Abstract
Chronic kidney disease and hypertension are both important global public health challenges and strongly interrelated. The current estimated prevalence of CKD ranges between 8 and 16 % of adult population worldwide. Hypertension is ranked the leading risk factor for death and disability-adjusted life years lost in 2010 and nearly a billion (26.4 %) of the adult population has hypertension. The relationship between hypertension and chronic kidney disease is complex. Hypertension can cause chronic kidney disease and modify chronic kidney disease progression but at the same time, can be a consequence of chronic kidney disease. According to the latest data from United States Renal Data system, up to 25 % of chronic kidney disease was attributed to hypertension. Overall, the prevalence of hypertension is very high among CKD subjects, approaching over 90 % and the prevalence increased with worsening estimated glomerular filtration rate and CKD stages. Proteinuria is a key determinant of hypertension in CKD. The prevalence of hypertension in CKD shows significant racial disparities and varies by the etiology of kidney disease, age, body mass index, socioeconomic status, education level, and lifestyle. Suboptimal blood control is frequently observed in CKD, especially among those at the highest risk of adverse outcomes and may be partly explained by poor medication adherence. High blood pressure was associated with the development and progression of CKD. Patients with CKD are considered at high risk of cardiovascular disease and mortality and hypertension is one of the key risk factors mediating this risk. There are compelling data from observational studies as well as randomized clinical trials to support blood pressure lowering as a key therapeutic strategy in reducing adverse renal and cardiovascular outcomes in CKD. However, the ideal blood pressure target that confers the greatest renal and cardiovascular benefit has remained uncertain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Blood pressure lowering
- Chronic kidney disease
- Prevalence
- Masked hypertension
- Proteinuria
- Cardiovascular mortality
- End-stage renal disease
Prevalence of Chronic Kidney Disease and Its Determinants
Chronic kidney disease (CKD ) is defined as persistent kidney damage, which is often reflected by either a reduction in glomerular filtration rate or increased urine albumin excretion, or both. CKD is now a worldwide public health challenge. According to the 2010 Global Burden of Disease study, CKD is ranked 18th in the list of causes of total number of global deaths with an estimated annual death rate of 16.3 per 100,000 [1]. The prevalence of CKD varies across different countries and regions and is estimated to range between 8 and 16 % of the adult population [2–9]. The prevalence of CKD has shown a steady global increase over the years. For example, in the USA, the prevalence of CKD was estimated to be around 10 % between 1988 and 1994 and it increased to 13.1 % between 1999 and 2004 [4]. The prevalence estimates of CKD differ slightly depending on the GFR estimating equations used [4, 10]. The rise in CKD prevalence and incidence has been attributed to an aging population as well as an increased prevalence of hypertension, diabetes, and obesity, all of which are recognized as important global health challenges [4, 11, 12].
According to the 2010 United States Renal Data System (USRDS ) Annual Data Report, the leading causes of kidney failure in the USA are diabetes, hypertension, and glomerulonephritis. Hypertension and diabetes account for 99 and 153 per million population of incident cases of end-stage renal disease (ESRD ), respectively [13]. The prevalence of stage 3 or higher CKD in diabetics in the USA exceeds 15 % [13]. Over 5 % of people with newly diagnosed type 2 diabetes already have CKD and an estimated 40 % of diabetics will develop CKD in their time course [14]. Data from World Health Organization showed that the number of individuals with diabetes is currently around 154 million globally and is projected to double within the next 20 years. The increase is most notable in less developed countries, where the number of diabetic patients could rise from 99 million to 286 million by 2025 [15]. China and India are projected to have 139 million people with diabetes in 2025 [16]. A parallel increase in the global incidence and prevalence of CKD due to diabetic nephropathy is therefore anticipated. CKD in diabetes is associated with an increased risk of progression to ESRD [17].
The aging population also in part explains an increase in the incidence of hypertension, diabetes, and CKD worldwide. The prevalence of CKD was reported to be 7.4 % among women aged 18–39 years and increased to 18.0 and 24.2 % among those aged 60–69 and 70 years or above, respectively, in the Chinese general population [2]. Similar parallel increase in CKD prevalence with aging was observed across the USA, Canada, and Europe although the absolute prevalence differed across countries [4, 8, 18]. Cardiovascular disease is also an important cause of CKD.
Definition of Hypertension
The American Heart Association defines hypertension as a blood pressure of 140/90 mmHg or more, measured in clinic setting, based on two readings, 5 min apart and sitting in chair and confirmed with elevated reading in contralateral arm.
Prevalence of Hypertension in CKD
Hypertension is very common in CKD, with a prevalence estimated around 60–95 % in CKD stage 3–5 [19–22]. Hypertension has a complex interrelationship with CKD. Hypertension is an important modifiable cause for CKD as well as a consequence of CKD. Nearly a billion of the adult population (around 26.4 %) in 2000 had hypertension (defined as >140/90 mmHg) and this proportion is projected to increase by about 60 % to 1.56 billion by 2025 (24 % in developed countries and 80 % in developing regions such as Africa and Latin America) [23]. A corresponding global increase in the prevalence of CKD is therefore anticipated. The Global Burden of Disease Study identified elevated blood pressure as the leading risk factor, among 67 studied, for death and disability-adjusted life years lost during 2010 [24]. According to the USRDS Annual Data report for 2014, up to 25 % of CKD was attributed to hypertension. Of the 10.7 % of Medicare patients diagnosed with CKD in 2013, nearly half also have diabetes, and over 92 % also have hypertension [25]. The rate of ESRD caused by hypertension has grown 8.1 % since 2000, to 99.1 per million population. In contrast, the incident rate of diabetic ESRD fell 1.5 % between 2007 and 2008, to 153 per million population—a rate nearly unchanged from that of 2000 while that of ESRD due to glomerulonephritis has fallen 23.4 %, to 23.7 per million population [25].
The prevalence of hypertension in CKD shows racial disparities in being higher among non-Hispanic blacks versus whites or Mexican Americans, as reported in several cohorts. In the Modification of Diet in Renal Disease (MDRD) study, the prevalence of hypertension was higher in blacks versus whites [93 % versus 81 %] [26]. Similarly, in Chronic Renal Insufficiency Cohort (CRIC ) study, hypertension was reported in 93 % of African Americans versus 80 % of whites although Hispanics show a greater risk for CKD and ESRD compared to non-Hispanics [27]. Socioeconomic status and lifestyle also influence the prevalence of hypertension in CKD patients. Patients with poorer socioeconomic status, lower income, and education level showed a higher prevalence of hypertension [27].
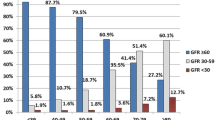
The prevalence of hypertension increases with worsening estimated glomerular filtration rate (eGFR ), as shown in CRIC study [27] (see Fig. 1.1). The prevalence of hypertension was 92 % for those with eGFR <30 ml/min per 1.73 m2 and 67 % for those with eGFR >60ml/min per 1.73 m2. The prevalence estimates for hypertension among CKD patients in China, India, and Mexico were similar to those reported in CRIC cohort [28–30].
Prevalence of hypertension in different CKD stages [27]
The prevalence of hypertension varies by the etiology of CKD. CKD due to diabetic nephropathy showed the highest prevalence of hypertension independent of kidney function [27]. Hypertension was also more frequent among patients with polycystic kidney disease, renal artery stenosis when compared to glomerulonephritis, tubulointerstitial disease, or chronic pyelonephritis [31]. Albuminuria was an important, independent risk factor for hypertension as shown by pooled data from National Health and Nutrition Examination Survey (NHANES) III and NHANES 1999–2005 [32, 33]. In the United States National Kidney Foundation’s Kidney Early Evaluation Program (KEEP), decreasing GFR by 10 ml/min/1.73 m2, increasing age, obesity, African American race, and microalbuminuria were all associated with an increased prevalence of hypertension [22]. Urine albumin to creatinine ratio greater than 6.67 mg/g in men and above 15.24 mg/g in women resulted in doubling the risk of developing hypertension when accounting for baseline blood pressure, body mass index, and creatinine [34]. Higher albumin/creatinine ratios, even within the normal range, are independently associated with increased risk for development of hypertension in the general population [35]. Proteinuria was shown to be one of the most important correlates of systolic blood pressure in older men, especially with ambulatory and home systolic blood pressure [36]. The association between albuminuria and hypertension may be mediated via an increased inflammation, endothelial dysfunction, and renal sodium handling [37].
