Abstract
Gene-directed enzyme prodrug therapy (GDEPT) is a promising therapeutic approach for treating cancers of various phenotypes. This strategy is independent of various other chemotherapeutic drugs used for treating cancers where the drugs are mainly designed to target endogenous cellular mechanisms, which are different in various cancer subtypes. In GDEPT an external enzyme, which is different from the cellular proteins, is expressed to convert the injected prodrug in to a toxic metabolite, that normally kill cancer cells express this protein. Theranostic imaging is an approach used to directly monitor the expression of these gene therapy enzymes while evaluating therapeutic effect. We recently developed a dual-GDEPT system where we combined mutant human herpes simplex thymidine kinase (HSV1sr39TK) and E. coli nitroreductase (NTR) enzyme, to improve therapeutic efficiency of cancer gene therapy by simultaneously injecting two prodrugs at a lower dose. In this approach we use two different prodrugs such as ganciclovir (GCV) and CB1954 to target two different cellular mechanisms to kill cancer cells. The developed dual GDEPT system was highly efficacious than that of either of the system used independently. In this chapter, we describe the complete protocol involved for in vitro and in vivo imaging of therapeutic cancer gene therapy evaluation.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Bioluminescence
- Gene-directed enzyme prodrug therapy
- Herpes simplex thymidine kinase
- In vivo imaging
- Firefly luciferase
1 Introduction
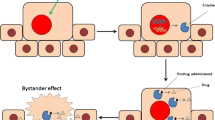
Gene-directed enzyme prodrug therapy (GDEPT) is a promising therapeutic strategy, in which enzyme-coding genes are introduced into cancer cells to convert nontoxic prodrugs into active cytotoxic compounds. HSV1-TK/GCV and NTR/CB1954 are two different GDEPT systems currently in different stages of clinical trials [1]. Active GCV compound of HSV1-TK/GCV GDEPT strategy inhibits DNA synthesis in actively dividing cancer cells, whereas, active compound of CB1954 in NTR/CB1954 GDEPT systems exerts cytotoxicity to cells with all stages of cell cycle. Further, these two GDEPT strategies have potential bystander effect and therefore extend the cytotoxicity to the adjacent cells, which are not expressing the GDEPT system and ensure the complete removal of tumor. But the limitation is the need of higher level of expression GDEPT enzymes to achieve therapeutic conversion of prodrugs in to active metabolites. Most of the gene therapy systems suffer from poor gene delivery efficiency. Hence to improve therapeutic efficiency with minimum level of gene expression we combined two different therapeutic genes target two different cellular mechanisms to kill cancer cells. The combination of these two GDEPT systems seems highly potential in terms of enhanced therapy and reduction in the amount of prodrugs needed for treatment. We showed the enhanced therapeutic effect of HSV1-TK-NTR fusion dual-GDEPT system in triple negative breast cancer cells such as SUM159 and MDA-MB-231 [2]. Additionally, it was highly efficient against metastatic tumor in mice model [3]. In this chapter we describe the protocol details by which the fusion HSV1-TK-NTR clone was generated and how it was evaluated for cancer gene therapy in cells and human xenograft models in mice. Further, we provide different methods to image and evaluate the expression of this fusion protein in living animals by theranosti c imaging.
Theranostic imaging combines treatment of specific disease and evaluating the therapeutic efficacy of particular drug simultaneously [4]. In conventional approach, separate set of agents will be administered for treatment, and thereafter imaging agents or contrasting substances will be given just before diagnostic imaging of particular disease. In GDEPT , therapeutic genes used for treating cancer can be tracked by designing imaging substrates, which monitor therapeutic gene expression while injected prodrugs can treat cancer. Further, when two genes are fused, multiple modality imaging could be used to evaluate the therapeutic efficiency of either of the gene separately or in combination (see Fig. 1). Theranostic imaging applications facilitates saving time by evaluating therapeutic efficacy of any therapeutic systems while measuring the expression level of delivered therapeutic genes, as it combines both diagnosis and therapy together. Additionally, personalized therapy in cancer has been widely accepted as a promising treatment strategy. By applying theranostic imaging, personalized therapy could be adopted as it reveals the aftermath of therapy right away. Theranostic imaging also helps to understand the problems of innate immunity while therapeutic evaluation are attempted.
