Abstract
The central nervous system is comprised of multiple cell types including neurons, glia, and other supporting cells that may differ dramatically in levels of signaling pathway activation. Immunohistochemistry in conjunction with drug interference are powerful tools that allow evaluation of signaling pathways in different cell types of the mouse central nervous system in vivo. Here we provide detailed protocols for immunohistochemistry to evaluate three essential components in the PI3K pathway in mouse brain: Pten, p-Akt, and p-4ebp1, and for rapamycin treatment to modulate mTOR signaling in vivo.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
The phosphoinositide 3-kinase (PI3K) pathway is an essential signaling cascade that regulates multiple processes in the central nervous system including proliferation, survival, metabolism, and cell migration [1]. When extracellular growth signals are relayed to the PI3K pathway through receptor tyrosine kinases, AKT is fully activated by recruitment to the cell membrane by binding of its pleckstrin homology domain (PH domain) to increased levels of PIP3 and subsequent phosphorylation at amino acid residue threonine 308 by PDK1 and serine 473 by mTORC2 complex [2, 3]. Among a variety of downstream targets, mTORC1 is a master regulator of cell growth and proliferation as well as many other cellular processes [4]. mTORC1 promotes cell growth and proliferation through phosphorylation of two of its downstream effectors, the S6 kinases (S6Ks) and 4E-BPs which enhance ribosome assembly and mRNA translation. Upon activation by growth factors, mTORC1 limits the extent of upstream growth factor signaling by a negative feedback loop through IRS-1 phosphorylation by S6K1 [5, 6]. PTEN is the major negative regulator of the PI3K pathway. Inactivation of PTEN results in unopposed and constitutive activation of the PI3K-AKT-mTORC1 pathway in the presence of upstream pathway activation. Increases in phosphorylation of AKT serine 473 and threonine 308, phospho-S6 serine 235/236 (substrate of S6K), and phospho-4EBP1 threonine 37/46 are often used as the readouts of pathway activation. Pten loss in the mouse brain could result in diverse phenotypes such as neuronal hypertrophy and premature differentiation, astrocyte hypertrophy, or increased neural stem cell self-renewal and proliferation given different cellular and developmental contexts [7–14].
Rapamycin is a small-molecule inhibitor of mTOR complexes. It inhibits mTORC1 activity in acute treatment while it also inhibits mTORC2 activity in long-term or high-dose treatment by interfering with its assembly [15, 16]. In many settings, inhibition of mTORC1 by rapamycin releases the negative feedback loop and results in enhanced PI3K signaling to AKT [17], although this is not always observed.
Here we provide detailed immunohistochemistry protocols for Pten, p-Akt, and p-4EBP1 in mouse brain and a protocol for rapamycin treatment of mice to manipulate PI3K signaling in vivo.
2 Materials
2.1 Immunohistochemistry for Pten, p-Akt, and p-4EBP1 in FFPE Tissue
-
1.
Anti-Pten antibody, rabbit monoclonal, Cell Signaling #9559.
-
2.
Anti-p-Akt (Ser473) antibody, rabbit polyclonal, Cell Signaling #9271.
-
3.
Anti-p-4EBP1 (T37/46) antibody, rabbit monoclonal, Cell Signaling #2855.
-
4.
Biotinylated anti-rabbit-IgG, included in Elite ABC reagent kit, Vector Lab PK-6101.
-
5.
Elite ABC reagent, Vector Lab PK-6101.
-
6.
NovaRED kit, Vector lab SK-4800 (DAB kit SK-4100).
-
7.
Hematoxylin QS, Vector Lab H-3404.
-
8.
Permount, Fisher SP15-100.
-
9.
Tyramide Signal Amplification (TSA) Kit, Perkin Elmer NEL746A.
-
10.
Antigen-retrieval solution: 18 ml of 0.1 M citric acid, 82 ml of 0.1 M sodium citrate, 850 ml of H2O. