Abstract
Studies on clinical symptoms have documented correlations between post-traumatic stress disorder (PTSD) and sleep disturbances, with high prevalence for subjective sleep disturbances and small-to-moderate effects regarding objective sleep parameters. Studies on cortisol responses in PTSD have been equivocal, but there is evidence that central noradrenergic levels are increased in PTSD and these may subserve fragmentation of (REM) sleep and prolong wake after sleep onset. Such REM disruptions in PTSD may impair memory consolidation and/or emotional homeostasis as REM sleep deprivation has shown to impair fear extinction consolidation. More quantitative EEG and whole-brain neuroimaging work is needed at this stage to examine the relevant neural circuitry. Understanding the effects of heightened arousal on both task performance and sleep transitions may be essential to elucidating the neurophysiological commonalities of various PTSD symptom clusters.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Already in 1989, Ross et al. proposed that sleep disturbances, and in particular REM sleep disturbances, are the hallmark of post-traumatic stress disorder (PTSD) [1]. A quarter of a century later, evidence from clinical studies, experimental human studies, and animal models is converging that sleep disturbances may represent more than secondary PTSD symptoms and may be the hallmark of “a heightened vulnerability to maladaptive stress responses” [2]. Clinical data are unequivocal and show a high incidence of a wide range of subjective sleep disruptions in PTSD patients [3, 4], from repetitive nightmares to insomnia [5] and also more “physiological” sleep disorders as sleep apnea and periodic limb movements [6]. Objective sleep disturbances have been less pronounced, but disentanglement of confounding factors such as comorbid depression and substance abuse, as well as gender and age, has revealed small-to-moderate effects with increased density of rapid eye movement (REM) sleep and reduced slow-wave sleep (SWS) [7]. However, high prevalence does not tell us anything about the causality of such sleep disturbances regarding the development or maintenance of PTSD, and longitudinal studies are relatively rare. Mellman et al. has shown that REM sleep fragmentation in the month after a motor vehicle accident predicted PTSD severity weeks later [8] and reports that insomnia is a frequent residual symptom after effective PTSD treatment [9] and that specific treatment for nightmares alleviates PTSD symptom severity [10] suggests that sleep disturbances may indeed play a critical role in the development of PTSD. To explore the possible mechanisms and neural circuitry, this chapter will focus on the proposed role of heightened arousal.
Arousal and the Locus Coeruleus Noradrenalin System
The Research Domain Criteria (RDoC) project of the National Institute of Mental Health (NIMH) provides a framework for the study of psychiatric disorders, which comprises five domains containing multiple constructs. These currently include positive and negative valence systems, cognitive systems, systems for social processes, and arousal/regulatory systems. The latter domain includes sleep and circadian rhythms, with arousal being a related but separate construct: it is naturally related to sleep and wakefulness as arousal differs throughout the circadian rhythm, yet there are also relevant arousal shifts within wakefulness, which are defined as “a continuum of sensitivity of the organism to stimuli, both external and internal” within the RDoC framework.
The inclusion of stimuli into this definition of arousal highlights that the construct extends mere vigilance and incorporates context- and task-related information processing. This is highly relevant since there is now a large body of work supporting the notion that the noradrenergic system, with the locus coeruleus (LC) in the brainstem as the main output center projecting throughout cortical and subcortical regions, does more than just regulating arousal and is in fact a critical system for optimal task performance and neural gain [11]. This is best illustrated by the inverted U shape associated with increased tonic activity of the LC as shown in Fig. 19.1. Low tonic LC activity results in inattentive, non-alert behavior, whereas high tonic LC activity results in reduced attention through increased orienting behavior, distractibility, and scanning of the environment. Optimal performance is achieved at intermediate levels of tonic LC activity , with phasic LC activity in response to relevant stimuli preceding the required motor responses [11].
