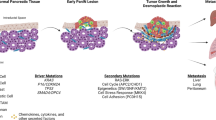
Abstract
Pancreatic cancer tumor microenvironment (TME), simply defined as the noncancerous desmoplastic reaction, is considered a key player in all aspects of tumor growth, and progression. The dismal prognosis of pancreatic cancer and disappointing clinical trials has drawn our attention to the TME, particularly to the tumor-stromal interactions. While a myriad of molecular, pathological, and clinical features contribute to the lethality of pancreatic cancer, local invasiveness and distant metastases is a hallmark and leading cause of mortality and morbidity in this ominous cancer. Cancer-associated stromal cells including stellate cells have been implicated in epithelial mesenchymal transition (EMT), a process involved in invasion and metastases. In addition, the pre-metastatic niche, immune evasion, and enhancement of angiogenesis have been attributed to these cells. Interactions of the tumor stromal complex operate as a command and logistics center for pancreatic cancer cells, triggering and maintaining invasiveness and metastases. Understanding and modulating these interactions is a promising strategy to tame one of the most aggressive human cancers to date.
Similar content being viewed by others
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014;74(11):2913–21.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. https://doi.org/10.3322/caac.21208. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24399786%0A.
Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo C F-d, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP, Je M. Is there a survival difference for R1 resections versus locally advanced Unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257(4):2–7.
Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10(4):511–8.
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15028824%5Cn, http://www.nejm.org/doi/pdf/10.1056/NEJMoa032295.
Das S, Batra SK. Pancreatic cancer metastasis: are we being pre-EMTed? Curr Pharm Des. 2015;21(10):1249–55.
Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008;149(2):319–28.
Wilson JS, Pirola RC, Apte MV. Stars and stripes in pancreatic cancer: role of stellate cells and stroma in cancer progression. Front Physiol. 2014;5 FEB(February):1–11.
Nielsen MFB, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol. 2016;22:2678.
Pandol SJ, Edderkaoui M. What are the macrophages and stellate cells doing in pancreatic adenocarcinoma? Front Physiol. 2015;6(May):125. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4432577&tool=pmcentrez&rendertype=abstract.
Xu Z, Pothula SP, Wilson JS, Apte MV. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol. 2014;20(32):11216–29.
Wörmann SM, Diakopoulos KN, Lesina M, Algül H. The immune network in pancreatic cancer development and progression. Oncogene. 2014;33(23):2956–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23851493.
Kabashima-Niibe A, Higuchi H, Takaishi H, Masugi Y, Matsuzaki Y, Mabuchi Y, et al. Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013;104(2):157–64.
Sarkar FH, Li Y, Wang Z, Kong D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009;64(5):489–500.
Amit M, Gil Z. Macrophages increase the resistance of pancreatic adenocarcinoma cells to gemcitabine by upregulating cytidine deaminase. Oncoimmunology. 2013;2(12):e27231. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24498570%5Cn, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3912006.
Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, Wilson JS. Pancreatic cancer: the microenvironment needs attention too. Pancreatology. 2015;15(4):S32–8.
Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–76.
Horimoto Y, Polanska UM, Takahashi Y, Orimo A. Emerging roles of the tumor-associated stroma in promoting tumor metastasis. Cell Adhes Migr. 2012;6(3):193–202.
von Ahrens D, Bhagat TD, Nagrath D, Maitra A, Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol. 2017;10(1):76. Available from: http://jhoonline.biomedcentral.com/articles/10.1186/s13045-017-0448-5.
Galvan JA, et al. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Brit J Cancer. 2015;112(12):1944–50.
Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fi broblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–96. https://doi.org/10.1016/j.addr.2015.07.007.
Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: are there any therapeutic targets? Cancer Lett. 2014;343(2):147–55. https://doi.org/10.1016/j.canlet.2013.09.039.
Pillarisetty VG. The pancreatic cancer microenvironment: an immunologic battleground. Oncoimmunology. 2014;3(8):e950171. https://doi.org/10.4161/21624011.2014.950171%5Cn. Available from: http://www.tandfonline.com/doi/full/10.4161/21624011.2014.950171?mobileUi=0&#.VNzp0JjF9ZI%5Cn, http://www.tandfonline.com/doi/pdf/10.4161/21624011.2014.950171.
Mielgo A, Schmid MC. Impact of tumour associated macrophages in pancreatic cancer. BMB Rep. 2013;46(3):131–8.
Porembka MR, Mitchem JB, Belt BA, Hsieh C-S, Lee H-M, Herndon J, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61(9):1373–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22215137%5Cn, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3697836.
Greten TF. Myeloid-derived suppressor cells in pancreatic cancer: more than a hidden barrier for antitumour immunity? Gut. 2014;63(11):2014–6.
Maity G, Mehta S, Haque I, Dhar K, Sarkar S, Banerjee SK, et al. Pancreatic tumor cell secreted CCN1/Cyr61 promotes endothelial cell migration and aberrant neovascularization. Sci Rep. 2014;4:4995. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24833309.
