Abstract
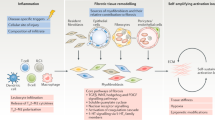
Human fibrotic diseases constitute a major health problem worldwide owing to the large number of affected individuals, the incomplete knowledge of the fibrotic process pathogenesis, the marked heterogeneity in their etiology and clinical manifestations, the absence of appropriate and fully validated biomarkers, and, most importantly, the current void of effective disease-modifying therapeutic agents. The fibrotic disorders encompass a wide spectrum of clinical entities including systemic fibrotic diseases such as systemic sclerosis (SSc), sclerodermatous graft vs. host disease, and nephrogenic systemic fibrosis, as well as numerous organ-specific disorders including radiation-induced fibrosis and cardiac, pulmonary, liver, and kidney fibrosis. Although their causative mechanisms are quite diverse and in several instances have remained elusive, these diseases share the common feature of an uncontrolled and progressive accumulation of fibrotic tissue in affected organs causing their dysfunction and ultimate failure. Despite the remarkable heterogeneity in the etiologic mechanisms responsible for the development of fibrotic diseases and in their clinical manifestations, numerous studies have identified activated myofibroblasts as the common cellular element ultimately responsible for the replacement of normal tissues with nonfunctional fibrotic tissue. Critical signaling cascades, initiated primarily by transforming growth factor-β (TGF-β), but also involving numerous cytokines and signaling molecules which stimulate profibrotic reactions in myofibroblasts, offer potential therapeutic targets. Here, we briefly review the current knowledge of the molecular mechanisms involved in the development of tissue fibrosis and point out some of the most important challenges to research in the fibrotic diseases and to the development of effective therapeutic approaches for this often fatal group of disorders. Efforts to further clarify the complex pathogenetic mechanisms of the fibrotic process should be encouraged to attain the elusive goal of developing effective therapies for these serious, untreatable, and often fatal disorders.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214(2):199–210. doi:10.1002/path.2277
Rosenbloom J, Castro SV, Jimenez SA (2010) Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med 152(3):159–166. doi:10.7326/0003-4819-152-3-201002020-00007
Rockey DC, Bell PD, Hill JA (2015) Fibrosis – a common pathway to organ injury and failure. N Engl J Med 373(1):96. doi:10.1056/NEJMc1504848
Varga J, Abraham D (2007) Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 117(3):557–567. doi:10.1172/jci31139
Gabrielli A, Avvedimento EV, Krieg T (2009) Scleroderma. N Engl J Med 360(19):1989–2003. doi:10.1056/NEJMra0806188
Ho YY, Lagares D, Tager AM et al (2014) Fibrosis – a lethal component of systemic sclerosis. Nat Rev Rheumatol 10(7):390–402. doi:10.1038/nrrheum.2014.53
Cowper SE, Su LD, Bhawan J et al (2001) Nephrogenic fibrosing dermopathy. Am J Dermatopathol 23(5):383–393. doi:10.1111/j.0303-6987.2005.0320e.x
Mendoza FA, Artlett CM, Sandorfi N et al (2006) Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 35(4):238–249. doi:10.1016/j.semarthrit.2005.08.002
Piera-Velazquez S, Mendoza FA, Jimenez SA (2016) Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med 5(4). doi:10.3390/jcm5040045
Jimenez SA, Hitraya E, Varga J (1996) Pathogenesis of scleroderma. Collagen. Rheum Dis Clin N Am 22(4):647–674
Rosenbloom J, Mendoza FA, Jimenez SA (2013) Strategies for anti-fibrotic therapies. Biochim Biophys Acta 1832(7):1088–1103. doi:10.1016/j.bbadis.2012.12.007
Castelino FV, Varga J (2014) Emerging cellular and molecular targets in fibrosis: implications for scleroderma pathogenesis and targeted therapy. Curr Opin Rheumatol 26(6):607–614. doi:10.1097/bor.0000000000000110
