Abstract
Secretion is the cellular process present in every organism that delivers soluble proteins and cargoes to the extracellular space. In eukaryotes, conventional protein secretion (CPS) is the trafficking route that secretory proteins undertake when are transported from the endoplasmic reticulum (ER) to the Golgi apparatus (GA), and subsequently to the plasma membrane (PM) via secretory vesicles or secretory granules. This book chapter recalls the fundamental steps in cell biology research contributing to the elucidation of CPS; it describes the most prominent examples of conventionally secreted proteins in eukaryotic cells and the molecular mechanisms necessary to regulate each step of this process.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- ER
- Ribosome
- SRP
- Translocon
- COPII
- COPI
- SNARE
- Golgi
- TGN
- Secretory vesicles
- Secretory granules
- Plasma membrane
- Regulated secretion
1 Introduction
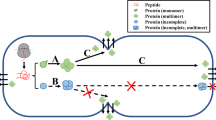
Cell secretion is a fundamental physiological process present both in prokaryotes and eukaryotes that delivers soluble proteins and cargoes to the outside. The need to expel substances to the extracellular space is instructive for a multitude of purposes: growth, cell homeostasis, cytokinesis , defense, structural maintenance, hormone release, and neurotransmission among others. While prokaryotic cells excrete cellular waste and other substances through translocons localized to the limiting cell membranes and secrete effector molecules to other cells through dedicated organs [1], eukaryotes rely on different cellular mechanisms. Eukaryotic cells not only have the characteristic of enclosing the genetic information into a specialized compartment (the nucleus), but they also have the peculiarity of carrying several different organelles across the cytoplasm which are functionally interconnected via a multitude of transport routes that constitute the secretory pathway. Selective cargo transport among compartments is mediated by different vesicular carriers that bud from a donor membrane and fuse with another [2]. Both soluble cargoes and membrane proteins are firstly translocated in the endoplasmic reticulum (ER) from where they are transported either to other organelles or secreted to the extracellular space [3, 4]. When we focus on the latter case, the best characterized mechanism of transport in eukaryotes is the conventional protein secretion (CPS): the transport route that delivers proteins from the ER to the Golgi apparatus (GA), then to the trans-Golgi network (TGN ) , and subsequently to the plasma membrane (PM) . The TGN is the organelle where proteins destined to be secreted are segregated from lysosomal/vacuolar enzymes and sorted in budding secretory vesicles or secretory granules [5]. When secretory vesicles and granules are released from the tubular elements of the TGN , they are transported at different rates along the cytoskeletal filaments and across the cytoplasm toward the plasma membrane with which they fuse, discharging their content to the outside. Importantly, integral PM proteins are delivered and integrated to the plasma membrane through membrane fusion by the same trafficking route. Secretory vesicles and secretory granules are distinct vesicular carriers employed in constitutive and regulated secretion , respectively. While constitutive secretion is constantly undergoing in every eukaryotic cell, regulated secretion is additionally present in special types of animal cells only (e.g., endocrine and exocrine cells, neurons), and it is exclusively triggered by extracellular stimuli [5, 6]. Both constitutive and regulated secretion are included in the CPS, and for both these types of secretion the ER-Golgi-TGN segment of the transport route is identical (Fig. 1). Although individual steps of CPS show a certain degree of variability among different organisms, the basic mechanisms hold true in every eukaryotic cell. The discovery of major principles of cell secretion started in the 1950s.
Schematic representation of conventional protein secretion in eukaryotes. Secretory proteins are translocated in the ER upon (1) signal sequence recognition by the signal recognition particle (SRP) ; (2) SRP interaction with its receptor SR; and (3) transport through the translocon and into the ER lumen. In the ER, the signal sequence is cleaved off, and proteins are folded by molecular chaperons (not shown), and packed in COPII vesicles upon receptor-ligand interaction. COPII vesicles are delivered to the ERGIC (in animals) or to the cis-Golgi (in yeasts and plants). Escaped ER luminal proteins are retrotransported from the ERGIC or from the cis-Golgi to the ER via COPI vesicles. PM proteins and secreted proteins are transported via cisternal maturation to the TGN , whereas integral Golgi proteins are retrieved via intra-Golgi COPI -mediated transport, although another model has been proposed. At the TGN , proteins destined to be secreted are sorted in secretory vesicles (SVs) or immature secretory granules (ISGs). SVs are constitutively delivered toward the PM , whereas ISGs accumulate in the cytoplasm. Upon the arrival of specific stimuli, ISGs form mature secretory granules (MSGs) that are transported to the PM
2 Conventional Protein Secretion: A Historic Perspective
The elucidation of cell secretion has been paved between the 1940s and 1950s, when major advances in electron microscopy were accomplished by Keith Porter, Albert Claude, and George Palade at the Rockefeller University. The discovery of the endoplasmic reticulum (initially called “lace-like reticulum”) in culture cells from chicken embryos [7], and the evidence that in cells synthesizing secretory proteins the majority of the ribosomes is attached to the ER membrane [8, 9], led George Palade to set crucial experiments to investigate the meaning of the ER-ribosome interaction. In an elegant combination of biochemistry , cell fractionation, and electron microscopy Palade and Philip Siekevitz showed that the microsomal fraction isolated from liver or pancreatic cells is almost homogeneously composed of ribosome-bound ER-membrane vesicles [10, 11]. Because Palade recognized that exocrine pancreatic cells of guinea pig contain an exceptionally developed network of ER membranes and produce massive amounts of digestive enzymes at the same time, this system was used for following key experiments in which Palade and Siekevitz demonstrated that the ribosomes are the exclusive site of protein synthesis [12, 13]. Soon after, performing in vivo labeling with radioactive C14-leucine to track the subcellular localization of newly synthesized digestive enzymes, Palade and Siekevitz showed that the pancreatic enzyme chymotrypsin is primarily detected in the microsomal fraction and synthesized by ER-bound ribosomes [14, 15]. These results led Palade to hypothesize that nascent polypeptide chains are driven across the ER-limiting membrane and into its lumen, which was demonstrated few years later by Palade, Siekevitz, and Colvin Redman using a microsomal fraction prepared from pigeon pancreas. Using radioactive amino acids, they analyzed the subcellular localization of the secreted enzyme amylase, which was initially associated with the ER-bound ribosomes. After longer incubation, microsomes were treated with sodium deoxycholate (a compound capable to solubilize membranes) and labeled amylase was detected in the soluble fraction, demonstrating that the newly synthesized enzyme was transported from the ribosomes into the microsomal lumen [16]. Similar results were obtained by Redman and David Sabatini using hepatic microsomes where secretory proteins were released upon puromycin treatment [17], and thus the rough ER (RER) was ascertained to be the site of secretory protein synthesis.
The functional link between the RER and the Golgi apparatus within the secretory pathway was demonstrated during the same years by Palade, Lucien Caro, and James Jamieson by using electron microscopic autoradiography and innovative pulse-chase experiments. These methods allowed the scientists to track in time and follow within cells the whole transport route of secretory proteins. The autoradiographic images obtained by intravenous injections of H3-leucine showed that after ~5 minutes the labeling was localized mostly to the endoplasmic reticulum , at ~20 minutes in the elements of the Golgi complex, and after one hour in the zymogen granules [18]. Moreover, the data highlighted that the zymogen granules were formed in the Golgi region by a progressive concentration of secretory products [18]. In order to better define the role of the Golgi and its surrounding vesicular elements, Palade and Jamieson used pancreatic tissue slices incubated in vitro that allowed shorter pulse labeling and a better resolution with respect to the in vivo situation. By using isopycnic centrifugation in a linear sucrose density gradient smooth-surfaced microsomes (representing mostly the peripheral, vesicular elements of the Golgi complex) and zymogen granules were separated from the rough microsomes (consisting of RER membranes). Labeled proteins appeared initially in the rough microsomes, but shortly after they were more abundantly detected to the smooth ones, reaching the peak of concentration in this fraction after 7 minutes chase incubation. Moreover, after 17 and 37 minutes the zymogen granules were half maximally and maximally labeled, respectively [19–21]. These results not only provided the first indication that vesicles could have been the shuttling elements responsible for intracellular trafficking among compartments, they additionally proved that the Golgi apparatus (discovered in 1898 by Camillo Golgi) was an authentic cell organelle, and not just an artifact produced by cell fixation (an issue discussed at length at the time [22]), having a specific role in cell secretion. Thus, the major cellular structures involved in this process had been finally related to specific cellular functions, although the biochemical and molecular mechanisms underlying the individual steps where still unknown.
In 1971 Günter Blobel and David Sabatini postulated that protein translocation in the ER lumen was dependent on the presence of a specific amino acid sequence at the amino-terminal portion of the nascent polypeptide chain. They also speculated that the putative “signal sequence” would have been capable to recruit a “binding factor” able to guide the ribosome to the ER membrane [23]. Intriguing results were obtained in 1972 by the laboratories of Philip Leder and Cesar Milstein using cell-free translation systems producing immunoglobulin light chains that were 6–8 amino acids longer than the normal secreted version [24, 25], leading to hypothesizing the cleavage of the putative signal sequence after translation. The final proof of the existence of the signal sequence (or “signal peptide ”) was provided few years later by Günter Blobel and Bernhard Dobberstein. Rough microsomes isolated from canine pancreatic cells were added to a cell-free protein-synthesizing mixture supplemented by exogenous mRNA of the immunoglobulin light chain. Subsequently, ribosomes were detached from the ER membranes with a detergent and collected. The isolated ribosomes, carrying unfinished proteins, were transferred in a suitable media where they resumed synthesis of interrupted polypeptide chains without starting new rounds of translation due to the presence of aurintricarboxylic acid (an inhibitor of initiation but not elongation of polypeptide synthesis). Initially the shorter, processed chains appeared, resulting from the completion of peptides in advanced stages of translation. However, few minutes later the in vitro-synthesizing system completed longer chains too, demonstrating that the enzyme responsible for the cleavage of the signal sequence resides in the ER [26]. When rough microsomes, producing only the short version of the protein, were treated with the proteolytic enzymes trypsin and chymotrypsin (which rarely enter the microsomes) the polypeptide chains were not digested, confirming that the newly synthesized secretory proteins are immediately sequestered and driven into the microsomal lumen when translation starts. Instead, when the in vitro system was set to produce the non-secreted protein globin the digestion with trypsin and chymotrypsin occurred, indicating that this protein did not slip into the microsomes [27]. Moreover, when unprocessed light chains were added after the microsomes, they did not lose the signal sequence, demonstrating that its removal occurs during translation and not afterwards [27]. These studies showed that secretory protein precursors enclose the information for their own translocation across the ER membrane.
Since translocation across lipid bilayers was abolished by extracting the microsomal membranes with high-ionic-strength buffers, and it was rescued by adding back the salt extract [28], it became clear that there was a cytosolic component playing a crucial role in the process of protein translocation. In 1980 the signal recognition particle (SRP) was discovered by Günter Blobel and Peter Walter from canine pancreatic cell microsomes. SRP, initially named “signal recognition protein,” was purified from the salt extract using hydrophobic chromatography SDS-gel electrophoresis revealed that SRP is a multimeric complex formed by six subunits of 9, 14, 19, 54, 68, and 72 kDa, respectively [29]. Moreover, SRP was shown to selectively associate with ribosomes engaged in the synthesis of secretory proteins [30, 31]. The association occurs through the binding of the 54 kDa subunit to the signal peptide (typically 7–12 hydrophobic amino acids) of nascent polypeptide chains emerging from the ribosome , which causes temporary arrest of translation [32–37]. In addition to the six different polypeptide components, SRP contains a 7S RNA molecule required for both structural and functional properties, that also represents the backbone to which the six subunits associate [38]. Thus, SRP was recognized to be a ribonucleoprotein (RNP) and was therefore renamed “signal recognition particle ” [38].
The ribosome attachment to the ER membrane is mediated by the interaction between SRP and an integral ER-membrane protein, the SRP-receptor (SR), first found by Bernhard Dobberstein and David Meyer the same year of SRP discovery (i.e., 1980). Initially the cytosolic portion of SR was identified [39, 40]; afterwards the protein was intracellularly localized in vivo with a specific antibody via immunofluorescence [41], and the apparent full size determined to be 72 kDa [41–44]. Few years later, it was shown that SR actually consists of two subunits, the previously identified SRα of 72 kDa and SRβ of 30 kDa [45]. The interaction between SRP and SR is GTP dependent, and both SRP and SR are displaced from the ribosome upon GTP hydrolysis. GTP hydrolysis is additionally required by the ribosome for chain elongation, but not for the polypeptide movement across the ER membrane. Remarkably, the SRP-dependent mechanism of protein targeting is present in all three kingdoms of life. Homologues of SRP and SR have been found also in prokaryotes, where they mediate protein secretion to the periplasmic space through the translocons localized to the inner membrane [46–49].
The vectorial transfer of secretory proteins into the ER lumen can proceed as a consequence of the positional shift of ribosomes on dedicated ER membrane sites [46, 50–52]. The existence of specific locations (“aqueous channels”) on the ER membrane through which secretory proteins enter the ER was already postulated in 1975 by Blobel and Dobberstein [26]. In a review of 1986, about the mechanism of protein translocation across the ER membrane, Walter and Lingappa coined the term “translocon” to identify the sites where polypeptide chains would have crossed the ER membrane to gain access to the lumen [53]. The existence of protein-conducting channels in the ER membrane was demonstrated by electrophysiological techniques. Rough microsomal vesicles were fused on one side (cis) of a planar lipid bilayer separating two aqueous chambers. At low puromycin concentration, single channels with a conductance of 220 picosiemens (pS) were observed. Increasing amounts of puromycin added to the cis side caused a large increase of membrane conductance, until it was abolished when salt concentration reached levels at which ribosomes detach from the vesicles, demonstrating that the ribosome attachment is required for the channel opening [54, 55]. The proteins that form the translocon were identified by photocross-linking using photoreactive probes that were incorporated into nascent polypeptide chains of various lengths. The chains were synthesized by an in vitro translation system supplemented with truncated mRNAs. Upon photolysis, the nascent chain was photocross-linked to specific ER membrane proteins adjacent to the nascent chain throughout translocation [56–59]. Afterwards, the translocon components that formed photoadducts with nascent chains were purified, reconstituted into proteoliposomes, and shown to execute the transfer [60–63].
3 Protein Translocation in the Endoplasmic Reticulum
The channel of the translocon is formed by the Sec61 complex, consisting of the heterotrimer Sec61α, Sec61β, and Sec61γ in mammals [62, 63]. The prefix “Sec” was chosen because the first isolated component Sec61α is homologous to the budding yeast Saccharomyces cerevisiae Sec61p protein, which was identified in a previous screening for secretory mutants that led to the isolation of 23 fundamental genes of the secretory pathway [64, 65]. The α- and γ-subunits are highly conserved, and both are essential for the function of the channel and for cell viability, whereas the β-subunit is dispensable. The Sec61 complex is the essential element for protein translocation, and the α-subunit alone forms the pore [63]. The same holds true in yeast, where the homologous components of the Sec61 complex are Sec61p, Sbh1p, and Sss1p [66], and in prokaryotes, where the bacterial heterotrimeric translocation pore complex (subunits SecY, SecE, and SecG) of plasma membrane translocons mediates secretion of different substances to the periplasmic space [67, 68]. Several integral ER membrane proteins can associate to the Sec61 complex to perform translocation, although the function of some of them is not fully clarified. In mammals, the associated proteins that mediate translocation are: (a) the translocation-associated membrane protein (TRAM) [61]; (b) the translocon-associated protein complex TRAP, a heterotetramer consisting of subunits α, β, γ, δ [62, 69]; (c) the oligosaccharyl transferase complex (OST), responsible for N-glycosylation in the ER, whose core complex is a heterotetramer formed by ribophorin I (66 kDa), ribophorin II (63/64 kDa), OST48 (48 kDa), and DAD1 (10 kDa) [70–72]; (d) the signal peptidase complex (SPC), responsible for the cleavage of the signal sequence in the ER lumen, consisting of five subunits, whose names SPC12, SPC18, SPC21, SPC22/23, and SPC25 indicate the respective molecular size [73]; and (e) the Sec62/Sec63 complex [74, 75]. As well as in mammals, the function of Sec61, OST, and SP complexes has been well characterized in yeast [66, 76, 77]. Depending on which associated components work in concert with the Sec61 complex, two different mechanisms of protein translocation in eukaryotes occur: co- or post-translationally. The co-translational mechanism is present in all cell types and occurs both for soluble and membrane proteins. The targeting phase requires the interaction of SRP with the signal sequence of a nascent polypeptide chain. Subsequently, the interaction between SRP and SR mediates the ribosome-channel alignment. During translocation of membrane proteins, specific polypeptide sequences do not enter the channel, but protrude from the ribosome-channel junction into the cytosol, generating a cytosolic domain [78]. In several, if not all organisms, some proteins are translocated after completion of their synthesis, therefore “post-translationally,” and they are not completely folded after their release from the ribosome [79]. Post-translational translocation is more frequently occurring in simpler organisms like bacteria and yeast. In S. cerevisiae the heterotetrameric Sec62/Sec63 complex specifically mediates post-translational translocation in concert with the cytosolic chaperon Hsp70, the Sec61 complex, and the luminal chaperone Kar2p/BiP in an ATP-dependent manner [79–84], Instead, the co-translational mechanism requires the function of the Sec61 complex only and it is instead GTP dependent [85]. Although in mammals translocation seems to occur preferentially co-translationally [85, 86], posttranslational mechanisms have been shown for specific kinds of proteins. In fact, the SRP-dependent pathway, although ubiquitous, is inaccessible for those proteins carrying a single transmembrane domain (TMD) on their C-terminal portion, because they are released from the ribosome before the TMD emerges from the ribosomal tunnel. These peptides, called tail-anchored proteins (TA), are involved in a wide range of cellular processes and include the SNAREs (involved in vesicular traffic), several translocon components, structural Golgi proteins, and enzymes located in almost every membrane. Thus, TAs are inserted in the ER membrane post-translationally both in higher eukaryotes and yeast. Cross-linking experiments revealed that the cytosolic TMD recognition complex TRC40 (previously known as Asna-1) interacts post-translationally with TAs in a TMD-dependent manner and mediates their targeting to the ER membrane [87, 88]. A conserved three-protein complex composed of Bat3, TRC35, and Ubl4A facilitates the TA protein capture by TRC40 [90]. Homologues of TRC40 are conserved in many species, including S. cerevisiae where it is termed Get3 [90]. TRC40 delivers TAs to an ER receptor composed of the tryptophan-rich basic protein (WRB) [91] and the calcium-modulating cyclophilin ligand (CAML) [92], mammalian equivalents of the yeast components Get1 and Get2, respectively [86, 93].