Obesity was an important predictor of hypertension among CKD patients. In the MDRD study, body mass index was a strong predictor of hypertension among patients with a GFR of 25–55 ml/min per 1.73 m2 [26]. Likewise, both the KEEP (78.6 % versus 60.3 %) and NHANES study (52 % versus 30.8 %) showed a greater prevalence of hypertension among obese subjects as compared to non-obese subjects [22]. Obesity was also an independent risk factor for CKD [38].
Suboptimal blood pressure control was common in CKD, especially those at the highest risk of adverse outcomes due to diabetes or albuminuria [39]. The NHANES data showed that elevated serum creatinine level was strongly related to suboptimal treatment of high blood pressure [20]. Only 36 % of subjects met the blood pressure target of <140/90 while only 14 % of treated hypertensive individuals met the older blood pressure target of <130/85 proposed for individuals with kidney disease [20]. In the KEEP program for people at high risk for CKD, the prevalence (86 %), awareness (80 %), and treatment (70 %) of hypertension were high but good blood control was achieved in only 13 % [40]. Poor medication adherence was associated with 23 % increased risk of uncontrolled hypertension [41]. These data suggest poorly controlled hypertension is associated with much of the high burden of CKD and there is a need to improve blood pressure control in CKD.
Clinic Blood Pressure and Ambulatory Blood Pressure
The diagnosis and control of hypertension critically depend on accuracy of blood pressure measurements. Clinic blood pressure frequently overestimates and underestimates true blood pressure in hypertensive general population and this is also observed in patients with CKD. Misclassification of blood pressure control at the office was observed in 1 of 3 hypertensive patients with CKD, suggesting that ambulatory-based control rates were far better than office-based rates [42]. Using ambulatory blood pressure monitoring as the reference standard, home blood pressure monitoring showed the best diagnostic performance for hypertension compared with routine or standardized clinic measurements in CKD. One week-averaged home blood pressure >140/80 mmHg was associated with awake ambulatory blood pressure >130/80, a threshold considered as hypertensive in the CKD population [43]. White coat hypertension is defined as an elevated clinic blood pressure but controlled blood pressure out of clinics. Masked hypertension is defined as controlled blood pressure in clinic setting with elevated blood pressure out of clinics. According to a systematic analysis including six studies with 980 CKD subjects, the overall prevalence of white coat hypertension was 18.3 % (range, 10–28 %) and masked hypertension 8.3 % (range, 5–28 %). Notably, 40.4 % of subjects with CKD considered to have normal or controlled blood pressure in fact had masked uncontrolled hypertension (range, 26–54 %) while 30 % of subjects with CKD that were thought to have hypertension had normotension at home [44]. Similarly, a high rate of masked hypertension was observed in the follow-up study of the African American Study of Kidney Disease (AASK ) cohort; of the 61 % subjects with controlled clinic blood pressure, 70 % had masked hypertension [45]. This location-dependent hypertension, namely masked hypertension and white coat hypertension, has been shown to predict prognosis in patients with hypertension. Masked hypertension carries a risk equivalent to sustained hypertension, whereas white coat hypertension carries a risk almost equivalent to normotension [46]. In the general population, the risk of CKD was significantly increased in sustained hypertension, masked hypertension, and white coat hypertension [47]. The AASK study showed that target organ damage was more common in subjects with sustained or masked hypertension [45]. CKD patients with masked hypertension were more likely to progress to end-stage renal disease [48]. The prevalence rates of masked hypertension depend very much on the definitions used to define masked hypertension. Conventionally, masked uncontrolled hypertension was defined as clinic blood pressure >140/90 mmHg or daytime ambulatory blood pressure ≥135/85 mmHg. In a recent study of 333 veterans with CKD, the prevalence of masked uncontrolled hypertension was 26.7 % defined by daytime ambulatory blood pressure, 32.8 % by 24-hour ambulatory blood pressure and more than doubled to 56.1 % if defined by daytime or nighttime ambulatory blood pressure [49].