Thannickal VJ, Henke CA, Horowitz JC et al (2014) Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the National Heart, Lung, and Blood Institute. Am J Pathol 184(6):1643–1651. doi:10.1016/j.ajpath.2014.02.003
Karsdal MA, Manon-Jensen T, Genovese F et al (2015) Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 308(10):G807–G830. doi:10.1152/ajpgi.00447.2014
Denton CP (2015) Systemic sclerosis: from pathogenesis to targeted therapy. Clin Exp Rheumatol 33(4 Suppl 92):S3–S7
Gabbiani G (1981) The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res 54:183–194
Desmouliere A (1995) Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int 19(5):471–476. doi:10.1006/cbir.1995.1090
McAnulty RJ (2007) Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol 39(4):666–671. doi:10.1016/j.biocel.2006.11.005
Hinz B, Phan SH, Thannickal VJ et al (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170(6):1807–1816. doi:10.2353/ajpath.2007.070112
Hinz B, Phan SH, Thannickal VJ et al (2012) Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180(4):1340–1355. doi:10.1016/j.ajpath.2012.02.004
Hu B, Phan SH (2013) Myofibroblasts. Curr Opin Rheumatol 25(1):71–77. doi:10.1097/BOR.0b013e32835b1352
Kirk TZ, Mark ME, Chua CC et al (1995) Myofibroblasts from scleroderma skin synthesize elevated levels of collagen and tissue inhibitor of metalloproteinase (TIMP-1) with two forms of TIMP-1. J Biol Chem 270(7):3423–3428
Abraham DJ, Eckes B, Rajkumar V et al (2007) New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep 9(2):136–143. doi:10.1007/s11926-007-0008-z
Gilbane AJ, Denton CP, Holmes AM (2013) Scleroderma pathogenesis: a pivotal role for fibroblasts as effector cells. Arthritis Res Ther 15(3):215. doi:10.1186/ar4230
Kendall RT, Feghali-Bostwick CA (2014) Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5:123. doi:10.3389/fphar.2014.00123
Laurent GJ, Chambers RC, Hill MR et al (2007) Regulation of matrix turnover: fibroblasts, forces, factors and fibrosis. Biochem Soc Trans 35(Pt 4):647–651. doi:10.1042/bst0350647
Wells RG, Discher DE (2008) Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal 1(10):pe13. doi:10.1126/stke.110pe13
Hinz B (2009) Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep 11(2):120–126
Parker MW, Rossi D, Peterson M et al (2014) Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124(4):1622–1635. doi:10.1172/jci71386
Rittié L (2015) Another dimension to the importance of the extracellular matrix in fibrosis. J Cell Commun Signal 9(1):99–100. doi:10.1007/s12079-015-0282-x
Postlethwaite AE, Shigemitsu H, Kanangat S (2004) Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol 16(6):733–738
Humphreys BD, Lin SL, Kobayashi A et al (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176(1):85–97. doi:10.2353/ajpath.2010.090517
Duffield JS (2014) Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124(6):2299–2306. doi:10.1172/JCI72267
Kramann R, Humphreys BD (2014) Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol 34(4):374–383. doi:10.1016/j.semnephrol.2014.06.004
Kramann R, Schneider RK, DiRocco DP et al (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16(1):51–66. doi:10.1016/j.stem.2014.11.004
Onuora S (2015) Connective tissue diseases: adipocyte-myofibroblast transition: linking intradermal fat loss to skin fibrosis in SSc. Nat Rev Rheumatol 11(2):63. doi:10.1038/nrrheum.2014.223
Marangoni RG, Korman BD, Wei J et al (2015) Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 67(4):1062–1073. doi:10.1002/art.38990
Kruglikov IL, Scherer PE (2016) Dermal adipocytes: from irrelevance to metabolic targets? Trends Endocrinol Metab 27(1):1–10. doi:10.1016/j.tem.2015.11.002
Martins V, Gonzalez De Los Santos F, Wu Z et al (2015) FIZZ1-induced myofibroblast transdifferentiation from adipocytes and its potential role in dermal fibrosis and lipoatrophy. Am J Pathol 185(10):2768–2776. doi:10.1016/j.ajpath.2015.06.005