4 The COPII -Mediated ER Exit
Nascent secretory and membrane proteins are translocated or inserted at the ER, eventually glycosylated, and then folded through the action of a multitude of molecular chaperons and cofactors that ensure conformation quality and fidelity. When the protein-folding capacity of the ER is unable to sustain a sufficient rate of folding, the accumulation of misfolded proteins triggers a multitude of signaling pathways collectively termed unfolded protein response (UPR) that increases the folding capacity. However, when problems persist, misfolded polypeptides are degraded through the action of the ER-associated degradation (ERAD) pathway, and the mutated and/or misfolded proteins are retro-translocated to the cytosol to be degraded by the 26S proteasome machinery [94, 95].
When membrane and soluble proteins reach the correct conformation and are not ER-resident proteins, they exit the ER. In all eukaryotic cells, the best characterized mechanism of ER exit is the COPII -mediated transport, whose components were all identified after a screening for yeast secretory mutants [64]. The coat protein complex II (COPII ) assembles on specific locations of the ER membrane, called ER-exit sites (ERES), from which COPII -coated vesicles bud off [96]. ERES are also known as transitional elements (TEs) or transitional ER (tER). The number, size, and dynamics of ERES vary among cell types and organisms; however, these organized export sites are present in most eukaryotic cells [97]. The assembly of COPII starts with the recruitment of the cytosolic small GTPase Sar1 (secretion-associated RAS-related 1) to the ER membrane [98, 99], where it is activated through the action of the guanine nucleotide exchange factor (GEF) Sec12, an integral ER membrane protein that catalyzes GDP/GTP exchange [100, 101]. An activated, GTP-bound Sar1 inserts its N-terminal helix into the ER membrane, inducing initial membrane curvature [102–104] alongside with the recruitment of the cytosolic Sec23/Sec24 heterodimer [105]. The Sar1-Sec23-Sec24 complex is recognized and bound by the Sec13/Sec31 heterotetramer, which forms the outer layer of the COPII cage [106–109]. Transmembrane cargo proteins are recognized and bound by Sec24, whereas soluble cargoes bind specific receptors that span the ER membrane. Multiple adjacent Sec13/Sec31 subcomplexes drive membrane bending and vesicle fission using the energy of GTP hydrolysis [110, 111]. Sec23 serves as a bridge between Sar1 and Sec24 and is a GTPase-activating protein (GAP) that stimulates Sar1 GTP hydrolysis [99], which is additionally needed for vesicle uncoating after release [111]. There is evidence that Sec31 interacts directly with Sar1 to promote Sec23 GAP activity [112]. In addition to the six core COPII components, Sec16 is involved in ERES maintenance and COPII -mediated ER export. Sec16 localizes to the ERES independent of Sec23/24 and Sec13/31, and its localization depends on Sar1 activity [113]. Sec16 has been shown to bind several COPII components and seems to serve as scaffold protein that concentrates, organizes, and stabilizes COPII proteins [114–116]. However, the precise Sec16 function is still not fully understood.
Since most COPII subunits have one or more paralogues [117], and since COPII transport is assisted by several different accessory proteins (e.g., 14-3-3, PX-RICS, Deshavelled) depending on the cell type [118–121], the result is a high number of molecularly different COPII -coated vesicles with tissue specificities and selectivity for different cargo molecules. The number and size of ERES, together with the expression levels of COPII components, may play a major role in the secretion rate in different tissues. One of the biggest open questions regarding COPII -mediated transport is how large-sized cargoes can be lodged inside vesicles which are typically of 60–100 nm in diameter. Procollagen fibrils (PC), composed of rigid triple helices of up to 400 nm in length, represent one of the most abundant secreted cargoes in animal cells , since collagen composes approximately 25 % of the whole-body protein content, and is fundamental for almost all cell-cell interactions [122]. There are several lines of evidence indicating that collagen secretion is COPII dependent. Depletion of Sec13 [123], disruption of Sec24D [124], mutation of Sec23A [125], loss-of-function of the Sedlin gene (a TRAPPI complex component interacting with Sar1 at the ER-Golgi interface) [126], and depletion of Sar1A and Sar1B [127] all block collagen secretion, leading to severe diseases. Cryomicroscopical data suggest a significant level of flexibility of the COPII cage, which in vitro can assemble on flatter membranes, forming larger cages that could accommodate procollagen fibrils [128, 129]. Recently, a potential mechanism for giant COPII-carriers biogenesis has been proposed, which involves TANGO1-mediated packing. TANGO1/Mia3 is a transmembrane protein identified from a screening for secretory mutants in Drosophila S2 cells, and shown to localize to early Golgi cisternae and to the ERES [130, 131]. Knockdown of TANGO1 with siRNA severely inhibits ER export of PC VII. TANGO1 interacts with Sec23A and Sec24C through its cytoplasmic proline-rich domain (PRD), and binds PC VII via its luminal SH3 domain [132]. cTAGE5 is the partner of TANGO1 in PC VII secretion; it is anchored to the ERES and interacts via its PRD with Sec23A, Sec24C, and Sec12 [133, 134]. Cullin3 (an E3 ligase), and its specific adaptor protein KLHL12, ubiquitinates SEC31. In mouse embryonic fibroblasts, Cul3 knockdown inhibits collagen IV secretion, and overexpression of KLHL12 increases secretion of PC I in the human fibroblast cell line IMR-90. The model proposes that TANGO1-cTAGE5 pack collagens in ERES enriched with Sec23/24 to the inner coat shell, and Cul3-KLHL12 mediate the assembly of a large outer layer composed of Sec13/31-ubiquitin. The final result would be the formation of a giant COPII-carrier carrier for procollagen export from the ER [122]. However, the evidence that TANGO1 interacts with the conserved syntaxin 5-binding protein Sly1, which in turn interacts with the ER-specific t-SNAREs syntaxin-17 and syntaxin-18 (involved in membrane fusion), leads to formulate a second hypothesis: a membrane domain of the ERGIC (ER-Golgi-intermediate compartment) could be recruited to the ERES, and the resulting fusion would promote the elongation of the PC VII-enriched domain into a tubular uncoated bud, while the TANGO1-cTAGE5-Sec12-Sec23/24 complex would remain at the neck [122].
5 The ER-Golgi Interface and COPI Vesicles
Passive incorporation of soluble cargoes into COPII vesicles can occur [135–138], whereas membrane proteins and receptors require diacidic or dihydrophobic motifs in their cytosolic domains for efficient transport through the interaction with multiple binding sites of Sec24 [139–142]. It is still unclear in mammals whether COPII vesicles are transported to the ERGIC along microtubules (from the plus- to minus-end), since contrasting results have been so far collected [117]. The directionality and fidelity of COPII vesicle transport and fusion with either the ERGIC or the cis-Golgi (depending on the organism) are mediated by the concerted action of RAB GTPases, tethering factors, and integral membrane SNARE proteins. In mammalian cells, RAB1 and the tethering factors p115, GM130, GRASP65, and the TRAPPI complex orchestrate the tethering [143–150]. TRAPPI-mediated RAB1 activation recruits p115, generating a localized signal to tether COPII vesicles, and TRAPPI binds directly Sec23 [151, 152]. Fusion of COPII -tethered vesicles depends on a set of four SNAREs: syntaxin-5, membrin/GS27, BET1, and Sec22B [153–155]. Additionally, the syntaxin 5-binding protein Sly1 is required for this vesicle fusion step [156] and may serve to coordinate the vesicle tethering and fusion. All fusion events between membranes require the correct pairing of specific cognate SNAREs on the vesicle surface and on the acceptor membrane. SNAREs (soluble N-ethylmaleimide-sensitive factor adaptor protein receptors) are tail-anchored proteins that contain a conserved membrane-proximal heptad repeat sequence known as the SNARE motif. The trans-assembly of motifs into a four-helix bundle drives the fusion between lipid bilayers [157–161]. In mammals, COPII vesicles reach first the ER-Golgi-intermediate compartment (ERGIC), alternatively termed vesicular tubular cluster (VTC), which is a distinct organelle respect to the Golgi and is absent in yeasts and plants [97]. While in animal cells the Golgi apparatus is a relatively stationary organelle, in plant cells the Golgi is instead highly mobile and moves with a speed of up to 4 μm/sec. [162]. Golgi stacks in plant cells move extensively along both the ER tubules and actin filaments (which are aligned to each other) throughout the cytoplasm. The movement relies on actomyosin motors, and displays a distinctive stop-and-go pace [162–167]. The plant ER-Golgi interface is spatially reduced (around 500 nm), and the two compartments are tightly coupled, as demonstrated by using optical tweezers [168]. The plant Golgi receives budding COPII vesicles from the ERES in a cytoskeleton-independent manner [169] within the so called secretory unit model, in which the two compartments are embedded in a ribosome-free surrounding matrix [170–174]. While plant COPI vesicles (the retrograde Golgi-to-ER carrier) have been biochemically isolated and localized in situ [175], visualization of COPII in plant tissues is rare (although observed) even when ultra-rapid cryofixing techniques are employed [170, 176–178]. Thus, it is a matter of debate whether COPII -mediated transport in higher plants can additionally occur via coated-tubular connections [179].