Ambulatory blood pressure was superior to clinic blood pressure measurements in correlating with end-organ damage [36]. Ambulatory blood pressure showed a stronger association with proteinuria [36] and echocardiographic left ventricular hypertrophy [50, 51] than clinic blood pressure in CKD population. In keeping with these observations, a prospective cohort study conducted in 217 veterans with CKD showed that 24 h ambulatory systolic blood pressure provided additional prognostic value for composite cardiovascular endpoint of myocardial infarction, stroke, and mortality beyond clinic blood pressure, indicating the importance of ambulatory blood pressure in predicting clinical outcomes in CKD (see Fig. 1.2) [48]. Clinic blood pressure above goal and ambulatory blood pressure at goal identify a low risk condition while clinic blood pressure at goal and ambulatory blood pressure above goal are both associated with higher cardio-renal risk similar to that observed in patients with both clinic and ambulatory blood pressure above goal [52]. Furthermore, the definitions used to classify patients as having hypertension or normotension can influence the risk for being classified as having masked hypertension in favor of white coat hypertension. A meta-analysis has shown that when the thresholds for classification of clinic and ambulatory blood pressure are equal, the risk for diagnosis of masked hypertension is less (risk ratio of 0.74). When the clinic blood pressure threshold is higher and home blood pressure threshold is lower, the risk (risk ratio of 1.36) for diagnosing masked hypertension is greater [44]. More recent analysis by Agarwal and co-workers showed that clinic blood pressure was a good determinant of masked uncontrolled hypertension with area under the receiver-operating characteristics curve of 0.82 (95 % confidence intervals, 0.76–0.87). Home blood pressure was no better than clinic blood pressure in diagnosing masked uncontrolled hypertension in CKD. Thus, the use of home blood pressure to diagnose masked uncontrolled hypertension was not supported. Nevertheless, ambulatory blood pressure showed short-term reproducibility in diagnosing masked uncontrolled hypertension and may detect a phenotype with increased cardiovascular risk [49].
Hypertension and Kidney Outcomes in Chronic Kidney Disease
There are compelling data from observational studies that high blood pressure was associated with the development and progression of CKD [53–55]. Lowering blood pressure has also been a key treatment strategy in slowing the progression of CKD. However, the blood pressure threshold level of which this risk was increased remains controversial. The NHANES study showed an eightfold higher risk of an elevated serum creatinine among those with hypertension versus those with a normal blood pressure [20]. In the Multiple Risk Factor Intervention Trial, systolic and diastolic blood pressure was identified as strong risk factors for progression to ESRD, independent of age, race, income, use of medication for diabetes mellitus, history of myocardial infarction, serum cholesterol concentration, and cigarette smoking as well as baseline serum creatinine and proteinuria over an average follow-up duration of 16 years [53]. Those with blood pressure more than 210/120 mmHg showed at least a 20-fold increased risk of ESRD than those with blood pressure less than 120/80 mmHg. The estimated risk of ESRD associated with systolic blood pressure was also greater than that with diastolic blood pressure when both systolic and diastolic blood pressure were considered together. The risk appeared to start at systolic blood pressure of 140 mmHg rather than 130 mmHg and was the highest among those with systolic blood pressure of 150 mmHg or above [53].
The KEEP cohort showed that high systolic blood pressure accounted for most of the risk of progression to ESRD [56]. There is also evidence that the risk of ESRD appeared to increase even with modest elevation of blood pressure and the observed relationship did not appear to be due to confounding by clinically evident baseline kidney disease. Notably, among subjects with eGFR over 60 ml/min per 1.73 m2, those with blood pressure of 12–129/80–84 mmHg were 62 % more likely to develop ESRD while those with blood pressure of 130–139/85–89 mmHg were 98 % more likely to develop ESRD compared to those with blood pressure below 120/80 mmHg [57]. Several earlier clinical trials also established that lower blood pressure levels were associated with slower progression of CKD among subjects with proteinuria [55, 58].
On the other hand, post-hoc analysis from the Homocysteinemia in Kidney and ESRD (HOST ) study , a double-blind randomized controlled trial in 2056 subjects with advanced CKD (mean eGFR 18 ml/min per 1.73 m2) showed no graded association between systolic blood pressure and cardiovascular events or ESRD. However, those with systolic blood pressure >157 mmHg were associated with an increased risk of ESRD [59]. A systematic analysis putting together 11 randomized controlled trials of 9287 patients with CKD concluded that a more intensive blood pressure lowering reduced the risk of composite kidney failure events by 17 % and the risk of ESRD alone by 18 %, especially those with proteinuria. However, intensive blood pressure lowering appeared to have no effect on kidney failure among those who did not have proteinuria [60]. Another systematic review of three large trials in this field, namely the MDRD study [58, 61, 62], Ramipril Efficacy in Nephropathy-2 (REIN-2) study [63], and the AASK study [64–66] including 2272 CKD subjects failed to show any significant benefit by lowering blood pressure to less than 125/75 to 130/80 as compared to 140/90 mmHg. However, subgroup analysis suggested that a lower target may benefit in subjects with proteinuria [67].
