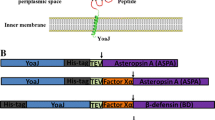
Abstract
Recombinant expression of heterologous proteins in E. coli is well established for a wide range of proteins, although in many cases, purifying soluble and properly folded proteins remains challenging (Sorensen and Mortensen, J Biotechnol 115:113–128, 2005; Correa and Oppezzo, Methods Mol Biol 1258:27–44, 2015). Proteins that contain disulfide bonds (e.g., cytokines, growth factors) are often particularly difficult to purify in soluble form and still need optimizing of protocols in almost every step of the process (Berkmen, Protein Expr Purif 82:240–251, 2012; de Marco, Microb Cell Fact 11:129, 2012). Expression of disulfide bonded proteins in the periplasm of E. coli is one approach that can help to obtain soluble protein with the correct disulfide bridges forming in the periplasm. This offers the appropriate conditions for disulfide formation although periplasmic expression can also result in low expression levels and incorrect folding of the target protein (Schlapschy and Skerra, Methods Mol Biol 705:211–224, 2011). Generation of specific antibodies often requires a specific antigenic sequence of a protein in order to get an efficient immune response and minimize cross-reactivity of antibodies. Larger proteins like GST (Glutathione-S-transferase) or MBP (maltose binding protein) as solubilizing fusion partners are frequently used to keep antigens soluble and immunize animals. This approach has the disadvantage that the immune response against the fusion partner leads to additional antibodies that need to be separated from the antigen-specific antibodies. For both classes of proteins mentioned above, a protocol has been developed and optimized using the human version of small ubiquitin-like modifier 3 (SUMO3) protein and its corresponding protease SenP2. This chapter describes the experimental steps for expression, purification, refolding, and cleavage that are applicable to both disulfide-bonded proteins with a defined structure and random protein fragments for antibody generation or larger peptides with defined sequence that are difficult express on their own.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sorensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115:113–128
Correa A, Oppezzo P (2015) Overcoming the solubility problem in E. coli: available approaches for recombinant protein production. Methods Mol Biol 1258:27–44
Berkmen M (2012) Production of disulphide-bonded proteins in Escherichia coli. Protein Expr Purif 82:240–251
de Marco A (2012) Recent contributions in the field of the recombinant expression of disulphide bonded proteins in bacteria. Microb Cell Fact 11:129
Schlapschy M, Skerra A (2011) Periplasmic chaperones used to enhance functional secretion of proteins in E. coli. Methods Mol Biol 705:211–224
Panavas T, Sanders C, Butt TR (2009) SUMO fusion technology for enhanced protein production in prokaryotic and eukaryotic expression systems. Methods Mol Biol 497:303–317
Reverter D, Lima CD (2006) Structural basis for SENP2 protease interactions with SUMO precursors and conjugated substrates. Nat Struct Mol Biol 13:1060–1068
Mukhopadhyay D, Dasso M (2007) Modification in reverse: the SUMO proteases. Trends Biochem Sci 32:286–295
Marblestone JG, Edavettal SC, Lim Y et al (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15:182–189
Peciak K, Tommasi R, Choi J et al (2014) Expression of soluble and active interferon consensus in SUMO fusion expression system in E. coli. Protein Expr Purif 99:18–26
Hoffmann A, Müller MQ, Gloser M et al (2010) Recombinant production of bioactive human TNF-alpha by SUMO-fusion system—high yields from shake-flask culture. Protein Expr Purif 72:238–243
Lu Q, Burns MC, McDevitt PJ et al (2009) Optimized procedures for producing biologically active chemokines. Protein Expr Purif 65:251–260
Dümmler A, Lawrence A-M, de Marco A (2005) Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb Cell Fact 4:34
Scholz J, Besir H, Strasser C et al (2013) A new method to customize protein expression vectors for fast, efficient and background free parallel cloning. BMC Biotechnol 13:12
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this protocol
Cite this protocol
Besir, H. (2017). A Generic Protocol for Purifying Disulfide-Bonded Domains and Random Protein Fragments Using Fusion Proteins with SUMO3 and Cleavage by SenP2 Protease. In: Burgess-Brown, N. (eds) Heterologous Gene Expression in E.coli. Methods in Molecular Biology, vol 1586. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-6887-9_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6887-9_9
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-6885-5
Online ISBN: 978-1-4939-6887-9
eBook Packages: Springer Protocols