Resovi A, Pinessi D, Chiorino G et al (2014) Current understanding of the thrombospondin-1 interactome. Matrix Biol 37:83–91. doi:10.1016/j.matbio.2014.01.012
Murphy-Ullrich JE, Sage EH (2014) Revisiting the matricellular concept. Matrix Biol 37:1–14. doi:10.1016/j.matbio.2014.07.005
Iozzo RV, Schaefer L (2015) Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42:11–55. doi:10.1016/j.matbio.2015.02.003
Kramann R, DiRocco DP, Humphreys BD (2013) Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J Pathol 231(3):273–289
Roberts AB, Sporn MB, Assoian RK et al (1986) Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A 83(12):4167–4171
Sporn MB, Roberts AB (1989) Transforming growth factor-beta. Multiple actions and potential clinical applications. JAMA 262(7):938–941
Santibanez JF, Quintanilla M, Bernabeu C (2011) TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 121(6):233–251. doi:10.1042/cs20110086
Moses HL, Roberts AB, Derynck R (2016) The discovery and early days of TGF-beta: a historical perspective. Cold Spring Harb Perspect Biol 8(7). doi:10.1101/cshperspect.a021865
Fujio K, Komai T, Inoue M et al (2016) Revisiting the regulatory roles of the TGF-beta family of cytokines. Autoimmun Rev 15(9):917–922. doi:10.1016/j.autrev.2016.07.007
Goumans MJ, Liu Z, ten Dijke P (2009) TGF-beta signaling in vascular biology and dysfunction. Cell Res 19(1):116–127. doi:10.1038/cr.2008.326
Medici D, Potenta S, Kalluri R (2011) Transforming growth factor-beta2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J 437(3):515–520. doi:10.1042/bj20101500
van Meeteren LA, ten Dijke P (2012) Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res 347(1):177–186. doi:10.1007/s00441-011-1222-6
Border WA, Noble NA (1994) Transforming growth factor beta in tissue fibrosis. N Engl J Med 331(19):1286–1292. doi:10.1056/nejm199411103311907
Pohlers D, Brenmoehl J, Loffler I et al (2009) TGF-beta and fibrosis in different organs – molecular pathway imprints. Biochim Biophys Acta 1792(8):746–756. doi:10.1016/j.bbadis.2009.06.004
Varga J, Whitfield ML (2009) Transforming growth factor-beta in systemic sclerosis (scleroderma). Front Biosci (Schol Ed) 1:226–235
Jimenez SA, Castro SV, Piera-Velazquez S (2010) Role of growth factors in the pathogenesis of tissue fibrosis in systemic sclerosis. Curr Rheumatol Rev 6(4):283–294
Biernacka A, Dobaczewski M, Frangogiannis NG (2011) TGF-beta signaling in fibrosis. Growth Factors 29(5):196–202. doi:10.3109/08977194.2011.595714
Lafyatis R (2014) Transforming growth factor beta – at the centre of systemic sclerosis. Nat Rev Rheumatol 10(12):706–719. doi:10.1038/nrrheum.2014.137
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 12(6):325–338. doi:10.1038/nrneph.2016.48
Miyazono K, Olofsson A, Colosetti P et al (1991) A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J 10(5):1091–1101
Taipale J, Miyazono K, Heldin CH et al (1994) Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol 124(1–2):171–181
Murphy-Ullrich JE, Poczatek M (2000) Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 11(1–2):59–69
Sheppard D (2005) Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev 24(3):395–402. doi:10.1007/s10555-005-5131-6
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425(6958):577–584. doi:10.1038/nature02006
ten Dijke P, Hill CS (2004) New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29(5):265–273. doi:10.1016/j.tibs.2004.03.008
Moustakas A, Heldin CH (2005) Non-Smad TGF-beta signals. J Cell Sci 118(Pt 16):3573–3584. doi:10.1242/jcs.02554
Wilkes MC, Leof EB (2006) Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem 281(38):27846–27854. doi:10.1074/jbc.M603721200
Jimenez SA, Gaidarova S, Saitta B et al (2001) Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J Clin Invest 108(9):1395–1403. doi:10.1172/jci12347
Bujor AM, Asano Y, Haines P et al (2011) The c-Abl tyrosine kinase controls protein kinase C delta-induced Fli-1 phosphorylation in human dermal fibroblasts. Arthritis Rheum 63(6):1729–1737. doi:10.1002/art.30284