Inverted U shape describing the relationship between tonic LC activity and task performance , showing optimal performance at intermediate levels of tonic LC activity, allowing phasic LC activity in response to cued targets requiring a motor response (From Aston-Jones et al. [12], reproduced with kind permission from Elsevier)
Increased central noradrenergic concentrations have been observed in PTSD patients in wakefulness [13], as well as in 24-h urinary measures in larger samples [14]. If this is related to increased tonic LC output, PTSD patients should show impaired performance in tasks probing for attention and/or general information processing speed . This is indeed the case: a recent meta-analysis on 4108 PTSD patients, trauma-exposed controls, and healthy controls has revealed a Cohen’s d of around −0.5 for attention and working memory tasks and a Cohen’s d of around −0.6 for information processing speed (both reduced in PTSD patients) [15]. Moreover, another meta-analysis on the P3 response as measured with EEG in PTSD revealed that the P3a to trauma-related distractor stimuli was higher in PTSD patients [16]. One study has provided evidence that the (frontal) P3 to irrelevant distractor stimuli is also increased with PTSD, which would indicate higher distractibility and tonic LC activity as in Fig. 19.1, and that this was related to both hyperarousal and re-experiencing symptom clusters [17].
The LC-Noradrenalin System and Sleep
Another important role for the LC-noradrenalin system is related to the transitioning of stages within sleep: noradrenergic output of the LC is highest in wakefulness and lower in non-REM sleep and reaches its nadir in REM sleep [18]. REM sleep discontinues the moment noradrenergic LC cells (and serotonergic cells in the dorsal raphe nucleus) start firing [19], reason why the LC is also referred to as a REM off switch. It is also worth noting that firing of just 10% of neurons in LC suffices to maintain normal cortical function [20]. This suggests that also minor increases in noradrenalin levels during REM sleep, which may not even be detectable peripherally, could already have an effect on REM sleep and potentially, the SWS-REM cycles seen throughout the night.
A blunted circadian rhythm of 3-methoxy-4-hydroxyphenylglycol (MHPG) [21], the central metabolite of noradrenalin, has also been reported in PTSD. Regarding sleep, although nightmares and insomnia are frequently reported symptoms in PTSD [6], polysomnographic studies on PTSD (a combination of EEG, electromyography, and electrooculography) have been rather inconclusive up until recently. Inconsistencies in the data were largely resolved by a meta-analysis that controlled for confounding factors that affect sleep (gender, age, comorbid depression, and substance abuse) and demonstrated that the amount of slow-wave sleep (SWS) amount is reduced, whereas light sleep stage 1 and REM density are increased in PTSD [7]. Note, however, that effects are modest at best. The question is whether increased REM density is due to an increased number of phasic REM bursts or disrupted fragments of tonic REM sleep; but having shorter and more frequent REM sleep periods within 2 weeks after a motor vehicle accident has been reported to be a significant predictor of PTSD symptomatology months later [8]. Finally, also in line with a role for the LC-noradrenalin system in dysregulating (REM) sleep in PTSD is the relative success of the noradrenergic alpha1-antagonist prazosin. When administered before sleep, prazosin seems to have positive effects on total and REM sleep length (and nightmare amelioration) [22], although these effects have not yet been observed with polysomnographic recordings [23].
Furthermore, increased noradrenergic output may in parallel affect SWS . Using microinjections in cholinergic nuclei in the basal forebrain in rodents, Cape and Jones were able to show that noradrenalin microinjections, compared to serotonergic and Ringer’s microinjections, decreased both SWS and REM percentages (both time and transitions into this stage) while increasing wakefulness [24]. Noradrenalin caused an increase in gamma and a reduction in delta activity in the EEG, with electromyography (EMG) being similarly affected by both serotonin and noradrenalin [24].
In a comprehensive review on the role of noradrenalin in sleep mentation, Gottesmann [25] proposed that it is the near absence of noradrenalin in the forebrain, specifically the nucleus accumbens, that gives rise to the maximal dopamine release taking place in REM sleep [26], which could explain increased levels of hallucinatory mental activity typical for REM sleep. A speculation worth mentioning is that increased noradrenergic levels in this region and adjacent subcortical structures such as amygdala and hypothalamus may result in increased physiological arousal and trigger emotional memory traces. However, the role of noradrenalin heavily depends on receptor subtypes (e.g., postsynaptic α1 or β, or presynaptic – inhibitory – α2 receptor function). See Berridge et al. [18] and Broese et al. [27] for reviews on noradrenergic modulation of arousal and sleep.
A Role for Cortisol in the Relationship Between Arousal and the LC System?