These observations are in contrast to the Systolic Blood Pressure Intervention Trial (SPRINT ) which is a randomized controlled trial that examined >9000 hypertensive subjects with an increased cardiovascular risk but without diabetes. Among those with baseline CKD, the number of subjects showing ≥50 % decline in GFR or ESRD did not differ between intensive treatment (target 120 mmHg) versus standard treatment (target 140 mmHg). However, among those with no CKD at baseline, those randomized to intensive treatment showed more adverse renal outcomes compared to standard treatment [68]. This finding adds to the growing uncertainties of target blood pressure for kidney protection in high risk subjects with and without CKD. Although the JNC 7 recommended a target blood pressure of less than 130/80 mmHg for CKD subjects [69], more recent recommendations from JNC 8 acknowledged the limitations of available evidence and suggest a target of less than 140/90 mmHg be used [70]. The CRIC study showed that having longitudinal blood pressure values and time-updated systolic blood pressure greater than 130 mmHg may be more strongly associated with CKD progression than analyses based on a single baseline systolic blood pressure [71]. Given the clinical uncertainties and the potential for harm, it appears that a BP target of <140/90 mmHg in the clinic or <135/85 mmHg at home appears reasonable even among those with CKD.
Hypertension and Cardiovascular Outcomes in CKD
Blood pressure is an important determinant of cardiovascular risk in the general population [72] and lowering blood pressure reduces cardiovascular events in this population [73, 74]. A previous meta-analysis showed that lowering blood pressure was the main target to lower major cardiovascular event risk in hypertensive subjects [74]. Earlier study demonstrated the importance of blood pressure reduction in CKD. In the MDRD study, each 10 mmHg increase in follow-up systolic blood pressure increased the risk of hospitalization for cardiovascular and cerebrovascular disease by 35 % [61]. However, it remains controversial as to what ideal blood pressure target should be in terms of cardiovascular protection in CKD. A recent systematic analysis including 26 trials with 152,290 participants of which 30,295 subjects had CKD (defined as having eGFR <60 ml/min per 1.73 m2) confirmed that blood pressure lowering significantly reduced the risk of major cardiovascular events by about one-sixth (for each 5 mmHg reduction in systolic blood pressure) in those with CKD (hazard ratio, 0.83, 95 % confidence intervals, 0.76–0.90) and the risk reduction was very comparable to those without CKD (hazard ratio, 0.83, 95 % confidence intervals, 0.79–0.88) [75]. Specifically, the systematic analysis showed that blood pressure lowering per se, rather than a particular anti-hypertensive drug class, was associated with cardiovascular risk reduction [75]. Notably, these observations are in keeping with the SPRINT trial showing that among adults with hypertension but without diabetes, lowering systolic blood pressure to a target goal of less than 120 mmHg significantly reduced the risk of fatal or non-fatal cardiovascular events and death from all causes as compared with the standard goal of less than 140 mmHg [68]. On the other hand, two retrospective cohort studies raised uncertainties with lower blood pressure targets [76, 77]. One study examined elderly veterans with CKD and initially uncontrolled hypertension and found higher mortality among subjects treated to a systolic blood pressure of less than 120 mmHg versus 120–139 mmHg (hazard ratio, 1.7, 95 % confidence intervals, 1.63–1.78) [77]. Another retrospective analysis of a large diverse cohort of hypertensive subjects observed a U-shaped curve for the risk associated with both achieved systolic and diastolic blood pressure in relation to a composite endpoint of all-cause mortality or ESRD [76]. Thus, while lowering blood pressure is recognized as one of the core strategies in reducing cardiovascular risk in CKD, the blood pressure target that confers the largest cardiovascular benefit has remained uncertain. Randomized trials rather than observational studies should inform clinical practice. Thus, guidelines such as the JNC 8 have taken the position that BP lowering to <140/90 mmHg measured in the clinic, even among those with CKD, appears reasonable.
Conclusions
Both hypertension and CKD are important global health problems and are strongly interrelated. Hypertension may cause CKD and modify the outcomes of CKD. On the other hand, hypertension may also result from CKD. High blood pressure increases the onset and progression of CKD and worsens the clinical outcomes of CKD patients. More attention is needed to raise awareness of hypertension and improve blood pressure control among CKD population.
References
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–22.
Jha V, Wang AY, Wang H. The impact of CKD identification in large countries: the burden of illness. Nephrol Dial Transplant. 2012;27 (Suppl 3):iii32–8.
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47.
Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13(6):621–30.
Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371(9631):2173–82.
Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17(8):2275–84.
Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117.
Varma PP, Raman DK, Ramakrishnan TS, Singh P, Varma A. Prevalence of early stages of chronic kidney disease in apparently healthy central government employees in India. Nephrol Dial Transplant. 2010;25(9):3011–7.
Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J kidney Dis. 2010;56(3):486–95.
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72.
Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–70.
Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. United States Renal Data System 2008 Annual Data Report. Am J kidney Dis. 2009;53(1 Suppl):S1–374.
Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care. 2009;32(12):2225–9.
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–31.
World Health Organization. Preventing chronic diseases: a vital investment. World Health Organization; 2005.
Jones CA, Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH. Epidemic of end-stage renal disease in people with diabetes in the United States population: do we know the cause? Kidney Int. 2005;67(5):1684–91.
Arora P, Vasa P, Brenner D, Iglar K, McFarlane P, Morrison H, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. Can Med Assoc J. 2013;185(9):E417–23.
Kalaitzidis R, Li S, Wang C, Chen SC, McCullough PA, Bakris GL. Hypertension in early-stage kidney disease: an update from the Kidney Early Evaluation Program (KEEP). Am J kidney Dis. 2009;53(4 Suppl 4):S22–31.
Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988-1994). Arch Intern Med. 2001;161(9):1207–16.
Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. Am J kidney Dis. 2011;57(1 Suppl 1):A8. e1-526.
Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999-2004. Am J kidney Dis. 2008;51(4 Suppl 2):S30–7.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60.
Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66(1 Suppl 1):Svii, S1–305.
Buckalew Jr VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis. 1996;28(6):811–21.
Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J kidney Dis. 2010;55(3):441–51.
Zheng Y, Cai GY, Chen XM, Fu P, Chen JH, Ding XQ, et al. Prevalence, awareness, treatment, and control of hypertension in the non-dialysis chronic kidney disease patients. Chin Med J. 2013;126(12):2276–80.
Obrador GT, Garcia-Garcia G, Villa AR, Rubilar X, Olvera N, Ferreira E, et al. Prevalence of chronic kidney disease in the Kidney Early Evaluation Program (KEEP) Mexico and comparison with KEEP US. Kidney Int Suppl. 2010;116:S2–8.
Singh AK, Farag YM, Mittal BV, Subramanian KK, Reddy SR, Acharya VN, et al. Epidemiology and risk factors of chronic kidney disease in India - results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14:114.
Ridao N, Luno J, de Vinuesa SG, Gomez F, Tejedor A, Valderrabano F. Prevalence of hypertension in renal disease. Nephrol Dial Transplant. 2001;16 Suppl 1:70–3.
Peralta CA, Hicks LS, Chertow GM, Ayanian JZ, Vittinghoff E, Lin F, et al. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005;45(6):1119–24.
Inker LA, Coresh J, Levey AS, Tonelli M, Muntner P. Estimated GFR, albuminuria, and complications of chronic kidney disease. J Am Soc Nephrol. 2011;22(12):2322–31.
Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D’Agostino RB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111(11):1370–6.
Takase H, Sugiura T, Ohte N, Dohi Y. Urinary albumin as a marker of future blood pressure and hypertension in the general population. Medicine (Baltimore). 2015;94(6), e511.
Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46(3):514–20.
Romero CA, Peixoto AJ, Orias M. Estimated GFR or albuminuria: which one is really associated with resistant hypertension? Semin Nephrol. 2014;34(5):492–7.
Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, et al. Association between body mass index and CKD in apparently healthy men. Am J kidney Dis. 2005;46(5):871–80.
Fraser SD, Roderick PJ, McIntyre NJ, Harris S, McIntyre CW, Fluck RJ, et al. Suboptimal blood pressure control in chronic kidney disease stage 3: baseline data from a cohort study in primary care. BMC Fam Pract. 2013;14:88.
Sarafidis PA, Li S, Chen SC, Collins AJ, Brown WW, Klag MJ, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med. 2008;121(4):332–40.
Schmitt KE, Edie CF, Laflam P, Simbartl LA, Thakar CV. Adherence to antihypertensive agents and blood pressure control in chronic kidney disease. Am J Nephrol. 2010;32(6):541–8.
Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, Banegas JR, et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: A 5,693-patient cross-sectional analysis from Spain. Am J kidney Dis. 2013;62(2):285–94.
Andersen MJ, Khawandi W, Agarwal R. Home blood pressure monitoring in CKD. Am J kidney Dis. 2005;45(6):994–1001.
Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2009;4(3):656–64.
Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20–7.
Hansen TW, Kikuya M, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25(8):1554–64.
Kanno A, Metoki H, Kikuya M, Terawaki H, Hara A, Hashimoto T, et al. Usefulness of assessing masked and white-coat hypertension by ambulatory blood pressure monitoring for determining prevalent risk of chronic kidney disease: the Ohasama study. Hypertens Res. 2010;33(11):1192–8.
Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(7):1175–80.
Agarwal R, Pappas MK, Sinha AD. Masked Uncontrolled Hypertension in CKD. J Am Soc Nephrol. 2016;27:924–32.
Tucker B, Fabbian F, Giles M, Thuraisingham RC, Raine AE, Baker LR. Left ventricular hypertrophy and ambulatory blood pressure monitoring in chronic renal failure. Nephrol Dial Transplant. 1997;12(4):724–8.
Peterson GE, de Backer T, Gabriel A, Ilic V, Vagaonescu T, Appel LJ, et al. Prevalence and correlates of left ventricular hypertrophy in the African American Study of Kidney Disease Cohort Study. Hypertension. 2007;50(6):1033–9.
Minutolo R, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, et al. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: a multicenter prospective cohort study. Am J Kidney Dis. 2014;64(5):744–52.
Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–8.
Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41(6):1341–5.
Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139(4):244–52.
Peralta CA, Norris KC, Li S, Chang TI, Tamura MK, Jolly SE, et al. Blood pressure components and end-stage renal disease in persons with chronic kidney disease: the Kidney Early Evaluation Program (KEEP). Arch Intern Med. 2012;172(1):41–7.
Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165(8):923–8.
Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330(13):877–84.
Palit S, Chonchol M, Cheung AK, Kaufman J, Smits G, Kendrick J. Association of BP with death, cardiovascular events, and progression to chronic dialysis in patients with advanced kidney disease. Clin J Am Soc Nephrol. 2015;10(6):934–40.
Lv J, Ehteshami P, Sarnak MJ, Tighiouart H, Jun M, Ninomiya T, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185(11):949–57.
Lazarus JM, Bourgoignie JJ, Buckalew VM, Greene T, Levey AS, Milas NC, et al. Achievement and safety of a low blood pressure goal in chronic renal disease. The Modification of Diet in Renal Disease Study Group. Hypertension. 1997;29(2):641–50.
Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123(10):754–62.
Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365(9463):939–46.
Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, et al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis. 2006;48(5):739–51.
Appel LJ, Wright Jr JT, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–29.
Wright Jr JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–31.
Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154(8):541–8.
Group SR, Wright Jr JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162(4):258–65.
MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–74.
Murray CJ, Lauer JA, Hutubessy RC, Niessen L, Tomijima N, Rodgers A, et al. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet. 2003;361(9359):717–25.
Turnbull F, Blood Pressure Lowering Treatment Trialists Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–35.
Blood Pressure Lowering Treatment Trialists Collaboration, Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ. 2013;347:f5680.
Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J Am Coll Cardiol. 2014;64(6):588–97.
Kovesdy CP, Lu JL, Molnar MZ, Ma JZ, Canada RB, Streja E, et al. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern Med. 2014;174(9):1442–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Wang, A.YM. (2016). Epidemiology of Hypertension in Chronic Kidney Disease. In: Singh, A., Agarwal, R. (eds) Core Concepts in Hypertension in Kidney Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-6436-9_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6436-9_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-6434-5
Online ISBN: 978-1-4939-6436-9
eBook Packages: MedicineMedicine (R0)