Lawler S, Feng XH, Chen RH et al (1997) The type II transforming growth factor-beta receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem 272(23):14850–14859
Galliher AJ, Schiemann WP (2007) Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res 67(8):3752–3758. doi:10.1158/0008-5472.can-06-3851
Pannu J, Asano Y, Nakerakanti S et al (2008) Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum 58(8):2528–2537. doi:10.1002/art.23698
Caraci F, Gili E, Calafiore M et al (2008) TGF-beta1 targets the GSK-3 beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res 57(4):274–282. doi:10.1016/j.phrs.2008.02.001
Andrianifahanana M, Wilkes MC, Gupta SK et al (2013) Profibrotic TGFbeta responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. FASEB J 27(11):4444–4454. doi:10.1096/fj.12-224907
Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22(4):233–241. doi:10.1080/08977190412331279890
Mulloy B, Rider CC (2015) The bone morphogenetic proteins and their antagonists. Vitam Horm 99:63–90. doi:10.1016/bs.vh.2015.06.004
Bi J, Ge S (2014) Potential roles of BMP9 in liver fibrosis. Int J Mol Sci 15(11):20656–20667. doi:10.3390/ijms151120656
Li RX, Yiu WH, Tang SC (2015) Role of bone morphogenetic protein-7 in renal fibrosis. Front Physiol 6:114. doi:10.3389/fphys.2015.00114
Zeisberg M, Hanai J, Sugimoto H et al (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9(7):964–968. doi:10.1038/nm888
Weiskirchen R, Meurer SK, Gressner OA et al (2009) BMP-7 as antagonist of organ fibrosis. Front Biosci (Landmark Ed) 14:4992–5012
Weiskirchen R, Meurer SK (2013) BMP-7 counteracting TGF-beta1 activities in organ fibrosis. Front Biosci (Landmark Ed) 18:1407–1434
Miyazono K, Kusanagi K, Inoue H (2001) Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 187(3):265–276. doi:10.1002/jcp.1080
Miyazawa K, Shinozaki M, Hara T et al (2002) Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells 7(12):1191–1204
Meng XM, Chung AC, Lan HY (2013) Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 124(4):243–254. doi:10.1042/CS20120252
Miyazono K, Kamiya Y, Morikawa M (2010) Bone morphogenetic protein receptors and signal transduction. J Biochem 147(1):35–51. doi:10.1093/jb/mvp148
Mueller TD, Nickel J (2012) Promiscuity and specificity in BMP receptor activation. FEBS Lett 586(14):1846–1859. doi:10.1016/j.febslet.2012.02.043
Bai S, Shi X, Yang X et al (2000) Smad6 as a transcriptional corepressor. J Biol Chem 275(12):8267–8270
Wang S, Hirschberg R (2004) Bone morphogenetic protein-7 signals opposing transforming growth factor beta in mesangial cells. J Biol Chem 279(22):23200–23206. doi:10.1074/jbc.M311998200
Yan X, Liu Z, Chen Y (2009) Regulation of TGF-beta signaling by Smad7. Acta Biochim Biophys Sin Shanghai 41(4):263–272
Motazed R, Colville-Nash P, Kwan JT et al (2008) BMP-7 and proximal tubule epithelial cells: activation of multiple signaling pathways reveals a novel anti-fibrotic mechanism. Pharm Res 25(10):2440–2446. doi:10.1007/s11095-008-9551-1
Wang S, Hirschberg R (2003) BMP7 antagonizes TGF-beta-dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol 284(5):F1006–F1013. doi:10.1152/ajprenal.00382.2002
Wang SN, Lapage J, Hirschberg R (2001) Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J Am Soc Nephrol 12(11):2392–2399
Wang S, de Caestecker M, Kopp J et al (2006) Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol 17(9):2504–2512. doi:10.1681/ASN.2006030278
Munoz-Felix JM, Gonzalez-Nunez M, Martinez-Salgado C et al (2015) TGF-beta/BMP proteins as therapeutic targets in renal fibrosis. Where have we arrived after 25 years of trials and tribulations? Pharmacol Ther 156:44–58. doi:10.1016/j.pharmthera.2015.10.003
Kessler D, Dethlefsen S, Haase I et al (2001) Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem 276(39):36575–36585. doi:10.1074/jbc.M101602200