Closely linked to both sleep regulation and PTSD symptomatology is the hypothalamus-pituitary-adrenal gland (HPA) axis that orchestrates the stress response and releases glucocorticoids into the bloodstream that provide negative feedback to the hippocampus. Corticotropin-releasing hormone (CRH) is released by the hypothalamus and causes an increase in adrenocorticotropic hormone (ACTH) in the pituitary, which in turn release glucocorticoids from the adrenal gland. Peripheral cortisol is at its trough during early sleep, slowly rises during the progression of the night, peaks in a cortisol-awakening response, and declines during the day. It is of note that cortisol levels are highest in the second half of the night [28] that is richest in REM sleep.
Reduced nocturnal cortisol levels have been observed in PTSD, albeit not consistently [6]. However, there is also evidence from animal models [29] and from a study in motor vehicle victims [30] that blunted cortisol responses after trauma exposure are a risk factor for PTSD. It has been proposed that, in case of reduced cortisol responses to acute stress, the noradrenergic system consequently becomes hyperactive as a homeostatic consequence, increasing the probability of PTSD development [31]. Closer to the central processes regulated by CRH is ACTH, and increased ACTH levels during the night and the awakening response were observed in PTSD and, importantly, correlated to sleep fragmentation and reduced SWS activity [32]. Interestingly, different ACTH/cortisol ratios have been shown in PTSD throughout the circadian cycle [32]. Such variability adds to the complex process of disentangling the multiple modulations of cortisol and reconciling the opposing findings; a meta-analysis on this topic seems warranted. In the meantime, continuous blood sampling during sleep in PTSD [32] appears helpful for extracting more information on ACTH and catecholamines, which might provide more unambiguous effects.
Disrupted Sleep and Disrupted Emotional Memory Consolidation/Homeostasis
The next question is then if the disruption of REM sleep impairs the consolidation of certain memories, given the role of non-REM sleep in declarative memory consolidation [33], and/or emotional homeostasis [34]. Both animal and human models have started to experimentally test this notion by employing analogous models that probe for core disrupted processes in PTSD, i.e., fear conditioning and extinction.
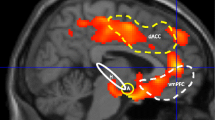
Fear extinction is an associative learning process central to PTSD [35, 36]. In an experimental setting, this is modeled by pairing neutral stimuli with an aversive event (fear conditioning). Subsequently presenting these stimuli alone elicits a fear response that normally extinguishes over time (fear extinction). Extinction learning and consolidation is impaired in PTSD patients [37,38,39]. As a result, fear conditioning and extinction has been a robust model for scientific advancement on neurocognitive mechanisms underlying PTSD in both preclinical and clinical studies, leading authoritative reviews to conclude that this is one of the most successful models for translating data from animal models to human subjects and psychiatric patients (see review by Milad et al. [40]). Moreover, using functional magnetic resonance imaging (fMRI), limbic and paralimbic regions such as the amygdala, hippocampus, dorsal anterior cingulate cortex (dACC), and ventromedial prefrontal cortex (vmPFC) have been repeatedly demonstrated to subserve fear extinction in healthy individuals (for a systematic review, see Sehlmeyer et al. [41]). Critically, these regions show abnormal fMRI activity in PTSD patients during symptom provocation [42] and during extinction and recall of extinction [38]. The dACC (excitatory influence over amygdala) and the more ventrally located vmPFC (inhibitory influence over amygdala) activity ratio during fear conditioning and extinction , as well as recall of extinction, has been identified in an extensive review on biological studies of PTSD as a promising functional imaging marker of PTSD [43].
There is a large body of preclinical data showing that the amygdala, hippocampus, and medial prefrontal cortex (mPFC) interact through (intracranially recorded) theta oscillations during fear conditioning and extinction [35]. Research in humans has shown abnormal event-related potentials during fear conditioning and extinction in PTSD patients with surface EEG [39], and excess of frontal midline oscillations (in vmPFC) in the theta range (4–8 Hz) during affectively loaded stimuli has recently been proposed as a novel marker of PTSD [44]. One unanswered question is whether hippocampal theta in rodents translates to hippocampal theta in humans – or to hippocampal rhythmic slow activity in the 1.5–3 Hz range as shown by Bodizs et al. [45] with intracranial foramen ovale electrodes in epilepsy patients.
