Abstract
Allelic ratio of an SNP has been used for prenatal diagnosis of fetal trisomy 21 by MALDI-TOF mass spectrometry (MS). Because MALDI-TOF MS is challenging in quanepsication performance, pyrosequencing was proposed to replace MS for better quanepsication of allelic ratios. To achieve a simple and a rapid clinical diagnosis, PCR with a high-pH buffer (HpH buffer) was developed to directly amplify amniotic fluid without any step of genomic DNA extraction. By the established assay, 114 samples of amniotic fluid were directly amplified and individually analyzed by the pyrosequencing of five SNPs of each sample; the allelic ratios of euploid heterozygotes were thus calculated to determine the cutoff values of prenatal diagnosis of trisomy 21. The panel of five SNPs is much high in heterozygosity, and at least one heterozygote was found in each of the 114 samples, and 86 % of the samples have at least two heterozygotes in the panel, giving a nearly 100 % sensitivity of the assay. By using the cutoff values of each SNP, 20 pre-diagnosed clinical samples were detected as trisomy 21 carriers with the confidence level over 99 %, indicating that our method and karyotyping analysis are consistent in results. In conclusion, this pyrosequencing-based approach, coupled with the direct amplification of amniotic fluid, is accurate in quantitative genotyping and is simple in operation. We believe that the approach could be a promising alternative to karyotyping analysis in prenatal diagnosis.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
One of the major contents of prenatal diagnosis is to detect fetal chromosomal aneuploidies. Currently, karyotyping is the preferred test method for fetal trisomy; however, it involves lengthy procedures for culturing amniotic fluid and chorionic villus cells before analysis, increasing parental stress during the wait for a culture result. With a PCR-based method, a rapid prenatal diagnosis can be achieved by using a small amount of specimens as a detection target [1–3].
As a normal fetus is different from a trisomy 21 fetus in genomic copy number of chromosome 21, a method enabling the prenatal diagnosis should be able to quanepsy the copy number of chromosomes. Since each chromosome is from a parent, paired chromosomes should have many polymorphisms ; thus, the allelic ratio of a euploid heterozygote should be 1:1, while the allelic ratio will be 2:1 or 1:2 for a trisomy 21 fetus. Consequently, a heterozygous SNP on chromosome 21 can be a diagnostic biomarker of a trisomy 21 patient. Quantitative detection of allelic ratios was thus proposed by Lo’s research group [4, 5]. The detection was based on a single base-extension assay coupled with MALDI-TOF mass spectrometry (MS), and the quanepsication was achieved by calculating the relative yield of each allele peak in an MS tracing of an SNP [6–8]. However, it was found that the data from MALDI-TOF MS was not so quantitative [7]. Although the quantitative performance of MALDI-TOF MS was improved by using the peak height ratio in MS tracings [9], unlike ESI-MS, MALDI-TOF MS is still challenging in quanepsication. In addition, a mass spectrometer used for the assay should be specific to the determination of DNA molecules; for the moment, only iPLEX Gold MALDI-TOF MS system is available in the market, limiting the wide application of this strategy to the clinical screening of Down’s syndrome.
Pyrosequencing is a sequencing-by-synthesis method which is based on the bioluminometric detection of inorganic pyrophosphate (PPi) coupled with multiple enzymatic reactions. In a pyrogram, the relative intensity of each peak is proportional to the number of incorporated nucleotides. As only one base species is extended at a time, the intensity of each peak is proportional to the template amounts incorporated with the dispensed dNTP; hence, two peaks with the equal height should appear in a pyrogram for an SNP if no homogenous region is adjacent to the SNP. Consequently, two peaks with the ratio of 1:2 or 2:1 would be observed for a trisomy 21 fetus when the SNP of interest is located on chromosome 21.
Like most molecule diagnostic methods, pyrosequencing still requires a PCR step to supply enough templates for sequencing [10–12]. Conventionally, a purification process is necessary to extract target DNAs from biological samples before amplification. However, DNA extraction is costly and laborious and most importantly increases the risk of cross-contamination; hence, it is preferred to develop an amplification method directly using raw samples (e.g., blood or amniotic fluid). Although amnio-PCR has been successfully developed for amplifying small tandem repeat markers on chromosome 21 from uncultured amniotic fluid, pretreatment steps involving cell lysis and pH adjustment are still required before amplification [13, 14]. To avoid complex pretreatment, a novel “HpH buffer”-based PCR , which was previously developed to directly amplify target DNA from whole blood, was proposed for replacing amnio-PCR. It was found that amniotic fluid could be used as starting material of “HpH buffer”-based PCR. Based on the simplified amplification, prenatal diagnosis of trisomy 21 was successfully achieved by calculating the peak height ratio of two alleles.
