Abstract
The bacille Calmette–Guérin (BCG) vaccine is one of the oldest and most widely used vaccines. The demonstration of its protective efficacy against tuberculosis (TB) in historical British Medical Research Council trials led to routine BCG vaccination in many parts of the world. Today, BCG vaccination policies vary across countries. BCG given once only at birth is the vaccination schedule currently practiced in most countries. In response to declining TB incidence rates, a number of countries have shifted from universal to selective BCG vaccination. Many countries have stopped BCG revaccination as evidence accumulates over its failure to confer additional protection. BCG vaccination of the HIV-infected is contraindicated, although neonatal BCG vaccination in TB-endemic countries may be continued until vaccination in HIV-exposed infants can be selectively deferred. Although BCG has demonstrated substantial protection against serious forms of TB, its protective effect against pulmonary TB is partial and variable. Without better vaccines and improved diagnostic and treatment tools, it may be difficult to meet the TB-related Millennium Development Goals. Concerted efforts are required to integrate scientific advancement and political mobilization in vaccine development, clinical trials, and field implementation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Bacille Calmette–Guérin (BCG) vaccine
- Selective vaccination
- HIV infection
- Efficacy
- Healthcare workers (HCW)
1 Historical Background
Bacille Calmette–Guérin (BCG), a live attenuated vaccine, is one of the oldest vaccines in the world. The original BCG strain was lost during World War I (1914–1918) (Lagrange 1984). Derived from a virulent strain of Mycobacterium bovis through 230 subcultures over 13 years by French investigators Calmette and Guérin (Sakula 1983), the first dose of BCG was orally given to a human volunteer in 1921 (Calmette 1931a; Weill-Hallé and Turpin 1925). The safety of BCG was severely challenged when contaminated BCG killed 72 children in Germany from 1929 to 1930 (Calmette 1931b; Lange; 1930; Moegling; 1935).
With early evidence for efficacy among student nurses in Norway (Heimbeck 1936), BCG was increasingly used in Europe. The demonstration of high efficacy of BCG against tuberculosis (TB) in trials initiated by the British Medical Research Council (BMRC) in the 1950s led to routine BCG vaccination in the majority of the world (WHO 1972; Hart and Sutherland 1977). Since BCG was incorporated into the Expanded Program on Immunization’s (EPI) infant vaccination schedule in 1974, use of BCG has globally expanded.
Owing to the lack of protection shown in BCG trials conducted by the United States Public Health Service (USPHS) in the early 1950s (Comstock and Palmer 1966), the USA has adopted a policy of selective BCG vaccination among high-risk populations and based their TB control strategy partly on rapid diagnosis and early treatment of TB disease and partly on preventive treatment of infected individuals (CDC 1996). The Netherlands has followed suit.
2 Global Use of BCG
As the only licensed vaccine against TB, BCG is one of the most widely used vaccines in the world. In the 1990s, approximately 100 million children received BCG vaccine every year (WHO 1997). Based on WHO/UNICEF estimates , global coverage of BCG vaccination during infancy further increased from 81 % in 1990 to 88 % in 2009 (WHO 2010b). More than 120 million doses are now used every year (Ritz et al. 2008).
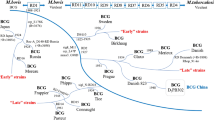
BCG vaccination policies vary across different countries by the BCG strain (Ritz and Curtis 2009) as well as the vaccination schedule (Brewer et al. 1995). Four BCG strains account for more than 90 % of the vaccines currently in use: Pasteur-1173 P2, Copenhagen-1331, Glaxo-1077, and Tokyo-172. The Pasteur strain is currently the international reference strain of vaccine (Milstien and Gibson 1990). BCG vaccination schedules can be classified into four main groups (Fine et al. 1999): vaccination once only at birth, vaccination once only in childhood or adolescence, multiple doses involving boosters, and selective vaccination among high-risk groups. Vaccination once only at birth is the schedule currently recommended by the WHO EPI) and practiced in most countries. A study by Ritz and Curtis found that 44 % of countries reportedly used more than one BCG strain within a 5-year period (Ritz and Curtis 2009). To help clinical interpretation of diagnostic tests as well as the design and evaluation of new TB vaccines, Zwerling and coworkers have created the first searchable online database of global BCG vaccination policies and practices which also captures any applicable changes (Zwerling et al. 2011).
BCG vaccination policies have changed with TB incidence. To improve cost-effectiveness, nine countries have shifted from universal to selective BCG vaccination in response to declining TB incidence rates: Spain in 1981; Denmark in 1986; Austria in 1990; Germany in 1998; and Isle of Man, Slovenia, UK, Finland, and France between 2005 and 2007 (Zwerling et al. 2011). According to the International Union against Tuberculosis and Lung Disease (IUATLD) , universal BCG vaccination may be stopped when an efficient notification system is in place with one of the following conditions: (1) the average annual notification rate of smear-positive pulmonary TB is less than 5 per 100,000, (2) the average annual notification rate of tuberculous meningitis in children under 5 years of age is less than 1 per 10 million population over the previous 5 years, or (3) the average annual risk of tuberculous infection is less than 0.1 % (IUATLD 1994).
Over 30 countries have stopped BCG revaccination as increasing evidence demonstrated lack of additional protection (Zwerling et al. 2011).