Recent work has shown that sleep benefits memory consolidation [33] and that REM sleep may play an essential role in emotional memory consolidation [34]. Critically, animal studies and studies on human subjects have demonstrated that specific REM deprivation impairs fear extinction consolidation [46, 47], see Fig. 19.2. Moreover, theta coherence in REM sleep predicted subsequent fear expression at recall of conditioning in rodents [48], whereas pontine wave quality in REM sleep in rodents was highly correlated with subsequent extinction memory recall [49]. This makes REM sleep, characterized by theta oscillations and pontine waves (or the human equivalents), relevant for plasticity [50], an important brain state for PTSD.
Relevance of REM sleep deprivation for fear extinction consolidation . (a) In rats, REM sleep deprivation (RSD) immediately after extinction learning (0–6 h, red arrow) impaired extinction consolidation manifest in increased freezing to light stimuli that acted as the extinguished stimulus. This was not seen in the control group (C), and REM sleep deprivation later after extinction learning (6–12 h, orange arrow, different groups) did not have the same effects (From Fu et al. [46], reproduced with kind permission from Elsevier). (b) A whole night of REM sleep deprivation (blue arrows) in young human subjects specifically affected the consolidation of the extinguished stimulus in the REM sleep deprivation (REMD) group, but not of the safety stimulus (or the unextinguished stimulus – not depicted). Note that the trials per task were a priori binned into blocks to reduce analysis flexibility and that a group × time interaction over all blocks had a trend for significance in this small pilot study, suggesting rather large effect sizes (From Spoormaker et al. [47], reproduced with kind permission from John Wiley and Sons)
Quantitative EEG and Neuroimaging in PTSD Patients
The reduction in delta sleep and increase in REM density (and the tendency for REM fragmentation) in PTSD patients are in accord with heightened arousal during sleep; however quantitative EEG findings have not been unequivocal in this regard. High-frequency beta (16–30 Hz) and gamma (>40 Hz) can be taken as indices of central arousal [51], but the handful studies conducted on this topic have shown conflicting results, varying from increased to decreased beta and increased to decreased delta (for a review, see [2]). Quantitative EEG may not be optimal to evaluate group differences due to either an increased amount of artifacts (e.g., different EMG tone in PTSD) or to the fact that differences may be more subcortically pronounced. Moreover, arousals may not constitute a single entity with a well-defined neural pathway; intracranial work in epileptic patients has shown that the neural correlates of arousals widely differ dependent on sleep stage and whether they were spontaneous or nociceptive induced [52]. The thalamus showed more stereotypical responses, but these were related to different patterns of cortical arousals [52].
Other neuroimaging approaches may also be helpful to characterize sleep and understand the neurobiology of REM disruptions in sleep in PTSD patients. In a pilot study employing positron-emission tomography, Ebdlahad et al. observed widespread activity increases in REM sleep in PTSD patients compared to depressed patients, among others, in thalamus, limbic, and paralimbic circuitry [53]. In wakefulness, depressed patients showed increased metabolism in several subcortical and (para)limbic regions. Interestingly, the dorsal anterior cingulate was increased during both wakefulness and REM sleep in PTSD compared to depressed patients [53]. In a small proof-of-concept study, we showed that EEG/fMRI data of subjects falling asleep were also feasible to acquire PTSD patients (N = 6 in both the PTSD group and in the trauma-exposed control group), with extra care to be taken for EEG data quality given a seemingly high number of arousals and EMG events in both groups (Vermetten, van Liempt et al. unpublished data).
Conclusion
Objective readouts are converging that in PTSD, both sleep and wakefulness are characterized by heightened arousal. Sleep characteristics of PTSD are reduced SWS and increased stage 1 sleep and REM density, whereas reduced attention, increased distractibility, and reduced information processing speed may well reflect heightened arousal during wakefulness. Animal and human models of PTSD employing fear extinction have revealed that REM sleep causally affects the ability to consolidate extinction, suggesting that disturbed sleep through increased arousal may play a causal role in the development or maintenance of PTSD. Future work should focus on advanced neuroimaging methodology to more closely examine the effects of noradrenalin and imbalances in subcortical circuitry on sleep and arousal in PTSD.
References
Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707.
Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170:372–82.
Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23:377–407.