COPI mediates retrograde transport of receptors and soluble proteins from the cis-Golgi (from the ERGIC in mammals) back to the ER along microtubules. The coat protein complex I (COPI ), or “coatomer,” is a heptameric (α, β, β′, g, δ, ε, ζ) complex, where the γ-COP, δ-COP, ζ -COP, and β-COP subunits constitute the inner coat layer, and α-COP, β-COP, and ε-COP form the outer shell [180–182]. Upon activation by ADP-ribosylation factor guanine nucleotide exchange factors (ARF-GEFs), the myristoylated membrane-anchored ARF1 GTPase recruits the COPI subunits to the Golgi membranes [183, 184]. Subunits α-COP, β′-COP, γ-COP, and δ-COP recognize sorting motifs on the cytosolic domain of membrane cargoes and mediate the load of soluble proteins into nascent COPI vesicles. ARF GTPase-activating proteins (GAPs) bind cytoplasmic signals on cargo proteins, γ-COP, β′-COP, and ARF1. Stimulation of the GTPase activity of ARF1 by GAPs leads to the release of ARF1 from the complex and to the dissociation of GAPs and the coat subunits [185]. COPI vesicles deliver ER receptors (recycled for new rounds of transport) and luminal ER proteins that escape through bulk flow via COPII vesicles. Luminal ER proteins classically carry a KDEL motif (in animals and yeast) or an HDEL motif (in plants) within their C-terminal domain, which represent the retrograde sorting signals recognized by dedicated Golgi receptors (Erd2 in yeast and plants; KDELRs in mammals). Targeting of COPI vesicles to the ER requires the multisubunit DSL1 tethering complex, and the SNARE proteins syntaxin-18, Sec20, Slt1, and Sec22B [186, 187].
6 The Golgi Apparatus, the TGN , and the Rab GTPase-Mediated Secretory Vesicle Formation
In most eukaryotes the Golgi apparatus (or Golgi complex) consists of a series of stacked cisternae, with a cis to trans polar orientation. The cisternae are kept adjacent by structural proteins present in the surrounding ribosome-free matrix [188], and by heterotypic tubular connections [189, 190]. In mammals the Golgi includes 4-8 cisternae, each of them 0.7–1.1 μm wide and 10–20 nm thick. Multiple Golgi stacks can be laterally interconnected by tubules, forming the so-called Golgi ribbon. In several lower eukaryotes, like the budding yeasts S. cerevisiae and Pichia pastoris , or in the fruit fly Drosophila melanogaster, the Golgi is formed by individual cisternae scattered throughout the cytoplasm, which can occasionally associate but do not form stacks, although polar features are maintained [188]. Single stacks are present both in higher plants (e.g., Arabidopsis thaliana , tobacco), and algae (e.g., Chlamydomonas reinhardtii). Depending on the enrichment of specific enzymes, three major regions can be recognized within one Golgi complex: cis, medial, and trans [188]. Juxtaposed to the Golgi trans-most cistemae, a pleiomorphic, tubular-vesicular compartment is present: the trans-Golgi-network (TGN) [191, 192]. In plant cells the TGN has been shown to additionally hold the role of early endosome (EE, the first compartment reached by endocytosed molecules) [193–195], whereas in animals the TGN and EE are distinct compartments. Two models have been proposed for secretory protein transport through the Golgi complex: (1) anterograde COPI -vesicular transport between stable cisternae; and (2) cisternal progression/maturation [196]. Detection of cargoes and bidirectional transport by distinct populations for COPI vesicles support the first scenario [196]; however, exclusive retrograde transport for COPI is supported by the detection of the KDEL receptor, resident Golgi proteins, and glycosylation enzymes. The cisternal maturation model is currently preferred because, among other reasons, it explains how transport of large cargoes is achieved [196]. In this view, the cisternae continuously mature from cis-to-trans, and secretory proteins are transported along the anterograde flow, and up to the TGN . The anterograde maturation is the net result from COPII vesicle entry and secretory vesicles exit on the respective cis and trans sides. Homotypic fusion of COPII vesicles gives rise to newly formed cis-cisternae, while the trans-most cisternae mature into a TGN . Intra-Golgi retrieval of integral Golgi proteins from older to younger cisternae occurs via COPI vesicles and through the heterotypic tubular connections. The Golgi is the organelle where glycosylation of soluble cargoes, membrane proteins, and lipids is completed, and where polysaccharide synthesis occurs. The cis-to-trans polarity in the distribution of Golgi glycosylation enzymes was discovered by cytochemical staining based on different enzymatic activity among cisternae, and it reflects the sequence of oligosaccharide processing reactions [188, 196, 197].
At the TGN , proteins are sorted toward three different destinations: PM, endosomes, and lytic compartments. These trafficking routes differ in terms of adaptors, effector molecules, and sorting signals involved. Formation of secretory vesicles delivered to the PM is GTP dependent, requires either ARF GTPases or Rab GTPases, and may be mediated by clustering of specific lipids on TGN subdomains. However, the molecular mechanisms and the sorting signals for TGN -to-PM delivery are far less understood in comparison to COPII -, COPI - and clathrin-mediated vesicle transports.
The heterotetrameric adaptor protein complexes (APs) are the most well-characterized cargo adaptors at the TGN . Five APs have been identified in higher eukaryotes, and three of them (AP-1, AP-3, and AP-4) sort proteins at the TGN . APs bind membrane cargoes and receptors via their μ subunit, and contribute to form coated carriers. AP-1 and AP-3 interact with clathrin, whereas AP-4 does not [198]. While AP-3 is involved in lysosomal/vacuolar sorting and traffic, AP-1 and AP-4 mediate polar transport of basolateral-located proteins in epithelial cells [199, 200], and both AP-1 and AP-4 require the function of ARF1. The PM of epithelial cells is polarized into apical and basolateral domains, and each of them contain distinct set of proteins carrying specific functions. Protein sorting at the TGN contributes to polar delivery of apical/basolateral proteins, and to the asymmetric localization of signaling receptors that determine planar cell polarity (PCP) of epithelia [201]. Tyrosine-based motifs and dileucine motifs at the C-terminal domain are canonical sorting signals for basolateral-targeted proteins, whereas apical sorting determinants are diversified and vaguely defined [198, 201]. However, apical determinants promote partitioning into glycosphingolipid- and cholesterol-rich membrane microdomains (i.e., lipid rafts) at the TGN , from where carriers arise [201–203].
In yeast, a unique adaptor complex, termed “exomer,” mediates protein transport directly from the TGN to the PM. Exomer is a heterotetramer consisting of two copies of Chs5p and two copies of the ChAPs family proteins (Chs6, Bud7p, Bch1p, and Bch2p). Chs5p binds to the small GTPase Arf1, whereas the ChAPs are responsible for cargo binding and sorting [204–208]. Exomer regulates trafficking of chitin synthase III (Chs3p) and Fus1p from the TGN to the PM [204, 205, 209, 210]. No known homologs of exomer have been found in metazoans as yet.
Secretory vesicles in yeast are transported to the cell surface through the function of the Sec4 GTPase [211], whose homolog in plants is RabE1 [212]. In plants, secretory vesicles deliver hemicelluloses and pectins to the plant apoplast from the TGN /EE [193], a transport route mediated by the protein ECHIDNA (ECH), which interacts with the Rab GTPases YIP4a and YIP4b [213, 214]. On the contrary, cellulose is synthesized by plasma membrane-localized cellulose synthase complexes [215]. ECH also specifically mediates the targeting of the auxin influx carrier AUX1 from the TGN to the PM, but not the transport of the auxin influx carriers LAX1-3 and of the efflux carrier PIN3 [216]. In contrast to animals, secretion in plants is fundamental for cytokinesis , since plants have evolved a unique mechanism of cell division. Instead of forming a contractile ring that constricts the plasma membrane, dividing plant cells target secretory vesicles to the center of the division plane, where they fuse with one another to form the cell plate. Afterwards, the cell plate fuses with the parental PM on both sides [217, 218]. This mechanism requires the targeting and function of the PM-located plant-specific syntaxin KNOLLE, the Sec1-like protein KEULE, and the t-SNARE AtSNAP-33 [219–222].
After budding, vesicles are delivered to the PM by motor-mediated transport along a cytoskeletal track (microtubules or actin), in which kinesins have been shown to be implicated [203, 223]. The tethering factor that mediates fusion of secretory vesicles and secretory granules with the PM is the exocyst complex, formed by eight components: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84, whose functions are conserved among eukaryotes [224–226].
7 Secretory Granules and Regulated Secretion
Animal cells where regulated secretion is present include endocrine and exocrine cells, epithelial cells, mast cells, platelets, large granular lymphocytes, neutrophils, and neurons. Secretion of insulin from endocrine pancreatic β-cells, secretion of zymogen from exocrine pancreatic cells to digest food, secretion of growth hormone from GH cells of the pituitary gland, and the release of neurotransmitters at the synapses are only few examples of regulated secretion. Secretory granules contain massive amounts of cargoes, which accumulate first in subdomains of the TGN , and are later released as immature secretory granules (ISGs) that accumulate in the cytoplasm. In endocrine cells the concentration factor from the ER to secretory granules may be as high as 200-fold, whereas in constitutive secretory vesicles there is at most a 2-fold concentration of secretory products then in the ER [5]. Biogenesis of mature secretory granules (MSGs) involves specific mechanisms of protein sorting, pro-hormone processing, and vesicle fusion. Specific sorting signals and domains in regulated secretory proteins (RSPs) are needed to direct them into the regulated secretory pathway, and for their segregation from constitutive secreted proteins at the TGN . Cell-type-specific composition of RSPs in the TGN has an important role to determine how the RSPs are sorted into ISGs. Lipid rafts are implicated in RSP sorting at the TGN and specific SNAREs are required for either MSG formation and for their fusion with the PM [6, 227].