Xie D, Xie K. Pancreatic cancer stromal biology and therapy. Genes Dis. 2015;2(2):133–43. https://doi.org/10.1016/j.gendis.2015.01.002.
Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Löhr JM, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121(4):699–705.
Grzesiak JJ, Ho JC, Moossa AR, Bouvet M. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas. 2007;35(4):293–301.
Neesse A, Algül H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64(9):1476–84. Available from: http://gut.bmj.com/lookup/doi/10.1136/gutjnl-2015-309304%5Cn, http://www.ncbi.nlm.nih.gov/pubmed/25994217.
Whipple CA. Tumor talk: understanding the conversation between the tumor and its microenvironment. Cancer Cell Microenviron. 2015;2(2):e773.
Hamada S, Masamune A, Shimosegawa T. Alteration of pancreatic cancer cell functions by tumor-stromal cell interaction. Front Physiol. 2013;4 NOV(November):1–7.
Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Molecular mechanism of pancreatic cancer – understanding proliferation, invasion, and metastasis. Langenbeck’s Arch Surg. 2010;395:295–308.
Schlitter AM, Segler A, Steiger K, Michalski CW, Jäger C, Konukiewitz B, et al. Molecular, morphological and survival analysis of 177 resected pancreatic ductal adenocarcinomas (PDACs): identification of prognostic subtypes. Sci Rep. 2017;7(December 2016):41064. Available from: http://www.nature.com/articles/srep41064.
Grage-Griebenow E, Schäfer H, Sebens S. The fatal alliance of cancer and T cells: how pancreatic tumor cells gather immunosuppressive T cells. Oncoimmunology. 2014;3(June):e29382. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4126073&tool=pmcentrez&rendertype=abstract.
Clark CE, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–27.
Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3- dependent manner. Cancer Res. 2013;73(10):3007–18.
Masamune A, Shimosegawa T. Pancreatic stellate cells: a dynamic player of the intercellular communication in pancreatic cancer. Clin Res Hepatol Gastroenterol. 2015;39:S98–103. https://doi.org/10.1016/j.clinre.2015.05.018.
Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145(5):1121–32. https://doi.org/10.1053/j.gastro.2013.07.025.
Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol. 2014;92(6): 543–52. https://doi.org/10.1038/icb.2014.22.
Hu J, Jo M, Eastman BM, Gilder AS, Bui JD, Gonias SL. UPAR induces expression of transforming growth factor β And interleukin-4 in cancer cells to promote tumor-permissive conditioning of macrophages. Am J Pathol. 2014;184(12):3384–93. https://doi.org/10.1016/j.ajpath.2014.08.003.
Ma Y, Hwang RF, Logsdon CD, Ullrich SE. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013;73(13):3927–37.
Sainz B, Martín B, Tatari M, Heeschen C, Guerra S. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Cancer Res. 2014;74(24):7309–20.
Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63(5):513–28.
Grage-Griebenow E, Jerg E, Gorys A, Wicklein D, Wesch D, Freitag-Wolf S, et al. L1CAM promotes enrichment of immunosuppressive T cells in human pancreatic cancer correlating with malignant progression. Mol Oncol. 2014;8(5):982–97.
Lutz ER, Kinkead H, Jaffee EM, Zheng L. Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology. 2014;3(11):e962401. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4292514&tool=pmcentrez&rendertype=abstract.
Ma Y, Ullrich SE. Intratumoral mast cells promote the growth of pancreatic cancer. Oncoimmunology. 2013;2(October):10–2.
Martínez-Bosch N, Fernández-Barrena MG, Moreno M, Ortiz-Zapater E, André S, Gabius H-J, Hwang RF, Poirier F, Munné-Collado J, Iglesias M, Navas C, Guerra C, Fernández-Zapico ME, Navarro P. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and hedgehog signaling activation Neus. Cancer Res. 2014;74(13):3512–24.
Martínez-Bosch N, Navarro P. Targeting Galectin-1 in pancreatic cancer: immune surveillance on guard. Oncoimmunology. 2014;3(8):e952201. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4292238&tool=pmcentrez&rendertype=abstract.
Mace TA, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells: a viable target for reducing immunosuppression in the tumor microenvironment. Oncoimmunology. 2013;2(7):e24891. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3782129&tool=pmcentrez&rendertype=abstract.
Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25(1):76–84. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:new+england+journal#2.
Grosse-Steffen T, et al. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol. 2012.
Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44.
Soares KC, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38(1):1–11.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Hamada S, et al. Regulators of epithelial mesenchymal transition in pancreatic cancer. Front Physiol. 2012;3:254.
Kikuta K, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403(3–4):380–4.
Froeling FEM, et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175(2):636–48.
Beuran M, et al. The epithelial to mesenchymal transition in pancreatic cancer: a systematic review. Pancreatology. 2015;15(3):217–25.
Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, et al. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery (United States). 2013;154(5):946–54. https://doi.org/10.1016/j.surg.2013.05.004.
Karnevi E, Rosendahl AH, Hilmersson KS, Saleem MA, Andersson R. Impact by pancreatic stellate cells on epithelial-mesenchymal transition and pancreatic cancer cell invasion: adding a third dimension in vitro. Exp Cell Res. 2016;346(2):206–15. https://doi.org/10.1016/j.yexcr.2016.07.017.
