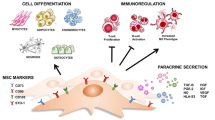
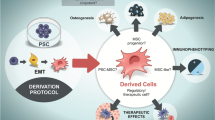
Abstract
Mesenchymal stem cells (MSCs) provide an opportunity to bring the field of regenerative medicine to realization. A lot of clinical trials are presently trying to establish their applicability in real-world scenarios. Some of the biggest challenges encountered in bringing MSCs from bench to bedside are the number of MSCs required, their procurement from various sources, and the batch-to-batch variability. This often leads to inconclusive results within and between different studies. Therefore, we have hereby proposed a simple protocol to source mesenchymal stem cells through differentiation of embryonic stem cells.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
de Wert G, Mummery C (2003) Human embryonic stem cells: research, ethics and policy. Hum Reprod 18(4):672–682
Orlando G, Gianello P, Salvatori M, Stratta RJ, Soker S, Ricordi C, Dominguez-Bendala J (2014) Cell replacement strategies aimed at reconstitution of the beta-cell compartment in type 1 diabetes. Diabetes 63(5):1433–1444. doi:10.2337/db13-1742
Wang X, Lazorchak AS, Song L, Li E, Zhang Z, Jiang B, Xu RH (2015) Immune modulatory mesenchymal stem cells derived from human embryonic stem cells through a trophoblast-like stage. Stem Cells 34(2):380–391. doi:10.1002/stem.2242
Baraniak PR, McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regen Med 5(1):121–143. doi:10.2217/rme.09.74
Law S, Chaudhuri S (2013) Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells 2(1):22–38
Jung Y, Bauer G, Nolta JA (2012) Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells 30(1):42–47. doi:10.1002/stem.727
Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT (2014) Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med 12:8. doi:10.1186/1479-5876-12-8
Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J (2012) Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy 14(2):147–152. doi:10.3109/14653249.2011.623691
Can A, Balci D (2011) Isolation, culture, and characterization of human umbilical cord stroma-derived mesenchymal stem cells. Methods Mol Biol 698:51–62. doi:10.1007/978-1-60761-999-4_5
Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE (2006) Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108(6):2114–2120. doi:10.1182/blood-2005-11-011650
Lin Y, Hogan WJ (2011) Clinical application of mesenchymal stem cells in the treatment and prevention of graft-versus-host disease. Adv Hematol 2011:427863. doi:10.1155/2011/427863
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this protocol
Cite this protocol
Sequiera, G.L., Sareen, N., El-Rub, E.A., Dhingra, S. (2017). Derivation of Mesenchymal Stem Cells from Embryonic Stem Cells: A Non-Variable and Inexhaustive Source of Adult Stem Cells. In: Di Nardo, P., Dhingra, S., Singla, D. (eds) Adult Stem Cells. Methods in Molecular Biology, vol 1553. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-6756-8_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6756-8_2
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-6754-4
Online ISBN: 978-1-4939-6756-8
eBook Packages: Springer Protocols