References
Wooldridge K (2009) Bacterial secreted proteins: secretory mechanisms and role in pathogenesis. Caister Academic Press, Norfolk, VA
Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116:153–166. doi:10.1016/S0092-8674(03)01079-1
Palade GE (1975) Intracellular aspects of the process of protein synthesis. Science 189:347–358. doi:10.1126/science.1096303
Kelly RB (1985) Pathways of protein secretion in eukaryotes. Science 230:25–32. doi:10.1126/science.2994224
Burgess TL, Kelly RB (1987) Constitutive and regulated secretion of proteins. Annu Rev Cell Biol 3:243–293. doi:10.1146/annurev.cb.03.110187.001331
Tooze SA, Martens GJ, Huttner WB (2001) Secretory granule biogenesis: rafting to the SNARE. Trends Cell Biol 11:116–122. doi:10.1016/S0962-8924(00)01907-3
Porter KR, Claude A, Fullam EF (1945) A study of tissue culture cells by electron microscopy: methods and preliminary observations. J Exp Med 81:233–246. doi:10.1084/jem.81.3.233
Palade GE (1955) A small particulate component of the cytoplasm. J Biophys Biochem Cytol 1:59–68. doi:10.1083/jcb.1.1.59
Palade GE (1955) Studies on the endoplasmic reticulum: II. Simple dispositions in cells in situ. J Biophys Biochem Cytol 1:567–582. doi:10.1083/jcb.1.6.567
Palade GE, Siekevitz P (1956) Liver microsomes: an integrated morphological and biochemical study. J Biophys Biochem Cytol 2:171–200. doi:10.1083/jcb.2.2.171
Palade GE, Siekevitz P (1956) Pancreatic microsomes: an integrated morphological and biochemical study. J Biophys Biochem Cytol 2:671–690. doi:10.1083/jcb.2.6.671
Siekevitz P, Palade GE (1958) A cytochemical study on the pancreas of the guinea pig. I. Isolation and enzymatic activities of cell fractions. J Biophys Biochem Cytol 4:203–218. doi:10.1083/jcb.4.2.203
Siekevitz P, Palade GE (1958) A cytochemical study on the pancreas of the guinea pig. II. Functional variations in the enzymatic activity of microsomes. J Biophys Biochem Cytol 4:309–318. doi:10.1083/jcb.4.3.309
Siekevitz P, Palade GE (1958) A cytochemical study on the pancreas of the guinea pig. III. In vivo incorporation of leucine-1-C14 into the proteins of cell fractions. J Biophys Biochem Cytol 4:557–566. doi:10.1083/jcb.4.5.557
Siekevitz P, Palade GE (1960) A cytochemical study on the pancreas of the guinea pig. 5. In vivo incorporation of leucine-l-C 14 into the chymotrypsinogen of various cell fractions. J Biophys Biochem Cytol 7:619–630. doi:10.1083/jcb.7.4.619
Redman CM, Siekevitz P, Palade GE (1966) Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J Biol Chem 241:1150–1158
Redman CM, Sabatini DD (1966) Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci U S A 56:608–615. doi:10.1073/pnas.56.2.608
Caro LG, Palade GE (1964) Protein synthesis, storage, and discharge in the pancreatic exocrine cell – an autoradiographic study. J Cell Biol 20:473–495. doi:10.1083/jcb.20.3.473
Jamieson JD, Palade GE (1966) Role of the Golgi complex in the intracellular transport of secretory proteins. Proc Natl Acad Sci U S A 55:424–431. doi:10.1073/pnas.55.2.424
Jamieson JD, Palade GE (1967) Intracellular transport of secretory proteins in pancreatic exocrine cell. I Role of peripheral elements of Golgi complex. J Cell Biol 34:577–596. doi:10.1083/jcb.34.2.577
Jamieson JD, Palade GE (1967) Intracellular transport of secretory proteins in the pancreatic exocrine cell. II Transport to condensing vacuoles and zymogen granules. J Cell Biol 34:597–615. doi:10.1083/jcb.34.2.597
Farquhar MG, Palade GE (1981) The Golgi apparatus (complex)-(1954–1981)-from artifact to center stage. J Cell Biol 91:77–103. doi:10.1083/jcb.91.3.77s
Blobel G, Sabatini D (1971) Dissociation of mammalian polyribosomes into subunits by puromycin. In: Manson LA (ed) Biomembranes. Springer, Berlin, pp 193–195
Swan D, Aviv H, Leder P (1972) Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A 69:1967–1971. doi:10.1073/pnas.69.7.1967
Milstein C, Brownlee GG, Harrison TM, Mathews MB (1972) A possible precursor of immunoglobulin light chains. Nat New Biol 239:117–120. doi:10.1038/newbio239117a0
Blobel G, Dobberstein B (1975) Transfer of proteins across membranes. I Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol 67:835–851. doi:10.1083/jcb.67.3.835
Blobel G, Dobberstein B (1975) Transfer of proteins across membranes. II Reconstitution of functional rough microsomes from heterologous components. J Cell Biol 67:852–862. doi:10.1083/jcb.67.3.852
Warren G, Dobberstein B (1978) Protein transfer across microsomal membranes reassembled from separated membrane components. Nature 273:569–571. doi:10.1038/273569a0
Walter P, Blobel G (1980) Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A 77:7112–7116. doi:10.1073/pnas.77.12.7112
Walter P, Ibrahimi I, Blobel G (1981) Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol 91:545–550. doi:10.1083/jcb.91.2.545
Walter P, Blobel G (1981) Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol 91:551–556. doi:10.1083/jcb.91.2.551
Walter P, Blobel G (1981) Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol 91:557–561. doi:10.1083/jcb.91.2.557
Gilmore R, Blobel G (1983) Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell 35:677–685. doi:10.1016/0092-8674(83)90100-9
Kurzchalia TV, Wiedmann M, Girshovich AS, Bochkareva ES, Bielka H, Rapoport TA (1986) The signal sequence of nascent preprolactin interacts with the 54K polypeptide of the signal recognition particle. Nature 320:634–636. doi:10.1038/320634a0
Krieg UC, Walter P, Johnson AE (1986) Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A 83:8604–8608. doi:10.1073/pnas.83.22.8604
Siegel V, Walter P (1988) Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: analysis of biochemical mutants of SRP. Cell 52:39–49. doi:10.1016/0092-8674(88)90529-6
Bernstein HD, Poritz MA, Strub K, Hoben PJ, Brenner S, Walter P (1989) Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature 340:482–486. doi:10.1038/340482a0
Walter P, Blobel G (1982) Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 299:691–698. doi:10.1038/299691a0
Meyer DI, Dobberstein B (1980) A membrane component essential for vectorial translocation of nascent proteins across the endoplasmic reticulum: requirements for its extraction and reassociation with the membrane. J Cell Biol 87:498–502. doi:10.1083/jcb.87.2.498
Meyer DI, Dobberstein B (1980) Identification and characterization of a membrane component essential for the translocation of nascent proteins across the membrane of the endoplasmic reticulum. J Cell Biol 87:503–508. doi:10.1083/jcb.87.2.503
Meyer DI, Louvard D, Dobberstein B (1982) Characterization of molecules involved in protein translocation using a specific antibody. J Cell Biol 92:579–583. doi:10.1083/jcb.92.2.579
Meyer DI, Krause E, Dobberstein B (1982) Secretory protein translocation across membranes-the role of the “docking protein”. Nature 297:647–650. doi:10.1038/297647a0
Gilmore R, Blobel G, Walter P (1982) Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol 95:463–469. doi:10.1083/jcb.95.2.463
Gilmore R, Walter P, Blobel G (1982) Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol 95:470–477. doi:10.1083/jcb.95.2.470
Tajima S, Lauffer L, Rath VL, Walter P (1986) The signal recognition particle receptor is a complex that contains two distinct polypeptide chains. J Cell Biol 103:1167c1178. doi:10.1083/jcb.103.4.1167
Keenan RJ, Freymann DM, Stroud RM, Walter P (2001) The signal recognition particle. Annu Rev Biochem 70:755–775. doi:10.1146/annurev.biochem.70.1.755
Römisch K, Webb J, Herz J, Prehn S, Frank R, Vingron M, Dobberstein B (1989) Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature 340:478–482. doi:10.1038/340478a0
Poritz MA, Bernstein HD, Strub K, Zopf D, Wilhelm H, Walter P (1990) An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science 250:1111–1117. doi:10.1126/science.1701272
Wolin SL (1994) From the elephant to E. coli: SRP-dependent protein targeting. Cell 77:787–790
Connolly T, Gilmore R (1986) Formation of a functional ribosome-membrane junction during translocation requires the participation of a GTP-binding protein. J Cell Biol 103:2253–2261. doi:10.1083/jcb.103.6.2253
Connolly T, Gilmore R (1989) The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell 57:599–610. doi:10.1016/0092-8674(89)90129-3
Connolly T, Rapiejko PJ, Gilmore R (1991) Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science 252:1171–1173. doi:10.1126/science.252.5009.1171
Walter P, Lingappa VR (1986) Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol 2:499–516. doi:10.1146/annurev.cb.02.110186.002435
Simon SM, Blobel G, Zimmerberg J (1989) Large aqueous channels in membrane vesicles derived from the rough endoplasmic reticulum of canine pancreas or the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A 86:6176–6180. doi:10.1073/pnas.86.16.6176
Simon SM, Blobel G (1991) A protein-conducting channel in the endoplasmic reticulum. Cell 65:371–380. doi:10.1016/0092-8674(91)90455-8
Krieg UC, Johnson AE, Walter P (1989) Protein translocation across the endoplasmic reticulum membrane: identification by photocross-linking of a 39-kDa integral membrane glycoprotein as part of a putative translocation tunnel. J Cell Biol 109:2033–2043. doi:10.1083/jcb.109.5.2033
Wiedmann M, Görlich D, Hartmann E, Kurzchalia TV, Rapoport TA (1989) Photocrosslinking demonstrates proximity of a 34 kDa membrane protein to different portions of preprolactin during translocation through the endoplasmic reticulum. FEBS Lett 257:263–268. doi:10.1016/0014-5793(89)81549-2
High S, Görlich D, Wiedmann M, Rapoport TA, Dobberstein B (1991) The identification of proteins in the proximity of signal-anchor sequences during their targeting to and insertion into the membrane of the ER. J Cell Biol 113:35–44. doi:10.1083/jcb.113.1.35