BCG vaccination policies have also changed as a result of HIV infection. Being live attenuated, BCG can cause invasive and disseminated BCG disease among the immunocompromised, especially HIV-infected subjects. Before 2007, WHO recommended routine vaccination in TB-endemic countries in the absence of symptoms of HIV infection (WHO 2004). Since 2007, in response to evidence of an unacceptably higher risk of disseminated BCG disease among HIV-infected vaccinees (WHO 2007a), WHO has recommended that BCG vaccination of HIV-infected children be discontinued in TB-endemic countries (WHO 2007b). The IUATLD has suggested that neonatal BCG vaccination in TB-endemic areas be continued until programs are in place for selective deferral of BCG vaccination in infants exposed to HIV (Hesseling et al. 2008).
3 Efficacy of BCG
Most of the variations regarding global use of BCG across different countries may have stemmed from the partial and variable efficacy of BCG.
3.1 Initial Experience and Historical Cohort Trials
With preliminary evidence for the utility of BCG in protecting against TB among student nurses in the 1930s (Heimbeck 1936) and American Indians in the 1940s (Aronson 1948a, b), BMRC and USPHS set up major trials in the early 1950s to further evaluate the efficacy of BCG against TB. Instead of drawing conclusive evidence for the protective efficacy of BCG, BMRC and USPHS demonstrated completely opposing findings. The use of Copenhagen strain against tuberculin-negative adolescents in BMRC trials efficaciously protected against TB, whereas the Park or Tice strains given to tuberculin-negative subjects by the USPHS demonstrated little protection. Two hypotheses were put forward to explain the discrepancy. One attributed the differences to variation between BCG strains (Hart 1967). The other considered environmental factors, especially environmental mycobacteria (Palmer and Long 1966). To evaluate these hypotheses, a large trial involving all age groups started in 1968 to compare two well-established BCG strains (“Paris/Pasteur” vs. “Danish/Copenhagen ”) in the Chingleput area of South India, where there is a high prevalence of environmental mycobacteria. A companion trial in an area in northern India with little environmental mycobacterial exposure was conceived but unfortunately aborted due to political unrest. The Chingleput trial revealed no evidence for the efficacy of either vaccine against pulmonary TB (Baily 1980; ICMR/WHO Scientific Group 1980). This set the scene for a series of clinical trials (WHO 1972; Bettag et al. 1964; Comstock et al. 1974, 1976; Comstock and Webster 1969; Frimodt-Moller et al. 1973; Rosenthal et al. 1961; Stein and Aronson 1953; Tripathy 1987; Vandiviere et al. 1973) and observational studies (Blin et al. 1986; Camargos et al. 1988; Canetti et al. 1972; Jin et al. 1989; Miceli et al. 1988; Murtagh 1980; Orege et al. 1993; Padungchan et al. 1986; Pönnighaus et al. 1992; Rodrigues et al. 1991; Shapiro et al. 1985; Tidjani et al. 1986; Wunsch Filho et al. 1990) that evaluated the efficacy of BCG in different populations of the world .
3.2 Efficacy and Impact of BCG Vaccination
Most studies were conducted among participants initially vaccinated during infancy and young childhood rather than adulthood. Vaccine efficacy rates are generally greatest within the few years following vaccination and most consistent against serious forms of TB in infants and young children (CDC 1996; Rieder 2008). Data are too few for evaluating the protective efficacy of BCG against other forms of extrapulmonary TB. On the other hand, the protective efficacy of BCG vaccination against pulmonary TB is highly heterogeneous with efficacy estimates ranging from below 0 % to approximately 80 % across clinical trials and observational studies (WHO 1972; Bettag et al. 1964; Blin et al. 1986; Comstock et al. 1974, 1976; Comstock and Webster 1969; Frimodt-Moller et al. 1973; Miceli et al. 1988; Orege et al. 1993; Putrali et al. 1983; Pönnighaus et al. 1992; Rodrigues et al. 1991; Shapiro et al. 1985; Stein and Aronson 1953; Tripathy 1987; Vandiviere et al. 1973).
The protective efficacy of BCG against TB has been evaluated by several meta-analyses (Brewer 2000; Colditz et al. 1994, 1995). For BCG vaccination during infancy, the summary protective efficacy was 65 % (95 % confidence interval (CI) 12–86 %) against death from TB, 64 % (95 % CI, 30–82 %) against TB meningitis, 78 % (95 % CI 58–88 %) against disseminated TB, 83 % (95 % CI 58–93 %) against laboratory-confirmed TB, 74 % (95 % CI, 62–83 %) against any TB case when estimated from randomized controlled trials, and 52 % (95 % CI 38–64 %) against any TB case when estimated from case–control studies (Colditz et al. 1995). These findings corroborated summary protective effects of BCG against severe forms of TB demonstrated by another meta-analysis (Rodrigues et al. 1993): 86 % (95 % CI 65–95 %) against miliary or meningeal TB according to randomized controlled trials and 75 % (95 % CI 61–84 %) according to case–control studies.
Assuming that TB meningitis is approximately 1 % of the annual risk of infection, it has been estimated that every 12,500–16,667 BCG vaccinations during infancy will prevent one case of TB meningitis among children under 5 years (Fine et al. 1999). Assuming an annual risk of infection from 0.5 to 1 %, a risk of primary disease from 1 to 5 %, and that BCG vaccination in infancy confers 50 % protection against childhood TB, it has been estimated that 267–2667 vaccinations will prevent one case of childhood TB (Fine et al. 1999).