Huang X, Yang N, Fiore VF et al (2012) Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47(3):340–348. doi:10.1165/rcmb.2012-0050OC
Hayashida T, Decaestecker M, Schnaper HW (2003) Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17(11):1576–1578. doi:10.1096/fj.03-0037fje
Thorin E, Clozel M (2010) The cardiovascular physiology and pharmacology of endothelin-1. Adv Pharmacol 60:1–26. doi:10.1016/b978-0-12-385061-4.00001-5
Kawanabe Y, Nauli SM (2011) Endothelin. Cell Mol Life Sci 68(2):195–203. doi:10.1007/s00018-010-0518-0
Shi-Wen X, Denton CP, Dashwood MR et al (2001) Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol 116(3):417–425. doi:10.1046/j.1523-1747.2001.01256.x
Xu SW, Howat SL, Renzoni EA et al (2004) Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem 279(22):23098–23103. doi:10.1074/jbc.M311430200
Jing J, Dou TT, Yang JQ et al (2015) Role of endothelin-1 in the skin fibrosis of systemic sclerosis. Eur Cytokine Netw 26(1):10–14. doi:10.1684/ecn.2015.0360
Abraham D, Ponticos M, Nagase H (2005) Connective tissue remodeling: cross-talk between endothelins and matrix metalloproteinases. Curr Vasc Pharmacol 3(4):369–379. doi:10.2174/157016105774329480
Park SH, Saleh D, Giaid A et al (1997) Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med 156(2 Pt 1):600–608. doi:10.1164/ajrccm.156.2.9607123
Ross B, D’Orleans-Juste P, Giaid A (2010) Potential role of endothelin-1 in pulmonary fibrosis: from the bench to the clinic. Am J Respir Cell Mol Biol 42(1):16–20. doi:10.1165/rcmb.2009-0175TR
Kim KK, Chapman HA (2007) Endothelin-1 as initiator of epithelial-mesenchymal transition: potential new role for endothelin-1 during pulmonary fibrosis. Am J Respir Cell Mol Biol 37(1):1–2. doi:10.1165/rcmb.2007-0001ED
Widyantoro B, Emoto N, Nakayama K et al (2010) Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 121(22):2407–2418. doi:10.1161/circulationaha.110.938217
Cipriani P, Di Benedetto P, Ruscitti P et al (2015) The endothelial-mesenchymal transition in systemic sclerosis is induced by endothelin-1 and transforming growth factor-beta and may be blocked by macitentan, a dual endothelin-1 receptor antagonist. J Rheumatol 42(10):1808–1816. doi:10.3899/jrheum.150088
Wermuth PJ, Li Z, Jimenez SA (2016) Stimulation of TGF-β1-induced endothelial-to-mesenchymal transition and tissue fibrosis by endothelin-1 (ET-1): a novel profibrotic effect of ET-1. PLoS One 11(9):e0161988. doi:10.1371/journal.pone.0161988
Igarashi A, Nashiro K, Kikuchi K et al (1995) Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J Invest Dermatol 105(2):280–284
Grotendorst GR (1997) Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev 8(3):171–179
Sato S, Nagaoka T, Hasegawa M et al (2000) Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol 27(1):149–154
Leask A, Abraham DJ (2003) The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol 81(6):355–363. doi:10.1139/o03-069
Shi-Wen X, Leask A, Abraham D (2008) Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev 19(2):133–144. doi:10.1016/j.cytogfr.2008.01.002
Ponticos M, Holmes AM, Shi-wen X et al (2009) Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum 60(7):2142–2155. doi:10.1002/art.24620
Ruperez M, Rodrigues-Diez R, Blanco-Colio LM et al (2007) HMG-CoA reductase inhibitors decrease angiotensin II-induced vascular fibrosis: role of RhoA/ROCK and MAPK pathways. Hypertension 50(2):377–383. doi:10.1161/hypertensionaha.107.091264
Betsholtz C (2003) Biology of platelet-derived growth factors in development. Birth Defects Res C Embryo Today 69(4):272–285. doi:10.1002/bdrc.10030
Alvarez RH, Kantarjian HM, Cortes JE (2006) Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc 81(9):1241–1257. doi:10.4065/81.9.1241
Farooqi AA, Waseem S, Riaz AM et al (2011) PDGF: the nuts and bolts of signalling toolbox. Tumour Biol 32(6):1057–1070. doi:10.1007/s13277-011-0212-3