Thrift RN, Andrews DW, Walter P, Johnson AE (1991) A nascent membrane protein is located adjacent to ER membrane proteins throughout its integration and translation. J Cell Biol 112:809–821. doi:10.1083/jcb.112.5.809
Nicchitta CV, Blobel G (1990) Assembly of translocation-competent proteoliposomes from detergent-solubilized rough microsomes. Cell 60:259–269. doi:10.1016/0092-8674(90)90741-V
Görlich D, Hartmann E, Prehn S, Rapoport TA (1992) A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature 357:47–52. doi:10.1038/357047a0
Görlich D, Prehn S, Hartmann E, Kalies KU, Rapoport TA (1992) A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell 71:489–503. doi:10.1016/0092-8674(92)90517-G
Görlich D, Rapoport TA (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75:615–630. doi:10.1016/0092-8674(93)90483-7
Novick P, Field C, Schekman R (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21:205–215. doi:10.1016/0092-8674(80)90128-2
Deshaies RJ, Schekman R (1987) A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol 105:633–645. doi:10.1083/jcb.105.2.633
Hartmann E, Sommer T, Prehn S, Görlich D, Jentsch S, Rapoport TA (1994) Evolutionary conservation of components of the protein translocation complex. Nature 367:654–657. doi:10.1038/367654a0
Brundage L, Hendrick JP, Schiebel E, Driessen AJ, Wickner W (1990) The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell 62:649–657. doi:10.1016/0092-8674(90)90111-Q
Akimaru J, Matsuyama S, Tokuda H, Mizushima S (1991) Reconstitution of a protein translocation system containing purified SecY, SecE, and SecA from Escherichia coli. Proc Natl Acad Sci U S A 88:6545–6549. doi:10.1073/pnas.88.15.6545
Hartmann E, Görlich D, Kostka S, Otto A, Kraft R, Knespel S, Bürger E, Rapoport TA, Prehn S (1993) A tetrameric complex of membrane proteins in the endoplasmic reticulum. Eur J Biochem 214:375–381. doi:10.1111/j.1432-1033.1993.tb17933.x
Kelleher DJ, Kreibich G, Gilmore R (1992) Oligosaccharyltransferase activity is associated with a protein complex composed of ribophorins I and II and a 48 kd protein. Cell 69:55–65. doi:10.1016/0092-8674(92)90118-V
Kelleher DJ, Gilmore R (1997) DAD1, the defender against apoptotic cell death, is a subunit of the mammalian oligosaccharyltransferase. Proc Natl Acad Sci U S A 94:4994–4999. doi:10.1073/pnas.94.10.4994
Nilsson I, Kelleher DJ, Miao Y, Shao Y, Kreibich G, Gilmore R, von Heijne G, Johnson AE (2003) Photocross-linking of nascent chains to the STT3 subunit of the oligosaccharyltransferase complex. J Cell Biol 161:715–725. doi:10.1083/jcb.200301043
Evans EA, Gilmore R, Blobel G (1986) Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci U S A 83:581–585. doi:10.1073/pnas.83.3.581
Meyer HA, Grau H, Kraft R, Kostka S, Prehn S, Kalies KU, Hartmann E (2000) Mammalian Sec61 is associated with Sec62 and Sec63. J Biol Chem 275:14550–14557. doi:10.1074/jbc.275.19.14550
Tyedmers J, Lerner M, Bies C, Dudek J, Skowronek MH, Haas IG, Heim N, Nastainczyk W, Volkmer J, Zimmermann R (2000) Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc Natl Acad Sci U S A 97:7214–7219. doi:10.1073/pnas.97.13.7214
Böhni PC, Deshaies RJ, Schekman RW (1988) SEC11 is required for signal peptide processing and yeast cell growth. J Cell Biol 106:1035–1042. doi:10.1083/jcb.106.4.1035
Dempski RE Jr, Imperiali B (2002) Oligosaccharyl transferase: gatekeeper to the secretory pathway. Curr Opin Chem Biol 6:844–850. doi:10.1016/S1367-5931(02)00390-3
Mothes W, Heinrich SU, Graf R, Nilsson I, von Heijne G, Brunner J, Rapoport TA (1997) Molecular mechanism of membrane protein integration into the endoplasmic reticulum. Cell 89:523–533. doi:10.1016/S0092-8674(00)80234-2
Ng DT, Brown JD, Walter P (1996) Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol 134:269–278. doi:10.1083/jcb.134.2.269
Hansen W, Garcia PD, Walter P (1986) In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell 45:397–406. doi:10.1016/0092-8674(86)90325-9
Chirico WJ, Waters MG, Blobel G (1988) 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 332:805–810. doi:10.1038/332805a0
Deshaies RJ, Sanders SL, Feldheim DA, Schekman R (1991) Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 349:806–808. doi:10.1038/349806a0
Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA (1995) Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell 81:561–570. doi:10.1016/0092-8674(95)90077-2
Hanein D, Matlack KES, Jungnickel B, Plath K, Kalies KU, Miller KR, Rapoport TA, Akey CW (1996) Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell 87:721–732. doi:10.1016/S0092-8674(00)81391-4
Rapoport TA (2007) Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450:663–669. doi:10.1038/nature06384
Johnson N, Powis K, High S (2013) Post-translational translocation into the endoplasmic reticulum. Biochim Biophys Acta 1833:2403–2409. doi:10.1016/j.bbamcr.2012.12.008
Stefanovic S, Hegde RS (2007) Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell 128:1147–1159. doi:10.1016/j.cell.2007.01.036
Favaloro V, Spasic M, Schwappach B, Dobberstein B (2008) Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J Cell Sci 121:1832–1840. doi:10.1242/jcs.020321
Mariappan M, Li X, Stefanovic S, Sharma A, Mateja A, Keenan RJ, Hegde RS (2010) A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature 466:1120–1124. doi:10.1038/nature09296
Schuldiner M, Metz J, Schmid V, Denic V, Rakwalska M, Schmitt HD, Schwappach B, Weissman JS (2008) The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134:634–645. doi:10.1016/j.cell.2008.06.025
Vilardi F, Lorenz H, Dobberstein B (2011) WRB is the receptor for TRC40/Asna1-mediated insertion of tail-anchored proteins into the ER membrane. J Cell Sci 124:1301–1307. doi:10.1242/jcs.084277
Yamamoto Y, Sakisaka T (2012) Molecular machinery for insertion of tail-anchored membrane proteins into the endoplasmic reticulum membrane in mammalian cells. Mol Cell 48:387–397. doi:10.1016/j.molcel.2012.08.028
Mariappan M, Mateja A, Dobosz M, Bove E, Hegde RS, Keenan RJ (2011) The mechanism of membrane-associated steps in tail-anchored protein insertion. Nature 477:61–66. doi:10.1038/nature10362
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. doi:10.1126/science.1209038
Howell SH (2013) Endoplasmic reticulum stress responses in plants. Annu Rev Plant Biol 64:477–499. doi:10.1146/annurev-arplant-050312-120053
Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII–a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77:895–907. doi:10.1016/0092-8674(94)90138-4
Brandizzi F, Barlowe C (2013) Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol 14:382–392. doi:10.1038/nrm3588
Nakano A, Muramatsu M (1989) A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol 109:2677–2691. doi:10.1083/jcb.109.6.2677
Yoshihisa T, Barlowe C, Schekman R (1993) Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 259:1466–1468. doi:10.1126/science.8451644
Nakano A, Brada D, Schekman R (1988) A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J Cell Biol 107:851–863. doi:10.1083/jcb.107.3.851
Barlowe C, Schekman R (1993) SEC12 encodes a guanine-nucleotide- exchange factor essential for transport vesicle budding from the ER. Nature 365:347–349. doi:10.1038/365347a0
Goldberg J (1998) Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 95:237–248. doi:10.1016/S0092-8674(00)81754-7
Huang M, Weissman JT, Beraud-Dufour S, Luan P, Wang C, Chen W, Aridor M, Wilson IA, Balch WE (2001) Crystal structure of Sar1-GDP at 1.7 Å resolution and the role of the NH2 terminus in ER export. J Cell Biol 155:937–948. doi:10.1083/jcb.200106039
Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R (2005) Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122:605–617. doi:10.1016/j.cell.2005.07.025
Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T (1998) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93:263–275. doi:10.1016/S0092-8674(00)81577-9
Bi X, Corpina RA, Goldberg J (2002) Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419:271–277. doi:10.1038/nature01040
Stagg SM, Gürkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE (2006) Structure of the Sec13/31 COPII coat cage. Nature 439:234–238. doi:10.1038/nature04339
Fath S, Mancias JD, Bi X, Goldberg J (2007) Structure and organization of coat proteins in the COPII cage. Cell 129:1325–1336. doi:10.1016/j.cell.2007.05.036
Stagg SM, LaPointe P, Razvi A, Gürkan C, Potter CS, Carragher B, Balch WE (2008) Structural basis for cargo regulation of COPII coat assembly. Cell 134:474–484. doi:10.1016/j.cell.2008.06.024
Miller EA, Beilharz TH, Malkus PN, Lee MC, Hamamoto S, Orci L, Schekman R (2003) Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114:497–509. doi:10.1016/S0092-8674(03)00609-3
Sato K, Nakano A (2005) Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol 12:167–174. doi:10.1038/nsmb893
Bi X, Mancias JD, Goldberg J (2007) Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell 13:635–645. doi:10.1016/j.devcel.2007.10.006
Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ (2006) Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic 7:1678–1687. doi:10.1111/j.1600-0854.2006.00493.x
Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS (2005) Sec16 is a determinant of transitional ER organization. Curr Biol 15:1439–1447. doi:10.1016/j.cub.2005.06.065
Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, Stephens DJ (2009) Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci 122:2924–2934. doi:10.1242/jcs.044032
Whittle JR, Schwartz TU (2010) Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat. J Cell Biol 190:347–361. doi:10.1083/jcb.201003092
Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R (2011) COPII and the regulation of protein sorting in mammals. Nat Cell Biol 14:20–28. doi:10.1038/ncb2390
O’Kelly I, Butler MH, Zilberberg N, Goldstein SA (2002) Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell 111:577–588. doi:10.1016/S0092-8674(02)01040-1
Nakamura T, Hayashi T, Nasu-Nishimura Y, Sakaue F, Morishita Y, Okabe T, Ohwada S, Matsuura K, Akiyama T (2008) PX-RICS mediates ER-to-Golgi transport of the N-cadherin/beta-catenin complex. Genes Dev 22:1244–1256. doi:10.1101/gad.1632308
Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A (2006) Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 133:1767–1778. doi:10.1242/dev.02347
Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M (2009) Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol 11:286–294. doi:10.1038/ncb1836
Malhotra V, Erlmann P (2015) The pathway of collagen secretion. Annu Rev Cell Dev Biol. doi:10.1146/annurev-cellbio-100913-013002
Townley AK, Feng Y, Schmidt K, Carter DA, Porter R, Verkade P, Stephens DJ (2008) Efficient coupling of Sec23–Sec24 to Sec13–Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J Cell Sci 121:3025–3034. doi:10.1242/jcs.031070
Sarmah S, Barrallo-Gimeno A, Melville DB, Topczewski J, Solnica-Krezel L, Knapik EW (2010) Sec24D dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS One 5, e10367. doi:10.1371/journal.pone.0010367
Boyadjiev SA, Kim SD, Hata A, Haldeman-Englert C, Zackai EH, Naydenov C, Hamamoto S, Schekman RW, Kim J (2011) Cranio-lenticulo-sutural dysplasia associated with defects in collagen secretion. Clin Genet 80:169–176. doi:10.1111/j.1399-0004.2010.01550.x
Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, Beznoussenko GV, Mironov AA, Mironov A Jr, Zelante L, Piemontese MR, Notarangelo A, Malhotra V, Vertel BM, Wilson C, De Matteis MA (2012) Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science 337:1668–1672. doi:10.1126/science.1224947
Nogueira C, Erlmann P, Villeneuve J, Santos AJ, Martínez-Alonso E, Martínez-Menárguez JÁ, Malhotra V (2014) SLY1 and Syntaxin 18 specify a distinct pathway for procollagen VII export from the endoplasmic reticulum. Elife 3, e02784. doi:10.7554/eLife.02784
Bacia K, Futai E, Prinz S, Meister A, Daum S, Glatte D, Briggs JA, Schekman R (2011) Multibudded tubules formed by COPII on artificial liposomes. Sci Rep 1:17. doi:10.1038/srep00017
Zanetti G, Prinz S, Daum S, Meister A, Schekman R, Bacia K, Briggs JA (2013) The structure of the COPII transport-vesicle coat assembled on membranes. Elife 2, e00951. doi:10.7554/eLife.00951
Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, Malhotra V (2006) Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439:604–607. doi:10.1038/nature04377
Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA, Horne-Badovinac S (2013) A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell 24:159–168. doi:10.1016/j.devcel.2012.12.005
Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V (2009) TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell 136:891–902. doi:10.1016/j.cell.2008.12.025
Saito K, Yamashiro K, Ichikawa Y, Erlmann P, Kontani K, Malhotra V, Katada T (2011) cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell 22:2301–2308. doi:10.1091/mbc.E11-02-0143
Saito K, Yamashiro K, Shimazu N, Tanabe T, Kontani K, Katada T (2014) Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export. J Cell Biol 206:751–762. doi:10.1083/jcb.201312062
Wieland FT, Gleason ML, Serafini TA, Rothman JE (1987) The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50:289–300. doi:10.1016/0092-8674(87)90224-8
Denecke J, Botterman J, Deblaere R (1990) Protein secretion in plant cells can occur via a default pathway. Plant Cell 2:51–59. doi:10.1105/tpc.2.1.51
Phillipson BA, Pimpl P, daSilva LL, Crofts AJ, Taylor JP, Movafeghi A, Robinson DG, Denecke J (2001) Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13:2005–2020. doi:10.1105/TPC.010110
Thor F, Gautschi M, Geiger R, Helenius A (2009) Bulk flow revisited: transport of a soluble protein in the secretory pathway. Traffic 10:1819–1830. doi:10.1111/j.1600-0854.2009.00989.x
Kappeler F, Klopfenstein DR, Foguet M, Paccaud JP, Hauri HP (1997) The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem 272:31801–31808. doi:10.1074/jbc.272.50.31801
Nishimura N, Balch WE (1997) A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277:556–558. doi:10.1126/science.277.5325.556
Contreras I, Yang Y, Robinson DG, Aniento F (2004) Sorting signals in the cytosolic tail of plant p24 proteins involved in the interaction with the COPII coat. Plant Cell Physiol 45:1779–1786. doi:10.1093/pcp/pch200
Hanton SL, Renna L, Bortolotti LE, Chatre L, Stefano G, Brandizzi F (2005) Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 17:3081–3093. doi:10.1105/tpc.105.034900
Hay JC, Chao DS, Kuo CS, Scheller RH (1997) Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell 89:149–158. doi:10.1016/S0092-8674(00)80191-9
Cao X, Ballew N, Barlowe C (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J 17:2156–2165. doi:10.1093/emboj/17.8.2156
Allan BB, Moyer BD, Balch WE (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289:444–448. doi:10.1126/science.289.5478.444
Moyer BD, Allan BB, Balch WE (2001) Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic 2:268–276. doi:10.1034/j.1600-0854.2001.1o007.x
Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M, Ferro-Novick S (2001) TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell 7:433–442. doi:10.1016/S1097-2765(01)00190-3
Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G (2002) Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol 157:45–62. doi:10.1083/jcb.200112127
Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz EM, Ferro-Novick S, Reinisch KM (2008) The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell 133:1202–1213. doi:10.1016/j.cell.2008.04.049
Wong M, Munro S (2014) Membrane trafficking. The specificity of vesicle traffic to the Golgi is encoded in the golgin coiled-coil proteins. Science 346:1256898. doi:10.1126/science.1256898
Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S (2007) TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature 445:941–944. doi:10.1038/nature05527
Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P, Ferro-Novick S (2011) Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 473:181–186. doi:10.1038/nature09969
Rowe T, Dascher C, Bannykh S, Plutner H, Balch WE (1998) Role of vesicle-associated syntaxin 5 in the assembly of pre-Golgi intermediates. Science 279:696–700. doi:10.1126/science.279.5351.696
Xu D, Joglekar AP, Williams AL, Hay JC (2000) Subunit structure of a mammalian ER/Golgi SNARE complex. J Biol Chem 275:39631–39639. doi:10.1074/jbc.M007684200
Lowe SL, Peter F, Subramaniam VN, Wong SH, Hong W (1997) A SNARE involved in protein transport through the Golgi apparatus. Nature 389:881–884. doi:10.1038/39923
Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Südhof TC (2002) Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell 2:295–305. doi:10.1016/S1534-5807(02)00125-9
Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362:318–324. doi:10.1038/362318a0
Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395:347–353. doi:10.1038/26412
Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92:759–772. doi:10.1016/S0092-8674(00)81404-X
Parlati F, McNew JA, Fukuda R, Miller R, Söllner TH, Rothman JE (2000) Topological restriction of SNARE-dependent membrane fusion. Nature 407:194–198. doi:10.1038/35025076
Südhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323:474–477. doi:10.1126/science.1161748
Nebenfuhr A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA. (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121:1127–1142. doi:10.1104/pp.121.4.1127
Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15:441–447. doi:10.1046/j.1365-313X.1998.00208.x
Avisar D, Prokhnevsky AI, Makarova KS, Koonin EV, Dolja VV (2008) Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol 146:1098–1108. doi:10.1104/pp.107.113647
Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV (2008) Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol 146:1109–1116. doi:10.1104/pp.107.113654
Prokhnevsky AI, Peremyslov VV, Dolja VV (2008) Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc Natl Acad Sci U S A 105:19744–19749. doi:10.1073/pnas.0810730105
Sparkes IA, Teanby NA, Hawes C (2008) Truncated myosin XI tail fusions inhibit peroxisome, Golgi, and mitochondrial movement in tobacco leaf epidermal cells: a genetic tool for the next generation. J Exp Bot 59:2499–2512. doi:10.1093/jxb/ern114
Sparkes IA, Ketelaar T, Ruijter NC, Hawes C (2009) Grab a Golgi: laser trapping of Golgi bodies reveals in vivo interactions with the endoplasmic reticulum. Traffic 10:567–571. doi:10.1111/j.1600-0854.2009.00891.x
Brandizzi F, Snapp EL, Roberts AG, Lippincott-Schwartz J, Hawes C (2002) Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell 14:1293–1309. doi:10.1105/tpc.001586
Kang BH, Staehelin LA (2008) ER-to-Golgi transport by COPII vesicles in Arabidopsis involves a ribosome-excluding scaffold that is transferred with the vesicles to the Golgi matrix. Protoplasma 234:51–64. doi:10.1007/s00709-008-0015-6
daSilva LLP, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F (2004) Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16, 1753–1771. doi:10.1105/tpc.022673
Stefano G, Renna L, Chatre L, Hanton SL, Moreau P, Hawes C, Brandizzi F (2006) In tobacco leaf epidermal cells, the integrity of protein export from the endoplasmic reticulum and of ER export sites depends on active COPI machinery. Plant J 46:95–110. doi:10.1111/j.1365-313X.2006.02675.x