A wide spectrum of therapeutic reporter genes are investigated for the past two decades, and many of them reached clinical trials. Herpes simplex virus 1-thymidine kinase (HSV1-TK), E. coli Nitroreductase (NTR), and cytosine deaminase (CD) are the major GDEPT therapeutic reporters investigated in details. Additionally, for the first time we developed a fusion form of two therapeutic genes which hold the potentials of reporter genes and studied them to reveal the enhanced therapeutic efficacy by imaging [3]. Other than these enzyme-based reporters, many transporters and receptors, such as sodium iodide symporter (NIS), somatostatin receptor type 2 has also been investigated as therapeutic reporters [5]. Imaging modalities such as PET, MRI, and optical imaging are generally applied to monitor the therapeutic efficacy of gene therapy vectors. HSV1-TK has been thoroughly evaluated using PET imaging. [18F]-Fluoro-3-hydroxymethyl butyl guanine ([18F]-FHBG) and [18F]-fluoro-5-ethyl-1-beta-d-arabinofuranosyluracil ([18F]-FEAU) labeled with fluorine-18 and fluoro-5-iodo-1-beta-d-arabinofuranosyluracil (FIAU) labeled with Iodine-124 are mainly used to image the therapeutic value of HSV1-TK using PET imaging [6]. Alternatively, MRI imaging agents like 5-methyl-5,6-dihydrothymidine (5-MDHT) have been developed and investigated to evaluate HSV1-TK therapeutic gene [7]. Additionally, to evaluate the extent of gene delivery, fusion genes such as GFP-HSV1-TK and FLuc-HSV1-TK has been used to image through optical imaging modalities [8]. Activity NTR could be imaged through fluorescence microscopy by applying CytoCy5S dye, which fluoresce when reduced by NTR enzyme [9]. Altogether, imaging modalities are chosen based on the type of reporter genes to be investigated.
Delivery vehicles are the important component in the successful execution of gene therapy. Plasmid vectors, viral vectors, synthetic liposomes, and nanoparticles have so far been used as vehicles of choice in gene therapy. All of these vehicles have advantages and limitations depending on the gene of interest and target organs involved. Plasmid vectors along with liposome complexes were initially employed for successful execution of gene delivery, but they were not efficient delivery vehicles to deliver genes in animal models. However, plasmid vehicles in assistance with other delivery vehicles such as super paramagnetic iron oxide nanoparticles and functionalized silica nanoparticles have shown promising delivery of nucleic acids and siRNAs [10]. Viral vectors have revolutionized the field of gene therapy by enabling the selective delivery of therapeutic genes in small animals and humans. Viruses specifically attack their host by introducing and stably maintaining their genomes by using the replication machineries of host cells. Manipulations of viral genomes allowed them to be efficiently introduced and stably integrated into host genome without causing any pathogenicity. Many types of viral vectors are developed from viruses’ derived different families. Adenovirus, Adeno-associated virus (AAV ), and Lentivirus are most common viruses adopted to develop viral vectors. Viral vectors differ with each other in terms of infectivity, efficiency in gene transfer, and maintenance in host cells [11]. Host immunity-associated restriction of viral vectors lead to reduction in the efficiency of gene therapy [12]. Adenoviral vectors are developed from adenoviruses that have nonenveloped double-stranded DNA, not stably integrating into human genomes and will not multiply with host cells. However, efficient gene transfer, high titer production of vectors, and capacity of transducing transgenes of more than 30 kb made them one of the most commonly used viral vectors in gene therapy [13]. Adenoviral vector usage has been put in hold after the death of a man in 1999. Since it caused upper respiratory infections, many patients already have antibodies against adenovirus that limits the usage of adenoviral vectors for gene therapy and vaccination. Further, rapid clearance of adenovirus from blood also hampered the use of adenoviral vectors in gene therapy [14]. Nevertheless recent developments in improving the targeting efficiency and sustained release of viral vectors appear to be promising for novel cancer gene therap y [15]. Adeno-associated viral (AAV ) vectors are developed from AAV belongs to parvoviridae family. AAV can package and deliver a linear single-stranded DNA genome with the help of a helper virus such as adenovirus and herpesvirus. AAV can infect on both dividing and nondividing host cells, develop minimal host immune response, maintain long-term transgene expressions, and it is nonpathogenic in nature. All these properties made this vector suitable for gene delivery in small animal models and humans [12]. Since the first application of AAV vector to introduce cystic fibrosis transmembrane regulator (CFTR) gene to treat cystic fibrosis in humans [16], many attempts were taken to treat several other diseases. In cancer, AAV mediated delivery of HSV1-TK showed high level of anti-tumor activity [17]. Although a few successful clinical trials are reported, investigations are on to improve the potentiality of AAV for efficient gene delivery.