Observed findings regarding BCG efficacy may suggest that BCG is particularly effective in preventing hematogenous spread of Mycobacterium tuberculosis (Balasubramanian et al. 1994; Marsh et al. 1997), but not so efficient in preventing the establishment of lung infection following exposure (Sutherland and Lindgren 1979). However, there is increasing evidence from observational studies that BCG can also reduce the risk of infection (Diel et al. 2011; Eisenhut et al. 2009; Eriksen et al. 2010; Leung et al. 2015; Roy et al. 2014; Soysal et al. 2005) and aid bacillary clearance during treatment of pulmonary disease (Jeremiah et al. 2010).
Besides differences in methodology (Rieder 2008), a number of hypotheses have been proposed to explore the highly variable efficacy of BCG against pulmonary TB (Fine et al. 1999; Lambert et al. 2009; Rieder 2008). These include differences in the BCG vaccine, the virulence between M. tuberculosis strains, and the stages in the TB epidemic. There are also variations in host factors such as nutritional status, exposure to environmental mycobacteria, ultraviolet light exposure, and genetics underlying susceptibility. Table 20.1 summarizes the arguments for and against such hypotheses. Although there is still no consensus, partial protection conferred by environmental mycobacterial exposure may offer a biologically plausible explanation that partly addresses why BCG efficacy tends to be higher in temperate regions than tropical regions and, in particular, rural areas with greater exposure to environmental mycobacteria.
3.3 Efficacy of BCG Revaccination
In addition to neonatal BCG vaccination , many countries have a tradition of repeated BCG vaccination (Fine et al. 1999). Some administer multiple doses of BCG at infancy, school entry, and graduation, whereas some (as in Hungary and Russia) have recommended up to five doses of BCG from birth to 30 years of age. Although it is debatable whether the protective effect of BCG against pulmonary disease may last more than 15 years after vaccination (Sterne et al. 1998), studies in Malawi, Brazil, Chile, Hong Kong, and other countries have suggested the lack of additional protection from BCG boosters (Karonga Prevention Trial Group 1996; Dantas et al. 2006; Rodrigues et al. 2005; Sepulveda et al. 1992; Springett and Sutherland 1994; Tala-Heikkilä et al. 1998). Over 30 countries have ceased BCG revaccination, whereas 16 still administer BCG boosters (Zwerling et al. 2011). A lack of additional protection from BCG revaccination may be partly explained by a diluting effect from continuous exposure to TB and environmental mycobacteria (Fine 1995).
3.4 Efficacy of BCG Vaccination Among Healthcare Workers (HCW)
It is contentious whether BCG may confer appreciable protection against TB among HCW. Assuming a workplace incidence of TB infection greater than 0.06 % per year, a decision analysis involving tuberculin-negative HCW showed that treatment of LTBI decreased the number of TB cases by 9 %, whereas BCG vaccination decreased the number by 49 % (Marcus et al. 1997). Another decision analysis among HCW exposed to multidrug-resistant TB (MDR-TB) also suggested that BCG was beneficial (Stevens and Daniel 1996). However, a review by Brewer and Colditz (1995) suggested that methodological flaws in the published literature would preclude a meta-analysis of efficacy of BCG among HCW. The United States CDC has suggested that BCG be considered among tuberculin-negative HCW when there is a high risk of exposure to multidrug-resistant TB and the risk cannot be effectively contained by comprehensive TB infection control measures (CDC 1997).
3.5 Efficacy of BCG Against Other Mycobacterial Diseases
BCG has been shown to confer protection against leprosy (Abel et al. 1990; Brown et al. 1968; Fine 1988; Fine et al. 1986; Lwin et al. 1985). The cessation of BCG vaccination was followed by a higher incidence of peripheral lymphadenitis due to environmental mycobacteria in Sweden (Romanus et al. 1995) and M. avium in the Czech Republic (Trnka et al. 1994). It has been postulated that infection with one species of mycobacterium triggers a cellular immune response that acts more swiftly in killing other mycobacteria subsequently encountered.
4 Need for Better Vaccines
BCG is insufficient for protecting against pulmonary TB, which contributes to the main public health burden of TB. A highly efficacious vaccine may be required for improving TB treatment efficacy and combating drug-resistant TB . HIV infection has fuelled the TB epidemic. Matched case–control studies suggest that HIV infection may abrogate the protective effect of BCG against extrapulmonary TB (Arbeláez et al. 2000). A safer and more effective TB vaccine is needed for HIV-infected subjects.
Four types of candidate TB vaccines have been proposed on the basis of a comprehensive TB vaccination strategy (Lambert et al. 2009): (1) a priming vaccine that induces the host immunity in preparation for subsequent boosters, (2) a boosting vaccine that enhances the immunity induced by the priming vaccine, (3) a vaccine for postexposure administration among healthy adolescents or adults, and (4) a therapeutic vaccine that can increase TB treatment efficacy or shorten TB treatment duration.
Reflecting new approaches in vaccinology , candidate TB vaccines can be classified into several categories (Lambert et al. 2009): (1) live attenuated vaccines (such as recombinant or recombinant attenuated M. tuberculosis), (2) live vectored subunits (such as adenovirus or Modified Vaccinia Ankara), (3) recombinant proteins in adjuvants, (4) DNA vaccines, and (5) whole-cell killed mycobacteria (such as M. vaccae).
Without safer and more effective vaccines alongside better tools for the diagnosis and treatment of TB, it may be difficult to meet the Millennium Development Goals of halving the global TB prevalence and death rates by 2015 relative to their levels in 1990 and eliminating TB as a public health problem by 2050 (WHO 2010a). Modeling studies have demonstrated that neonatal vaccination with a preexposure vaccine that is 60 % effective would reduce 39 % of the TB incidence by 2050 in southern Asia (Abu-Raddad et al. 2009). The same model shows that mass vaccination with such a preexposure vaccine can achieve a much bigger impact on TB transmission and prevent 84 % new cases (85.9 million) and 81 % deaths (14.5 million) (Abu-Raddad et al. 2009).
By April 2009, the development pipeline for TB vaccines had included seven candidates tested in humans, including two nonreplicating viral-vectored vaccines in Phase IIb trial in infants and in HIV-infected adults (Beresford and Sadoff 2010). By the end of 2010, 10 vaccine candidates had entered different phases of trials (5 in Phase I, 2 in Phase II, 2 in Phase IIb, and 1 in Phase III trials) (WHO 2010a). By August 2014, a total of 15 vaccine candidates were in clinical trials, including recombinant BCGs, attenuated M. tuberculosis strains, recombinant viral-vectored platforms, protein/adjuvant combinations, and mycobacterial extracts (WHO 2014).
5 Future Perspectives
TB vaccine development is onerous and challenging. Although the deciphering of the whole genome of M. tuberculosis (Cole et al. 1998) and the significant progress on sequencing that of BCG have facilitated the development of better vaccines, it is by no means easy to evaluate the efficacy of new vaccines in developing countries in the presence of confounding factors that can boost the host immunity, such as TB disease, BCG vaccination, and environmental mycobacteria.
Major obstacles include (1) major gaps in scientific knowledge: inadequate understanding of natural host immunity and bacillary latency, the lack of surrogate markers for vaccine-induced immunity in humans, and a dearth of more natural nonhuman primate models of TB infection; (2) underfunding of expensive TB research work; and (3) the need for sustained political commitment in making future vaccines affordable, accessible, and available in areas that need them most (Beresford and Sadoff 2010).
Science alone is inadequate for combating TB (Beresford and Sadoff 2010; Migliori et al. 2007). Concerted efforts are required to integrate scientific advancement and political mobilization to ensure that substantial TB-related morbidity and mortality can be effectively avoided by mass vaccination campaigns using better TB vaccines.
Abbreviations
- BCG:
-
Bacille Calmette–Guérin
- BMRC:
-
British Medical Research Council
- CI:
-
Confidence interval
- HCW:
-
Healthcare workers
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- IUATLD:
-
International Union against Tuberculosis and Lung Disease
- NRAMP:
-
Natural resistance-associated macrophage protein
- TB:
-
Tuberculosis
- UNICEF:
-
United Nations Children’s Fund
- USPHS:
-
United States Public Health Service
- WHO:
-
World Health Organization
References
Abel, L., Cua, V. V., Oberti, J., Lap, V. D., Due, L. K., Grosset, J., et al. (1990). Leprosy and BCG in southern Vietnam. Lancet, 335(8704), 1536.
Abu-Raddad, L. J., Sabatelli, L., Achterberg, J. T., Sugimoto, J. D., Lonjini, I. M., Jr., Dye, C., et al. (2009). Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proceedings of the National Academy of Sciences U.S.A., 106, 13980–13985.
Arbeláez, M. P., Nelson, K. E., & Muñoz, A. (2000). BCG vaccine effectiveness in preventing tuberculosis and its interaction with human immunodeficiency virus infection. International Journal of Epidemiology, 29, 1085–1091.
Aronson, J. D. (1948a). BCG vaccination among American Indians. American Review of Tuberculosis, 57, 96–99.
Aronson, J. D. (1948b). Protective vaccination against tuberculosis with special reference to BCG vaccination. American Review of Tuberculosis, 58, 255–281.
Baily, G. V. (1980). Tuberculosis prevention trial, Madras. Indian Journal of Medical Research, 72(Suppl.), 1–74.
Balasubramanian, V., Wiegeshaus, E. H., Taylor, B. T., & Smith, D. W. (1994). Pathogenesis of tuberculosis: Pathway to apical localization. Tubercle and Lung Disease, 75, 168–178.
Bellamy, R., Ruwende, C., Corrah, T., McAdam, K. P. W. J., Whittle, H. C., & Hill, A. V. S. (1998). Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. New England Journal of Medicine, 338, 640–644.
Beresford, B., & Sadoff, J. C. (2010). Update on research and development pipeline: Tuberculosis vaccines. Clinical Infectious Diseases, 50(Suppl. 3), S178–S183.
Bettag, O. L., Kaluzny, A. A., Morse, D., & Radner, D. B. (1964). BCG study at a state school for mentally retarded. Diseases of the Chest, 45, 503–507.
Blin, P., Delolme, H. G., Heyraud, J. D., Charpak, Y., & Sentilhes, L. (1986). Evaluation of the protective effect of BCG vaccination by a case-control study in Yaounde, Cameroon. Tubercle, 67, 283–288.
Brahmajothi, V., Pitchappan, R. M., Kakkanaiah, V. N., Sashidhar, M., Rajaram, K., Ramu, S., et al. (1991). Association of pulmonary tuberculosis and HLA in south India. Tubercle, 72, 123–132.
Brewer, T. F. (2000). Preventing tuberculosis with bacillus Calmette-Guérin vaccine: A meta-analysis of the literature. Clinical Infectious Diseases, 31(Suppl. 3), S64–S67.
Brewer, T. F., & Colditz, G. A. (1995). Bacille Calmette-Guérin vaccination for the prevention of tuberculosis in health care workers. Clinical Infectious Diseases, 20, 136–142.
Brewer, T. F., Wilson, M. E., & Nardell, E. A. (1995). BCG immunization: Review of past experience, current use, and future prospects. Current Clinical Topics in Infectious Diseases, 15, 253–270.
Brown, J. A., Stone, M. M., & Sutherland, I. (1968). B.C.G. vaccination of children against leprosy in Uganda: Results at end of second follow-up. British Medical Journal, 1, 24–27.
Brown, C. A., Brown, I. N., & Swinburne, S. (1985). The effect of oral Mycobacterium vaccae on subsequent responses of mice to BCG sensitization. Tubercle, 66, 251–260.
Calmette, A. (1931a). Preventive vaccination against tuberculosis with BCG. Proceedings of the Royal Society of Medicine, 24, 1481–1490.
Calmette, A. (1931b). Epilogue of the catastrophe of Lubeck. La Presse Médicale, 2, 17–18.
Camargos, P. A., Guimaraes, M. D., & Antunes, C. M. (1988). Risk assessment for acquiring meningitis tuberculosis among children not vaccinated with BCG: A case-control study. International Journal of Epidemiology, 17, 193–197.
Canetti, G., Sutherland, I., & Svandova, E. (1972). Endogenous reactivation and exogenous reinfection: Their relative importance with regard to the development of non-primary tuberculosis. Bulletin of the International Union Against Tuberculosis, 47, 116–134.
Center for Disease Control and Prevention. (1997). Immunization of health-care workers: Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). Morbidity and Mortality Weekly Report Recommendation and Reports, 46, 1–42.
Center for Disease Control and Prevention (CDC). (1996). The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report Recommendation and Reports, 45, 1–18.
Colditz, G. A., Brewer, T. F., Berkey, C. S., Wilson, M. E., Burdick, E., Fineberg, H. V., et al. (1994). Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Journal of the American Medical Association, 271, 698–702.
Colditz, G. A., Berkey, C. S., Mosteller, F., Brewer, T. F., Wilson, M. E., Burdick, E., et al. (1995). The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: Meta-analyses of the published literature. Pediatrics, 96, 29–35.
Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., et al. (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature, 393, 537–544.
Comstock, G. W. (1988). Identification of an effective vaccine against tuberculosis. American Review of Respiratory Disease, 138, 479–480.
Comstock, G. W., & Edwards, P. Q. (1972). An American view of BCG vaccination, illustrated by results of a controlled trial in Puerto Rico. Scandanavian Journal of Respiratory Disease, 53, 207–217.
Comstock, G. W., & Palmer, C. E. (1966). Long-term results of BCG vaccination in the southern United States. American Review of Respiratory Disease, 93, 171–183.
Comstock, G. W., & Webster, R. G. (1969). Tuberculosis studies in Muscogee County, Georgia. VII. A twenty-year evaluation of BCG vaccination in a school population. American Review of Respiratory Disease, 100, 839–845.
Comstock, G. W., Livesay, V. T., & Woolpert, S. F. (1974). Evaluation of BCG vaccination among Puerto Rican children. American Journal of Public Health, 64, 283–291.
Comstock, G. W., Woolpert, S. F., & Livesay, V. T. (1976). Tuberculosis studies in Muscogee County, Georgia. Twenty-year evaluation of a community trial of BCG vaccination. Public Health Reports, 91, 276–280.
Dannenberg, A. M., Bishai, W. R., Parrish, N., Ruiz, R., Johnson, W., Zook, B. C., et al. (2000). Efficacies of BCG and vole bacillus (Mycobacterium microti) vaccines in preventing clinically apparent pulmonary tuberculosis in rabbits: A preliminary report. Vaccine, 19, 796–800.
Dantas, O. M. S., Ximenes, R. A. D. A., de Albuquerque, M. D. F. P. M., da Silva, N. L. C. L., Montarroyos, U. R., & de Souza, W. V. (2006). A case-control study of protection against tuberculosis by BCG revaccination in Recife, Brazil. International Journal of Tuberculosis Lung Disease, 10, 536–541.
Diel, R., Loddenkemper, R., Niemann, S., Meywald-Walter, K., & Nienhaus, A. (2011). Negative and positive predictive value of a whole-blood interferon-γ release assay for developing active tuberculosis: An update. American Journal of Respiratory Critical Care Medicine, 183, 88–95.
Eisenhut, M., Paranjothy, S., Abubakar, I., Bracebridge, S., Lilley, M., Mulla, R., et al. (2009). BCG vaccination reduces risk of infection with Mycobacterium tuberculosis as detected by gamma interferon release assay. Vaccine, 27, 6116–6120.
Eriksen, J., Chow, J. Y., Mellis, V., Whipp, B., Walters, S., Abrahamson, E., et al. (2010). Protective effect of BCG vaccination in a nursery outbreak in 2009: Time to reconsider the vaccination threshold? Thorax, 65, 1067–1071.
Indian Council of Medical Research (ICMR). (1999). Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Tuberculosis Research Centre (ICMR), Chennai.Indian Journal of Medical Research, 110, 56–69.
Fine, P. E. (1988). BCG vaccination against tuberculosis and leprosy. British Medical Bulletin, 44, 691–703.
Fine, P. E. (1995). Variation in protection by BCG: Implications of and for heterologous immunity. Lancet, 346, 1339–1345.
Fine, P. E., Maine, N., Ponnighaus, J. M., Clarkson, J. A., & Bliss, L. (1986). Protective efficacy of BCG against leprosy in Northern Malawi. Lancet, 2, 499–502.
Fine, P. E. M., Carneiro, I. A. M., Milstein, J. B., & Clements, C. J. (1999). Issues relating to the use of BCG in immunization programmes: A discussion document (World Health Organization/Vaccines &Biologicals/99.23). Geneva: World Health Organization.
Frimodt-Moller, J., Acharyulu, G. S., & Pilli, K. K. (1973). Observation concerning the protective effect of BCG vaccination in the rural population of South India: Report number 4. Bulletin of the International Union Against Tuberculosis, 48, 40–52.
Goldfeld, A. E., Delgado, J. C., Thim, S., et al. (1998). Association of an HLA-DQ allele with clinical tuberculosis. Journal of the American Medical Association, 279, 226–228.
Hart, P. D. (1967). Efficacy and applicability of mass B.C.G. vaccination in tuberculosis control. British Medical Journal, 1, 587–592.
Hart, P. D., & Sutherland, I. (1977). BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. British Medical Journal, 2, 293–295.
Heimbeck, J. (1936). Tuberculosis in hospital nurses. Tubercle, 18, 97–99.
Hesseling, A. C., Cotton, M. F., von Reyn Fordham, C., Graham, S. M., Gie, R. P., & Hussey, G. D. (2008). Consensus statement on the revised World Health Organization recommendations for BCG vaccination in HIV-infected infants. International Journal of Tuberculosis Lung Disesase, 12, 1376–1379.
ICMR/WHO Scientific Group. (1980) Vaccination against tuberculosis (World Health Organization Technical Report Series, 651).
International Union Against Tuberculosis and Lung Disease. (1994). Criteria for discontinuation of vaccination programmes using Bacille Calmette-Guerin (BCG) in countries with a low prevalence of tuberculosis. A statement of the International Union Against Tuberculosis and Lung Disease. Tubercle and Lung Disease, 75, 179–180.
Jeremiah, K., Praygod, G., Faurholt-Jepsen, D., Range, N., Andersen, A. B., Grewal, H. M., et al. (2010). BCG vaccination status may predict sputum conversion in patients with pulmonary tuberculosis: A new consideration for an old vaccine? Thorax, 65, 1072–1076.
Jin, B. W., Hong, Y. P., & Kim, S. J. (1989). A contact study to evaluate the BCG vaccination programme in Seoul. Tubercle, 70, 241–248.
Jouanguy, E., Altare, F., Lamhamedi, S., Revy, P., Emile, J. F., Newport, M., et al. (1996). Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. New England Journal of Medicine, 335, 1956–1961.
Jouanguy, E., Lamhamedi-Cherradi, S., Altare, F., Fondanèche, M. C., Tuerlinckx, D., Blanche, S., et al. (1997). Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guérin infection and a sibling with clinical tuberculosis. Journal of Clinical Investigation, 100, 2658–2664.
Karonga Prevention Trial Group. (1996). Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet, 348, 17–24.
Khor, C. C., Vannberg, F. O., Chapman, S. J., Guo, H., Wong, S. H., Walley, A. J., et al. (2010). CISH and susceptibility to infectious diseases. New England Journal of Medicine, 362, 2092–2101.
Lagrange, P. H. (1984). The bacillus of Calmette Guerin. Bulletin de l'Institut Pasteur, 82, 53–65.
Lambert, P., Hawkridge, T., & Hanekom, W. A. (2009). New vaccines against tuberculosis. Clinical Chest Medicine, 30, 811–826.
Lange, B. (1930). Investigations to clarify the causes of infant diseases following Calmette vaccination. Zeitschr Tuberkulose, 59, 1–18.
Leung, C. C., Yam, W. C., Ho, P. L., Yew, W. W., Chan, C. K., Law, W. S., et al. (2015). T-Spot.TB outperforms tuberculin skin test in predicting development of active tuberculosis among household contacts. Respirology, 20, 496–503.
Lwin, K., Sundaresan, T., Gyi, M. M., Bechelli, L. M., Tamondong, C., Garbajosa, P. G., et al. (1985). BCG vaccination of children against leprosy: Fourteen-year findings of the trial in Burma. Bulletin of the World Health Organization, 63, 1069–1078.
Marcus, A. M., Rose, D. N., Sacks, H. S., & Schechter, C. B. (1997). BCG vaccination to prevent tuberculosis in health care workers: A decision analysis. Preventive Medicine, 26, 201–207.
Marsh, B. J., von Reyn, C. F., Edwards, J., Ristola, M. A., Bartholomew, C., Brindle, R. J., et al. (1997). The risks and benefits of childhood bacille Calmette-Guérin immunization among adults with AIDS. International MAC study groups. AIDS, 11, 669–672.
Miceli, I., de Kantor, I. N., Colaiacovo, D., Peluffo, G., Cutillo, I., Gorra, R., et al. (1988). Evaluation of the effectiveness of BCG vaccination using the case-control method in Buenos Aires, Argentina. International Journal of Epidemiology, 17, 629–634.
Migliori, G. B., Loddenkemper, R., Blasi, F., & Raviglione, M. C. (2007). 125 years after Robert Koch’s discovery of the tubercle bacillus: The new XDR-TB threat. Is “science” enough to tackle the epidemic? European Respiratory Journal, 29, 423–427.
Milstien, J. B., & Gibson, J. J. (1990). Quality control of BCG vaccine by WHO: A review of factors that may influence vaccine effectiveness and safety. Bulletin of the World Health Organization, 68, 93–108.
Moegling, A. (1935). The epidemiology of Luebeck infant tuberculosis. Arb Reichsges-Amt, 69, 1–24.
Murtagh, K. (1980). Efficacy of BCG. Lancet, 1, 423.
Orege, P. A., Fine, P. E., Lucas, S. B., Obura, M., Okelo, C., & Okuku, P. (1993). Case-control study of BCG vaccination as a risk factor for leprosy and tuberculosis in western Kenya. International Journal of Leprosy and Other Mycobacterial Diseases, 61, 542–549.
Orme, I. M., & Collins, F. M. (1984). Efficacy of Mycobacterium bovis BCG vaccination in mice undergoing prior pulmonary infection with atypical mycobacteria. Infection and Immunity, 44, 28–32.
Padungchan, S., Konjanart, S., Kasiratta, S., Daramas, S., & ten Dam, H. G. (1986). The effectiveness of BCG vaccination of the newborn against childhood tuberculosis in Bangkok. Bulletin of the World Health Organization, 64, 247–258.
Palmer, C. E., & Long, M. W. (1966). Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. American Review of Respiratory Disease, 94, 553–568.
Pönnighaus, J. M., Msosa, E., Gruer, P. J. K., Liomba, N. G., Fine, P. E., Sterne, J. A., et al. (1992). Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet, 339, 636–639.
Putrali, J., Sutrisna, B., Rahayoe, N. (1983). A case-control study of the effectiveness of BCG vaccination in children in Jakarta, Indonesia. In Proceeding I of the Eastern Regional Tuberculosis Conference of IUAT, 1983, Jakarta, Indonesia. pp. 194–200.
Rieder, H. L. (2008). BCG vaccination. In P. D. O. Davies, P. F. Barnes, & S. B. Gordon (Eds.), Clinical tuberculosis (4th ed.). London: Hodder & Stoughton Ltd.
Ritz, N., & Curtis, N. (2009). Mapping the global use of different BCG vaccine strains. Tuberculosis (Edinburgh, Scotland), 89, 248–251.
Ritz, N., Hanekom, W. A., Robins-Browne, R., Britton, W. J., & Curtis, N. (2008). Influence of BCG vaccine strain on the immune response and protection against tuberculosis. Federation of European Microbiological Society Microbiological Review, 32, 821–841.
Rodrigues, L. C., Gill, O. N., & Smith, P. G. (1991). BCG vaccination in the first year of life protects children of Indian subcontinent ethnic origin against tuberculosis in England. Journal of Epidemiology and Community Health, 45, 78–80.
Rodrigues, L. C., Diwan, V. K., & Wheeler, J. G. (1993). Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: A meta-analysis. International Journal of Epidemiology, 22, 1154–1158.
Rodrigues, L. C., Pereira, S. M., Cunha, S. S., Genser, B., Ichihara, M. Y., de Brito, S. C., et al. (2005). Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: The BCG-REVAC cluster-randomised trial. Lancet, 366, 1290–1295.
Romanus, V., Hallander, O., Wåhlén, P., Olinder-Nielsen, A. M., Magnusson, P. H. W., & Juhlin, I. (1995). Atypical mycobacteria in extrapulmonary disease among children. Incidence in Sweden from 1969 to 1990, related to changing BCG-vaccination coverage. Tuberculosis Lung Disease, 76, 300–310.
Rosenthal, S. R., Loewinsohn, E., Graham, M. L., Liveright, D., Thorne, M. G., & Johnson, V. (1961). BCG vaccination against tuberculosis in Chicago. A twenty-year study statistically analyzed. Pediatrics, 28, 622–641.
Roy, A., Eisenhut, M., Harris, R. J., Rodrigues, L. C., Sridhar, S., Habermann, S., et al. (2014). Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: Systematic review and meta-analysis. BMJ, 349, g4643.
Sakula, A. (1983). BCG: Who were Calmette and Guérin? Thorax, 38, 806–812.
Sepulveda, R. L., Parcha, C., & Sorensen, R. U. (1992). Case-control study of the efficacy of BCG immunization against pulmonary tuberculosis in young adults in Santiago, Chile. Tuberculosis Lung Disease, 73, 372–377.
Shapiro, C., Cook, N., Evans, D., Willett, W., Fajardo, I., Koch-Eser, D., et al. (1985). A case-control study of BCG and childhood tuberculosis in Cali, Colombia. International Journal of Epidemiology, 14, 441–446.
Smith, D., Harding, G., Chan, J., Edwards, M., Hank, J., Muller, D., et al. (1979). Potency of 10 BCG vaccines as evaluated by their influence on the bacillemic phase of experimental airborne tuberculosis in guinea-pigs. Journal of Biological Standardization, 7, 179–197.
Smith, D., Wiegeshaus, E., & Balasubramanian, V. (2000). An analysis of some hypotheses related to the Chingelput bacille Calmette-Guérin trial. Clinical Infectious Diseases, 31(Suppl 3), S77–S80.
Soysal, A., Millington, K. A., Bakir, M., Dosanjh, D., Aslan, Y., Deeks, J. J., et al. (2005). Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: A prospective community-based study. Lancet, 366, 1443–1451.
Springett, V. H., & Sutherland, I. (1994). A re-examination of the variations in the efficacy of BCG vaccination against tuberculosis in clinical trials. Tuberculosis Lung Disease, 75, 227–233.
Stein, S. C., & Aronson, J. D. (1953). The occurrence of pulmonary lesions in BCG-vaccinated and unvaccinated persons. American Review of Tuberculosis, 68, 695–712.
Sterne, J. A., Rodrigues, L. C., & Guedes, I. N. (1998). Does the efficacy of BCG decline with time since vaccination? International Journal of Tuberculosis Lung Disease, 2, 200–207.
Stevens, J. P., & Daniel, T. M. (1996). Bacille Calmette Guérin immunization of health care workers exposed to multidrug-resistant tuberculosis: A decision analysis. Tuberculosis Lung Disease, 77, 315–321.
Sutherland, I., & Lindgren, I. (1979). The protective effect of BCG vaccination as indicated by autopsy studies. Tubercle, 60, 225–231.
Tala-Heikkilä, M. M., Tuominen, J. E., & Tala, E. O. (1998). Bacillus Calmette-Guérin revaccination questionable with low tuberculosis incidence. American Journal Respiratory Critical Care Medicine, 157, 1324–1327.
ten Dam, H. G. (1993). BCG vaccination. In L. B. Reichman & E. S. Hershfield (Eds.), Tuberculosis: A comprehensive international approach. New York: Mercel Dekker, Inc.
ten Dam, H. G., & Pio, A. (1982). Pathogenesis of tuberculosis and effectiveness of BCG vaccination. Tubercle, 63, 225–233.
Tidjani, O., Amedome, A., & Ten Dam, H. G. (1986). The protective effect of BCG vaccination of the newborn against childhood tuberculosis in an African community. Tubercle, 67, 269–281.
Tripathy, S. P. (1983). The case for B.C.G. Annals of the National Academies of Medical Sciences, 19, 11–21.
Tripathy, S. (1987). Fifteen-year follow-up of the Indian BCG prevention trial. Bulletin of the International Union Against Tuberculosis and Lung Disease, 62, 69–72.
Trnka, L., Danková, D., & Svandová, E. (1994). Six years’ experience with the discontinuation of BCG vaccination. 4. Protective effect of BCG vaccination against the Mycobacterium avium intracellulare complex. Tuberculosis Lung Disease, 75, 348–352.
Vandiviere, H. M., Dworski, M., Melvin, I. G., Watson, K. A., & Begley, J. (1973). Efficacy of bacillus Calmette-Guérin and isoniazid-resistant bacillus Calmette-Guérin with and without isoniazid chemoprophylaxis from day of vaccination. II. Field trial in man. American Review of Respiratory Disease, 108, 301–313.
Weill-Hallé, B., & Turpin, R. (1925). First Trials of TB vaccination of children with Bacillus Calmette-Guérin (BCG). Bulletin de la Société médicale des hôpitaux de Paris, 49, 1589–1601.
Wilson, M. E., Fineberg, H. V., & Colditz, G. A. (1995). Geographic latitude and the efficacy of bacillus Calmette-Guérin vaccine. Clinical Infectious Diseases, 20, 982–991.
WHO. (1972). BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Bulletin of the World Health Organization, 46, 371–385.
WHO. (1979). Trial of BCG vaccines in south India for tuberculosis prevention: First report—Tuberculosis Prevention Trial. Bulletin of the World Health Organization, 57, 819–827.
WHO. (1997). EPI information system. Global summary, August. Geneva: World Health Organization. (WHO/EPI/GEN/98.10).
WHO. (2004). BCG vaccine. WHO position paper. The Weekly Epidemiological Record, 79, 27–38.
WHO. (2007a). Global Advisory Committee on Vaccine Safety, 29–30 November 2006. The Weekly Epidemiological Record, 82, 18–24.
WHO. (2007b). Revised BCG vaccination guidelines for infants at risk for HIV infection. The Weekly Epidemiological Record, 82, 193–196.
WHO. (2010a) The global plan to stop TB 2011–2015. Geneva: World Health Organization. (WHO/HTM/STB/2010.2).
WHO. (2010b) WHO vaccine-preventable diseases monitoring system, global summary. Geneva: World Health Organization. (WHO/IVB/2010).
WHO. (2014). Global tuberculosis report 2014. Geneva: World Health Organization. (WHO/HTM/TB/2014.08).
Wunsch Filho, V., de Castilho, E. A., Rodrigues, L. C., & Huttly, S. R. A. (1990). Effectiveness of BCG vaccination against tuberculous meningitis: A case-control study in Sao Paulo, Brazil. Bulletin of the World Health Organization, 68, 69–74.
Zwerling, A., Behr, M. A., Verma, A., Brewer, T. F., Menzies, D., & Pai, M. (2011). The BCG world atlas: A database of global BCG vaccination policies and practices. PLoS Medicine, 8, e1001012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this chapter
Cite this chapter
Chang, K.C., Leung, C.C. (2017). BCG Immunization: Efficacy, Limitations, and Future Needs. In: Lu, Y., Wang, L., Duanmu, H., Chanyasulkit, C., Strong, A., Zhang, H. (eds) Handbook of Global Tuberculosis Control. Springer, Boston, MA. https://doi.org/10.1007/978-1-4939-6667-7_20
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6667-7_20
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4939-6665-3
Online ISBN: 978-1-4939-6667-7
eBook Packages: MedicineMedicine (R0)