Bonner JC (2004) Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15(4):255–273. doi:10.1016/j.cytogfr.2004.03.006
Yamakage A, Kikuchi K, Smith EA et al (1992) Selective upregulation of platelet-derived growth factor alpha receptors by transforming growth factor beta in scleroderma fibroblasts. J Exp Med 175(5):1227–1234
Tallquist M, Kazlauskas A (2004) PDGF signaling in cells and mice. Cytokine Growth Factor Rev 15(4):205–213. doi:10.1016/j.cytogfr.2004.03.003
Olson LE, Soriano P (2009) Increased PDGFR alpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16(2):303–313. doi:10.1016/j.devcel.2008.12.003
Czochra P, Klopcic B, Meyer E et al (2006) Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol 45(3):419–428. doi:10.1016/j.jhep.2006.04.010
Ogawa S, Ochi T, Shimada H et al (2010) Anti-PDGF-B monoclonal antibody reduces liver fibrosis development. Hepatol Res 40(11):1128–1141. doi:10.1111/j.1872-034X.2010.00718.x
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316
Lam AP, Flozak AS, Russell S et al (2011) Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol 45(5):915–922. doi:10.1165/rcmb.2010-0113OC
Beyer C, Schramm A, Akhmetshina A et al (2012) Beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71(5):761–767. doi:10.1136/annrheumdis-2011-200568
Niehrs C (2012) The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13(12):767–779. doi:10.1038/nrm3470
Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
Wei J, Fang F, Lam AP et al (2012) Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum 64(8):2734–2745. doi:10.1002/art.34424
Bergmann C, Distler JH (2016) Canonical Wnt signaling in systemic sclerosis. Lab Investig 96(2):151–155. doi:10.1038/labinvest.2015.154
Nusse R (2005) Wnt signaling in disease and in development. Cell Res 15(1):28–32. doi:10.1038/sj.cr.7290260
Huang H, He X (2008) Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol 20(2):119–125. doi:10.1016/j.ceb.2008.01.009
Konigshoff M, Balsara N, Pfaff EM et al (2008) Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One 3(5):e2142. doi:10.1371/journal.pone.0002142
He W, Dai C, Li Y et al (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20(4):765–776. doi:10.1681/asn.2008060566
He W, Zhang L, Ni A et al (2010) Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A 107(49):21110–21115. doi:10.1073/pnas.1004708107
Trensz F, Haroun S, Cloutier A et al (2010) A muscle resident cell population promotes fibrosis in hind limb skeletal muscles of mdx mice through the Wnt canonical pathway. Am J Physiol Cell Physiol 299(5):C939–C947. doi:10.1152/ajpcell.00253.2010
Wei J, Melichian D, Komura K et al (2011) Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum 63(6):1707–1717. doi:10.1002/art.30312
Bafico A, Liu G, Yaniv A et al (2001) Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/arrow. Nat Cell Biol 3(7):683–686. doi:10.1038/35083081
Pinzone JJ, Hall BM, Thudi NK et al (2009) The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113(3):517–525. doi:10.1182/blood-2008-03-145169
Echelard Y, Epstein DJ, St-Jacques B et al (1993) Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75(7):1417–1430. doi:10.1016/0092-8674(93)90627-3
Rohatgi R, Milenkovic L, Corcoran RB et al (2009) Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci U S A 106(9):3196–3201. doi:10.1073/pnas.0813373106
Rohatgi R, Scott MP (2007) Patching the gaps in hedgehog signalling. Nat Cell Biol 9(9):1005–1009. doi:10.1038/ncb435
Xie J, Murone M, Luoh SM et al (1998) Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391(6662):90–92. doi:10.1038/34201
Dahmane N, Sanchez P, Gitton Y et al (2001) The sonic hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development 128(24):5201–5212
Thayer SP, di Magliano MP, Heiser PW et al (2003) Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425(6960):851–856. doi:10.1038/nature02009
Horn A, Palumbo K, Cordazzo C et al (2012) Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum 64(8):2724–2733. doi:10.1002/art.34444