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–61. https://doi.org/10.1016/j.cell.2011.11.025.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23.
Guan J, Zhang H, Wen Z, Gu Y, Cheng Y, Sun Y, et al. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014;345(1):132–9. https://doi.org/10.1016/j.canlet.2013.12.006.
Xia X, Wu W, Huang C, Cen G, Jiang T, Cao J, et al. SMAD4 and its role in pancreatic cancer. Tumor Biol. 2014;36(1):111–9.
Lohr M, et al. Transforming growth factor-beta 1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61(2):550–5.
Yamada S, Fujii T, Shimoyama Y. SMAD4 expression predicts local spread and treatment failure in resected pancreatic cancer. Pancreas. 2015;44:1–5.
Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–13. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19273710.
Hagopian MM, Brekken RA. Stromal TGFβR2 signaling: a gateway to progression for pancreatic cancer. Mol Cell Oncol. 2015;2(3). http://doi.org/10.4161/23723556.2014.975606.
Nagathihalli NS, Castellanos JA, Vansaun MN, Dai X, Ambrose M, Guo Q, et al. Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells. Oncotarget. 2016;7(40):1–11.
Birtolo C, Pham H, Morvaridi S, Chheda C, Go VLW, Ptasznik A, et al. Cadherin-11 is a cell surface marker up-regulated in activated pancreatic stellate cells and is involved in pancreatic cancer cell migration. Am J Pathol. 2017;187(1):146–55. https://doi.org/10.1016/j.ajpath.2016.09.012.
Ohuchida K, Mizumoto K, Murakami M, Qian L, Sato N, Nagai E, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal. Interactions. 2004;1:3215–22.
Heinrich EL, Arrington AK, Ko ME, Luu C, Lee W, Lu J, et al. Paracrine activation of chemokine receptor CCR9 enhances the invasiveness of pancreatic cancer cells. Cancer Microenviron. 2013;6(3):241–5.
Lu J, Zhou S, Siech M, Habisch H, Seufferlein T, Bachem MG. Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway. Br J Cancer. 2014;110(2):409–20. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3899756&tool=pmcentrez&rendertype=abstract.
Leca J, Martinez S, Lac S, Nigri J, Secq V, Rubis M, et al. Cancer-associated fibroblast-derived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness. J Clin Invest. 2016;126(9):1–17.
Schneiderhan W, Diaz F, Fundel M, Zhou S, Siech M, Hasel C, et al. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120(Pt 3):512–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17227797.
Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta. 2010;1803(1):103–20.
Nagakawa Y, Aoki T, Kasuya K, Tsuchida A, Koyanagi Y. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24(11854622):169–78. Available from: http://www.hubmed.org/display.cgi?uids=11854622.
Tjomsland V, Pomianowska E, Aasrum M, Sandnes D, Verbeke CS, Gladhaug IP. Profile of MMP and TIMP expression in human pancreatic stellate cells: regulation by IL-1α and TGFβ and implications for migration of pancreatic cancer cells. Neoplasia (United States). 2016;18(7):447–56. https://doi.org/10.1016/j.neo.2016.06.003.
Tjomsland V, Sandnes D, Pomianowska E, Aasrum M, Christoffersen T, Gladhaug IP. TGFβ/IL-1R1 regulation of human pancreatic stellate cells: reduced MMP activity and inhibition of migration of pancreatic cancer cells in a collagen matrix model. Pancreatology. 2015;15(3):S17. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1424390315001921.
Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177(5):2585–96. Available from: http://www.sciencedirect.com/science/article/pii/S0002944010603082.
Gong H. Analysis of intercellular signal transduction in the tumor microenvironment. BMC Syst Biol. 2013;7 Suppl 3:S5.
Storck H, et al. Ion channels in control of pancreatic stellate cell migration. Oncotarget. 2017;8(1):769–84.
Acknowledgment
We would like to acknowledge the contributions of the authors of the excellent research studies and comprehensive reviews that were cited herein. We apologize to any authors whose work we omitted due to space limitations. We are grateful to Mohamed Mohameden Ibrahim Elamir for his unwavering support, and motivation. This work was supported by the NIH/NCI CA136526, Mayo Clinic Pancreatic SPORE P50 CA102701, and Mayo Clinic Center for Cell Signaling in Gastroenterology P30 DK84567 and Mayo Clinic Cancer.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media LLC
About this entry
Cite this entry
Olson, R.L.O., Forner, J.V., Navarro, P., Fernandez-Zapico, M.E., Elamir, A.M. (2018). Role of Tumor-Stromal Interactions in Pancreatic Cancer Invasion and Metastases. In: Neoptolemos, J., Urrutia, R., Abbruzzese, J., Büchler, M. (eds) Pancreatic Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-6631-8_89-1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6631-8_89-1
Received:
Accepted:
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-6631-8
Online ISBN: 978-1-4939-6631-8
eBook Packages: Springer Reference Biomedicine and Life SciencesReference Module Biomedical and Life Sciences