Lentiviral vectors are other gene delivery vehicles under investigation for gene therapy applications. Lentiviral vectors are developed from single-stranded RNA viruses belong to retroviridae family. Murine leukemia viruses (MLV)-based vectors are the most commonly used lentiviral vectors. Lentiviral vectors are designed by replacing gag, pol, and env genes of MLV genomes with expression cassette containing specific promoter and gene to be transferred and expressed. Therapeutic genes are stably integrated in dividing and quiescent cells with lentiviral vectors. Lentiviral particles are produced by co-transfecting three different vectors such as packaging vector, transfer, and envelope-encoding vectors in HEK293FT cells. Third generation lentiviral vectors are further improved with more safety features in which they replaced tat-independent constitutive promoter in transfer vector [18]. Modern lentiviral vectors are developed further to yield insignificant insertional mutagenesis that was a major setback in γ-retroviral vectors previously used for gene therapy. Lentiviral vectors are used to treat many immune-associated diseases such as X-SCID and melanoma. In cancer immunotherapy, it has been used to induce anti-tumor immune response. With the desirable outcomes it generated in clinics, lentiviral vectors seem a potential delivery vehicle for the successful treatment of major diseases including cancer. Adenovirus, lentivirus, and adeno-associated viral vectors are the important vectors that are used for the gene therapy in clinical trials associated with major diseases. Poxvirus, herpes virus, and alpha viruses are also investigated to introduce alternative gene delivery vectors. Because of side effects they caused, other viral vector may take several years to actively take part in the mainstream gene therapy missions.
2 Materials
2.1 Expression of NTR, HSV1-TK, and HSV1-TK-NTR Fusion
-
1.
Plasmid vectors pcPur-NTR, pcPur-HSV1-TK, and pcPur-HSV1-TK-NTR (see Fig. 2 and Note 1 ).
-
2.
MDA-MB-231 cells (ATCC®, Manassas, VA).
-
3.
Dulbecco’s modified Eagle’s medium (DMEM) with 10 % fetal bovine serum and 1 % penicillin and streptomycin solution (GIBCO BRL, Frederick, MD).
-
4.
Lipofectamine 2000 and serum free OptiMEM medium (Life Technologies, Grand Island, NY).
-
5.
37 °C Incubator with 5 % CO2 (Thermo scientific, Sunnyvale, CA).
2.2 Evaluation of NTR Gene Expression
-
1.
CytoCy5S dye (a quenched substrate of NTR enzyme fluoresce upon reduction by NTR enzyme).
-
2.
Fluorescent microscope with Cy5 and/or TxRed filters (Leica Microsystems, Buffalo Grove, IL).
-
3.
Fluorescent assisted cell sorter (FACS) (BD Biosciences, San Jose, CA).
2.3 3H-Penciclovir (3H-PCV) Uptake
-
1.
3H-PCV (specific activity 14.9 Ci/mmol) (Moravek Biochemicals, La Brea, CA, USA).
-
2.
Scintillation fluid (Cytoscint).
-
3.
Scintillation counter (Beckman Coulter, Brea, CA).
-
4.
0.1 N NaOH.
-
5.
Ice cold PBS (GIBCO BRL, Frederick, MD).
-
6.
MDA-MB-231 cells (ATCC, Manassas, VA).
-
7.
DMEM Medium, FBS, Penicillin, and streptomycin for cell culture (GIBCO BRL, Frederick, MD).
-
8.
Bio-Rad protein assay kit (Bio-Rad, Hercules, CA).
2.4 Therapeutic Evaluation in Cells
-
1.
MDA-MB-231 cells stably expressing NTR or HSV1-TK or HSV1-TK-NTR fusion.
-
2.
Ganciclovir (GCV) and CB1954 (Sigma, St. Louis, MO).
-
3.
A colorimetric assay reagent for cell activity, 3-(4,5-dimethylthiczol-2-yl)-2,5-diphenyltetrazolium bromide (MTT).
-
4.
Infinite-1000 (Tecan-Safire) spectrofluorometer.
-
5.
Nanodrop (Thermo scientific, Sunnyvale, CA).
2.5 Therapeutic Evaluation in Small Animal Model
-
1.
MDA-MB-231 cells stably coexpressing HSV1-TK, NTR, and HSV1-TK-NTR fusion proteins along with firefly luciferase-EGFP (FLuc-EGFP ).
-
2.
d-Luciferin (Biosynth, Itasca, IL).
-
3.
Nude mice (nu/nu) (Charles River, San Diego, CA).
-
4.
MicroPET/CT (Inveon, Siemens, Malvern, PA, USA).
-
5.
Optical imaging i nstrument with cooled CCD camera and living image in vivo imaging software (Perkin Elmer, Waltham, MA).
3 Methods
3.1 Generation of Stable Cells Coexpressing NTR or HSV1-TK or HSV1-TK-NTR Fusion with FLuc-EGFP
-
1.
Plasmids (5 μg each) expressing fusion proteins of NTR or HSV1-TK or HSV1-TK-NTR, and FLuc-EGFP fusion proteins were co-transfected in MDA-MB-231 cells in 10 cm plate plated at 70 % confluence 24 h before transfection by using lipofectamine 2000 transfection agent using manufacturer suggested protocol. The cells were incubated at 37 °C with 5 % CO2 for 24 h.
-
2.
24 h after transfection, cells were trypsinized and plated to a 1:3 subculture ratio in 10 cm plates and puromycin (Life Technologies, Grand Island, NY) was added to a final concentration of 500 ng/mL.
-
3.
The cells were further incubated for 48 h. The dead cells were washed and added fresh medium with 500 ng/mL of puromycin. At this stage more than 80 % of cells died. The steps were repeated till no further cell death was observed. The cells were FACS sorted for EGFP expression and CytoCy5S reduction, and plated at low dilution (2000 cells/10 cm plate in 10 mL medium with 500 ng/mL puromycin).
-
4.
The cells were incubated further for 2 weeks with medium change once in every 3 days.
-
5.
At this stage colonies of 2–3 mm in diameter were formed from individual cells.
-
6.
Single colony of transfected cells were selected and expanded by plating first in 12-well plate followed by 10 cm plate.
-
7.
The cells expanded from several colonies that express NTR or HSV1-TK or HSV1-TK-NTR with FLuc-EGFP fusion proteins were tested for the identification of clones that express equal level of sensor fusion proteins by immunoblot analysis using HSV1-TK antibody and mRNA expression by real time qRT-PCR (see Note 2 ).
-
8.
The identified stable clones of cells were used for further in vitro and in vivo therapeutic evaluations.
3.2 Expression and Functional Analysis of NTR Using CytoCy5S Dye
-
1.
CytoCy5S is a quenched substrate of NTR enzyme. When this dye is reduced by the catalytic action of NTR enzyme, the resulting reduced fluorescent product is retained inside the cells.
-
2.
CytoCy5S dye was used to check the expression of NTR in MDA-MB-231 cells stably expressing NTR, and HSV1-TK-NTR proteins.
-
3.
To check the function of NTR, MDA-MB-231 stable cells expressing NTR, and HSV1-TK-NTR were plated in 12 well culture and treated with CytoCy5S dye (1 μg/mL) 24 h after initial plating and incubated further at 37 °C for 2 h.
-
4.
The cells were washed with PBS before viewed in fluorescent microscope under Cy5 or TxRed filter (see Fig. 3 and Note 3 ).
-
5.
Alternatively, MDA-MB-231 cells after CytoCy5S treatment can be analyzed with standard FACS analysis in live cell suspension to confirm the expression of NTR in NTR and HSV1-TK-NTR fusion.
3.3 Functional Analysis of HSV1-TK Expression by 3H-PCV Uptake
-
1.
HSV1-TK phosphorylates 3H-PCV and retains phosphorylated 3H-PCV inside the cells; therefore the level of 3H-PCV uptakes by cells indicates the amount of functional HSV1-TK present in cells.
-
2.
3H-PCV-uptake assay was done to confirm the functionality of HSV1-TK in MDA-MB-231 cells stably expressing HSV1-TK and HSV1-TK-NTR fusion protein.
-
3.
Stable MDA-MB-231 cells expressing HSV1-TK and HSV1-TK-NTR fusion protein were plated to 70–80 % confluence in 12-well culture plate.
-
4.
After 24 h incubation at 37 °C and 5 % CO2, 3H-PCV (0.5 μCi/well) was added to each well and incubated further at 37 °C for 3 h.
-
5.
3H-PCV treated MDA-MB-231 cells were washed twice with PBS after aspirating the medium (The standard protocols described to handle radio-active compounds should be followed).
-
6.
To each well, 1 mL of 0.05 N NaOH was added and kept at RT for 10 min for complete cell lysis.
-
7.
Protein amount from every well was measured using Bio-Rad protein assay kit.
-
8.
Cell lysate from each well was added with 10 mL scintillation fluid (Cytoscint) and the radioactivity was recorded in scintillation counter.
-
9.
The functionality of HSV1-TK was assessed using the percentage conversion of 3H-PCV/mg protein/total count.
-
10.
Appropriate negative control (untransfected cells) should be used to subtract the background activity from endogenous thymidine kinase.
3.4 Therapeutic Evaluation of NTR, HSV1-TK, and HSV1-TK-NTR Fusion in Cells
-
1.
To evaluate the therapeutic efficiency of NTR, HSV1-TK, and HSV1-TK-NTR fusion, MDA-MB-231 stable cells expressing NTR, HSV1-TK, and HSV1-TK-NTR along with FLuc-EGFP fusion protein were used.
-
2.
Stable cells were seeded in 12-well culture plate to a confluence of 60–70 % (1.0 × 105 cells/well in 12 well plate and 0.5 × 105 cells/well in 24 well plates).
-
3.
The cells were treated with GCV (1 μg/mL) and CB1954 (10 μM) individually or in combinations 24 h after initial plating of the cells. The medium was changed to 2 % FBS at the time of treatment.
-
4.
The cells were analyzed for induced apoptotic cells by PI-staining-based FACS analysis [3], trypan blue exclusion assay and MTT assay [19]. The analysis was performed once in every 24 h for up to 4 days.
-
5.
The ratios of apoptotic cells measured from different assays were compared with controls (untreated stable cells and cell without therapeutic gene and treated with drug combinations) to estimate therapeutic evaluation of different gene therapy vectors.
3.5 Therapeutic Evaluation of NTR, HSV1-TK, and HSV1-TK-NTR Fusion in Subcutaneous and Metastatic Tumor Xenografts in Nude Mice by Optical (Bioluminescence and Fluorescence) and microPET Imaging
3.5.1 Therapeutic Optical Bioluminescence Imaging of NTR, HSV1-TK, and HSV1-TK-NTR Fusion in Nude Mice Model
-
1.
Nude mice handling was done following Institutional Animal Care and Use Committee guidelines.
-
2.
Generated MDA-MB-231 stable cells coexpressing NTR or HSV1-TK or HSV1-TK-NTR with FLuc-EGFP fusion proteins were used for the study.
-
3.
MDA-MB-231 stable cells expressing NTR or HSV1-TK or HSV1-TK-NTR and FLuc-EGFP fusion proteins were trypsinized and collected in PBS on day 1.
-
4.
Cell numbers in PBS suspension were evaluated using hemocytometer.
-
5.
Stable cell suspension in PBS was adjusted to a concentration of 5 millions/50 μL.
-
6.
Stable cell preparation in PBS must be implanted within 2 h after trypsinization (see Note 4 ).
-
7.
To implant the stable cells, nude mice with an age group of 4–5 weeks was anesthetized with 2 % isofluorane with oxygen flow of 0.8–1 L/min (see Note 5 ).
-
8.
Stable cell suspension (5 millions/50 μL) was mixed with 50 μL of growth factor reduced matrigel just before implantation.
-
9.
Cell suspension mix was injected on either side of the lower flank and shoulder regions (five million cells per xenograft) for subcutaneous xenograft model. For metastatic model 0.5 × 105 cells in 200 μL PBS was tail vein injected slowly over 1 min time.
-
10.
Tumor growth was monitored periodically until it grows a volume of approximately 50–60 mm3 for subcutaneous xenograft model and by bioluminescence imaging for metastatic model. It may take 3–4 weeks for the tumor to grow.
-
11.
After tumor growth, animals were administered with d-Luciferin substrate (3 mg in 100 μL PBS) and optical images were captured with CCD camera 5 min after substrate injection. The same day animals were imaged with microPET/CT system using [18F]-FHBG injection (radioactive substrate for HSV1-TK).
-
12.
After imaging, animals were treated with GCV (40 mg/kg body weight) and CB1954 (40 mg/kg body weight) independently or in combinations to different groups animals for therapeutic evaluation. The animals were treated two times with an interval of 5 days. Prodrugs were treated intraperitoneally with a maximum volume of 250 μL in sterile physiological saline and 10 % PEG400 [3].
-
13.
Animals must be optically imaged every day through out the experimental period for the expressed FLuc-EGFP bioluminescen ce by injecting d-Luciferin (3 mg/animal).
-
14.
Images can be analyzed with Living Image analysis software. For analysis, regions of interest (ROI) were drawn over the area of signal acquired around the xenografts, and the signals must be defined as maximum photons per second per square centimeter per steradian (p/s/cm2/sr) (see Fig. 4a).
Fig. 4 Optical imaging of nude mice models bearing MDA-MB-231 tumor xenograft expressing NTR, and HSV1-TK-NTR fusion protein. (a) Optical images of nude mice bearing MDA-MB-231 tumor xenograft model captured in CCD camera after injecting with d-luciferin substrate for the coexpressed FLuc-EGFP over time to monitor tumor growth. (b) Fluorescent images acquired from nude mice model pretreated with CytoCy5S dye (NTR substrate) and followed over time after co-treated with GCV (40 mg/kg body weight) and CB1954 (40 mg/kg body weight) combination twice at a interval of 5 days
-
15.
FLuc signal indicates the volume of tumor, and therefore reduced FLuc signal in due course of time indicates the enhanced therapeutic efficiency of HSV1-TK-NTR fusion protein.
-
16.
Therapeutic evaluation was compared by the amount of FLuc signal captured in nude mice xenograft model expressing HSV1-TK-NTR fusion with nude mice xenograft model expressing NTR or HSV1-TK alone.
3.5.2 Therapeutic Evaluation of NTR, and HSV1-TK-NTR Fusion by Optical Fluorescence Imaging in Nude Mice Model
-
1.
For fluorescence imaging, same procedure described in Subheading 3.5.2 was followed except the CytoCy5S treatment for monitoring NTR instead of FLuc-EGFP for biolu minescence imaging.