D’Souza B, Miyamoto A, Weinmaster G (2008) The many facets of Notch ligands. Oncogene 27(38):5148–5167. doi:10.1038/onc.2008.229
Fortini ME (2009) Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16(5):633–647. doi:10.1016/j.devcel.2009.03.010
Borggrefe T, Liefke R (2012) Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle 11(2):264–276. doi:10.4161/cc.11.2.18995
Dees C, Tomcik M, Zerr P et al (2011) Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis 70(7):1304–1310. doi:10.1136/ard.2010.134742
Kavian N, Servettaz A, Weill B et al (2012) New insights into the mechanism of notch signalling in fibrosis. Open Rheumatol J 6:96–102. doi:10.2174/1874312901206010096
Louvi A, Artavanis-Tsakonas S (2012) Notch and disease: a growing field. Semin Cell Dev Biol 23(4):473–480. doi:10.1016/j.semcdb.2012.02.005
Distler A, Lang V, Del Vecchio T et al (2014) Combined inhibition of morphogen pathways demonstrates additive antifibrotic effects and improved tolerability. Ann Rheum Dis 73(6):1264–1268. doi:10.1136/annrheumdis-2013-204221
Beyer C, Schett G, Gay S et al (2009) Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res Ther 11(2):220. doi:10.1186/ar2598
Haase VH (2009) Pathophysiological consequences of HIF activation: HIF as a modulator of fibrosis. Ann N Y Acad Sci 1177:57–65. doi:10.1111/j.1749-6632.2009.05030.x
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148(3):399–408. doi:10.1016/j.cell.2012.01.021
Lokmic Z, Musyoka J, Hewitson TD et al (2012) Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol 296:139–185. doi:10.1016/b978-0-12-394307-1.00003-5
Distler JH, Jungel A, Pileckyte M et al (2007) Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum 56(12):4203–4215. doi:10.1002/art.23074
Sanchez-Elsner T, Botella LM, Velasco B et al (2001) Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem 276(42):38527–38535. doi:10.1074/jbc.M104536200
Higgins DF, Kimura K, Bernhardt WM et al (2007) Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117(12):3810–3820. doi:10.1172/jci30487
Liu RM, Desai LP (2015) Reciprocal regulation of TGF-beta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 6:565–577. doi:10.1016/j.redox.2015.09.009
Richter K, Konzack A, Pihlajaniemi T et al (2015) Redox-fibrosis: impact of TGFbeta1 on ROS generators, mediators and functional consequences. Redox Biol 6:344–352. doi:10.1016/j.redox.2015.08.015
Kliment CR, Oury TD (2010) Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 49(5):707–717. doi:10.1016/j.freeradbiomed.2010.04.036
Siani A, Tirelli N (2014) Myofibroblast differentiation: main features, biomedical relevance, and the role of reactive oxygen species. Antioxid Redox Signal 21(5):768–785. doi:10.1089/ars.2013.5724
Bourji K, Meyer A, Chatelus E et al (2015) High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic Biol Med 87:282–289. doi:10.1016/j.freeradbiomed.2015.07.002
Cucoranu I, Clempus R, Dikalova A et al (2005) NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97(9):900–907. doi:10.1161/01.res.0000187457.24338.3d
Hecker L, Vittal R, Jones T et al (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15(9):1077–1081. doi:10.1038/nm.2005
Amara N, Goven D, Prost F et al (2010) NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65(8):733–738. doi:10.1136/thx.2009.113456
Barnes JL, Gorin Y (2011) Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79(9):944–956. doi:10.1038/ki.2010.516
Crestani B, Besnard V, Boczkowski J (2011) Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 43(8):1086–1089. doi:10.1016/j.biocel.2011.04.003
Sancho P, Mainez J, Crosas-Molist E et al (2012) NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One 7(9):e45285. doi:10.1371/journal.pone.0045285
Jiang F, Liu GS, Dusting GJ et al (2014) NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol 2:267–272. doi:10.1016/j.redox.2014.01.012