Langhans M, Meckel T, Kress A, Lerich A, Robinson DG (2012) ERES (ER exit sites) and the “secretory unit concept”. J Microsc 247:48–59. doi:10.1111/j.1365-2818.2011.03597.x
Lerich A, Hillmer S, Langhans M, Scheuring D, van Bentum P, Robinson DG (2012) ER import sites and their relationship to ER exit sites: a new model for bidirectional ER-Golgi transport in higher plants. Front Plant Sci 3:143. doi:10.3389/fpls.2012.00143
Pimpl P, Movafeghi A, Coughlan S, Denecke J, Hillmer S, Robinson DG (2000) In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12:2219–2236. doi:10.1105/tpc.12.11.2219
Ritzenthaler C, Nebenführ A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14:237–261. doi:10.1105/tpc.010237
Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C (2007) Membrane dynamics in the early secretory pathway. Crit Rev Plant Sci 26:199–225. doi:10.1080/07352680701495820
Staehelin LA, Kang BH (2008) Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol 147:1454–1468
Robinson DG, Brandizzi F, Hawes C, Nakano A (2015) Vesicles versus tubes: is endoplasmic reticulum-Golgi transport in plants fundamentally different from other eukaryotes? Plant Physiol 168:393–406. doi:10.1104/pp.15.00124
Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE (1989) Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58:329–336. doi:10.1016/0092-8674(89)90847-7
Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orci L, Rothman JE, Wieland FT (1991) A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature 349:215–220. doi:10.1038/349215a0
Waters MG, Serafini T, Rothman JE (1991) ‘Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature 349:248–251. doi:10.1038/349248a0
Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE (1991) ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell 67:239–253. doi:10.1016/0092-8674(91)90176-Y
Orci L, Palmer DJ, Ravazzola M, Perrelet A, Amherdt M, Rothman JE (1993) Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature 362:648–652. doi:10.1038/362648a0
Rothman JE, Wieland FT (1996) Protein sorting by transport vesicles. Science 272:227–234. doi:10.1126/science.272.5259.227
Zink S, Wenzel D, Wurm CA, Schmitt HD (2009) A link between ER tethering and COP-I vesicle uncoating. Dev Cell 17:403–416. doi:10.1016/j.devcel.2009.07.012
Hong W (2005) SNAREs and traffic. Biochim Biophys Acta 1744:493–517. doi:10.1016/j.bbamcr.2005.03.014
Klumperman J (2011) Architecture of the mammalian Golgi. Cold Spring Harb Perspect Biol 3:a005181. doi:10.1101/cshperspect.a005181
Marsh BJ, Volkmann N, McIntosh JR, Howell KE (2004) Direct continuities between cisternae at different levels of the Golgi complex in glucose-stimulated mouse islet b cells. Proc Natl Acad Sci 101:5565–5570. doi:10.1073/pnas.0401242101
Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, San Pietro E, Beznoussenko GV, Polishchuk EV, Baldassarre M, Buccione R, Geerts WJ, Koster AJ, Burger KN, Mironov AA, Luini A (2004) Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol 6:1071–1081. doi:10.1038/ncb1180
Griffiths G, Pfeiffer S, Simons K, Matlin K (1985) Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol 101:949–964. doi:10.1083/jcb.101.3.949
Griffiths G, Simons K (1986) The trans Golgi network: sorting at the exit site of the Golgi complex. Science 234:438–443. doi:10.1126/science.2945253
Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, Jürgens G, de Vries SC, Robinson DG, Schumacher K (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22:1344–1357. doi:10.1105/tpc.109.072637
Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H + -ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18:715–730. doi:10.1105/tpc.105.037978
Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, Jiang L (2007) Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19:296–319. doi:10.1105/tpc.106.045708
Glick BS, Luini A (2011) Models for Golgi traffic: a critical assessment. Cold Spring Harb Perspect Biol 3:a005215. doi:10.1101/cshperspect.a005215
Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T (1995) Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci 108:1617–1627
Guo Y, Sirkis DW, Schekman R (2014) Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol 30:169–206. doi:10.1146/annurev-cellbio-100913-013012
Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W (2002) AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol 4:154–159. doi:doi:10.1038/ncb745
Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E (2012) The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell 22:811–823. doi:10.1016/j.devcel.2012.02.004
Bonifacino JS (2014) Adaptor proteins involved in polarized sorting. J Cell Biol 204:7–17. doi:10.1083/jcb.201310021
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572. doi:10.1038/42408
Bard F, Malhotra V (2006) The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol 22:439–455. doi:10.1146/annurev.cellbio.21.012704.133126
Wang CW, Hamamoto S, Orci L, Schekman R (2006) Exomer: a coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J Cell Biol 174:973–983. doi:10.1083/jcb.200605106
Sanchatjate S, Schekman R (2006) Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface. Mol Biol Cell 17:4157–4166. doi:10.1091/mbc.E06-03-0210
Barfield RM, Fromme JC, Schekman R (2009) The exomer coat complex transports Fus1p to the plasma membrane via a novel plasma membrane sorting signal in yeast. Mol Biol Cell 20:4985–4996. doi:10.1091/mbc.E09-04-0324
Paczkowski JE, Richardson BC, Strassner AM, Fromme JC (2012) The exomer cargo adaptor structure reveals a novel GTPase-binding domain. EMBO J 31:4191–4203. doi:10.1038/emboj.2012.268
Starr TL, Pagant S, Wang CW, Schekman R (2012) Sorting signals that mediate traffic of chitin synthase III between the TGN/endosomes and to the plasma membrane in yeast. PLoS One 7, e46386. doi:10.1371/journal.pone.0046386
Santos B, Snyder M (2003) Specific protein targeting during cell differentiation: polarized localization of Fus1p during mating depends on Chs5p in Saccharomyces cerevisiae. Eukaryot Cell 2:821–825. doi:10.1128/EC.2.4.821-825.2003
Trautwein M, Schindler C, Gauss R, Dengjel J, Hartmann E, Spang A (2006) Arf1p, Chs5p and the ChAPs are required for export of specialized cargo from the Golgi. EMBO J 25:943–954. doi:10.1038/sj.emboj.7601007
Goud B, Salminen A, Walworth NC, Novick PJ (1988) A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53:753–768. doi:10.1016/0092-8674(88)90093-1
Rutherford S, Moore I (2002) The Arabidopsis Rab GTPase family: another enigma variation. Curr Opin Plant Biol 5:518–528. doi:10.1016/S1369-5266(02)00307-2
Gendre D, Oh J, Boutté Y, Best JG, Samuels L, Nilsson R, Uemura T, Marchant A, Bennett MJ, Grebe M, Bhalerao RP (2011) Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc Natl Acad Sci U S A 108:8048–8053. doi:10.1073/pnas.1018371108
Gendre D, McFarlane HE, Johnson E, Mouille G, Sjödin A, Oh J, Levesque-Tremblay G, Watanabe Y, Samuels L, Bhalerao RP (2013) Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell 25:2633–2646. doi:10.1105/tpc.113.112482
McFarlane HE, Döring A, Persson S (2014) The cell biology of cellulose synthesis. Annu Rev Plant Biol 65:69–94. doi:10.1146/annurev-arplant-050213-040240
Boutté Y, Jonsson K, McFarlane HE, Johnson E, Gendre D, Swarup R, Friml J, Samuels L, Robert S, Bhalerao RP (2014) ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation. Proc Natl Acad Sci U S A 110:16259–16264. doi:10.1073/pnas.1309057110
Jürgens G (2005) Plant cytokinesis: Fission by fusion. Trends Cell Biol 15:277–283. doi:10.1016/j.tcb.2005.03.005
Müller S, Jürgens G (2015) Plant cytokinesis-No ring, no constriction but centrifugal construction of the partitioning membrane. Semin Cell Dev Biol. doi:10.1016/j.semcdb.2015.10.037
Lukowitz W, Mayer U, Jürgens G (1996) Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84:61–71. doi:10.1016/S0092-8674(00)80993-9
Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jürgens G (1997) The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol 139:1485–1493. doi:10.1083/jcb.139.6.1485
Assaad FF, Huet Y, Mayer U, Jürgens G (2001) The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE. J Cell Biol 152:531–543. doi:10.1083/jcb.152.3.531
Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jurgens G (2001) Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol 155:239–249. doi:10.1083/jcb.200107126
Hammer JA 3rd, Wu XS (2002) Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr Opin Cell Biol 14:69–75. doi:10.1016/S0955-0674(01)00296-4
TerBush DR, Maurice T, Roth D, Novick P (1996) The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15:6483–6494
Cai H, Reinisch K, Ferro-Novick S (2007) Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell 12:671–682. doi:10.1016/j.devcel.2007.04.005
Zárský V, Kulich I, Fendrych M, Pečenková T (2013) Exocyst complexes multiple functions in plant cells secretory pathways. Curr Opin Plant Biol 16:726–733. doi:10.1016/j.pbi.2013.10.013
Borgonovo B, Ouwendijk J, Solimena M (2006) Biogenesis of secretory granules. Curr Opin Cell Biol 18:365–370. doi:10.1016/j.ceb.2006.06.010
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Viotti, C. (2016). ER to Golgi-Dependent Protein Secretion: The Conventional Pathway. In: Pompa, A., De Marchis, F. (eds) Unconventional Protein Secretion. Methods in Molecular Biology, vol 1459. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3804-9_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3804-9_1
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3802-5
Online ISBN: 978-1-4939-3804-9
eBook Packages: Springer Protocols