2 Materials
-
1.
Taq polymerase was purchased from TaKaRa (Dalian, China).
-
2.
Bovine serum albumin (BSA), d-luciferin and adenosine 5′-phosphosulfate (APS), and apyrase VII were obtained from Sigma (St. Louis, MO).
-
3.
ATP sulfurylase and exo − Klenow fragment were obtained by gene engineering in our lab.
-
4.
Polyvinylpyrrolidone (PVP) and QuantiLum recombinant luciferase were purchased from Promega (Madison, WI).
-
5.
2′-Deoxyadenosine-5′-O-(1-thiotriphosphate) sodium salt (dATPαS), dGTP, dTTP, and dCTP were purchased from Mychem (San Diego, CA).
-
6.
Streptavidin sepharose beads was from GE Healthcare (Piscataway, NJ).
-
7.
PCR primers were designed by PyroMark Assay 2.0 and synthesized by Invitrogen Inc., Shanghai, China.
3 Methods
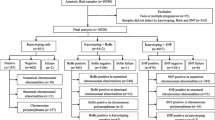
3.1 Sample Collection and Processing
-
1.
A set of 114 samples was collected from patients who came for an amniocentesis after the combination test indicated high risk for Down’s syndrome. Samples were obtained with informed consent and approval of the Ethics Committee.
-
2.
One milliliter of amniotic fluid was centrifuged at 10,000 × g for 10 min (see Note 1 ). Remove the supernatant and mix the remainder evenly. The concentrated amniotic fluid was moved to a 200-μL tube and heated at 94 °C for 15 min. Three microliter of the treated amniotic fluid (see Note 2 ) was used in a 50-μL reaction.
3.2 SNP Selection and Genotyping
The SNP information of chromosome 21 was obtained from NCBI database. In this study, we selected six SNP loci with a reported high heterozygosity in Chinese population: rs464783, rs767055, rs914195, rs914232, rs2254522, and rs243609. We also used another panel of six SNPs previously reported by our group: rs1053315, rs818219, rs2839110, rs1042917, rs35548026, and rs8130833 [15](see Note 3 ).
3.3 PCR Amplification
-
1.
A 50-μL reaction contained 5.35 μL of “HpH buffer” (see Note 4 ); 200 μmol/L each of dATP, dCTP, dGTP, and dTTP; 0.4 μmol/L of each primer; and 2.5 U of Taq polymerase.
-
2.
We initiated the reaction at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 72 °C for 30 s, 55–60 °C for 30 s, and a final extension at 72 °C for 7 min.
3.4 Single-Stranded DNA (ssDNA) Preparation
-
1.
Streptavidin-coated sepharose beads were used to capture biotinylated PCR products. After sedimentation and washing steps, purified double-stranded DNA was denatured by alkali to yield ssDNA .
-
2.
The immobilized biotinylated strand was then annealed with a sequencing primer under the condition of 80 °C for 5 min and 25 °C for 10 min before used as a sequencing template.
3.5 Pyrosequencing Reaction
-
1.
A 100 μL of pyrosequencing mixture contained 0.1 M Tris–HAc (pH 7.7), 2 mM EDTA, 10 mM Mg(Ac)2, 0.1 % BSA, 1 mM DTT, 2 μmol/L APS, 0.4 g/L PVP, 0.4 mM d-luciferin, 2 μmol/L ATP sulfurylase, 1.6 U/mL apyrase VII, 18 U/mL exo − Klenow fragment, and an appropriate amount of luciferase.
-
2.
Pyrosequencing was performed in a portable bioluminescence analyzer (Hitachi, Ltd., Japan) as we previously described [16, 17].
4 Method Validation
4.1 Investigation of “HpH Buffer” for PCR
An approach that amplified DNA directly from whole blood was developed by using “HpH buffer” [18]. To investigate whether or not this buffer could be used to amplify DNA from amniotic fluid directly, PCR with conventional PCR buffer (Fig. 1, lane 1) and HpH buffer (Fig. 1, lane 2) were individually performed using amniotic fluid as starting material. As shown in Fig. 1, the amount of amplicons from “HpH buffer” (lane 2) is much larger than that from conventional PCR buffer (lane 1), indicating that the PCR suppression from components in amniotic fluid was greatly blocked by “HpH buffer.” Although the amount of amplicons in lane 1 could be used for pyrosequencing, the intensity of peaks in a pyrogram was low. A higher peak intensity is preferred for better quanepsication performance; hence, it is better to concentrate amniotic fluid by centrifugation so as to improve PCR yield by increasing the initial template amount. After centrifugation at 10,000 × g for 10 min, 90 % of supernatant was removed, and the remaining was used for PCR. As shown in Fig. 1 (lane 3), the amount of amplicons was significantly increased, indicating that centrifugation could efficiently increase the amount of initial genomic DNA for PCR. Conventionally a process that lyses cells at a higher temperature could increase the amount of PCR templates. Lane 4 in Fig. 1 further indicated that the PCR yield was remarkably increased after a preheating step in addition to the centrifugation of amniotic fluid. Therefore, “HpH buffer” could be used for efficiently amplifying target DNA sequence from amniotic fluid directly.
Gel electropherograms of amplicons using conventional PCR buffer (lane 1) and “HpH buffer” (lanes 2–4) lane N: Blank control; lane 1: PCR with conventional PCR buffer by using raw amniotic fluid; lane 2: PCR with “HpH buffer” by using raw amniotic fluid; lane 3: PCR with “HpH buffer” by using concentrated amniotic fluid; lane 4: PCR with “HpH buffer” by using concentrated and preheated amniotic fluid; lane M: DNA markers
4.2 Investigation of the Effect of Template Amounts on PCR Yield
As the concentration of fetus cells varies greatly among different amniotic fluid samples, it is necessary to investigate the effect of template amounts on PCR yields. As shown in Fig. 2, the amount of amplicons that was sufficient for pyrosequencing could be obtained from the amplification of different volumes (ranging from 0.5 to 5 μL) of tenfold concentrated amniotic fluid. To get a reproducible result, a higher volume is preferred; thus, 3 μL of concentrated amniotic fluid in a 50-μL PCR reaction was used routinely. This means that a PCR assay needs 30 μL of raw amniotic fluid. If a triple detection is performed, around 0.1 mL of raw amniotic fluid will be required for an SNP assay; hence, 1 mL of raw amniotic fluid is enough for an assay with a panel of ten SNPs. Usually, 10 mL of raw amniotic fluid is needed for karyotyping. Therefore, our method requires only one-tenth of the amount of raw amniotic fluid in a karyotyping assay.
4.3 Selection of SNP Markers for Prenatal Diagnosis of Trisomy 21
As the proposed method is based on the allelic ratio of an SNP in a pyrogram, the heterozygosity of an SNP should be high in the population. At first, we selected six SNPs from the NCBI database with a heterozygosity over 40 % in the Chinese population and a panel of six SNPs from reported information with a population coverage of 92.9 % in Chinese. After 20 individual blood samples were genotyped by pyrosequencing, five SNPs with the highest heterozygosity were selected out of 12 SNPs for further study. Before being used as markers for prenatal diagnosis of trisomy 21, this panel of five SNPs was typed for 114 samples of amniotic fluid by using “HpH buffer”-based PCR and pyrosequencing. As shown in Table 1, the heterozygosities were between 0.421 and 0.589. In these 114 samples, it was found that at least one heterozygote appeared in each sample, and 86 % of samples had at least two heterozygotes, giving a 100 % heterozygote coverage; thus, the selected panel of SNPs can be a marker for prenatal diagnosis of trisomy 21. Although the allele frequency of SNP 4 is the lowest one in the five according to Table 2, the sensitivity (population coverage) will be decreased without SNP 4. For example, 99.1 % (113/114) of samples were covered by at least one heterozygote without SNP 4 in the panel. In addition, the number of samples with at least two heterozygotes decreased from 98 to 83 without the use of SNP 4. Therefore, SNP 4 should be included in the panel.
4.4 Cutoff Values of Allelic Ratios for Prenatal Diagnosis of Trisomy 21
In a pyrogram, the allelic ratio of an SNP should be close to 1 if no homogenous sequence exists around the SNP locus. However, this ratio may fluctuate with a target sequence; it is not a problem for SNP typing, but it may be problematic for the accurate determination of an allelic ratio, particularly for an SNP close to a homogenous region. In the selected five SNPs, the expected allelic ratios are 1, 3, 3, 1, and 1 for SNP 1, SNP 2, SNP 3, SNP 4, and SNP 5, respectively. To investigate whether the observed values of each allelic ratio were close to the expected values, the five SNPs in 94 euploid samples were detected by pyrosequencing, and the corresponding allelic ratio of every heterozygote was calculated for each SNP. As a small amount of PPi (due to the decomposition of dNTPs) may exist in dNTPs, each dNTP was dispensed twice, and a peak height was then calculated by subtracting the noise at the second dNTP dispensation from the signal at the first dNTP dispensation. The allelic ratio was the height ratio of two allele-specific peaks in a pyrogram. As shown in Table 2, the average observed allelic ratios of each SNP were 1.06, 2.91, 2.98, 0.86, and 0.82 for SNP 1, SNP 2, SNP 3, SNP 4, and SNP 5, respectively. As described in Ref. [7], the cutoff value of each SNP was calculated as the mean heterozygote allelic ratio ± [1.96 × SD]. The intervals of cutoff value were [0.88, 1.23], [2.54, 3.28], [2.63, 3.33], [0.64, 1.08], and [0.70, 0.94], respectively, and it was found that 95.5 % of detected allelic ratios fell within these intervals. Therefore, it is possible to employ these cutoff values for the discrimination of trisomy 21 fetus.
4.5 Genotyping Trisomy 21 Samples
With the cutoff values of each SNP, 20 samples, which were diagnosed as trisomy 21 carriers, were detected by pyrosequencing. The typical pyrograms of the five SNP loci for a euploid heterozygote and two types of trisomic heterozygotes are shown in Fig. 3. It was found that at least two heterozygotes were detected for each trisomic sample. The detected allelic ratios of all heterozygotes for 20 samples were out of the cutoff value intervals in Fig. 4, indicating that these samples were significantly different from euploidies. To further investigate the confidence level of detected results, Z-scores were employed and calculated by the formula of “Z-score = (the detected allelic ratio of a sample − the mean allelic ratio of euploid heterozygotes)/SD of the mean allelic ratio of heterozygotes.” The larger the Z-score is, the higher the degree of confidence level is. Further study showed that the low Z-score of sample no. 20 was due to its low signal intensity in the pyrogram, and this was overcome by increasing the template amount of pyrosequencing. Conventionally, there was more than one heterozygote in a sample; therefore, the accuracy of trisomy 21 discrimination would not be affected if the Z-score of only one heterozygote was not desirably high. The coherence between karyotyping analysis and our proposed method indicated that our method could be a promising alternative to karyotyping analysis in prenatal diagnosis.
5 Technical Notes
-
1.
Conventionally, a process that lyses cells at a higher temperature could increase the amount of PCR templates [19, 20]. Our result obtained from real-time PCR showed that preheating at 94 °C for 15 min gave a 100-fold increase of starting template amount.
-
2.
To get a reproducible result, a higher volume is preferred; thus, 3 μL of concentrated amniotic fluid in a 50 μL PCR reaction was used routinely. This means that a PCR assay needs 30 μL of raw amniotic fluid. If a triple detection is performed, around 0.1 mL of raw amniotic fluid will be required for an SNP assay.
-
3.
PCR primers were designed by PyroMark Assay 2.0 and synthesized by Invitrogen Inc., Shanghai. To simplify the name of each selected SNP, rs767055, rs914195, rs914232, rs2254522, and rs243609 were referred to as SNP 1, SNP 2, SNP 3, SNP 4, and SNP 5, respectively.
-
4.
HpH buffer had a pH (9.1–9.6) higher than that of a conventional PCR buffer (pH 8.5–8.8), aiming at weakening the interaction between blood inhibitors and genomic DNA.
References
Zhang Y, Liu H, Chen X, Xie X, Liu S, Wang H (2012) Noninvasive prenatal diagnosis of Down syndrome in samples from Southwest Chinese gravidas using pregnant plasma placental RNA allelic ratio. Genet Test Mol Biomarkers 16:1051–1057
Dutta UR, Pidugu VK, Goud VA, Dalal AB (2012) Mosaic Down syndrome with a marker: molecular cytogenetic characterization of the marker chromosome. Gene 495:199–204
Ghosh D, Sinha S, Chatterjee A, Nandagopal K (2012) Discerning non-disjunction in Down syndrome patients by means of GluK1-(AGAT)(n) and D21S2055-(GATA)(n) microsatellites on chromosome 21. Indian J Hum Genet 18:204–216
Tsui NB, Chiu RW, Ding C, El-Sheikhah A, Leung TN, Lau TK, Nicolaides KH, Lo YM (2005) Detection of trisomy 21 by quantitative mass spectrometric analysis of single-nucleotide polymorphisms. Clin Chem 51:2358–2362
Chow KC, Chiu RW, Tsui NB, Ding C, Lau TK, Leung TN, Lo YM (2007) Mass spectrometric detection of an SNP panel as an internal positive control for fetal DNA analysis in maternal plasma. Clin Chem 53:141–142
Huang DJ, Nelson MR, Zimmermann B, Dudarewicz L, Wenzel L, Spiegel R, Nagy B, Holzgreve W, Hahn S (2006) Reliable detection of trisomy 21 using MALDI-TOF mass spectrometry. Genet Med 8:728–734
Lo YM, Tsui NB, Chiu RW, Lau TK, Leung TN, Heung MM, Gerovassili A, Jin Y, Nicolaides KH, Cantor CR, Ding C (2007) Plasma placental RNA allelic ratio permits noninvasive prenatal chromosomal aneuploidy detection. Nat Med 13:218–223
Tsui NB, Wong BC, Leung TY, Lau TK, Chiu RW, Lo YM (2009) Non-invasive prenatal detection of fetal trisomy 18 by RNA-SNP allelic ratio analysis using maternal plasma SERPINB2 mRNA: a feasibility study. Prenat Diagn 29:1031–1037
Trewick AL, Moustafa JS, de Smith AJ, Froguel P, Greve G, Njolstad PR, Coin LJ, Blakemore AI (2011) Accurate single-nucleotide polymorphism allele assignment in trisomic or duplicated regions by using a single base-extension assay with MALDI-TOF mass spectrometry. Clin Chem 57:1188–1195
Zhang X, Wu H, Chen Z, Zhou G, Kajiyama T, Kambara H (2009) Dye-free gene expression detection by sequence-tagged reverse-transcription polymerase chain reaction coupled with pyrosequencing. Anal Chem 81:273–281
Huang H, Wu H, Xiao P, Zhou G (2009) Single-nucleotide polymorphism typing based on pyrosequencing chemistry and acryl-modified glass chip. Electrophoresis 30:991–998
Song Q, Wu H, Feng F, Zhou G, Kajiyama T, Kambara H (2010) Pyrosequencing on nicked dsDNA generated by nicking endonucleases. Anal Chem 82:2074–2081
Ogilvie CM, Donaghue C, Fox SP, Docherty Z, Mann K (2005) Rapid prenatal diagnosis of aneuploidy using quantitative fluorescence-PCR (QF-PCR). J Histochem Cytochem 53:285–288
Levett LJ, Liddle S, Meredith R (2001) A large-scale evaluation of amnio-PCR for the rapid prenatal diagnosis of fetal trisomy. Ultrasound Obstet Gynecol 17:115–118
Liu XQ, Wu HP, Bu Y, Zou BJ, Chen ZY, Zhou GH (2009) Quantitative pyrosequencing of heterozygous single nucleotide polymorphisms for rapid diagnosis of Down’s syndrome. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 26:331–335
Wu H, Wu W, Chen Z, Wang Z, Zhou G, Kajiyama T, Kambara H (2011) Highly sensitive pyrosequencing based on the capture of free adenosine 5′ phosphosulfate with adenosine triphosphate sulfurylase. Anal Chem 83:3600–3605
Chen Z, Fu X, Zhang X, Liu X, Zou B, Wu H, Song Q, Li J, Kajiyama T, Kambara H, Zhou G (2012) Pyrosequencing-based barcodes for a dye-free multiplex bioassay. Chem Commun (Camb) 48:2445–2447
Bu Y, Huang H, Zhou G (2008) Direct polymerase chain reaction (PCR) from human whole blood and filter-paper-dried blood by using a PCR buffer with a higher pH. Anal Biochem 375:370–372
Heung MM, Jin S, Tsui NB, Ding C, Leung TY, Lau TK, Chiu RW, Lo YM (2009) Placenta-derived fetal specific mRNA is more readily detectable in maternal plasma than in whole blood. PLoS One 4, e5858
Deng YH, Yin AH, He Q, Chen JC, He YS, Wang HQ, Li M, Chen HY (2011) Non-invasive prenatal diagnosis of trisomy 21 by reverse transcriptase multiplex ligation-dependent probe amplification. Clin Chem Lab Med 49:641–646
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Ye, H. et al. (2016). Prenatal Diagnosis of Trisomy 21 by Quantitatively Pyrosequencing Heterozygotes Using Amniotic Fluid as Starting Material of PCR. In: Zhou, G., Song, Q. (eds) Advances and Clinical Practice in Pyrosequencing. Springer Protocols Handbooks. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3308-2_26
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3308-2_26
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3306-8
Online ISBN: 978-1-4939-3308-2
eBook Packages: Springer Protocols