Paik YH, Kim J, Aoyama T et al (2014) Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal 20(17):2854–2872. doi:10.1089/ars.2013.5619
Piera-Velazquez S, Makul A, Jimenez SA (2015) Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor beta. Arthritis Rheumatol 67(10):2749–2758. doi:10.1002/art.39242
Spadoni T, Svegliati Baroni S, Amico D et al (2015) A reactive oxygen species-mediated loop maintains increased expression of NADPH oxidases 2 and 4 in skin fibroblasts from patients with systemic sclerosis. Arthritis Rheumatol 67(6):1611–1622. doi:10.1002/art.39084
Wang Y, Stricker HM, Gou D et al (2007) MicroRNA: past and present. Front Biosci 12:2316–2329
Jiang X, Tsitsiou E, Herrick SE et al (2010) MicroRNAs and the regulation of fibrosis. FEBS J 277(9):2015–2021. doi:10.1111/j.1742-4658.2010.07632.x
Bowen T, Jenkins RH, Fraser DJ (2013) MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J Pathol 229(2):274–285. doi:10.1002/path.4119
Rutnam ZJ, Wight TN, Yang BB (2013) miRNAs regulate expression and function of extracellular matrix molecules. Matrix Biol 32(2):74–85. doi:10.1016/j.matbio.2012.11.003
Zhu H, Luo H, Zuo X (2013) MicroRNAs: their involvement in fibrosis pathogenesis and use as diagnostic biomarkers in scleroderma. Exp Mol Med 45:e41. doi:10.1038/emm.2013.71
Babalola O, Mamalis A, Lev-Tov H et al (2013) The role of microRNAs in skin fibrosis. Arch Dermatol Res 305(9):763–776. doi:10.1007/s00403-013-1410-1
Pandit KV, Milosevic J (2015) MicroRNA regulatory networks in idiopathic pulmonary fibrosis. Biochem Cell Biol 93(2):129–137. doi:10.1139/bcb-2014-0101
He Y, Huang C, Zhang SP et al (2012) The potential of microRNAs in liver fibrosis. Cell Signal 24(12):2268–2272. doi:10.1016/j.cellsig.2012.07.023
Srivastava SP, Koya D, Kanasaki K (2013) MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT and EndMT. Biomed Res Int 2013:125469. doi:10.1155/2013/125469
Thum T (2014) Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol 11(11):655–663. doi:10.1038/nrcardio.2014.125
Razani B, Zhang XL, Bitzer M et al (2001) Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem 276(9):6727–6738. doi:10.1074/jbc.M008340200
Del Galdo F, Lisanti MP, Jimenez SA (2008) Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol 20(6):713–719
Gvaramia D, Blaauboer ME, Hanemaaijer R et al (2013) Role of caveolin-1 in fibrotic diseases. Matrix Biol 32(6):307–315. doi:10.1016/j.matbio.2013.03.005
Del Galdo F, Sotgia F, de Almeida CJ et al (2008) Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum 58(9):2854–2865. doi:10.1002/art.23791
Wang XM, Zhang Y, Kim HP et al (2006) Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 203(13):2895–2906. doi:10.1084/jem.20061536
Tourkina E, Richard M, Gooz P et al (2008) Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 294(5):L843–L861. doi:10.1152/ajplung.00295.2007
Jasmin JF, Mercier I, Dupuis J et al (2006) Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 114(9):912–920. doi:10.1161/circulationaha.106.634709
Li Z, Wermuth PJ, Benn BS et al (2013) Caveolin-1 deficiency induces spontaneous endothelial-to-mesenchymal transition in murine pulmonary endothelial cells in vitro. Am J Pathol 182(2):325–331. doi:10.1016/j.ajpath.2012.10.022
Acknowledgments
Partially supported by NIH grant AR 19616 to SAJ. We thank the expert assistance of Alana Pagano and Carol Kelly in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this protocol
Cite this protocol
Rosenbloom, J., Macarak, E., Piera-Velazquez, S., Jimenez, S.A. (2017). Human Fibrotic Diseases: Current Challenges in Fibrosis Research. In: Rittié, L. (eds) Fibrosis. Methods in Molecular Biology, vol 1627. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-7113-8_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7113-8_1
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7112-1
Online ISBN: 978-1-4939-7113-8
eBook Packages: Springer Protocols