Pillar G, Malhotra A, Lavie P. Post-traumatic stress disorder and sleep: what a nightmare! Sleep Med Rev. 2000;4:183–200.
Neylan TC, Marmar CR, Metzler TJ, Weiss DS, Zatzick DF, Delucchi KL, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155:929–33.
Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12:169–84.
Kobayashi I, Boarts JM, Delahanty D. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9.
Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701.
Nappi CM, Drummond SP, Hall JM. Treating nightmares and insomnia in posttraumatic stress disorder: A review of current evidence. Neuropharmacology. 2012;62:576–85.
Krakow B, Hollifield M, Johnston L, Koss M, Schrader R, Warner TD, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. 2001;286:537–45.
Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28(1):403–50.
Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46(9):1309–20.
Geracioti TDJ, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–30.
Wingenfeld K, Whooley MA, Neylan TC, Otte C, Cohen BE. Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the mind your heart study. Psychoneuroendocrinology. 2015;52:83–91.
Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105–40.
Johnson JD, Allana TN, Medlin MD, Harris EW, Karl A. Meta-analytic review of P3 components in posttraumatic stress disorder and their clinical utility. Clin EEG Neurosci. 2013;44(2):112–34.
Shucard JL, McCabe DC, Szymanski H. An event-related potential study of attention deficits in posttraumatic stress disorder during auditory and visual go/NoGo continuous performance tasks. Biol Psychol. 2008;79(2):223–33.
Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–97.
Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–93.
Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–93.
Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38:174–9.
Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–32.
Germain A, Richardson R, Moul DE, Mammen O, Haas G, Forman SD, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res. 2012;72:89–96.
Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–66.
Gottesmann C. The involvement of noradrenaline in rapid eye movement sleep mentation. Front Neurol. 2011;2:81.
Léna I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep – wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–9.
Broese M, Riemann D, Hein L, Nissen C. α-adrenergic receptor function, arousal and sleep: mechanisms and therapeutic implications. Pharmacopsychiatry. 2012;45:209–16.
Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–52.
Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–18.
Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48:940–7.
Zohar J, Juven-Wetzler A, Sonnino R, Cwikel-Hamzany S, Balaban E, Cohen H. New insights into secondary prevention in post-traumatic stress disorder. Dialogues Clin Neurosci. 2011;13:301–9.
van Liempt S, Arends J, Cluitmans PJ, Westenberg HG, Kahn RS, Vermetten E. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;38:155–65.
Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26.
Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48.
Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–63.
Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research – past, present, and future. Biol Psychiatry. 2006;60:376–82.
Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Therapy. 2007;45:2019–33.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82.
Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–92.
Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51.
Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865.
Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–69.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87.
Cohen JE, Shalev H, Admon R, Hefetz S, Gasho CJ, Shachar LJ, et al. Emotional brain rhythms and their impairment in post-traumatic patients. Hum Brain Mapp. 2013;34:1344–56.
Bódizs R, Kántor S, Szabó G, Szûcs A, Erõss L, Halász P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001;11:747–53.
Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, et al. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144:1186–92.
Spoormaker VI, Schröter MS, Andrade KC, Dresler M, Kiem S, Goya-Maldonado R, et al. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp. 2012;33:2362–76.
Popa D, Duvarci S, Popescu AT, Léna C, Paré D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–9.
Datta S, O’Malley MW. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J Neurosci. 2013;33:4561–9.
Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsáki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75:1001–7.
Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34.
Peter-Derex L, Magnin M, Bastuji H. Heterogeneity of arousals in human sleep: a stereo-electroencephalographic study. NeuroImage. 2015;123:229–44.
Ebdlahad S, Nofzinger EA, James JA, Buysse DJ, Price JC, Germain A. Comparing neural correlates of REM sleep in posttraumatic stress disorder and depression: a neuroimaging study. Psychiatry Res Neuroimaging. 2013;214(3):422–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media LLC
About this chapter
Cite this chapter
Spoormaker, V.I. (2018). PTSD, Arousal, and Disrupted (REM) Sleep. In: Vermetten, E., Germain, A., Neylan, T. (eds) Sleep and Combat-Related Post Traumatic Stress Disorder. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-7148-0_19
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7148-0_19
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-7146-6
Online ISBN: 978-1-4939-7148-0
eBook Packages: MedicineMedicine (R0)