathol. 2005;18(10):1329–35.
Koch CA, Gimm O, Vortmeyer AO, Al-Ali HK, Lamesch P, Ott R, et al. Does the expression of c-kit (CD117) in neuroendocrine tumours represent a target for therapy? Ann N Y Acad Sci. 2006;1073:517–26.
Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003;4(Suppl 1):S169–77.
Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–35.
Tan PK, Downey TJ, Spitznagel EL Jr, Xu P, Fu D, Dimitrov DS, Lempicki RA, Raaka BM, Cam MC. Evaluation of gene expression measurements from commercial microarray platforms. Nucleic Acids Res. 2003;31(19):5676–84.
Maitra A, Hansel DE, Argani P, Ashfaq R, Rahman A, Naji A, Deng S, Geradts J, Hawthorne L, House MG. Global expression analysis of well-differentiated pancreatic endocrine neoplasms using oligonucleotide microarrays. Clin Cancer Res. 2003;95:988–95.
Dilley WG, Kalyanaraman S, Verma S, Cobb JP, Laramie JM, Lairmore TC. Global gene expression in neuroendocrine tumours from patients with MEN-I syndrome. Mol Cancer. 2005;4(1):9.
Bloomston M, Durkin A, Yang I, Rojiani M, Rosemurgy AS, Enkmann S, Yeatman TJ, Zervos EE. Identification of molecular markers specific for pancreatic neuroendocrine tumours by genetic profiling of core biopsies. Ann Surg Oncol. 2004;11:413–9.
Duerr EM, Mizukami Y, Ng A, Xavier RJ, Kikuchi H, Deshpande V, Warshaw AL, Glickman J, Kulke MH, Chung DC. Defining molecular classifications and targets in gastroenteropancreatic neuroendocrine tumours through DNA microarray analysis. Endocr Relat Cancer. 2008;15(1):243–56.
Hansel DE, Rahman A, House M, Ashfaq R, Berg K, Yeo CJ, Maitra A. Met proto-oncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasms. Clin Cancer Res. 2004;10:6152–8.
Couvelard A, Hu J, Steers G, O’Toole D, Sauvanet A, Belghiti J, Bedossa P, Gatter K, Ruszniewski P, Pezzella F. Identification of potential therapeutic targets by gene-expression profiling in pancreatic endocrine tumours. Gastroenterology. 2006;131(5):1597–610.
Grützmann R, Saeger HD, Lüttges J, Schackert HK, Kalthoff H, Klöppel G, Pilarsky C. Microarray-based gene expression profiling in pancreatic ductal carcinoma: status quo and perspectives. Int J Color Dis. 2004;19(5):401–13.
Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumours are associated with distinctive pathologic features and clinical behaviour. Clin Oncol. 2006;24(29):4677–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this entry
Cite this entry
Delle Fave, G., Merola, E., Capurso, G., Festa, S., Piciucchi, M., Valente, R. (2018). Molecular Pathology of Pancreatic Endocrine Tumors. In: Neoptolemos, J., Urrutia, R., Abbruzzese, J., Büchler, M. (eds) Pancreatic Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-7193-0_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7193-0_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-7191-6
Online ISBN: 978-1-4939-7193-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences