Abstract
Spinal cord injury (SCI) results in thermoregulatory impairment related to the disruption in autonomic function. As a result, body core temperature generally increases at a greater rate during exercise in individuals with SCI compared to their able-bodied counterparts, placing them at elevated risk for heat-related illnesses. These effects are exacerbated during exercise in the heat. Conversely, body core temperature may decrease at a greater rate during exercise in cold environments. In this chapter, we first briefly describe the anatomy and physiology of normal (non-disrupted) thermoregulation. Next, we present evidence demonstrating that SCI results in impaired thermoregulation both at rest and during exercise. We then discuss the mechanisms behind why these impairments occur, particularly in terms of how disruptions in the sympathetic nervous system affect the various arms of the thermoregulatory negative feedback loop. Next, we discuss how the types of exercise available to individuals with SCI may present additional challenges to thermoregulation, and finally, we present strategies currently in use or under investigation for combatting these thermoregulatory challenges.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
It has long been recognized that spinal cord injury (SCI) results in thermoregulatory impairment. Early studies in animals with complete spinal cord transections observed dramatic and rapid reductions in body temperature during cold exposure (Sherrington 1924; Pembrey 1897). Later, investigators studying humans who had sustained SCI often referred to these patients as ‘poikilotherms’, those lacking the ability to maintain a constant body core temperature independent of ambient temperature (Holmes 1915; Downey et al. 1967; Attia and Engel 1983). In a lecture in 1875, Dr. Jonathan Hutchinson (1875) was the first to attribute these impairments to loss of autonomic control, stating, “The unusual combination of symptoms is explicable only by supposing the vasomotor completely paralysed, and at the same time the heart’s vigor much depressed.”
Following these early reports, subsequent investigations have attributed impaired thermoregulation following SCI to the loss of sympathetic innervation over a large portion of the body. As displayed in Fig. 7.1, body core temperature decreases as a result of the inability to constrict cutaneous blood vessels or activate shivering during exposure to cold ambient conditions. Conversely, due to an impaired ability to sweat and increase skin blood flow under conditions of heat gain, core temperature increases at a much faster rate in individuals with SCI compared to their able-bodied (AB) counterparts. These effects are exacerbated with exercise, as the cardiovascular system is not able to keep up with blood flow demands for maintaining both exercise and thermoregulation. Even under exercising conditions where AB individuals can easily maintain their core temperature, individuals with SCI will experience net heat gains, placing them at elevated risk of heat-related illness.
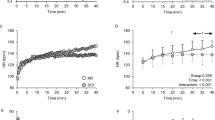
Individuals with intact nervous systems are able to maintain core temperature across a wide range of ambient temperatures. However, body temperature in spinal cord injured individuals can vary greatly with changes in ambient temperature. Data from Attia and Engel (1984) redrawn by Sawka et al. (1989)
During exercise, the increase in metabolic rate challenges the autonomic and cardiovascular systems to raise skin blood flow to divert warm blood to the skin surface for the dissipation of heat. Although individuals with SCI have difficulty maintaining body temperature at rest in cool conditions, this is not generally a challenge during exercise as metabolic heat production is able to offset heat loss to the environment (Boot et al. 2006; Dawson et al. 1994). As such, in this chapter we will focus on impairments in heat loss mechanisms, save for a short section summarizing the literature on individuals with SCI exercising in the cold.
7.2 Anatomy and Physiology of Normal Thermoregulation
Although a typical resting body core temperature for humans is about 37 °C, human internal temperature can fluctuate by 0.2 °C to 1.0 °C due to changes in the circadian rhythm (across a 24 h period) or during hormonal changes attending the menstrual cycle. Although no area of the brain has been identified in humans where the thermoregulatory “set point” resides, data from other species have suggested that mean body temperature is a regulated variable within the hypothalamus, with thermal inputs from the pre-optic area of the anterior hypothalamus and the posterior hypothalamus. Local blood temperature and neural inputs from the spinal cord and peripheral thermoreceptors are integrated within the hypothalamus in order to activate both behavioral and neural adjustments aimed at defending the internal set-point temperature. When body core and/or skin temperatures are increased, reflex responses are activated to initiate heat loss mechanisms, and when core or skin temperatures are decreased, reflex responses are activated to increase heat conservation mechanisms. This system operates as a proportional control system. That is, the more mean body temperature deviates from the set-point temperature, the greater the appropriate heat loss or heat conservation responses are activated. In addition to central mechanisms, when heat or cold is directly applied to the skin, mechanisms including local axon reflexes and vasodilator/vasoconstrictor substances from the blood vessels will alter cutaneous vascular tone, independent of reflex responses arising from the central nervous system.
In the absence of disability or disease, humans are extremely well adapted to living and exercising in warm environments. When exposed to a hot environment or in response to a rise in body heat content during exercise, a number of thermoregulatory adjustments are set into motion. The primary thermoregulatory reflexes include increasing both sweating and skin blood flow, served by an increased cardiac output and a redistribution of our limited blood supply. Under resting thermoneutral conditions, skin blood vessels maintain a low level of tonic vasoconstrictor tone, mediated by sympathetic adrenergic nerves releasing norepinephrine to α1 and α2 receptors. Small fluctuations in the internal or external environments are managed by modest increases or decreases in this basal level of vascular tone, allowing slight changes in the body core to skin temperature gradient. If body temperature continues to rise, tonic vasoconstrictor tone is withdrawn until a “threshold” for active vasodilation and sweating is achieved (Fig. 7.2). Active vasodilation and sweating are mediated by sympathetic cholinergic nerves, which release acetylcholine and an unknown cotransmitter to mediate sweating and active vasodilation, respectively (Kellogg et al. 1995). Current thinking is that vasoactive intestinal peptide (VIP) is the primary co-transmitter, although there is evidence both for and against this (Bennett et al. 2003; Wilkins et al. 2004). There is a well-established role for nitric oxide (NO) in active vasodilation (Kellogg et al. 1998; Wilkins et al. 2003), and it appears to be this component that is affected in conditions in which active vasodilation is diminished (Holowatz et al. 2003; Holowatz and Kenney 2007).
Skin blood flow (SkBF) responses in able-bodied subjects during passive whole-body heating and during low and high intensity exercise. During passive heating, initial increases in skin blood flow occur due to withdrawal of tonic vasoconstriction (VC). After a temperature threshold is reached (~0.4 °C above resting core temperature), further increases in SkBF are the result of active vasodilation (VD). At the onset of exercise, skin blood flow will initially decrease due to systemic sympathetic VC. Threshold for active vasodilation is shifted to a higher core temperature and peak SkBF will be reduced due to competition for blood flow between the active skeletal muscle and the skin
The rise in skin blood flow in able-bodied individuals during direct passive heating in the supine position has been reported to be as high as 4.5–7 L/min, greater than or equal to resting cardiac output. This capacity for increasing blood flow is higher than any other tissue with the exception of exercising skeletal muscle. In order to achieve these very high levels of skin blood flow, cardiac output is increased via a rise in heart rate, and blood flow is redirected from the splanchnic and renal circulations and inactive skeletal muscle. During direct passive heating (such as from a high environmental temperature or in a sauna or Jacuzzi), both central and peripheral thermoreceptors provide the stimulus for thermoregulatory responses. However, central blood temperature accounts for a much greater percentage of the integrated outflow, estimated to be 80–90 % of central reflex thermoregulatory drive. Only minimal reflexively mediated increases in skin blood flow and sweating will occur if skin temperature over the entire body is rapidly elevated (i.e., before central blood temperature rises). The clearest role for skin temperature is that it affects the internal temperature theshold at which skin blood flow begins to increase. The internal threshold temperature for increasing skin blood flow and sweating are lowered when skin temperature is high. That is, with a high skin temperature, heat loss mechanisms are initiated at a lower internal body temperature. That said, in the absence of exercise, it is difficult to raise internal temperature with neutral or cool skin temperatures. There have been attempts to independently control these variables by warming the insensate areas of skin in persons with SCI, which confirmed the affects of skin temperature on the heat loss thresholds described above (Freund et al. 1984; Tam et al. 1978a). Thus, the total integrated thermoregulatory efferent outflow is affected by the total surface area of sensate skin.
During exercise, much of the energy created by the high metabolic rate is in the form of heat that must be dissipated. This sets up a difficult cardiovascular challenge between the skeletal muscles and the skin to compete for a limited blood supply. At the onset of exercise there is a rapid vasoconstrictor response in skin, which is dependent on an intact adrenergic vasoconstrictor system (Fig. 7.2). As body core temperature continues to rise, a threshold is reached to activate active vasodilation and sweating. However, during exercise this threshold is shifted to a higher mean internal temperature than during passive heating. The influence of exercise on the internal threshold is dependent on the intensity of exercise, such that it is shifted to higher temperatures with increasing exercise intensity. This results in a lower absolute skin blood flow and sweat rate for a given body core temperature at higher exercise intensities, to the point where, at maximal exercise, skin blood flow will not increase much above baseline, despite elevations in core temperature. Under these conditions, exercise cannot be maintained for very long due to the very rapid rise in core temperature.
Skin temperature during exercise is dependent on the environmental conditions. Typically in a warm, dry environment, skin temperature decreases during exercise as heat is lost from the skin surface with the evaporation of sweat, despite the warmer blood perfusing the skin. However, skin temperature may increase along with blood temperature during exercise in a hot, humid environment in which evaporative cooling is no longer effective. Thermoregulation in this environment is extremely challenging and can prove to be quite dangerous.
Humans have more limited capacity to tolerate cold temperatures, with behavioral responses and prevention allowing survival in most cold environments. However, in all but the most extreme environmental conditions, body core temperature increases during exercise, even when performed in cold environments. The initial physiologic response to cold stress involves increasing cutaneous sympathetic adrenergic tone to reduce skin blood flow and minimize heat loss. This decreases the convective transfer of heat from the body core to the skin surface, affectively increasing the insulation of the body shell. With increased heat loss, body core temperature decreases further until a threshold for shivering, a form of cold induced thermogenesis, is achieved. Shivering is mediated through the alpha motor neurons and involves involuntary rhythmic contractions of skeletal muscle during which most of the metabolic activity is liberated as heat, as little external work is performed. Shivering typically starts in the torso then moves to the limbs (Bell, JAP, 1992). The extent and intensity of shivering depends on the severity of cold stress. Basal metabolic rate can also be increased in cold temperatures through increases in thyroid hormones. However, in general, both shivering and increased metabolic rate are of limited benefit, and our ability to tolerate cold is not as robust as it is to tolerate heat.
7.3 Evidence for Impaired Thermoregulation Following SCI
7.3.1 Importance of Level of SCI
Before beginning this section, we want to comment on the importance of considering injury level, as an individual’s magnitude of thermoregulatory impairment greatly depends on the level and completeness of injury (Price and Campbell 2003; Hopman et al. 1993a; Petrofsky 1992; Guttmann et al. 1958). For example, tetraplegia is associated with much greater thermal strain during both rest and exercise due to the absence of sweating over the entire body surface (Randall et al. 1966; Totel et al. 1971). By contrast, in paraplegics much of the upper body may have intact cutaneous and sudomotor systems allowing a relatively large body surface area for heat dissipation.
The sympathetic nerves exit the spinal cord at T1-L3. Therefore, tetraplegics (>C7) with complete SCI have loss of all sympathetic innervation, resulting in the largest impairments in thermoregulation. The heart is innervated by T1-T5, thus injuries above T6 result in impaired ability to increase heart rate and actively increase contractility. All increases in heart rate in individuals with complete lesions above T1 will be the result of vagal withdrawal, drastically reducing maximal heart rate during exercise (Van Loan et al. 1987; Coutts et al. 1983). Injuries above T5 result in loss of innervation to the splanchnic region (innervated by T5-L2), impairing ability to redistribute blood flow effectively (Rothe 1983). Additionally, loss of innervation to the kidneys and adrenals (innervated by T4-T11) may impact hormonal control of the circulation during exercise (Christensen and Galbo 1983) and passive heat stress (Minson et al. 1999).
Normell (1974) determined the regions of loss of cutaneous vasomotor and sweating function for a given lesion level. The skin of the face and neck are innervated by T1-T4. Therefore, tetraplegics may have little or no control of sympathetically-mediated vasodilation to the face despite having sensory control of these regions. The skin of the upper extremity is innervated by T5-T7 and the lower extremity by T10-L3.
Accordingly, grouping SCI into the following levels has been recommended when performing studies on thermoregulation (Hopman et al. 1993a; Guttmann 1976):
-
1.
Tetraplegic >C7
-
2.
High paraplegic: T1-T6
-
3.
Low paraplegic: T7-T12
Additionally, for the purposes of more fully presenting available data in individuals with SCI, we have added a “very low paraplegia” category, including individuals with lesions <T12. Thermoregulation in these individuals would be expected to be near-normal as nearly all sympathetic innervation is retained.
The majority of investigations on thermoregulation have studied individuals with complete injury in order to minimize variability. Accordingly, the majority of literature presented in this chapter refers to complete SCI. However, those with complete injuries only represent approximately 35 % of the SCI community (NSCISC 2015). Those with incomplete injuries may retain some sympathetic control and thus may be better able to thermoregulate. However, this should not be assumed since complete loss of autonomic innervation can occur even when sensory innervation is partially preserved (e.g., ASIA B or C) (Cariga et al. 2002).
Lastly, level of injury will have a significant impact on exercise intensities that can be achieved. Although paraplegics are able to thermoregulate better than tetraplegics, they typically can achieve higher exercise intensities, resulting in greater metabolic heat production. Thus, it is important to ensure thermal conditions are safe for all individuals with SCI, regardless of level or completeness of injury.
7.3.2 Evidence of Thermoregulatory Impairment
In the 1960s and 1970s, a series of studies were performed in paraplegic subjects in efforts to tease out the contributions of central and skin thermoreceptor inputs to thermoregulatory drive. These studies utilized passive heating or cooling of sensate and insensate skin in order to alter core temperature with or without sensory input from the skin thermoreceptors. As a result, these studies provided key insight into the impairments in thermoregulation experienced following SCI.
Under conditions of high ambient temperature, core temperature at rest is elevated in paraplegics (Petrofsky 1992; Yamasaki et al. 2001; Huckaba et al. 1976), and even more so in tetraplegics (Petrofsky 1992; Pollock et al. 1951) (Fig. 7.3, left panel). For a given core temperature, mean whole-body sweat rate is lower in paraplegics compared to AB subjects (Hopman et al. 1993a; Huckaba et al. 1976), and even lower in tetraplegics (Downey et al. 1976), generally as a function of surface area of insensate skin (Petrofsky 1992). Even in sensate skin, local sweat rates (Tam et al. 1978a, b) and skin blood flow (Freund et al. 1984; Tam et al. 1978b) are also lower for a given core temperature. Furthermore, the onset of sweating and increased skin blood flow is delayed to a higher core temperature (Tam et al. 1978a; Downey et al. 1976).
Core temperature responses in able-bodied and tetraplegic subjects during water immersion in 102.5 °F (left) or 66 °F (right) water. From Pollock et al. (1951)
In cold ambient conditions, core temperature decreases at a considerably greater rate in individuals with SCI compared to AB individuals, in whom body core temperature is generally well preserved (Pollock et al. 1951) (Fig. 7.3, right panel). SCI results in an impaired ability to vasoconstrict the skin microvessels or shiver below the level of the lesion, resulting in significant loss of heat to the environment and limited capability to offset that loss through metabolic heat production.
With the increased use of exercise as a rehabilitation tool for individuals with SCI through the 1970s to 1980s, studies began investigating thermoregulatory impairment during exercise. Similarly to passive heat stress, individuals with SCI generally experience a greater increase in core temperature during exercise relative to that of AB individuals (Table 7.1). Mean whole-body sweat rate and local sweat rate in sensate skin are both lower (Downey et al. 1976; Yaggie et al. 2002), and skin blood flow in sensate skin is lower (Freund et al. 1984). Typically in temperate environmental conditions, skin temperature in insensate skin increases (Gass and Camp 1984; Griggs et al. 2015), as they lack evaporative heat loss mechanisms. Skin temperature in sensate skin either remains unchanged or decreases (Gass et al. 1988), reflecting abilities to sweat, albeit to a lesser extent than in AB individuals. In higher ambient temperatures, the effects on skin temperature in insensate skin will be exacerbated (Price and Campbell 2003) and skin temperature in sensate skin will increase as a function of environmental temperature.
Table 7.1 summarizes the studies that have reported core temperature and/or skin temperature changes during exercise in SCI subjects, as compared to AB subjects. The left panel displays the level of lesion by environmental condition. As environmental temperature increases, the ability to maintain a thermoneutral body temperature diminishes. Similarly, the skin temperature more closely reflects the ambient temperature. Severity of the lesion is related to the severity of the thermoregulatory dysfunction, as represented by the number of arrows. The greatest variability in what has been reported in the literature is in low-level paraplegics exercising in temperate conditions. These individuals have been studied more commonly and generally have greater exercise capacities than their counterparts with higher level SCI. Thus, there has been greater variability in the duration and intensity of exercise studied, which will greatly affect heat production, and therefore thermoregulation.
7.4 Autonomic Mechanisms Underlying Thermoregulatory Impairment
As discussed previously, thermoregulation is a negative feedback system that relies on intact afferent and efferent pathways for proper function. Both of these branches are disrupted following SCI (Fig. 7.4). Therefore, while sensory afferent signals from regions innervated by nerves above the level of injury may function appropriately, the overall magnitude of sensory information entering the thermoregulatory centers in the hypothalamus will be greatly attenuated. Similarly, while the efferent signals leaving the hypothalamus may be appropriate, only a subset of these signals will reach the effector organs. Impairments may also exist in the transduction of these signals to the effector organs, namely the skin microvessels and sweat glands. As such, there are multiple places in the feedback system where problems may occur. The following section will present evidence for how SCI impairs each segment of the thermoregulatory pathway.
The thermoregulation reflex arc. Afferent input from central thermoreceptors in the brain and peripheral thermoreceptors in the skin, muscle, abdomen, and spinal cord is integrated in the thermoregulatory centers of the hypothalamus. Efferent output is then sent to the sweat glands and skin microvessels. Spinal cord injury causes disruptions in neural signaling of both the afferent and efferent arms of the reflex arc below the level of the injury. Additionally, there may be some alterations in how afferent input is integrated in the hypothalamus, and the sweat glands and skin microvessels may become abundant and/or less responsive to neurotransmitter release
7.4.1 Set-Point Theory
We will begin by discussing potential alterations in the thermoregulatory set point (in the hypothalamus), as from a historical perspective, this was the earliest theory to explain impaired thermoregulation. Early investigations observed that core temperature had to increase to a greater extent in individuals with SCI prior to the onset of sweating (compared to AB) (Pollock et al. 1951). This led to the theory that central thermoregulatory set-point must be increased to a higher core temperature (Downey et al. 1967; Attia and Engel 1983). Observations that sweating in sensate skin could be affected by altering skin temperatures also resulted in the suggestion that set-point could be reset to suit ambient temperature. Tam et al. (1978a) also proposed that the gain of the response may be reduced. However, these hypotheses were not able to be adequately explored as the technology did not yet exist to determine whether neural output for a given stimulus was decreased or whether the sensitivity of the effector organs was decreased (e.g., actually no change in central gain, but reduced peripheral sensitivity for a given neural outflow).
7.4.2 Reduced Afferent Input
A more likely explanation to why onset of sweating is shifted to a higher core temperature is that afferent input is reduced. As discussed previously, the thermoregulatory centers in the hypothalamus receive afferent input from both the central thermoreceptors (located in the brain and spinal cord) and skin thermoreceptors, and both impact thermoregulatory drive. With SCI, there is a loss of afferent input from spinal thermoreceptors below the lesion and from all skin thermoreceptors located in the dermatomes innervated by spinal nerves below the lesion, representing a sizable reduction in the input coming into the hypothalamus.
The concept of mean body temperature was developed in order to better quantify the afferent input that results in a given thermoeffector output. Commonly, it is considered that core temperature contributes ~80–90 % and skin temperature contributes ~10–20 % to determine the output (e.g., sympathetic neural activity to the cholinergic nerves innervating the skin). SCI results in a significant loss of skin afferent input (up to 90 % loss in a high-level tetraplegia), and a loss of the central thermoreceptors located in the isolated spinal cord. Furthermore, thermoreceptors in the skeletal muscle and abdomen can also contribute a portion to central drive. Therefore, total afferent input received by the hypothalamus (when including input signaled by local hypothalamic temperature) could be reduced by as much as ~30 %. Accordingly, the thermoregulatory centers will respond with equally reduced sympathetic output. For example, Petrofsky (1992) found that mean whole-body sweat rate during exercise was inversely correlated with surface area of insensate skin across AB, paraplegic, and tetraplegic subjects.
In support of this, reductions in sensate skin temperature have a much greater effect on modulating thermoregulatory drive (e.g., shivering) in subjects with SCI compared to AB subjects (Downey et al. 1967, 1971, 1973; Attia and Engel 1983) since the same surface area of skin will contribute a larger overall percentage to the total afferent input.
7.4.3 Reduced Efferent Output and Target Sensitivity
While much of the thermoregulatory impairment can be explained by reduced afferent input, SCI also results in impairments in the effector arm, that is, reduced sweating and skin blood flow for a given sympathetic output. Of course, sweating and skin blood flow will be largely absent in areas below the level of the lesion due to loss of sympathetic innervation. However, SCI is also associated with impairments in these mechanisms in sensate skin. These impairments may be explained by decrements in the neural signal as it travels down the peripheral nerves due to degeneration, differences in the neural-vascular transduction of output to the target areas, or diminished responses of the skin microvessels and sweat glands to a given level of stimulation. The lowered responses could be an aspect of the injury itself, secondary to cardiovascular adaptations attempting to maintain blood pressure (e.g. elevated circulating vasoconstrictors), or as a result of detraining and/or overall health (Johnson et al. 2014).
7.4.3.1 Insensate Skin
Following complete SCI, there is no transmission of signals from the brain to the isolated spinal cord. Even in individuals who have retained some sensory innervation (e.g., ASIA B or C), the autonomic neurons may still be damaged. By this theory, no centrally-mediated increases in sweating or skin blood flow should occur in response to elevations in core temperature. The majority of studies have supported this, detecting no sweating below the level of the lesion in response to elevations in core temperature, either as a result of high ambient temperature (Tam et al. 1978a; Guttmann et al. 1958; Pollock et al. 1951; Wyndham 1955), exercise (Petrofsky 1992), or a combination of both (Petrofsky 1992). Similarly, increases in skin blood flow below the level of the lesion are minimal (Normell 1974; Yamasaki et al. 2000, 2001; Theisen et al. 2001; Muraki et al. 1995, 1996a, b). However, in some individuals, sweating has been reported in insensate skin. Typically, it is sparse and not synchronous with sensate skin (Huckaba et al. 1976), but some individuals experience profuse sweating below the level of lesion. There are several potential explanations for these observations, as noted below.
Spinal Mass Reflex
Profuse sweating can be caused in insensate skin due to a spinal mass reflex. These occurrences are not related to thermoregulation, but instead induced by other noxious stimuli, such as bladder distension, muscle spasms, intestinal conditions, painful stimuli to the skin, or emotional stress (Head and Riddoch 1917; Guttmann and Whitteridge 1947). It is believed that the reflex occurs due to remapping of the sensory and cutaneous sympathetic neurons, much like autonomic dysreflexia, a condition in which noxious sensory stimuli will trigger sympathetic vasomotor nerves, resulting in marked and sudden increases in blood pressure (Erickson 1980; Silver 2000). The spinal mass reflex typically occurs more frequently in the first few months post-injury as, over time, there may be some degeneration of the peripheral nerves. Regardless, this reflex does not originate due to thermoregulatory challenges, and would contribute only modestly to the maintenance of core body temperature.
Peripheral Thermoreceptors
Some studies have reported detectable sweating below the level of the lesion in response to high ambient temperature (Randall et al. 1966; Silver et al. 1991). A few theories exist for why this may occur, in support of a local thermoregulatory reflex arc. One explanation for this is that sympathetic sudomotor neurons exiting the spinal cord above the lesion can travel via the paravertebral ganglia and innervate regions of insensate skin. Seckendorf and Randall (1961) were the first to come to this conclusion. They studied subjects with lesions between T3-T8 and so reasonably concluded that sweating in insensate skin must have originated from sudomotor nerves from T1 down to the level of the lesion. However, Randall et al. (1966), in order to ensure complete isolation of the sympathetic nerves, repeated this study in patients who all had complete transections above T1. Even in these subjects, there was some minimal, albeit sparse, sweating in response to high ambient temperatures. These results were confirmed in a later study by Silver et al. (1991). In all three of these studies, sweating ceased once patients were removed from the high ambient temperature despite core temperature remaining elevated (Silver et al. 1991). These authors concluded that there must be an intact thermoregulatory reflex arc below the lesion. Specifically, they suggested that the isolated spinal cord must be able to reflexively respond to input from skin thermoreceptors by stimulating the sympathetic sudomotor nerves, thereby inducing sweating. However, contrary to this hypothesis is the observation that there is no sudomotor response when the peroneal sympathetic nerves are electrically stimulated (Cariga et al. 2002; Reitz et al. 2002). Together, these observations argue in favor of a local thermoregulatory reflex arc that exists between the sensory nerves and sweat glands, independent of the spinal cord.
Skin Blood Flow and Local Skin Temperature
Under conditions of high ambient heat, small increases in skin blood flow in insensate skin may be observed, most likely due to a local thermal hyperemic response. Increases in local skin temperature results in vasodilation due to synthesis and release of nitric oxide (Kellogg et al. 1999; Minson et al. 2001) and endothelial-derived hyperpolarizing factors (Brunt and Minson 2012). However, although present, this response is greatly attenuated in insensate skin compared to sensate skin in both subjects with SCI and AB subjects (Nicotra et al. 2004; Van Duijnhoven et al. 2009). The magnitude of the response is also highly dependent on skin temperature (Choi et al. 2014; Houghton et al. 2006). Thus, little vasodilation will occur during exercise in temperate environments as skin temperature does not increase substantially under these conditions (and may decrease some in individuals with SCI) (Price and Campbell 1997, 1999a). In hot conditions, although a local thermal hyperemia response may occur, this will aid in thermoregulation only minimally due to the lack of temperature gradient between the skin and the environment.
7.4.3.2 Sensate Skin
Despite normally functioning neural pathways, impairments in sudomotor and vasomotor function may exist in sensate skin. Part of this is due to the anatomy of the sympathetic system—e.g., the sympathetic nerves innervating the face and neck exit the spinal cord at T1-T4, thus individuals with a high level of injury may have little to no sweating or increased skin blood flow in seemingly sensate regions of skin. However, sudomotor and vasomotor function is impaired even in sensate skin innervated by intact sympathetic nerves.
Sudomotor Function
SCI is associated with reduced sensitivity of the sweat glands to cholinergic agonists (Yaggie et al. 2002). Furthermore, these authors also showed a reduction in sweat gland density and drastically reduced sweat output per gland. These observations were even more dramatic in insensate skin. Interestingly, these effects in sensate areas were partially reversed in athletes with SCI, although chronic exercise was unable to return sweat gland characteristics back to that of AB individuals. It has been demonstrated that sweat gland function is improved following repeated exposures to heat stress, independent of changes in central thermoregulatory drive (Lorenzo and Minson 2010). Similarly, repeated injections of methacholine in the skin of paraplegics resulted in improved sweating in both sensate and insensate areas (Johnson and Johnson 1970). As such, individuals with SCI may experience a peripheral “detraining” of the sweat glands, which may precede changes in central thermoregulation (Ogawa and Asayama 1978).
Vasomotor Function
Sympathetically-mediated dilation in response to heat stress (known as ‘active vasodilation’) is primarily dependent on endothelial-derived vasodilators, such as nitric oxide (Kellogg et al. 1998; Wilkins et al. 2003; Shastry et al. 1998) and prostanoids (McCord et al. 2006). However, vascular tone is reflective of the balance between vasodilator and vasoconstrictor influences, thus vasodilation can be limited by the presence of vasoconstrictors. SCI is associated with widespread declines in vascular function and an increase in circulating vasoconstrictors, such as angiotensin-II (Groothuis et al. 2010) and endothelin-I (Thijssen et al. 2007). Accordingly, active vasodilation is attenuated even in the sensate skin of individuals with SCI (Freund et al. 1984; Muraki et al. 1996a). Although not yet shown experimentally, it is likely that active vasodilation is attenuated due to a combination of limitations imparted by increased circulating vasoconstrictors and impaired nitric oxide bioavailability. Many disease states/conditions, including SCI, are characterized by increased oxidative stress. Under these conditions, free radicals, such as superoxide, can combine with nitric oxide, thus reducing bioavailable nitric oxide and vasodilation. Importantly, exercise training improves nitric oxide bioavailability and NO-dependent vasodilation (Green et al. 2004; Hambrecht et al. 2003). As such, training status will impact active vasodilation in individuals with SCI and thus their ability to thermoregulate (as discussed in more depth below).
7.4.4 Cardiovascular Impairments that Affect Thermoregulation
Other cardiovascular autonomic impairments may also play a role in limiting thermoregulation. During exercise, these limitations become even more apparent due to competition for blood flow between the active skeletal muscles and the skin for thermoregulation. At high exercise intensities, this competition results in reduced skin blood flow and sweating in AB individuals, which is exaggerated in individuals with SCI.
As reviewed in Chap. 6 (Circulatory Responses during Submaximal and Maximal Exercise in SCI), SCI results in loss of sympathetic innervation to the heart and vasculature (depending on the level of injury), resulting in an impaired ability to increase cardiac output (Hopman et al. 1993a, b; Van Loan et al. 1987) and redistribute blood flow (Davis 1993; Hopman et al. 1992a, 1998; Kinzer and Convertino 1989; Hopman 1994). Loss of sympathetic innervation to the heart limits the relative increase in heart rate and contractility that can be achieved with exercise and/or systemic stress. Furthermore, in individuals with SCI, the ability to divert any additional blood flow away from inactive regions, such as the inactive skeletal muscle, towards active regions is impaired due to loss of sympathetically mediated vasoconstriction and loss of the muscle pump. Subsequently, blood may pool in these inactive regions, limiting venous return to the heart and further limiting cardiac output. In addition to this, individuals with SCI generally have reduced blood volume (Knutsson et al. 1973). As an overall result, the skin is supplied with insufficient blood flow to meet thermoregulatory demands.
Additionally, the splanchnic circulation, which is a highly compliant vascular bed with a large vasoconstrictor capacity, is integral in redistributing blood flow during exercise and heat stress, and is sympathetically innervated by T5-T12. Therefore, individuals with lesions above this range will have the hardest time redistributing blood flow and will likely be the most prone to heat-related illness. Individuals with loss of sympathetic innervation to the heart (above T4-T6), will also have additional challenges, as they lack the ability to (partially) compensate for reduced venous return by increasing heart rate and/or cardiac contractility (Hopman et al. 1993a; Mathias and Frankel 1988).
Another consideration when exercising in the heat is cardiac drift. During exercise in the heat, heart rate ‘drifts’ up over time as core temperature increases due to direct effects of heat on the sinoatrial node (Fritzsche et al. 1999; Coyle and González-Alonso 2001) and in order to compensate for loss of plasma volume (due to sweating) and the additional blood flow demands of the skin (Rowell 1986). In individuals with SCI, this effect can be seen during exercise even in temperate environmental conditions (Dawson et al. 1994; Fitzgerald et al. 1990; Theisen et al. 2001). For example, paraplegics typically experience higher heart rate compared to AB individuals while exercising in order to compensate for lower stroke volume (Hopman et al. 1992a, 1993a; Christensen and Galbo 1983; Theisen et al. 2001). If cardiac drift occurs, these individuals may reach maximal heart rate much sooner. At this point, they either reach exhaustion and will be forced to stop exercising early, or if they continue as some may be inclined to do in competition, they will greatly predispose themselves to heat-related illness. Individuals with lesions above T4 will be even worse off as their maximal heart rate is determined by vagal withdrawal and is therefore considerably lower than lower-level paraplegics or AB individuals (Van Loan et al. 1987; Coutts et al. 1983).
7.4.5 Medications
Individuals with SCI typically benefit from ongoing medical care to manage the loss of function and prevent more serious complications. As a result, the prevalence of chronic therapeutic medication use is high in the SCI population. The most common types of medications used include those for bladder control, antispasmodics, and pain relievers. Antibiotics and anti-inflammatory medications are often used intermittently. Additionally, anti-hypertensive medications are commonly utilized by people with paraplegia. Many of these can interfere with thermoregulation, and thus should be considered on an individual basis when making exercise and environmental recommendations.
Individuals with SCI can experience either spastic/overactive or flaccid/underactive bladders, depending on the injury. Anticholinergic medications (e.g., oxybutynin) are commonly prescribed for spastic/overactive bladders to promote smooth muscle relaxation, whereas flaccid/underactive bladders are commonly treated with cholinergic agonists (e.g., bethanochol) to promote bladder contraction and/or alpha adrenergic blockers (e.g., dibenyline, Flomax) to help relax the sphincter. All of these medications affect the sympathetic nervous system and thermoregulation, often in a competing manner. For example, there have been case studies of patients developing sudden hypotension and hypothermia (Tc < 36.0 °C) secondary to the massive sweat induction experienced from concomitant use of cholinergic agonists and alpha blockers (Berard et al. 1989). In opposition, anticholinergics will inhibit sweat production, which could be dangerous for someone exercising in the heat who already has impaired sweating capabilities. Consideration of what medications athletes utilize should be of primary importance given the opposing effects of these medications.
7.4.6 Compensatory Mechanisms
Although humans primarily rely on sweating and increases in skin blood flow for heat loss, respiratory heat loss can be significant under some circumstances, particularly during exercise (Mitchell et al. 1972). In individuals with SCI, respiratory heat loss may serve to compensate for the impaired ability to sweat and redistribute blood flow to the skin. In tetraplegics (C5-C8) with low sweating capacity, minute ventilation increased substantially while resting in the heat (38 °C, 20 %rh for up to 150 min); whereas no increases were observed in AB subjects (Totel 1974). In fact, linear increases of 2.4 L/min for each 1.0 °C increase in oral temperature were observed. These increases were due to increased respiratory rate with no changes in tidal volume, thus it was essentially a ‘panting’ mechanism. Observations of ‘panting’ have been reported in other studies in subjects with SCI, although increases in ventilation were not quantified (Guttmann et al. 1958).
7.5 Considerations Based on Exercise Modalities
The aforementioned impairments in thermoregulation mean that individuals with SCI will experience considerably higher core temperatures for a given metabolic rate compared to AB individuals. These differences are greatly exacerbated for exercise in the heat. The types of exercise modalities and sports that individuals with SCI participate in may also present some additional challenges to thermoregulation. As of 2016, there are now 22 sports included in the Summer Paralympic Games and another 5 included in the Winter Paralympic Games. These range from court sports, such as wheelchair basketball, tennis, and rugby, to power lifting, and to continuous-effort sports, such as road racing and triathlon. The specific thermoregulatory factors present in each sport should be considered. For example, wheelchair road racing requires athletes to be positioned in very compact racing chairs that are low to the ground. Their proximity to the ground while racing outside means that they will be exposed to higher radiant heat from the road surface.
The majority of the older studies on thermoregulation in individuals with SCI used continuous arm-cranking exercise; however, this may not be as applicable to field sports (Price and Campbell 1999b). Arm-crank ergometers require less force to maintain a given speed than wheelchair ergometers, although thermal strain may be higher, perhaps due to differences in propulsion biomechanics (Price and Campbell 1999b). Newer thermoregulatory studies have thus utilized wheelchair ergometers.
Many sports, including wheelchair basketball, rugby, and tennis require intermittent bouts of high-intensity exercise. Intermittent exercise results in increased thermal load compared to continuous exercise at matched overall intensities (Ekblom et al. 1971). Several of the studies presented in other sections attempted to be more applicable to wheelchair sports by utilizing intermittent sprint exercise (e.g., Griggs et al. 2015; Webborn et al. 2005; Goosey-Tolfrey et al. 2008a).
7.5.1 Upper vs. Lower Body Exercise
Even in able-bodied individuals, there are differences in the thermoregulatory responses to upper vs. lower body exercise (Sawka et al. 1989; Asmussen and Nielsen 1947; Nielsen 1968). Typically, upper body exercise results in greater heat production for a given absolute workrate or metabolic rate/VO2 compared to lower body exercise (Sawka et al. 1984). Due to lower overall muscle mass, the arm muscles must work at a higher percent of maximal intensity in order sustain similar workrates. Efficiency is also reduced at higher intensities (Zoladz et al. 1995), meaning a higher percentage of energy expended is given off as heat rather than used for external work, resulting in greater heat production. Furthermore, due to the reduced muscle mass of the arms compared to the legs, for a given metabolic rate, less afferent input from metaboreceptors and thermoreceptors in the skeletal muscle and venous system will be sent to the brain. This may require more metabolic heat production prior to initiation of heat loss mechanisms. Accordingly, some have suggested that the thermoregulatory set point in the hypothalamus is shifted to a higher core temperature with upper body exercise (Tam et al. 1978a; Asmussen and Nielsen 1947); however, (Sawka et al. 1984) found no difference in threshold or slope of the sweat response.
The cardiovascular system may also limit thermoregulation in upper body exercise. In individuals with SCI, the lack of the skeletal muscle pump limits cardiac output, in turn limiting blood flow that can be routed to the exercising muscles and to the skin for thermoregulation. Due to the smaller muscle mass of the arms, there is less of a need to redistribute blood flow to these muscles during exercise than with leg or whole-body exercise. In individuals with SCI who already have impaired abilities to redistribute blood flow due to loss of sympathetic vasoconstriction, loss of muscle pump does limit the rise in cardiac output. In fact, studies in which the muscle pump has been artificially created using functional electrical stimulation to the legs (Davis et al. 1990) or by wearing an anti-gravity suit (Hopman et al. 1992b) have shown increases in cardiac output and stroke volume in individuals with SCI during submaximal arm-crank exercise. Interestingly, no effects of the interventions were observed in AB subjects in either of these studies. AB individuals also experience greater plasma volume loss and hemoconcentration with upper body exercise for a given VO2 (Miles et al. 1983; Pimental et al. 1984), which will limit sweat loss.
One advantage of upper body exercise is that the arms have a higher surface area-to-mass ratio which should facilitate convective heat transfer. However, this does not make much difference when exercise is performed in air. It may, however, make a difference in water since conduction of heat is so much greater (~24× greater). Toner et al. (1984) demonstrated that subjects exercising in 26 °C and 33 °C water had lower core temperature during upper body exercise compared to lower body exercise at a matched VO2. However, this may not lead to substantial differences in thermoregulation in individuals with SCI, as convective heat loss is already reduced.
7.5.2 Training Status
Trained athletes with SCI are considerably better able to thermoregulate than non-trained individuals. In fact, some studies have shown that trained low-level paraplegics maintain core temperature during exercise in varied temperate conditions on par with AB athletes and have similar mean whole-body sweat loss (Price and Campbell 1999a) (although they still typically experience impaired thermoregulation in warm to hot environmental conditions). As it is almost impossible to exercise without a rise in core temperature, it is likely that much of this adaptation is secondary to an exercise heat-acclimation response.
Endurance exercise training results in earlier activation (shifted threshold to a lower body core temperature) of sweating and active vasodilation (Thomas et al. 1999) and thus elevated sweat rate and skin blood flow for any given body core temperature during exercise (Roberts et al. 1977). However, exercise training also results in functional adaptations to the cutaneous vasculature, namely, the cutaneous vasculature undergoes functional adaptations, such as increased endothelium-dependent dilation (Lenasi and Strucl 2004; Kvernmo et al. 1998; Vassalle et al. 2003). Collectively, these studies demonstrate that improved cardiovascular fitness is associated with a greater skin blood flow response to a given thermal stimuli. That said, there is also evidence that repeated exposure to heat stress, whether through passive heating or exercise heat stress, results in improved sensitivity of the skin and sweat glands to a given neural or chemical input (Lorenzo and Minson 2010).
SCI represents a massive “detraining” stimulus and many of the peripheral adaptations (e.g. sweat gland atrophy and vascular remodeling) may be largely in response to the sudden reduction in physical activity. The higher temperature thresholds required for sweating and active vasodilation are consistent with lower overall fitness following SCI. In paraplegics, long-term exercise training results in increased sweat gland density, increased sweat output per gland, and increased upper body overall sweat rates (Yaggie et al. 2002). Although not shown yet in individuals with SCI, in AB individuals, exercise training shifts the threshold for sweating and skin blood flow to lower temperatures and both are elevated at a given body core temperature. Under conditions of minimal external heat stress, this means paraplegic athletes should be generally able to thermoregulate sufficiently to remove metabolic heat within the “prescriptive zone”, that is, the range of ambient conditions that core temperature increases in proportion to the metabolic rate, independent of the environment (Lind 1963). It is not until the environmental temperatures are greater that trained paraplegics experience cardiovascular limitations to thermoregulation. Of course, complete tetraplegic athletes are unable to gain training thermoregulatory adaptations as intact sweating and vasodilator systems are required to achieve these adaptations.
7.6 Strategies for Mitigating Thermoregulatory Dysfunction
As discussed in previous sections, the thermoregulatory limitations of individuals with SCI presents a great challenge to exercise, particularly in warm environments. Individuals with SCI are at elevated risk of heat-related illness in competitive sports compared to the AB population (Price 2006, 2016). In addition to compromised thermoregulation, the impaired ability to sense elevated core temperature means that athletes with SCI are less likely to discontinue exercise at high core temperatures, increasing the propensity toward thermal injury. In addition, the majority of Paralympic competitions are held in hot environments, making it imperative to investigate strategies for combatting heat gain in this population. A variety of strategies have been investigated in the AB population. Evidence of their effectiveness in individuals with SCI is limited, but promising avenues have emerged in recent years.
We have focused this section on strategies for preventing heat-related illness in warm environments, but it is also important to consider strategies for improving thermoregulation during exercise in cold environments, particularly given the rising popularity of adaptive winter sports, such as skiing. Unfortunately, there have been no scientific studies to date to investigate strategies in cold environments. However, we propose some potential options in the last sub-section below.
7.6.1 Cooling
A variety of cooling methods have been investigated in the AB population to improve performance in hot environments. These include cooling vests (Duffield et al. 2003; Hasegawa et al. 2005), whole-body pre-cooling (Kay et al. 1999; White et al. 2003), and hand/foot cooling (Grahn et al. 2005; Livingstone et al. 1995; House et al. 1997; Giesbrecht et al. 2007). The idea behind all of these methods is that, by lowering skin temperature, the skin-to-core temperature gradient will be increased, allowing for greater heat dissipation while requiring less blood to be directed to the skin for that purpose. Blood can instead be routed to the exercising muscles and higher workrates can be maintained, thereby improving exercise performance. Lower skin and core temperature also delays the onset of sweating, preserving plasma volume (Marino 2002).
It has been postulated that cooling before or during exercise may even be more effective in the SCI population due to reduced sympathetically-mediated vasoconstriction, which would normally serve to route blood away from cooler areas of skin, limiting the benefit of cooling. However, despite a wealth of knowledge on the effects of cooling strategies in AB athletes, very few studies have been performed in athletes with SCI (see Griggs et al. (2014) for a recent review of all nine studies). Strategies in athletes with SCI are also limited by their biomechanics. Although many devices have been developed for AB athletes, many of them are bulky and impractical for wheelchair athletes utilizing adaptive equipment.
7.6.1.1 Microclimate Cooling Vests
A variety of microclimate cooling garments have been developed and tested for their efficacy in reducing heat strain and improving performance in the heat (Bennett et al. 1995). In individuals with SCI, the two primary types of garments that have been tested are vests containing either ice (e.g. Arctic Heat, Hillsdale, NJ) or renewable phase change material (e.g. Glacier Tek, Inc., West Melbourne, FL). Ice vests have the advantage of being slightly colder (0 °C vs. ~15 °C) and so have greater capacity to remove heat; however, phase change materials may maintain lower temperatures for longer and therefore be better able to attenuate rises in core temperature during long durations of exercise (Chou et al. 2008). Phase change materials tend to be more comfortable against the skin and they may be safer for individuals with SCI when covering insensate skin since they are less likely to cause skin breakdown secondary to local cutaneous vasoconstriction when compared to ice vests (House et al. 2013).
Data on the effectiveness of ice vests in the SCI population are mixed. In endurance-trained athletes with SCI, the ice vest tended to attenuate the rise in core temperature and to reduce heat strain over a 30 min wheelchair time trial in the heat (32.9 °C, 75 %rh), but these differences were minimal (rectal temperature reduced by only 0.3 °C) and non-significant (Armstrong et al. 1995). However, during intermittent high-intensity exercise in the heat (32 °C, 50 %rh), ice vests successfully reduced the rate at which core temperature increased (Webborn et al. 2005, 2010) and extended the volitional time to fatigue. Additionally, the authors noted that the self-selected workrates were slightly lower initially in the ice vest condition, but were better maintained throughout the exercise protocol, suggesting that cooling during exercise may allow athletes with SCI to better pace their efforts. This observation is important to consider in the SCI population, as their ability to pace may be impaired under normal conditions due to reduced afferent feedback.
Only one study has investigated the cooling abilities of renewable phase change vests in SCI individuals (Trbovich et al. 2014). These authors observed no effect of the vest on attenuating the rise in core temperature during 60 min of indoor team sport play (wheelchair basketball or rugby) in tetraplegic, paraplegic, or AB athletes. Therefore, despite studies demonstrating benefits in laboratory settings (Chou et al. 2008), the renewable phase change vest seems to be less effective in field settings and its utility remains questionable.
7.6.1.2 Refrigerated Headpieces
Refrigerated headpieces have also been designed. Transfer of heat from the head is the highest per surface area compared to other regions of the body, making it an ideal target. Additionally, the scalp lacks vasoconstrictor innervation (Nunneley et al. 1971), enhancing the cooling capacity of these devices. In AB subjects, cooling the head has been shown to be more effective at reducing core temperature than cooling 60 % of the rest of the body (Kissen et al. 1971). In athletes with SCI, cooling the head tended to attenuate the rise in core temperature during a 30-min wheelchair time trial in the heat (32.9 °C, 75 %rh), however these differences were non-significant (Armstrong et al. 1995). It may be the case that the cooling capacity of the headpiece was not able to keep up with the accelerated heat gain of the SCI subjects compared to AB subjects in previous head-cooling studies.
7.6.1.3 Hand/Foot Cooling
The hands and feet have a high abundance of arteriovenous anastomoses (AVAs), pathways that bypass the high resistance arterioles and capillaries of the papillary loops found in the superficial layer of skin. AVA’s are structurally linked arterioles and venules and are connected without a capillary, and thus do not contribute to nutritive blood flow. They often form a net-like structure that facilitates the transfer of heat. These pathways open under warm conditions, supplying blood to the venous plexus and facilitating heat loss through a countercurrent mechanism. Cooling of the hands and/or feet targets AVAs and has been shown to be effective at promoting heat loss in AB subjects during rest, exercise, and exercise recovery in the heat (Grahn et al. 2005; Livingstone et al. 1995; House et al. 1997). Devices that additionally utilize negative pressure to increase blood flow through AVAs have also been developed (e.g. Rapid Thermal Exchange; AVAcore, Inc., Palo Alto, CA), as the cold stimulus would generally cause vasoconstriction and closure of AVA pathways. However, these have not yet been employed in studies on athletes with SCI.
In SCI subjects, continuous cooling of the feet in a 32 °C 26 %rh environment reduced tympanic temperature at rest and attenuated the rise in tympanic temperature during exercise (1.0 °C increase vs. 1.6 °C increase with no cooling) (Hagobian et al. 2004). Foot cooling also had a slight effect in a comparator AB group, however core temperature under non-cooling conditions increased only minimally in this group. Another study utilized hand cooling in 10 °C water during a 10-min recovery period after 60 min of moderate-intensity intermittent wheelchair exercise in 30.8 °C 61 %rh heat (Goosey-Tolfrey et al. 2008b). In just 10 min, the reduction in core temperature was almost 0.5 °C greater in the hand cooling condition compared to no cooling. Additionally, this intervention improved subsequent performance on a 1k time trial. However, the authors noted that some subjects reported numbness and loss of dexterity, particularly when gripping the wheelchair, and in some case blisters, suggesting hand cooling may not be practical for sports where a high level of dexterity is required.
7.6.1.4 Pre-cooling
For sports in which cooling garments are either not allowed by regulations or would impede the biomechanics of wheelchair propulsion, pre-cooling may be a viable option. Whole-body pre-cooling (immediately before exercise) reduces core temperature and improves exercise performance in AB individuals, but these effects are typically brief (Bolster et al. 1999; Booth et al. 1997). In subjects with SCI, 20 min of pre-cooling using an ice vest reduced core temperature at rest by 0.3 °C and attenuated the rise in core temperature during 30-min of intermittent high-intensity exercise in 32 °C 50 %rh heat to a greater extent than cooling by the same method during exercise (Webborn et al. 2005, 2010). However, pre-cooling had no effect on exercise performance, measured by time to volitional fatigue.
7.6.2 Artificial Sweating/Water Spray
Water spray bottles are a much more commonly used and more cost-effective method of cooling compared to the devices previously described. Athletes with SCI commonly use water spray bottles in both practice and competition to mimic the sweating response that AB athletes experience.
To investigate whether water spray bottles actually affect core temperature and heat strain, Pritchett et al. (2010) studied seven paraplegic individuals during two matched trials involving incremental stages of arm-ergometer exercise with 1-min recovery periods between stages in 22 °C, designed to mimic wheelchair basketball games which typically take place in climate-controlled gymnasiums. In one trial, subjects sprayed themselves ad libitum with a water spray bottle during the 1-min recovery periods. Unfortunately, this resulted in no significant differences in core temperature (esophageal or rectal), mean skin temperature, or thermal sensation, although the authors did observe a slightly lower heart rate by the fourth stage of the water spray trial. Given the limitations of the study, their results do not rule out an advantage of using this strategy. For example, water spraying may be more effective in warmer or drier conditions. Given the large number of athletes with SCI who utilize this strategy and anecdotally notice a difference, it certainly warrants further study.
7.6.3 Heat Acclimation
In able-bodied individuals, heat acclimation is highly effective for reducing heat strain and improving exercise performance in hot environments. Heat acclimation, which typically consists of prolonged low-to-moderate intensity exercise in a hot environment (35–50 °C) for 7–14 consecutive days, has been extensively studied and shown to induce a number of “classical” adaptations. These include reductions in resting core temperature, improved thermoregulation (e.g. higher sweat rate and skin blood flow for a given core temperature secondary to a lowered threshold), and increased plasma volume (Taylor 2014; Périard et al. 2015; Sawka et al. 2000).
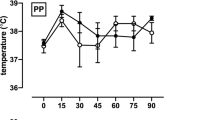
Despite the extensive benefits that have been shown in able-bodied individuals, only two studies have investigated whether heat acclimation could also be a viable strategy for improving thermoregulation and performance in individuals with SCI. Price et al. (2011) observed no effects of 7 days of exercise heat acclimation (30 min of moderate intensity exercise followed by 30 min of rest in the heat) in either paraplegics or tetraplegics, although perceived thermal strain was lower in the paraplegics. Conversely, Castle et al. (2013) demonstrated that 7 days of exercise heat acclimation (20 min of low-intensity arm crank exercise followed by 40 min of rest or target shooting practice) reduced core temperature both at rest (aural temperature from 36.3 to 36.0 °C) and during exercise in the heat (average aural temperature over 60 min of exercise: 37.2–36.7 °C) (Fig. 7.5). Estimated plasma volume increased by 1.5 %; however, increases of 5–7 % are common in able-bodied individuals undergoing 7+ days of heat acclimation (Sawka et al. 2000; Nielsen et al. 1993). No changes in sweat rate, either whole-body or by patch placed on T12 vertebra, or heart rate during exercise were observed. These results indicate that heat acclimation may be advantageous in individuals with SCI; however, further studies are required. For example, the authors suggested only partial heat acclimation may have occurred since they were limited in the duration and intensity of heat exposure sessions as all subjects were Paralympic target-shooting athletes who were competing soon after participating in the study. Longer durations and/or higher target core temperatures would likely induce greater adaptation.
From Castle et al. (2013). Mean ± SD aural temperature during 60 min heat acclimation sessions on day 1 and day 7 in five paralympic wheelchair-dependent target-shooting athletes. Following heat acclimation, subjects experienced a reduction in aural temperature both at rest (prior to entering the hot chamber) and during exercise in the heat
It may also be possible to gain the same benefits of exercise heat acclimation passively, e.g., using hot water immersion or sauna. One such study investigated the effects of 5 days of repeated hot water immersion in paraplegics (Gass and Gass 2001). The only significant change observed in paraplegic subjects was an expansion of plasma volume, estimated using the Dill and Costill (1974) method; however, there were also few significant changes observed in AB subjects (Gass and Gass 2001), suggesting that longer periods of heat exposure are required to elicit adaptation, particularly in response to passive heating.
Two recent studies describe the use of passive heating to improve macrovascular (Brunt et al. 2016a) and microvascular (Brunt et al. 2016b) function in sedentary individuals following 8 weeks of heat therapy. This strategy, utilizing hot water immersion, may prove to be useful for the improvement of vascular health in patients with SCI, but this remains to be studied.
It is also important to note the high degree of variability in responses in this population. The range of injuries and therefore disparate sweat rates and skin blood flow distribution across individuals needs to be considered. As such, extent of acclimation should be determined on an individual basis [for example, by hematocrit after a heat stress test as proposed by Racinais et al. (2012)] rather than applying the same protocols and ambient temperature regulations across entire teams.
7.6.4 Body-Weight-Supported Treadmill Training
Body-weight-supported treadmill (BWST) training is a form of rehabilitation in which patients participate in upright treadmill ambulation with the assistance of a harness and pulley system that is supporting the majority of the patient’s weight. It has previously been shown to improve ambulation (Hicks et al. 2005), partially reverse muscle atrophy (Giangregorio et al. 2006), and improve heart rate and blood pressure variability (Ditor et al. 2005) following SCI, among other advantages. Cotie et al. (2010) demonstrated that 4 weeks of BWST training lowers resting leg skin temperatures. The authors approached this question from the standpoint of utilizing BWST training to reduce the incidence of pressure ulcers, as these have been associated with higher skin temperatures (Bergstrom and Braden 1992; Fisher et al. 1978). Higher skin temperatures increase the metabolic demand of the tissue, increasing ischemic tissue damage; however, lower resting skin temperature could also prove advantageous for facilitating heat loss during exercise. Of course, future studies are required to determine whether BWST actually provides any thermoregulatory benefit during exercise and/or during heat exposure.
7.6.5 Strategies for Thermoregulation During Exercise in Cool Environments
Studies investigating strategies for thermoregulation in individuals with SCI have focused on preventing heat-related illness in warm environments. However, it is important to also consider how thermoregulatory impairment can be mitigated during exercise in cold environments. This issue is particularly timely given the rise in participation in winter sports like skiing by individuals with SCI.
The two main dangers during exposure to cold environments include the risk of hypothermia and frostbite. In terms of hypothermia, there are two main types: primary and secondary hypothermia. Primary hypothermia occurs when a person is subjected to extreme cold, whereas secondary refers to hypothermia in relatively mild or modest environmental conditions caused by an inability to adequately regulate body core temperature, as can occur with certain medications. Thus, the likely causes of hypothermia in an individual with SCI include inappropriate vasodilation (possibly medication induced), inappropriate sweating, or inadequate heat production through metabolism (Menard and Hahn 1991). In the latter case, this can be due to the lack of shivering below the lesion or due to reduced thyroid function, which has been reported in SCI (Bloch 1986). In both cases, the best practice is avoidance of extremely cold temperatures for those individuals with impaired thermoregulatory responses. In extreme cold conditions, frostbite can be a significant risk to the SCI athlete, especially in insensate areas of the skin. Direct contact with the metal on wheelchairs can increase this risk in both sensate and insensate areas. For athletes with SCI, it is important to customize clothing based on their personal thermoregulatory impairment, the intensity and duration of exercise they will be performing, and of course the environmental conditions. The transitions between exercise and recovery can pose risks for hypothermia as well, with rapid cooling and a drop in body core temperature if the individual was sweating or clothing became saturated. Thus, proper clothing is important to the individual with SCI as much as able-bodied athletes.
7.7 Conclusions
With the possible exception of very low level SCI in temperate conditions, overall thermoregulatory function is diminished in individuals with SCI during exposure to hot or cold environments. While a reduced capacity for vasoconstriction and shivering places these individuals at greater risk of hypothermia, a greater risk centers on the dangers of hyperthermia. The reduced ability to thermoregulate in hot environments places these individuals at much higher risk of heat-related illness and death during environmental extremes such as climatic heat waves and during exercise in a hot environment. The thermoregulatory challenge for this population is primarily due to the limited ability to adequately raise skin blood flow and actively sweat in order to match heat loss to heat gain from the environment or the metabolic heat produced during exercise. The total area of sensate to insensate skin is a strong predictor of their ability to tolerate a given thermal stress; however, thermoregulatory responses are diminished in sensate area of skin as well, although the exact reasons for this are not clear and likely vary between individuals. There seems to be increasing interest in finding novel approaches to protect SCI athletes from heat-related disorders during training and competition, however more research is needed to better determine ideal approaches. There is also a need to better understand the limits to how much adaptation is possible in people with SCI, so that exercise programs and heat acclimation paradigms could be designed to limit the risk in this vulnerable population.
References
Armstrong LE, Maresh CM, Riebe D, Kenefick RW, Castellani JW, Senk JM et al (1995) Local cooling in wheelchair athletes during exercise-heat stress. Med Sci Sports Exerc 27(2):211–216
Asmussen E, Nielsen M (1947) The regulation of the body-temperature during work performed with the arms and with the legs. Acta Physiol Scand 14(4):373–382
Attia M, Engel P (1983) Thermoregulatory set point in patients with spinal cord injuries (spinal man). Paraplegia 21(4):233–248
Attia M, Engel P (1984) Thermoregulatory set-point in paraplegics. In: Hales JR (ed) Thermal physiology. Raven Press, New York, pp. 79–82
Bennett BL, Hagan RD, Huey KA, Minson C, Cain D (1995) Comparison of two cool vests on heat-strain reduction while wearing a firefighting ensemble. Eur J Appl Physiol Occup Physiol 70(4):322–328
Bennett LAT, Johnson JM, Stephens DP, Saad AR, Kellogg DL (2003) Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552(1):223–232
Berard EJ, Boucand MH, Depassio J, Fyon JP (1989) Effects of bethanechol and adreno-blockers on thermoregulation in spinal cord injury. Paraplegia 27(1):46–50
Bergstrom N, Braden BA (1992) Aprospective study of pressure sore risk among institutionalized elderly. J Am Geriatr Soc 40(8):747–758
Bell DG, Tikuisis P, Jacobs I (1992) Relative intensity of muscular contraction during shivering. J Appl Physiol 72(6):2336–2342
Bloch RF (1986) Autonomic dysfunction. In: Block RF, Basbaum M (eds) Management of spinal cord injuries. Williams & Wilkins, Baltimore, pp. 149–163
Bolster DR, Trappe SW, Short KR, Scheffield-Moore M, Parcell AC, Schulze KM et al (1999) Effects of precooling on thermoregulation during subsequent exercise. Med Sci Sports Exerc 31(2):251–257
Boot CRL, Binkhorst RA, Hopman MTE (2006) Body temperature responses in spinal cord injured individuals during exercise in the cold and heat. Int J Sports Med 27(8):599–604
Booth J, Marino F, Ward JJ (1997) Improved running performance in hot humid conditions following whole body precooling. Med Sci Sports Exerc 29(7):943–949
Brunt VE, Minson CT (2012) KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590(15):3523–3534
Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT (2016a) Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594(18):5329–5342
Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT (2016b) Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol 121(3):716–723
Cariga P, Catley M, Mathias CJ, Savic G, Frankel HL, Ellaway PH (2002) Organisation of the sympathetic skin response in spinal cord injury. J Neurol Neurosurg Psychiatry 72(3):356–360
Castle PC, Kularatne BP, Brewer J, Mauger AR, Austen RA, Tuttle JA et al (2013) Partial heat acclimation of athletes with spinal cord lesion. Eur J Appl Physiol 113(1):109–115
Choi PJ, Brunt VE, Fujii N, Minson CT (2014) New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol 117(3):277–283
Chou C, Tochihara Y, Kim T (2008) Physiological and subjective responses to cooling devices on firefighting protective clothing. Eur J Appl Physiol 104(2):369–374
Christensen NJ, Galbo H (1983) Sympathetic nervous activity during exercise. Annu Rev Physiol 45:139–153
Cotie LM, Geurts CLM, Adams MME, MacDonald MJ (2010) Leg skin temperature with body-weight-supported treadmill and tilt-table standing training after spinal cord injury. Spinal Cord 49(1):149–153
Coutts KD, Rhodes EC, McKenzie DC (1983) Maximal exercise responses of tetraplegics and paraplegics. J Appl Physiol Respir Environ Exerc Physiol 55(2):479–482
Coyle EF, González-Alonso J (2001) Cardiovascular drift during prolonged exercise: new perspectives. Exerc Sport Sci Rev. 29(2):88–92
Davis GM (1993) Exercise capacity of individuals with paraplegia. Med Sci Sports Exerc 25(4):423–432
Davis GM, Servedio FJ, Glaser RM, Gupta SC, Suryaprasad AG (1990) Cardiovascular responses to arm cranking and FNS-induced leg exercise in paraplegics. J Appl Physiol 69(2):671–677
Dawson B, Bridle J, Lockwood RJ (1994) Thermoregulation of paraplegic and able bodied men during prolonged exercise in hot and cool climates. Paraplegia 32(12):860–870
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248
Ditor DS, Kamath MV, MacDonald MJ, Bugaresti J, McCartney N, Hicks AL (2005) Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J Appl Physiol 98(4):1519–1525
Downey JA, Chiodi HP, Darling RC (1967) Central temperature regulation in the spinal man. J Appl Physiol 22(1):91–94
Downey JA, Huckaba CE, Darling RC (1971) The effect of skin and central cooling on human thermoregulation. Int J Biometeorol 15(2):171–175
Downey JA, Huckaba CE, Myers SJ, Darling RC (1973) Thermoregulation in the spinal man. J Appl Physiol 34(6):790–794
Downey JA, Huckaba CE, Kelley PS, Tam HS, Darling RC, Cheh HY (1976) Sweating responses to central and peripheral heating in spinal man. J Appl Physiol 40(5):701–706
Duffield R, Dawson B, Bishop D, Fitzsimons M, Lawrence S (2003) Effect of wearing an ice cooling jacket on repeat sprint performance in warm/humid conditions. Br J Sports Med 37(2):164–169
Ekblom B, Greenleaf CJ, Greenleaf JE, Hermansen L (1971) Temperature regulation during continuous and intermittent exercise in man. Acta Physiol Scand 81(1):1–10
Erickson RP (1980) Autonomic hyperreflexia: pathophysiology and medical management. Arch Phys Med Rehabil 61(10):431–440
Fisher SV, Szymke TE, Apte SY, Kosiak M. Wheelchair cushion effect on skin temperature. Arch Phys Med Rehabil 1978 59(2):68–72
Fitzgerald PI, Sedlock DA, Knowlton RG (1990) Circulatory and thermal adjustments to prolonged exercise in paraplegic women. Med Sci Sports Exerc 22(5):629–635
Freund PR, Brengelmann GL, Rowell LB, Halar E (1984) Attenuated skin blood flow response to hyperthermia in paraplegic men. J Appl Physiol 56(4):1104–1109
Fritzsche RG, Switzer TW, Hodgkinson BJ, Coyle EF (1999) Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. J Appl Physiol 86(3):799–805
Gass GC, Camp EM (1984) The maximum physiological responses during incremental wheelchair and arm cranking exercise in male paraplegics. Med Sci Sports Exerc 16:355–359
Gass EM, Gass GC (2001) Thermoregulatory responses to repeated warm water immersion in subjects who are paraplegic. Spinal Cord 39(3):149–155
Gass GC, Camp EM, Nadel ER, Gwinn TH, Engel P (1988) Rectal and rectal vs. esophageal temperatures in paraplegic men during prolonged exercise. J Appl Physiol 64(6):2265–2271
Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM et al (2006) Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab 31(3):283–291
Giesbrecht GG, Jamieson C, Cahill F (2007) Cooling hyperthermic firefighters by immersing forearms and hands in 10 degrees C and 20 degrees C water. Aviat Space Environ Med 78(6):561–567
Goosey-Tolfrey VL, Diaper NJ, Crosland J, Tolfrey K (2008a) Fluid intake during wheelchair exercise in the heat: effects of localized cooling garments. Int J Sports Phys Perform 3(2):145–156
Goosey-Tolfrey V, Swainson M, Boyd C, Atkinson G, Tolfrey K (2008b) The effectiveness of hand cooling at reducing exercise-induced hyperthermia and improving distance-race performance in wheelchair and able-bodied athletes. J Appl Physiol 105(1):37–43
Grahn DA, Cao VH, Heller HC (2005) Heat extraction through the palm of one hand improves aerobic exercise endurance in a hot environment. J Appl Physiol 99(3):972–978
Green DJ, Maiorana A, O’Driscoll G, Taylor R (2004) Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561(1):1–25
Griggs KE, Price MJ, Goosey-Tolfrey VL (2014) Cooling athletes with a spinal cord injury. Sports Med 45(1):9–21
Griggs KE, Leicht CA, Price MJ, Goosey-Tolfrey VL (2015) Thermoregulation During Intermittent Exercise in Athletes With a Spinal-Cord Injury. Int J Sports Phys Perform 10(4):469–475
Groothuis JT, Thijssen DHJ, Rongen GA, Deinum J, Danser AHJ, Geurts ACH et al (2010) Angiotensin II contributes to the increased baseline leg vascular resistance in spinal cord-injured individuals. J Hypertens 28(10):2094–2101
Guttmann L (1976) Textbook of sport for the disabled. HM & M Publishers, Aylesbury
Guttmann L, Whitteridge D (1947) Effects of bladder distension on autonomic mechanisms after spinal cord injuries. Brain 70:361–404
Guttmann L, Silver J, Wyndham CH (1958) Thermoregulation in spinal man. J Physiol 142(3):406–419
Hagobian TA, Jacobs KA, Kiratli BJ, Friedlander AL (2004) Foot cooling reduces exercise-induced hyperthermia in men with spinal cord injury. Med Sci Sports Exerc 36(3):411–417
Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y et al (2003) Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107(25):3152–3158
Hasegawa H, Takatori T, Komura T, Yamasaki M (2005) Wearing a cooling jacket during exercise reduces thermal strain and improves endurance exercise performance in a warm environment. J Strength Cond Res 19(1):122–128
Head H, Riddoch G (1917) The automatic bladder, excessive sweating and some other reflex conditions in gross injuries of the spinal cord. Brain 40:188
Hicks AL, Adams MM, Martin Ginis K, Giangregorio L, Latimer A, Phillips SM et al (2005) Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 43(5):291–298
Holmes G (1915) The goulstonian lectures on spinal injuries of warfare II: the clinical symptoms of gunshot wounds to the spine. Br Med J 2(2865):815–821
Holowatz LA, Kenney WL (2007) Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 293(2):H1090–H1096
Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL et al (2003) Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284(5):H1662–H1667
Hopman MT (1994) Circulatory responses during arm exercise in individuals with paraplegia. Int J Sports Med 15(3):126–131
Hopman MT, Oeseburg B, Binkhorst RA (1992a) Cardiovascular responses in paraplegic subjects during arm exercise. Eur J Appl Physiol Occup Physiol 65(1):73–78
Hopman MT, Oeseburg B, Binkhorst RA (1992b) The effect of an anti-G suit on cardiovascular responses to exercise in persons with paraplegia. Med Sci Sports Exerc. 24(9):984–990
Hopman MT, Oeseburg B, Binkhorst RA (1993a) Cardiovascular responses in persons with paraplegia to prolonged arm exercise and thermal stress. Med Sci Sports Exerc 25(5):577–583
Hopman MT, Pistorius M, Kamerbeek IC, Binkhorst RA (1993b) Cardiac output in paraplegic subjects at high exercise intensities. Eur J Appl Physiol Occup Physiol 66(6):531–535
Hopman MT, Monroe M, Dueck C, Phillips WT, Skinner JS (1998) Blood redistribution and circulatory responses to submaximal arm exercise in persons with spinal cord injury. Scand J Rehabil Med 30(3):167–174
Houghton BL, Meendering JR, Wong BJ, Minson CT (2006) Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572(Pt 3):811–820
House JR, Holmes C, Allsopp AJ (1997) Prevention of heat strain by immersing the hands and forearms in water. J R Nav Med Serv 83(1):26–30
House JR, Lunt HC, Taylor R, Milligan G, Lyons JA, House CM (2013) The impact of a phase-change cooling vest on heat strain and the effect of different cooling pack melting temperatures. Eur J Appl Physiol 113(5):1223–1231
Huckaba CE, Frewin DB, Downey JA, Tam HS, Darling RC, Cheh HY (1976) Sweating responses of normal, paraplegic and anhidrotic subjects. Arch Phys Med Rehabil 57(6):268–274
Hutchinson J (1875) Clinical lecture on temperature and circulation after crushing of cervical spinal cord. Lancet 105:747–749
Johnson BB, Johnson RE (1970) Training of eccrine sweat glands in paraplegics. J Invest Dermatol 54(3):229–232
Johnson JM, Minson CT, Kellogg DL (2014) Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4:33–89
Kay D, Taaffe DR, Marino FE (1999) Whole-body pre-cooling and heat storage during self-paced cycling performance in warm humid conditions. J Sports Sci 17(12):937–944
Kellogg DL, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M et al (1995) Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77(6):1222–1228
Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM (1998) Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85(3):824–829
Kellogg DL, Liu Y, Kosiba IF, O’Donnell D (1999) Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86(4):1185–1190
Kinzer SM, Convertino VA (1989) Role of leg vasculature in the cardiovascular response to arm work in wheelchair-dependent populations. Clin Physiol 9(6):525–533
Kissen AT, Hall JF, Klemm FK (1971) Physiological responses to cooling the head and neck versus the trunk and leg areas in severe hyperthermic exposure. Aerosp Med 42(8):882–888
Knutsson E, Lewenhaupt-Olsson E, Thorsen M (1973) Physical work capacity and physical conditioning in paraplegic patients. Paraplegia 11(3):205–216
Kvernmo HD, Stefanovska A, Kirkebøen KA, Osterud B, Kvernebo K (1998) Enhanced endothelium-dependent vasodilatation in human skin vasculature induced by physical conditioning. Eur J Appl Physiol Occup Physiol 79(1):30–36
Lenasi H, Strucl M (2004) Effect of regular physical training on cutaneous microvascular reactivity. Med Sci Sports Exerc 36(4):606–612
Lind AR (1963) A physiological criterion for setting thermal environmental limits for everyday work. J Appl Physiol 18:51–56
Livingstone SD, Nolan RW, Keefe AA (1995) Heat loss caused by cooling the feet. Aviat Space Environ Med 66(3):232–237
Lorenzo S, Minson CT (2010) Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. J Appl Physiol 109(6):1736–1743
Marino FE (2002) Methods, advantages, and limitations of body cooling for exercise performance. Br J Sports Med 36(2):89–94
Mathias CJ, Frankel HL (1988) Cardiovascular control in spinal man. Annu Rev Physiol 50:577–592
McCord GR, Cracowski J-L, Minson CT (2006) Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291(3):R596–R602
Menard MR, Hahn G (1991) Acute and chronic hypothermia in a man with spinal cord injury: environmental and pharmacologic causes. Arch Phys Med Rehabil 72(5):421–424
Miles DS, Sawka MN, Glaser RM, Petrofsky JS (1983) Plasma volume shifts during progressive arm and leg exercise. J Appl Physiol Respir Environ Exerc Physiol 54(2):491–495
Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL (1999) Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol 276(1 Pt 2):R203–R212
Minson CT, Berry LT, Joyner MJ (2001) Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91:1619–1626
Mitchell JW, Nadel ER, Stolwijk JA (1972) Respiratory weight losses during exercise. J Appl Physiol 32(4):474–476
Muraki S, Yamasaki M, Ishii K, Kikuchi K, Seki K (1995) Effect of arm cranking exercise on skin blood flow of lower limb in people with injuries to the spinal cord. Eur J Appl Physiol Occup Physiol 71(1):28–32
Muraki S, Yamasaki M, Ishii K, Kikuchi K, Seki K (1996a) Relationship between core temperature and skin blood flux in lower limbs during prolonged arm exercise in persons with spinal cord injury. Eur J Appl Physiol Occup Physiol 72(4):330–334
Muraki S, Yamasaki M, Ehara Y, Kikuchi K, Seki K (1996b) Effect of maximal arm exercise on skin blood flux in the paralyzed lower limbs in persons with spinal cord injury. Eur J Appl Physiol Occup Physiol 74(5):481–483
Nicotra A, Asahina M, Mathias CJ (2004) Skin vasodilator response to local heating in human chronic spinal cord injury. Eur J Neurol 11(12):835–837
Nielsen B (1968) Thermoregulatory responses to arm work, leg work and intermittent leg work. Acta Physiol Scand 72(1):25–32
Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B (1993) Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 460:467–485
Normell LA (1974) Distribution of impaired cutaneous vasomotor and sudomotor function in paraplegic man. Scand J Clin Lab Invest Suppl 138:25–41
NSCISC (2015) NSCISC annual statistical report. United States National Spinal Cord Injury Statistical Center
Nunneley SA, Troutman SJ, Webb P (1971) Head cooling in work and heat stress. Aerosp Med 42(1):64–68
Ogawa T, Asayama M (1978) Frequency of sweat expulsion as indicator of sudomotor neural activity. In: Houdas Y, Guieu JD (eds) Trends in thermal physiology. Masson, Paris, pp. 105–107
Pembrey MS (1897) The temperature of man and animals after section of the spinal cord. Br Med J 2:883–884
Périard JD, Racinais S, Sawka MN (2015) Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand J Med Sci Sports 25(Suppl 1):20–38
Petrofsky JS (1992) Thermoregulatory stress during rest and exercise in heat in patients with a spinal cord injury. Eur J Appl Physiol Occup Physiol 64(6):503–507
Pimental NA, Sawka MN, Billings DS, Trad LA (1984) Physiological responses to prolonged upper-body exercise. Med Sci Sports Exerc 16(4):360–365
Pollock LJ, Boshes B, Chor H, Finkelman I, Arieff AJ, Brown CM (1951) Defects in regulatory mechanisms of autonomic function in injuries to spinal cord. J Neurophysiol 14(2):85–93
Price MJ (2006) Thermoregulation during exercise in individuals with spinal cord injuries. Sports Med 36:863–879
Price MJ (2016) Preparation of paralympic athletes; environmental concerns and heat acclimation. Front Physiol 6:302
Price MJ, Campbell IG (1997) Thermoregulatory responses of paraplegic and able-bodied athletes at rest and during prolonged upper body exercise and passive recovery. Eur J Appl Physiol Occup Physiol 76(6):552–560
Price MJ, Campbell IG (1999a) Thermoregulatory responses of spinal cord injured and able-bodied athletes to prolonged upper body exercise and recovery. Spinal Cord 37(11):772–779
Price MJ, Campbell IG (1999b) Thermoregulatory and physiological responses of wheelchair athletes to prolonged arm crank and wheelchair exercise. Int J Sports Med 20(7):457–463
Price MJ, Campbell IG (2003) Effects of spinal cord lesion level upon thermoregulation during exercise in the heat. Med Sci Sports Exerc 35(7):1100–1107
Price MJ, Kiratli BJ, Brand M (2011) The effects of a 7-day heat acclimation protocol in persons with paraplegia and tetraplegia. In. Proceedings of the 14th international conference on environmental ergonomics, Nafplio, Greece, July 2011
Pritchett RC, Bishop PA, Yang Z, Pritchett KL, Green JM, Katica CP et al (2010) Evaluation of artificial sweat in athletes with spinal cord injuries. Eur J Appl Physiol 109(1):125–131
Racinais S, Mohr M, Buchheit M, Voss SC, Gaoua N, Grantham J et al (2012) Individual responses to short-term heat acclimatisation as predictors of football performance in a hot, dry environment. Br J Sports Med 46(11):810–815
Randall WC, Wurster RD, Lewin RJ (1966) Responses of patients with high spinal transection to high ambient temperatures. J Appl Physiol 21(3):985–993
Reitz A, Schmid DM, Curt A, Knapp PA, Schurch B (2002) Sympathetic sudomotor skin activity in human after complete spinal cord injury. Auton Neurosci 102(1–2):78–84
Roberts MF, Wenger CB, Stolwijk JA, Nadel ER (1977) Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol Respir Environ Exerc Physiol 43(1):133–137
Rothe CF (1983) Reflex control of veins and vascular capacitance. Physiol Rev 63(4):1281–1342
Rowell LB (1986) Human circulation: regulation during physical stress. Oxford University Press, New York, 44 p
Sawka MN, Gonzalez RR, Drolet LL, Pandolf KB (1984) Heat exchange during upper- and lower-body exercise. J Appl Physiol Respir Environ Exerc Physiol 57(4):1050–1054
Sawka MN, Latzka WA, Pandolf KB (1989) Temperature regulation during upper body exercise: able-bodied and spinal cord injured. Med Sci Sports Exerc 21(5 Suppl):S132–S140
Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ (2000) Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc 32(2):332–348
Seckendorf R, Randall WC (1961) Thermal reflex sweating in normal and paraplegic man. J Appl Physiol 16:796–800
Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ (1998) Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85(3):830–834
Sherrington CS (1924) Notes on temperature after spinal transection, with some observations on shivering. J Physiol 58(6):405–424
Silver JR (2000) Early autonomic dysreflexia. Spinal Cord 38(4):229–233
Silver JR, Randall WC, Guttmann L (1991) Spinal mediation of thermally induced sweating. J Neurol Neurosurg Psychiatry 54(4):297–304
Tam HS, Darling RC, Cheh HY, Downey JA (1978a) Sweating response: a means of evaluating the set-point theory during exercise. J Appl Physiol Respir Environ Exerc Physiol 45(3):451–458
Tam HS, Darling RC, Cheh HY, Downey JA (1978b) The dead zone of thermoregulation in normal and paraplegic man. Can J Physiol Pharmacol 56(6):976–983
Taylor NAS (2014) Human Heat Adaptation. Compr Physiol 4:325–365
Theisen D, Vanlandewijck Y, Sturbois X, Francaux M (2001) Cutaneous vascular response and thermoregulation in individuals with paraplegia during sustained arm-cranking exercise. Int J Sports Med 22(2):97–102
Thijssen DHJ, Ellenkamp R, Kooijman M, Pickkers P, Rongen GA, Hopman MTE et al (2007) A causal role for endothelin-1 in the vascular adaptation to skeletal muscle deconditioning in spinal cord injury. Arterioscler Thromb Vasc Biol 27(2):325–331
Thomas CM, Pierzga JM, Kenney WL (1999) Aerobic training and cutaneous vasodilation in young and older men. J Appl Physiol 86(5):1676–1686
Toner MM, Sawka MN, Pandolf KB (1984) Thermal responses during arm and leg and combined arm-leg exercise in water. J Appl Physiol Respir Environ Exerc Physiol 56(5):1355–1360
Totel GL (1974) Physiological responses to heat of resting man with impaired sweating capacity. J Appl Physiol 37(3):346–352
Totel GL, Johnson RE, Fay FA, Goldstein JA, Schick J (1971) Experimental hyperthermia in traumatic quadriplegia. Int J Biometeorol 15(2):346–355
Trbovich M, Ortega C, Schroeder J, Fredrickson M (2014) Effect of a cooling vest on core temperature in athletes with and without spinal cord injury. Top Spinal Cord Inj Rehabil 20(1):70–80
Van Duijnhoven NTL, Janssen TWJ, Green DJ, Minson CT, Hopman MTE, Thijssen DHJ (2009) Effect of functional electrostimulation on impaired skin vasodilator responses to local heating in spinal cord injury. J Appl Physiol 106(4):1065–1071
Van Loan MD, McCluer S, Loftin JM, Boileau RA (1987) Comparison of physiological responses to maximal arm exercise among able-bodied, paraplegics and quadriplegics. Paraplegia. 25(5):397–405
Vassalle C, Lubrano V, Domenici C, L'Abbate A (2003) Influence of chronic aerobic exercise on microcirculatory flow and nitric oxide in humans. Int J Sports Med 24(1):30–35
Webborn N, Price MJ, PC C, Goosey-Tolfrey VL (2005) Effects of two cooling strategies on thermoregulatory responses of tetraplegic athletes during repeated intermittent exercise in the heat. J Appl Physiol 98(6):2101–2107
Webborn N, Price MJ, Castle P, Goosey-Tolfrey VL (2010) Cooling strategies improve intermittent sprint performance in the heat of athletes with tetraplegia. Br J Sports Med 44(6):455–460
White AT, Davis SL, Wilson TE (2003) Metabolic, thermoregulatory, and perceptual responses during exercise after lower vs. whole body precooling. J Appl Physiol 94(3):1039–1044
Wilkins BW, Holowatz LA, Wong BJ, Minson CT (2003) Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol 548(3):963–969
Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT (2004) Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol 97(4):1291–1298
Wyndham CH (1955) Thermoregulation in the human spinal cord isolated above the thoracic sympathetic outflow. S Afr J Med Sci 20:93
Yaggie JA, Niemi TJ, Buono MJ (2002) Adaptive sweat gland response after spinal cord injury. Arch Phys Med Rehabil 83(6):802–805
Yamasaki M, Shiokawa M, Choi SW, Muraki S (2000) Effect of acute heat exposure on skin blood flow of the paralyzed thigh in persons with spinal cord injury. Spinal Cord 38(4):224–228
Yamasaki M, Kim KT, Choi SW, Muraki S, Shiokawa M, Kurokawa T (2001) Characteristics of body heat balance of paraplegics during exercise in a hot environment. J Physiol Anthropol Appl Human Sci 20(4):227–232
Zoladz JA, Rademaker AC, Sargeant AJ (1995) Non-linear relationship between O2 uptake and power output at high intensities of exercise in humans. J Physiol 488(Pt 1):211–217
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 The American Physiological Society
About this chapter
Cite this chapter
Minson, C.T., Brunt, V.E. (2016). Thermoregulatory Considerations for the Performance of Exercise in SCI. In: Taylor, J. (eds) The Physiology of Exercise in Spinal Cord Injury. Physiology in Health and Disease. Springer, Boston, MA. https://doi.org/10.1007/978-1-4939-6664-6_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6664-6_7
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4939-6662-2
Online ISBN: 978-1-4939-6664-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)