-
2.
MDA-MB-231 stable cells expressing NTR and HSV1-TK-NTR fusion protein pretreated with CytoCy5S dye before implantation to monitor therapeutic effect of tumor implants before they develop in to tumor or post tumor growth by injecting 25 μg of CytoCy5S/animal in 200 μL of PBS by tail 4 h before fluorescence imaging.
-
3.
Nude mice model with implanted cells were subjected to fluorescence imaging immediately after injected with cells.
-
4.
Animals were treated with GCV (40 mg/kg body weight) and CB1954 (40 mg/kg body weight) the same day after imaging.
-
5.
Fluorescence images were captured every day throughout the experimental period.
-
6.
Images were analyzed as described earlier.
-
7.
CytoCy5S fluorescent signal is an indication of NTR enzyme expression at the time of imaging, hence any reduction in CytoCy5S signal is an indication of down-regulation of NTR expression due to cell death (see Fig. 4b and Note 6 ).
3.5.3 Therapeutic Evaluation of NTR, and HSV1-TK-NTR Fusion by microPET Imaging in Nude Mice Model
-
1.
Positron Emission Tomography/Computed tomography (PET/CT) imaging was done to evaluate therapeutic potential of HSV1-TK using [18F]-FHBG.
-
2.
MDA-MB-231 stable cells are implanted in nude mice as described above.
-
3.
PET/CT imaging was done before and 9 days after prodrug treatment.
-
4.
Nude mice bearing metastatic xenografts of MDA-MB-231 stable cells were anesthetized with isoflurane, and injected intravenously with 7.4–8.9 MBq of [18F]-FHBG in 200 μL volume.
-
5.
After 3 h, PET images were captured with 5 min static PET scans.
-
6.
CT scan also was done for anatomic localization.
-
7.
Image analysis was done with IRW software (Siemens).
-
8.
Data was corrected for partial volume effect and spillover using calibration factors obtained from scanning a cylinder containing phantoms of different sizes, and is presented as %ID/g.
-
9.
MicroPET/CT imaging is useful for monitoring metastatic tumor growth in the internal organs with tomographic information while showing therapeutic gene expression (HSV1-TK) by substrate accumulation.
-
10.
We used both bioluminescence and microPET/CT imaging to monitor therapeutic gene expression while monitoring tumor growth (see Fig. 5).
Fig. 5 Micro-PET/CT fusion images of nude mice bearing MDA-MB-231 metastatic xenograft in the lungs. Top panel showing images of nude mice before and after treated with vehicle control. The bottom panel showing images of animals before and after treated with two doses of GCV and CB1954 combination. Reproduced in part from Sekar et al. with permission from Theranostics, Ivyspring International Publisher Pty Ltd. [1]
4 Notes
-
1.
pcPur vector is a modified version of pcDNA3.1+ vector that express puromycin resistant gene instead of Neomycin.
-
2.
MDA-MB-231 stable cells expressing NTR, HSV1-TK, and HSV1-TK-NTR fusion should be periodically checked with CytoCy5S dye and/or 3H-PCV uptake assays to confirm the maintenance of transgenes expression.
-
3.
Reduced CytoCy5S (a Cy5-labeled quenched substrate) fluoresces at ‘red-shifted’ wavelengths at 638 nm.
-
4.
MDA-MB-231 stable cells should be implanted within 2 h from the time of trypsinization.
-
5.
Nude mice animals should be preferably 4–6 weeks old during the time of tumor implantation.
-
6.
Control tumors also yield autofluorescence signal at early time point due to low threshold of the target signal and it disappears over time.
References
Duarte S, Carle G, Faneca H, de Lima MC, Pierrefite-Carle V (2012) Suicide gene therapy in cancer: where do we stand now? Cancer Lett 324:160–170
Sekar TV, Foygel K, Willmann JK, Paulmurugan R (2013) Dual-therapeutic reporter genes fusion for enhanced cancer gene therapy and imaging. Gene Ther 20:529–537
Sekar TV, Foygel K, Ilovich O, Paulmurugan R (2014) Noninvasive theranostic imaging of HSV1-sr39TK-NTR/GCV-CB1954 dual-prodrug therapy in metastatic lung lesions of MDA-MB-231 triple negative breast cancer in mice. Theranostics 4:460–474
Kelkar SS, Reineke TM (2011) Theranostics: combining imaging and therapy. Bioconjug Chem 22:1879–1903
Penheiter AR, Russell SJ, Carlson SK (2012) The sodium iodide symporter (NIS) as an imaging reporter for gene, viral, and cell-based therapies. Curr Gene Ther 12:33–47
Buursma AR, Rutgers V, Hospers GA, Mulder NH, Vaalburg W, de Vries EF (2006) 18F-FEAU as a radiotracer for herpes simplex virus thymidine kinase gene expression: in-vitro comparison with other PET tracers. Nucl Med Commun 27:25–30
Bar-Shir A, Liu G, Greenberg MM, Bulte JW, Gilad AA (2013) Synthesis of a probe for monitoring HSV1-tk reporter gene expression using chemical exchange saturation transfer MRI. Nat Protoc 8:2380–2391
Ponomarev V, Doubrovin M, Serganova I, Vider J, Shavrin A, Beresten T, Ivanova A, Ageyeva L, Tourkova V, Balatoni J, Bornmann W, Blasberg R, Gelovani Tjuvajev J (2004) A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur J Nucl Med Mol Imaging 31:740–751
Bhaumik S, Sekar TV, Depuy J, Klimash J, Paulmurugan R (2012) Noninvasive optical imaging of nitroreductase gene-directed enzyme prodrug therapy system in living animals. Gene Ther 19:295–302
Jin S, Leach JC, Ye K (2009) Nanoparticle-mediated gene delivery. Methods Mol Biol 544:547–557
Howarth JL, Lee YB, Uney JB (2010) Using viral vectors as gene transfer tools (Cell Biology and Toxicology Special Issue: ETCS-UK 1 day meeting on genetic manipulation of cells). Cell Biol Toxicol 26:1–20
Daya S, Berns KI (2008) Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 21:583–593
Volpers C, Kochanek S (2004) Adenoviral vectors for gene transfer and therapy. J Gene Med 6:S164–S171
Green NK, Seymour LW (2002) Adenoviral vectors: systemic delivery and tumor targeting. Cancer Gene Ther 9:1036–1042
Kasala D, Choi JW, Kim SW, Yun CO (2014) Utilizing adenovirus vectors for gene delivery in cancer. Expert Opin Drug Deliv 11:379–392
Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R (1996) A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Human Gene Ther 7:1145–1159
Pan JG, Zhou X, Luo R, Han RF (2012) The adeno-associated virus-mediated HSV-TK/GCV suicide system: a potential strategy for the treatment of bladder carcinoma. Med Oncol 29:1938–1947
Escors D, Breckpot K (2010) Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp (Warsz) 58:107–119
Devulapally R, Sekar NM, Sekar TV, Foygel K, Massoud TF, Willmann JK, Paulmurugan R (2015) Polymer nanoparticles mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano 9:2290–2302
Acknowledgement
We thank the Department of Radiology and Canary Center at Stanford for their facilities and support. The funding support by National Institutes of Health (NIH grant R01 CA161091 and R21 CA185805 to R.P) is gratefully acknowledged. We also thank Dr. Sanjiv Sam Gambhir, Chairman, Department of Radiology, Stanford University, for his constant support. We gratefully acknowledge the use of the SCi3 Core Facility, Stanford University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Sekar, T., Paulmurugan, R. (2016). Theranostic Imaging of Cancer Gene Therapy. In: Kim, S. (eds) Bioluminescence. Methods in Molecular Biology, vol 1461. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3813-1_20
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3813-1_20
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3811-7
Online ISBN: 978-1-4939-3813-1
eBook Packages: Springer Protocols