Abstract
Multiple overlapping systemic and local inhibitory networks have evolved to prevent the unwanted deposition of mineral at ectopic sites. Fetuin-A is a liver-derived glycoprotein abundant in plasma that binds and stabilizes nascent mineral ion nuclei to form soluble colloidal high molecular weight complexes, called calciprotein particles (CPP). The binding of fetuin-A to mineral retards crystal ripening and precipitation from the aqueous phase, thereby facilitating the regulated clearance of mineral debris from the extracellular fluid. However, persistent disturbances in this humoral homeostatic system, as frequently seen in patients with Chronic Kidney Disease, may lead to the accumulation and aggregation of these nanoparticles in extraosseous tissues like the vasculature, driving inflammatory cascades, aberrant tissue remodeling, and functional impairment. Consistent with this conceptual framework, higher circulating CPP levels are associated with reduced renal function, increments in systemic inflammatory markers, derangements in bone morphogenetic cytokines, higher vascular calcification scores, aortic stiffening and an increased risk of death. This chapter describes optimized sample collection and preparative procedures for the isolation and enrichment of CPP from biological fluids. Methods for CPP quantitation are critically reviewed and detailed.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Fetuin-A
- Calciprotein particles
- Ultracentrifugation
- Gel filtration chromatography
- Immunoisolation
- Flow cytometry
1 Introduction
Even under physiological conditions, plasma is considered a metastable aqueous solution of calcium and phosphate, sustaining mineral precipitation once crystals are nucleated. Multiple systemic and local inhibitory networks have evolved to constrain mineral deposition to physiologic calcified tissues such as bone, cartilage, teeth (dentin, cementum, enamel) and inner ear otoconia. For ectopic calcification to occur, these inhibitory mechanisms must be compromised. Fetuin-A (also known as α2 -Heremans Schmid glycoprotein; AHSG) is a liver-derived, partially phosphorylated, glycoprotein that circulates in plasma at high concentration and has multiple roles in the regulation of matrix mineralization at both molecular and cellular levels [1]. Outstanding in this multifunctional repertoire is the ability of fetuin-A to bind and organize nascent mineral nuclei, forming colloidal high molecular weight complexes, termed calciprotein particle s (CPP), in an analogous manner to the solubilization of lipid cargo by apolipoproteins [2]. CPP formation limits further crystal growth, retards the ripening of amorphous mineral to more crystalline phases [3], and is thought to facilitate the rapid, immunologically inert, clearance of mineral debris from the extracellular fluid [4]. Removal of such debris may help to reduce ectopic deposition of precipitates within the tissues and undesirable downstream inflammatory sequelae. Elevated CPP levels, as seen in patients with Chronic Kidney Disease (CKD ), may reflect increased genesis of CPP from bone or calcifying tissues and/or reduced clearance due to impairment of renal elimination pathways or inadequate buffering through incorporation into bone.
CPP formation is a multistep process, starting with the aggregation of small clusters of fetuin-A -bound mineral called calciprotein monomers (similar to Posner clusters and other ion-association complexes), forming spherical nanoparticles, called primary CPP (approx. 50–100 nm in diameter), which contain amorphous calcium phosphate. These primary CPP undergo spontaneous rearrangement to more stable, densely packed, prolate needle-shaped particles, called secondary CPP (approx. 100–200 nm in diameter), that contain mineral in a crystalline phase [2, 3, 5, 6]. The primary-to-secondary CPP transition is dependent on time, fetuin-A concentration, the ionic activity of calcium, phosphate and magnesium, pH, temperature [2, 5, 7] in addition to other unidentified small molecule/protein modulators.
Arguably, the first in vivo evidence of CPP and their putative role in mineral trafficking comes from studies by Gersh [8, 9]. In the late 1930s, he reported the appearance of a colloidal mineral phase in the serum of dogs, following IV loading with supraphysiological amounts of calcium phosphate, which was rapidly cleared from the circulation by the reticuloendothelial system. Herrmann and colleagues elucidated the clearance pathway of fetuin-A -containing CPP in more contemporary studies, which appears principally mediated by the class A scavenger receptor [4]. In 2002, Price et al. identified a high molecular weight complex of fetuin-A, matrix Gla protein, and mineral (termed fetuin mineral complexes) in the serum of etidronate-treated rats [10]. The appearance of these particles after administration of various other bone-modulating agents in the same animals led these authors to speculate on a bone origin for CPP [11]. Importantly CPP were not detected in the serum of rats treated with vehicle. Heiss et al. made the first identification of CPP in humans, in the ascitic fluid of a dialysis patient with calcifying peritonitis (with albumin and fetuin-A as major protein components) [5]. Subsequently, Young et al. isolated relatively pleomorphic CPP-like mineraloprotein nanoparticles from 0.2 μm-filtered human and bovine serum [12]. Proteomic different these particles and related “granulations” isolated from different human body fluids (ascites, urine, cerebrospinal and synovial fluid) revealed a complement of proteins that varied with each fluid, but which consistently included fetuin-A, albumin, complement C3, α1-antitrypsin, prothrombin, and apolipoprotein A1 [13].
With respect to kidney disease, studies by Matsui et al. revealed the appearance of circulating CPP in rats with adenine-induced renal failure [14]. Again, CPP were undetectable in control animals. Importantly, as implied in earlier studies from Price and colleagues [15], elevations in serum CPP preceded aortic calcium accrual and histological evidence of arterial mineralization [14]. Treatment with alendronate completely abrogated the development medial calcification and serum CPP became undetectable within 5 weeks. Despite the disappearance of CPP from serum, total serum fetuin-A and hepatic levels (mRNA and protein) were unchanged compared to vehicle treated uremic rats. Intriguingly, further characterization of CPP isolated from these uremic rats demonstrated that virtually all of the fetuin-A circulating in CPP was in the fully phosphorylated state [14]. The functional significance of this protein modification remains obscure, as fetuin-A phosphorylation does not appear to be a requisite for the inhibition of mineral precipitation in solution [16, 17].
Despite the well-defined role of fetuin-A in the control of matrix mineralization, and the strong calcifying phenotype of fetuin-A knockout mice [18, 19], epidemiological studies evaluating the relationship between serum concentrations this protein, declining renal function, vascular calcification , and patient outcome have yielded somewhat conflicting findings (reviewed in [20]). The apparent disconnect between the clinical and preclinical evidence base may reflect the complex molecular heterogeneity of fetuin-A [21], which has largely been overlooked in epidemiological studies to date. Indeed, accumulating evidence indicates that ascertainment of specific subfractions present in serum, such as fetuin-A containing-CPP, may reveal important pathobiological relationships that are not evident with total fetuin-A determinations alone [17, 22–24]. Using fairly nonspecific insensitive methodology, CPP appear undetectable in the serum of healthy adults, but relatively abundant in patients with non-dialysis dependent CKD [17, 23], in whom levels are strongly associated with systemic inflammatory markers, bone resorption markers and aortic stiffness [17]. Circulating CPP load also appears increased in patients with chronic rheumatologic inflammatory disease but normal renal function and markedly so in a number of patients with calcific uremic arteriolopathy (calciphylaxis) [22, 25], where levels were found to track closely with serial changes in inflammatory cytokine concentrations. Other authors have correlated serum CPP levels with coronary calcification scores in CKD patients, and linked reductions in CPP with decrements in PTH following parathyroidectomy or instigation of cinacalcet therapy [23]. Proteomic analysis of the CPP fraction revealed a similar complement of proteins to those previously identified in CPP-like particles fetuin-A, albumin, fibrinogen, fibronectin, β-actin, immunoglobulin κ light chains, and apolipoprotein A1 [23].
In prospective analyses, higher serum CPP levels have emerged as a strong inflammation-associated predictor of an increased risk of all-cause mortality in the pre-dialysis CKD setting, while the transformation time of primary amorphous mineral containing-CPP to secondary crystalline-mineral containing CPP (calcification propensity) was associated with the risk of death, independent of conventional renal and cardiovascular risk factors [24]. More recently, increased calcification propensity has been associated with an increased risk of cardiovascular mortality and graft failure in renal transplant recipients [26, 27]. Thus, although more confirmatory data is awaited, disturbances in this humoral homeostatic system and resultant accumulation of these nanoparticles, in addition to the propensity for their formation in the uremic milieu, appear at least predictive of vascular dysfunction and poor patient outcome.
This chapter describes optimized sample collection and preparative procedures for the isolation of CPP from human biological fluids using centrifugation, gel filtration chromatography , and immuno-enrichment with magnetic beads. Methods for the quantitation of CPP in serum and other fluids are also discussed in detail.
2 Materials
2.1 Sample Collection
-
1.
BD Vacutainer™ SST™ (Becton Dickinson, Franklin Lakes, NJ, USA) or serum 10 mL venous blood collection tubes.
-
2.
TBS: 50 mM Tris–HCl, 140 mM NaCl pH-adjusted with 10 M NaOH to 7.40 at 37 °C (keep separate stocks at RT and 4 °C).
-
3.
Eppendorf Centrifuge 5702 R with A-4-38 swing-bucket rotor and appropriate tube adaptors (Eppendorf, Hamburg, Germany).
2.2 CPP Isolation
2.2.1 Centrifugal Fractionation
-
1.
Eppendorf Centrifuge 5430 R equipped with FA-45-24-11-HS rotor.
-
2.
Eppendorf Safe-lock 2.0 mL tubes.
-
3.
THP depletion buffer: 1 % CHAPS (w/v) in TBS.
-
4.
−80 °C freezer.
2.2.2 Size Exclusion Chromatography
-
1.
HiPrep 16/60 Sephacryl™ S-500 HR SEC column (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
-
2.
ÄKTA™ pure chromatography system (GE Healthcare Life Sciences).
-
3.
Ca-TBS: 50 mM Tris–HCl, 140 mM NaCl, 10 mM CaCl2 pH-adjusted with 10 M NaOH to 7.40 at 37 °C.
-
4.
Centrifugal filter units; Amicon Ultra-15 and 0.5 mL units with 100 kDa MWCO regenerated cellulose membrane (Millipore, Billerica, MA, USA).
2.2.3 CPP Immuno-Enrichment
-
1.
Exosome–Dynabeads® Streptavidin for Isolation/Detection (Life Technologies, Carlsbad, CA, USA).
-
2.
Goat Anti-AHSG polyclonal antibody for biotinylation and bead capture (Abcam, Cambridge, UK).
-
3.
EZ-Link™ Sulfo-NHS-Biotin kit (Thermo Scientific, Waltham, MA, USA).
-
4.
Zeba™ Spin Desalting Columns, 7K MWCO, 0.5 mL (Thermo Scientific).
-
5.
Magnet.
-
6.
Mixer.
-
7.
Flat-bottom microcentrifuge 2.0 mL tubes with screw caps (Neptune Scientific, San Diego, CA, USA).
-
8.
Isolation Buffer: TBS with 0.1 % bovine serum albumin (BSA), filtered through a 0.22 μm Millex-GP filter (Millipore).
2.3 SDS-PAGE/Western Blotting Analysis
-
1.
Micro BCA™ Protein Assay Kit (Thermo Scientific).
-
2.
Mouse Anti-AHSG monoclonal antibody [2H2] (Abcam) for flow cytometry and Western blotting.
-
3.
Goat Anti-APOA1 polyclonal antibody (LifeSpan Bioscience, Seattle, CA, USA).
-
4.
APEX™ Alexa Fluor® 488/647 Antibody Labeling Kits (Life Technologies).
-
5.
1× Laemmli Sample Buffer with 50 mM EDTA (Bio-Rad, Hercules, CA, USA).
-
6.
2-Mercaptoethanol reducing agent (Bio-Rad).
-
7.
Thermal Cycler (Bio-Rad).
-
8.
Mini-PROTEAN™ TGX Stain-Free precast gels 4–15 % (Bio-Rad).
-
9.
Mini-PROTEAN™ Tetra Cell Vertical Electrophoresis System (Bio-Rad).
-
10.
PowerPac Basic (Bio-Rad).
-
11.
10× Tris–HCl/Glycine/SDS Running Buffer (Bio-Rad).
-
12.
Trans-Blot Turbo Transfer System (Bio-Rad).
-
13.
Trans-Blot Turbo Transfer Pack, mini format 0.2 μm nitrocellulose (Bio-Rad).
-
14.
Precision Plus Protein WesternC Standards (Bio-Rad).
-
15.
Precision Protein StrepTactin-HRP conjugate (Bio-Rad).
-
16.
Mouse TrueBlot™ ULTRA: Anti-Mouse Ig HRP (Rockland, Limerick, PA, USA).
-
17.
Goat TrueBlot™ ULTRA: Anti-Goat Ig HRP (Rockland).
-
18.
SuperSignal West Dura Extended Duration Substrate (Thermo Scientific).
-
19.
ChemiDoc MP Imaging System (Bio-Rad).
-
20.
TBS-T: TBS with 0.05 % Tween 20 (v/v) (Bio-Rad).
-
21.
Blocker™ BLOTTO in TBS (Thermo Scientific).
2.4 CPP Characterization and Quantitation
-
1.
BD FACSVerse™ flow cytometer (Becton Dickinson).
-
2.
Precision™ Automated Microplate Pipetting System (BioTek, Winooski, VT, USA).
-
3.
EDTA-TBS: 100 mM Tris–HCl, 140 mM NaCl, 20 mM EDTA pH-adjusted with 10 M NaOH to 7.40 at 37 °C.
-
4.
Fetuin-A (AHSG) Human ELISA kit (Biovendor, Karasek, Czech Republic).
-
5.
DS2—2-plate ELISA processing system (Dynex Technologies, Chantilly, VA, USA).
3 Methods
3.1 Purification of CPP from Body Fluids
This section gives a simple method for obtaining crude CPP preparations from body fluids, which have been successfully applied to the isolation of CPP from blood (serum), urine and ascetic fluid/dialysate effluent. Pre-analytical conditions can impinge greatly on the quality of the sample obtained, so best sampling practice should be observed throughout. Given the sensitivity of mineralization biochemistry to changes in pH and ionic strength, cell-depleted samples are immediately diluted in buffered saline to maintain neutrality prior to isolation. Dilution also reduces sample viscosity and thus improves pelleting efficiency. A stepped centrifugation protocol is used to remove cells, dead cells, debris, and large protein aggregates/cryo-precipitates and to separate the CPP from the bulk fluid phase, which contains high concentrations of unbound proteins also present as bound components of CPP (e.g., fetuin-A , albumin). The pellet is then washed and resuspended in a small volume of buffered saline to concentrate the CPP present. Alternatively, size exclusion chromatography can be used to separate out CPP from major serum constituents including free monomeric fetuin-A. It is important to appreciate that the CPP-containing fraction obtained from these steps is likely to be contaminated to a variable extent by large extracellular vesicles (variously termed microparticles or microvescicles), apoptotic bodies and exosomes depending on the density of their cargo. Some protein/chromatin complexes/aggregates and, even denser lipoprotein particles, may also co-sediment or co-elute with this fraction. Thus, for some downstream applications, an additional selective purification step (e.g., magnetic bead-based immunoaffinity isolation) is needed to accomplish greater CPP enrichment. In our hands, ultrafiltration-based methods for CPP isolation from biological fluids have proved unreliable due to frequent membrane clogging, loss of buffering (serum retentates can become quite strongly alkaline) and poor recovery characteristics, and are not recommended for the isolation of CPP from biological fluids.
3.2 Sample Collection
3.2.1 Serum
-
1.
Collect blood into 10 mL plastic serum or SST tubes (see Note 1 ).
-
2.
Gently invert tubes at least five times to mix the activator with the blood (see Note 2 ).
-
3.
Allow samples to clot completely. Place capped tubes upright in a rack at room temperature: 60 min for plain serum and at least 30 min for SST tubes (see Note 3 ).
-
4.
Centrifuge blood tube in swinging-bucket rotor for 10 min at 1200 × g at room temperature (see Note 4 ).
-
5.
Transfer serum to a clean tube, dilute 1:2 in an appropriate volume of sterile-double filtered TBS and mix carefully with a pipet (see Note 5 ).
-
6.
Diluted serum is stable at room temperature for 4 h, up to 48 h at 2–4 °C. For long-term storage, aliquots should be frozen at −80 °C in safe-lock capped tubes. Do not store in the freezer at –20 °C.
3.2.2 Urine and Other Fluids (e.g., Peritoneal Dialysate Effluent, Ascites)
-
1.
Collect mid-stream second morning void or other fluid into a sterile 50 mL container (see Note 6 ).
-
2.
Centrifuge in swinging-bucket rotor for 10 min at 1200 × g at 4 °C to remove debris (see Note 7 ).
-
3.
Recover supernatant and dilute 1:1 in an appropriate volume of sterile-filtered TBS. Mix carefully.
-
4.
Diluted fluid should be used immediately or aliquots taken and stored frozen at −80 °C.
3.3 CPP Isolation
After the removal of large contaminants (Subheading 3.3.1), crude CPP isolates can be obtained by high-speed centrifugation (Subheading 3.3.2) or size exclusion chromatography (Subheading 3.3.3). Equivalent recoveries are achieved using either technique, but centrifugal isolation is likely to be more accessible to most users and is used routinely in our laboratory. Crude CPP isolates can be further enriched for CPP by bead-based immunoisolation (Subheading 3.3.4).
3.3.1 Removal of Large Contaminants
-
1.
Stored serum samples should be thawed at 37 °C to avoid the formation of cryoprecipitates. Mix all samples thoroughly after thawing.
-
2.
Centrifuge diluted samples for 30 min at 10,000 × g and 4 °C in an appropriate clean tube or bottle to pellet residual cell debris, cryoprecipitates, and other large contaminants (see Fig. 1a).
Fig. 1 The appearance of CPP-containing sediments and contaminating debris following centrifugation of human serum. (a) Fibrin/red cell debris following centrifugation of serum for 30 min at 10,000 × g and 4 °C (i) and after TBS wash (ii). (b) CPP-containing pellets after centrifugation of supernatant in (a) for 2 h at 30,000 × g and 4 °C (i) and TBS wash (ii). As noted in text, pellets are small and translucent or slightly off-white in colouration and difficult to visualize. Arrows denote position of pellet
-
3.
Carefully transfer the supernatant to a clean safe-lock capped tube, taking care not to contaminate the supernatant with the pellet. If isolating CPP from urine do not discard the pellet at this stage.
For urine samples only:
-
4.
Deplete polymeric THP from urine by resuspending pellets from step 3 in 1 % (w/v) CHAPS in TBS and re-spinning for 30 min at 10,000 × g and 4 °C (see Note 8 ).
-
5.
Pool supernatants from steps 3 and 4 to maximize CPP recovery.
3.3.2 CPP Isolation by Centrifugation
-
1.
Mark the outer side of each safe-lock capped tube with a pen to indicate the orientation of the tube in the rotor and help locate the pellet (see Note 9 ).
-
2.
Centrifuge for 2 h at 30,000 × g and 4 °C to sediment the CPP-containing fraction (see Note 10 and Fig. 1b).
-
3.
Remove and discard as much supernatant as possible, then carefully wash and resuspend the pellet in 100–200 μL ice-cold TBS using a pipette. If the pellet is not visible, resuspend by flushing the pipette tip up and down the side of the tube where the pellet should be located (bottom of the tube on the side of the mark). Add ice-cold TBS to fill the tube.
-
4.
Centrifuge for 1 h at 30,000 × g and 4 °C and resuspend the pellet in 100 μL warmed (37 °C) TBS.
-
5.
Pool aliquots of the same sample and store at −80 °C until analysis.
3.3.3 CPP Isolation by Size Exclusion Chromatography (SEC)
-
1.
Apply 3 mL aliquots of diluted sample to a HiPrep 16/60 Sephacryl™ S-500 HR column equilibrated with calcium-supplemented TBS (Ca-TBS).
-
2.
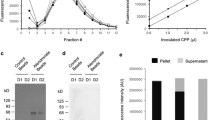
Elute column isocratically with Ca-TBS at a flow rate of 0.5 mL/min with monitoring at 280 and 400 nm (Fig. 2).
Fig. 2 CPP isolation by size exclusion chromatography. (a) Exemplar chromatographs showing the fractionation of fetuin-A -containing CPP from the fetuin-A monomer in human serum and serum spiked with buffered calcium and phosphate solutions (at the stated concentrations) monitored at 280 nm. CPP are eluted directly after the void volume (V 0). (b) Immunoblot of fetuin-A in eluted fractions
-
3.
Collect the 5 mL fraction eluting immediately after the void volume (see Note 11 ).
-
4.
Concentrate CPP-containing fraction, by passing eluate through a centrifugal filter unit (100 kDa MWCO). Collect the retentate.
-
5.
Pool as necessary and store at −80 °C until analysis.
3.3.4 CPP Immuno-Enrichment Using Magnetic Beads
Here, fetuin-A -containing CPP are selectively enriched from crude isolates by labeling particles with high-affinity biotinylated anti-Fetuin-A antibodies, which can then be captured using streptavidin-coated paramagnetic Dynabeads. Separation of this bound solid phase from the unbound phase is achieved with the use of a magnet.
-
1.
Titrate the volume of the pre-enriched CPP obtained from Subheading 3.3.2 or 3.3.3 to ~20 μg of total protein and make up to a final volume of 1 mL in Isolation Buffer in a clean safe-lock capped tube (see Note 12 ).
-
2.
Add 10 μg biotinylated anti-Fetuin-A antibody to the tube and mix.
-
3.
Incubate for 60 min at 2 °C with gentle mixing (see Note 13 ).
-
4.
Mark the outer side of each safe-lock capped tube as described in step 1, Subheading 3.3.2. Centrifuge for 1 h at 30,000 × g and 4 °C to pellet CPP and separate from unbound antibody.
-
5.
Remove and discard as much supernatant as possible then carefully wash and resuspend the pellet in 200 μL ice-cold Isolation Buffer using a pipette. If the pellet is not visible, resuspend by flushing the pipette tip up and down the side of the tube where the pellet should be located (bottom of the tube on the side of the mark).
-
6.
Repeat spin steps 4 and 5, and resuspend pellet in 1 mL Isolation Buffer.
-
7.
Transfer 50 μL beads into a flat-bottomed tube and wash beads with 300 μL of Isolation Buffer. Mix well (see Note 14 ).
-
8.
Place the tube on the magnet for 1 min and discard the supernatant.
-
9.
Remove the tube from the magnet and add 1 mL of the antibody-bound CPP complex mixture. Mix well and incubate the tube for 20 h at 4 °C with mixing.
-
10.
Centrifuge the tube in a microfuge for 5 s to collect the sample at the bottom of the tube.
-
11.
Wash the bead-bound CPP by adding 500 μL of Isolation Buffer. Mix gently by pipetting up and down.
-
12.
Place the tube on the magnet for 1 min and discard the supernatant.
-
13.
Remove the tube from the magnet and repeat wash with 500 μL of Isolation Buffer. Mix gently by pipetting.
-
14.
Repeat steps 9 and 10 twice.
-
15.
CPP-bound beads can now be denatured for SDS-PAGE/Western blotting (Subheading 3.5.1) or stained for flow cytometry (Subheading 3.5.2) to confirm successful isolation.
3.4 Confirming the Isolation of CPP
The techniques described in this section can be applied to bead-bound CPP isolates (Subheading 3.4.1). Conventional Western blotting and flow cytometry can be used to confirm the presence of protein markers ubiquitously present in CPP (e.g., fetuin-A , Apo-AI). The use of bead-bound CPP for staining for flow cytometry offers the advantage of be able to use a magnet to immobilize the bead for washing during separation of bound from unbound antibody, instead of more cumbersome and time-consuming centrifugation steps to pellet CPP after each wash step. It should be stressed that although Western blotting can be used to detect proteins present in purified CPP isolates, it does not confirm that they are necessarily of CPP origin and that contaminants are absent. Nonetheless, in our hands, markers of apoptotic bodies (e.g., calreticulin) and exosomes (e.g., CD63, CD81, CD9) are not usually detected in these preparations. Flow cytometry is more amenable to multiplexing targets and determining the relative abundance of various surface proteins. However, neither Western blotting nor flow cytometry of bead-bound CPP enable the user to discriminate between differences in the number of particles from changes in molecular composition/enrichment and therefore do not allow quantitation.
Analysis of particle size, morphology , crystallinity (e.g., diffraction studies), elemental composition (e.g., electron energy loss spectroscopy) and immunolabeling of free CPP can be made under cryo-TEM as reported previously (Fig. 3) [28]. An advantage of this EM modality is the avoidance of sample dehydration, collapse of the carbon (protein) matrix, and the use of chemical fixatives which can lead to artifactual appearances and compositional changes. Imaging studies can also help to assess sample heterogeneity and the presence of contaminants. Details of these EM methods are beyond the scope of this chapter and the interested reader is directed to other recent texts [29].
Cryo-TEM analysis of fetuin-A -containing CPP isolated from human serum. (a) Micrograph showing heterogeneous population of mineral-containing nanoparticles isolated from human serum by differential centrifugation and immuno-enrichment (scale bar = 100 nm). (b) Immunogold labeling of fetuin-A in mature secondary CPP (scale bar = 20 nm)
3.4.1 SDS-PAGE/Western Blotting Analysis of CPP
-
1.
Place the tube containing bead-bound CPP on the magnet for 1 min and discard the supernatant (see Note 15 ).
-
2.
Add 25 μL 2× reducing Laemmli Sample Buffer (containing 40 mM EDTA) and vortex vigorously for 30 s.
-
3.
Incubate at 95 °C for 10 min.
-
4.
Place the tube in the magnet and load supernatant and molecular weight standards onto 4–15 % TGX gels. Run for ~45 min at 200 V.
-
5.
Transfer to 0.2 μm nitrocellulose using Trans-Blot Turbo (3 min, 2.5 A, 25 V).
-
6.
Block the membranes in BLOTTO for 2 h at room temperature.
-
7.
Incubate overnight at 2 °C (with gentle mixing) with primary anti-Fetuin-A or anti-ApoAI antibodies diluted 1 in 1000 in BLOTTO.
-
8.
Wash extensively in TBS-T (6 × 10 min).
-
9.
Incubate with species-matched TrueBlot HRP-conjugated secondary antibody (1 in 2000) plus StrepTactin-HRP conjugate (1 in 10,000) in BLOTTO for 2 h at room temperature.
-
10.
Wash extensively in TBS-T (6 × 10 min).
-
11.
Develop signal using SuperSignal West Dura Extended Duration Substrate and image after 5 min incubation at room temperature protected from light.
3.4.2 Staining CPP for Flow Cytometry
-
1.
Prepare staining antibody mix: combine 1 μL APOAI antibody (AF647), 1 μL Fetuin-A antibody (AF488) in 20 μL Isolation Buffer (see Note 16 ). This is sufficient for ten labeling reactions.
-
2.
Add 2 μL staining antibody mix to 100 μL of bead-bound CPP from step 8, Subheading 3.4.1 in a flow tube and mix gently by pipetting (see Note 17 ).
-
3.
Incubate for 30 min at RT protected from light with mixing.
-
4.
Wash the bead-bound CPP by adding 300 μL of Isolation Buffer. Mix gently by pipetting.
-
5.
Place the tube on the magnet for 1 min and discard the supernatant.
-
6.
Repeat the steps 4 and 5 once and resuspend in the desired volume of Isolation Buffer for analysis. See Fig. 4 for an example of flow cytometric analysis of CPP bound to dyanbeads.
Fig. 4 Flow cytometric detection of CPP in serum from patient with CKD. CPP were pre-enriched by ultracentrifugation, bound via fetuin-A-biotin to streptavidin-coated dynabeads during an overnight incubation at 4 °C. Bound CPP were stained with the AF488/647-conjugated isotype antisera (grey), or specific antibodies to fetuin-A-AF488 (green; b) or ApoA1-AF647 (blue; c) and analysed on the FACSVerse. Events were gated on singlet bead/CPP complexes (G1) on FSC/SSC plots (panel a).
3.5 CPP Quantitation
Hamano et al. developed an ELISA-based protocol for measuring circulating CPP levels based on the apparent reduction in (total) serum fetuin-A concentrations after high-speed centrifugation of serum [23] (Fig. 5). Using this analytical approach, CPP were detectable in serum from CKD patients but not in serum from healthy individuals (NB, this is likely to reflect the relative insensitivity and imprecision of the method and does not indicate their absence in healthy sera) [23]. Moreover, whereas CPP levels were associated with the severity of coronary artery calcification on CT, total serum fetuin-A concentrations showed no significant association with calcification scores [23], underscoring the importance of considering CPP-associated fetuin-A levels rather than total serum fetuin-A. Our group has applied the same methodology (with some modifications) to the measurement of serum CPP in other CKD and non-CKD cohorts, where, consistent with their inflammatory potential in vitro [28], relationships between fetuin-A reduction ratios (RR) and systemic inflammatory status has been reproducibly observed [17, 22, 24].
CPP detection by serum fetuin-A reduction with high-speed centrifugation as originaly described by Hamano et al. [Ref. 23]. In health, CPP are undetectable and free fetuin-A does not sediment after centrifugation [16,000 × g 2 h, 4 °C]. In states of mineral stress, CPP are formed in extracellular fluid to inhibit mineral precipitation and are sedimented with centrifugation [16,000 × g 2 h, 4 °C], resulting in an apparent reduction in supernatant fetuin-A concentrations
A protocol for the determination of serum fetuin-A reduction ratios is given below. However, a number of theoretical and technical shortcomings of this procedure should be acknowledged from the outset. Overall, the technique lacks satisfactory analytical specificity or sensitivity. Fetuin-A may bind other high molecular weight species (possibly nonspecifically) that are sedimented in the same fraction (e.g., extracellular vesicles/cell debris), which may result in an over-estimation of circulating levels and give a false positive signal. In particular, heavily mineral-laden matrix vesicles (and other exosomal bodies) are likely to be pelleted in this fraction which may, in part, explain the apparent discrepancy of the proportion of fetuin-A present in the CPP-containing fraction in serum and that predicted from studies of simple saturated solutions (<5 %) [6]. Moreover, such a test readout does not provide a measure of particle number (rather of fetuin-A mass/occupancy), nor distinguish between primary and secondary CPP, which potentially have quite disparate biological effects. Indeed, theoretical considerations suggest that denser heavily crystalline mineral-laden secondary CPP may be relatively enriched by ultracentrifugation . The sensitivity of the analysis is also inherently constrained, as estimates are based on the small numerical difference in two values, both measured with error, following substantial (1 in 10,000), multistep dilution. Indeed, the relative abundance of fetuin-A in serum (~0.5 g/L), as well as its evident functional and structural heterogeneity, does not favor measurement of this protein by immunoassay. Furthermore, despite the crude nature of the separation, exceedingly low analytical imprecision (at the limit of attainability for ELISA) is an absolute requirement and necessitates the use of expensive high-precision pipetting systems (e.g., Precision™ Automated Microplate Pipetting Systems, BioTek; Liquidator 96, Mettler Toledo). Even then, adequate analytical control is difficult to achieve even in experienced hands due to variation in the manufacture of commercial ELISA kit.
An alternative analytical strategy is to quantitate the amount of mineral contained within the CPP-fraction. Standard colorimetric calcium assays (e.g., o-cresolphthalein complexone) are not sufficiently sensitive to detect the very small quantities of calcium typically found in this fraction (<1 μg/mL of serum [17, 23]). Acid-dissolved sediments can be analyzed for calcium by ultrasensitive graphite furnace atomic absorption spectrophotometry, as described by us previously [17], but instrumentation for this is not widely available and is not suitable for high-throughput analysis. Fluorometric-based assays using various proprietary calcium-sensitive probes are commercially available (e.g., Rhod Red or calcein) and demonstrate much greater sensitivity than their colorimetric counterparts. The use of fluorophore-conjugated bisphosphonate-based imaging agents (e.g., Osteosense, PerkinElmer) may enhance specificity for the mineral phase (e.g., OCP, or HAP) and permit direct measurement in fluids, (without the need for acid dissolution or separation) but lack validation in such quantitative applications. Indeed, a variety of mineral phases are likely to coexist in extracellular fluid that may not only differ in their affinity for probe binding but also complicates calibration and standardization of the readout. Qualitative and quantitative differences in the protein corona of mineral nanoparticles (e.g., primary vs. secondary CPP) may also significantly impact on probe binding and accessibility to the mineral core. Fundamentally, measurement of the mineral phase alone offers little advantage over protein-directed strategies and arguably even less analytical specificity given the abundance of calcium-containing contaminants that are present in the CPP-fraction. Like fetuin-A reduction ratios by ELISA, mineral detection does not yield information about absolute particle number or type. Furthermore, while significantly correlated, fetuin-A reduction ratios and CPP mineral content do not appear consistently related [17].
3.5.1 Determination of Fetuin-A Reduction Ratios in Serum
-
1.
Collect blood and prepare serum samples according to Subheading 3.2.1.
-
2.
Remove large insoluble debris/contaminants by centrifugation as described in steps 1–3 in Subheading 3.3.1.
-
3.
Dilute each sample supernatant 1 in 100 with TBS to a total volume of 2 mL (see Note 18 ).
-
4.
Combine 1 mL of each diluted supernatant with an equal volume of TBS containing 20 mM EDTA into a safe-lock tube and label “TF”—‘total fetuin-A ’(see Note 19 ).
-
5.
To the remaining 1 mL, mix with an equal volume of TBS and label “PF”—‘pellet fetuin-A ’.
-
6.
Mix both aliquots for 2 h at 4 °C.
-
7.
For “PF” samples, mark the outer side of tube with a pen to indicate the orientation of the tube in the rotor and help locate the pellet. Centrifuge for 2 h at 30,000 × g and 4 °C to sediment the CPP-containing fraction.
-
8.
Carefully transfer the supernatant to another clean tube and label “SF”—‘supernatant fetuin-A ’.
-
9.
Dilute TF and SF samples 1 in 10 in 1× Dilution Buffer and mix thoroughly.
-
10.
Measure fetuin-A concentration for each sample (in duplicate) using commercial ELISA kit according to the manufacturer’s instructions (see Note 20 ).
-
11.
Determine the fetuin-A reduction ratio for each sample according to the following formula:
$$ \left({\left[\mathrm{fetuin}-\mathrm{A}\right]}_{\mathrm{TF}}-{\left[\mathrm{fetuin}-\mathrm{A}\right]}_{\mathrm{SF}}\right)/{\left[\mathrm{fetuin}-\mathrm{A}\right]}_{\mathrm{TF}}\times 100 $$
4 Notes
-
1.
Blood collection tubes containing calcium-chelating anticoagulants (EDTA, citrate) which make result in the dissolution of CPP are not suitable for testing. Use of tubes with heparin-based anticoagulants is not recommended due to variable effects on CPP recovery and downstream techniques.
-
2.
Serum and SST tubes contain a powdered silica clot activator and a silicone-coated interior and must be inverted to ensure proper mixing and sample clotting. Improper mixing can result in a gelatinous/fibrinous serum sample which may interfere with pelleting efficiency and the recovery of CPP. Do not shake blood tubes vigorously as this can result in hemolysis. Heavily hemolysed samples are not suitable for analysis due to the formation of hemoglobin aggregates and hemoglobin–haptoglobin precipitation complexes which may contaminate the CPP-fraction. The release of large amounts of intracellular phosphate from lysed cells may also result in the ex vivo production of CPP (i.e., false positive) with prolonged storage.
-
3.
Clotting times may have to be extended for patients who are receiving anticoagulant therapy (e.g., heparin or Coumadin). Tubes must remain stoppered as changes in pH may occur with loss of dissolved CO2. Premature centrifugation may result in a gelatinous/fibrinous serum that is unsuitable for further processing. Re-spinning may result in loss of CPP. Excessive delays (>48 h) in separation from cells may result in further changes in pH (due to anaerobic metabolism of cells) and leakage of cell contents into serum (e.g., phosphate). Relatively short delays in centrifugation (<2 h) may also result in the release of contaminating extracellular vesicles.
-
4.
Always spin blood at room temperature to avoid the formation of cryoprecipitates and other insoluble protein aggregates.
-
5.
HEPES-buffered saline can be used as an alternative to TBS [50 mM HEPES, 140 mM NaCl pH-adjusted with 10 M NaOH to 7.40 at 37 °C]. A minimum volume of 2 mL of diluted serum (400 μL) is needed for reliable downstream analysis. Higher dilutions (1:10) can be employed with more viscous samples.
-
6.
CPP urinary load varies greatly between individuals and over time in the time in the same individual. To maximize recovery, it is recommended that at least 30 mL is collected to provide sufficient material for analysis. The use of “fresh” urine is desirable and samples should be processed within 4 h of collection to avoid bacterial growth/contamination. Ideally, urine samples should be stored on ice or at 4 °C until the preparative steps are complete.
-
7.
Pellets can be difficult to see and may be frankly invisible to the eye: to avoid potential contamination leave the bottom 10 mL of supernatant so not to disturb the pellet.
-
8.
Tamm-Horsfall protein (THP) is abundant in urine and can polymerize to form dense aggregates that trap particulates like CPP and which sediment at relatively low speeds.
-
9.
Safe-lock tubes should be used to prevent evaporative losses during high-speed centrifugation (even at low temperature). This is imperative if performing quantitative volume-based measurements.
-
10.
The CPP-containing pellet is typically very small and translucent or slightly off-white in colouration. In some instances, the pellet may not be visible.
-
11.
The void volume (V 0) can be determined using 1 μm diameter polystyrene sizing beads (Spherotech, Lake Forest, IL, USA).
-
12.
Estimation of total protein can be performed by Micro BCA™ Protein Assay Kit (Thermo Scientific).
-
13.
Adequate mixing is essential for successful CPP isolation. Use a mixer that provides titling and rotation of tubes to ensure beads do not settle during incubation. End-over-end mixing is suboptimal.
-
14.
This protocol has been optimized to achieve high CPP depletion efficiency from pre-enriched serum samples suitable for quantitation and morphological/elemental analysis by cryo-TEM or proteomic workflows. Fewer beads (20 μL per 1 mL) may be needed for some flow cytometry applications to maximize the coupling of particles per bead. The protocol can be scaled proportionately if larger or smaller input volumes are used.
-
15.
If analyzing unbound CPP from step 8, Subheading 3.4.2 for Western blotting, samples will first need to be concentrated using a microcentrifugal filter unit (e.g., Amicon 0.5 mL Ultracel 100K). Mix with an appropriate volume of 2× reducing Laemmli Sample Buffer (containing 40 mM EDTA), heat to 95 °C for 5 min and then process sample as for bead-bound CPP.
-
16.
Remove unreacted dye and protein aggregates from labelled antibody by centrifugation (10,000 g, 4 °C, 30 min) before use in staining.
-
17.
If staining unbound CPP from step 8, Subheading 3.4.2 for flow cytometry , labeled particles will need to be separated from unbound labeled antibody by centrifugation. Follow steps 4 and 5 in Subheading 3.4.1, but resuspend pellet in 100 μL Isolation Buffer after final wash.
-
18.
Note requirement for high-precision pipetting for dilution steps (<1.0 % for 10 μL).
-
19.
Direct detection of CPP-bound fetuin-A on the solid phase of the ELISA plate is unreliable, is nonstoichiometric and tends to underestimate total fetuin-A present due to the masking of epitopes. Estimates of total fetuin-A (TF) should therefore be based on samples following dissolution with calcium chelation (EDTA).
-
20.
Note requirement for very low analytical coefficients of variation (<2.5 %) to permit accurate derivation of fetuin-A reduction ratios.
References
Jahnen-Dechent W, Heiss A, Schafer C et al (2011) Fetuin-A regulation of calcified matrix metabolism. Circ Res 108(12):1494–1509
Heiss A, DuChesne A, Denecke B et al (2003) Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem 278(15):13333–13341
Heiss A, Jahnen-Dechent W, Endo H et al (2007) Structural dynamics of a colloidal protein-mineral complex bestowing on calcium phosphate a high solubility in biological fluids. Biointerphases 2(1):16–20
Herrmann M, Schafer C, Heiss A et al (2012) Clearance of fetuin-A-containing calciprotein particles is mediated by scavenger receptor-A. Circ Res 111(5):575–584
Heiss A, Eckert T, Aretz A et al (2008) Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem 283(21):14815–14825
Heiss A, Pipich V, Jahnen-Dechent W et al (2010) Fetuin-A is a mineral carrier protein: small angle neutron scattering provides new insight on Fetuin-A controlled calcification inhibition. Biophys J 99(12):3986–3995
Rochette CN, Rosenfeldt S, Heiss A et al (2009) A shielding topology stabilizes the early stage protein-mineral complexes of fetuin-A and calcium phosphate: a time-resolved small-angle X-ray study. Chembiochem 10(4):735–740
Gersh I (1938) The fate of colloidal calcium phosphate in the dog. Am J Physiol 121(3):589–594
Gersh I (1938) Histochemical studies on the fate of colloidal calcium phosphate in the rat. Anat Rec 70(3):331–349
Price PA, Thomas GR, Pardini AW et al (2002) Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J Biol Chem 277(6):3926–3934
Price PA, Caputo JM, Williamson MK (2002) Bone origin of the serum complex of calcium, phosphate, fetuin, and matrix Gla protein: biochemical evidence for the cancellous bone-remodeling compartment. J Bone Miner Res 17(7):1171–1179
Young JD, Martel J, Young D et al (2009) Characterization of granulations of calcium and apatite in serum as pleomorphic mineralo-protein complexes and as precursors of putative nanobacteria. PLoS One 4(5):e5421
Martel J, Young D, Young A et al (2011) Comprehensive proteomic analysis of mineral nanoparticles derived from human body fluids and analyzed by liquid chromatography-tandem mass spectrometry. Anal Biochem 418(1):111–125
Matsui I, Hamano T, Mikami S et al (2009) Fully phosphorylated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int 75(9):915–928
Price PA, Roublick AM, Williamson MK (2006) Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int 70(9):1577–1583
Schinke T, Amendt C, Trindl A et al (1996) The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem 271(34):20789–20796
Smith ER, Ford ML, Tomlinson LA et al (2012) Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant 27(5):1957–1966
Merx MW, Schafer C, Westenfeld R et al (2005) Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J Am Soc Nephrol 16(11):3357–3364
Schafer C, Heiss A, Schwarz A et al (2003) The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112(3):357–366
Cai MM, Smith ER, Holt SG (2015) The role of fetuin-A in mineral trafficking and deposition. Bonekey Rep 4:672
Smith ER, Holt SG (2010) Important differences in measurement of fetuin-A. Ann Intern Med 153(6):419, author reply 419–420
Smith ER, Cai MM, McMahon LP et al (2013) Serum fetuin-A concentration and fetuin-A-containing calciprotein particles in patients with chronic inflammatory disease and renal failure. Nephrology (Carlton) 18(3):215–221
Hamano T, Matsui I, Mikami S et al (2010) Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J Am Soc Nephrol 21(11):1998–2007
Smith ER, Ford ML, Tomlinson LA et al (2014) Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol 25(2):339–348
Cai MM, Smith ER, Brumby C et al (2013) Fetuin-A-containing calciprotein particle levels can be reduced by dialysis, sodium thiosulphate and plasma exchange. Potential therapeutic implications for calciphylaxis? Nephrology (Carlton) 18(11):724–727
Pasch A, Bachtler M, Smith ER et al (2014) Blood calcification propensity and cardiovascular events in hemodialysis patients in the EVOLVE trial. ASN Kidney Week
Keyzer CA, de Borst MH, van den Berg E et al (2015) Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol
Smith ER, Hanssen E, McMahon LP et al (2013) Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS One 8(4):e60904
Aronova MA, Leapman RD (2013) Elemental mapping by electron energy loss spectroscopy in biology. Methods Mol Biol 950:209–226
Acknowledgements
Thanks to Dr. Eric Hansen (Bio21 Electron Microscopy Unit, University of Melbourne) for technical assistance with the EM analyses. This work was supported by an unrestricted investigator-initiated grant from Amgen Australia Pty Ltd, Baxter Pty Ltd, the Kincaid-Smith Research Foundation, and a RMH Home Lottery Research Grant-in-aid.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Smith, E.R. (2016). The Isolation and Quantitation of Fetuin-A-Containing Calciprotein Particles from Biological Fluids. In: Hewitson, T., Smith, E., Holt, S. (eds) Kidney Research. Methods in Molecular Biology, vol 1397. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3353-2_15
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3353-2_15
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3351-8
Online ISBN: 978-1-4939-3353-2
eBook Packages: Springer Protocols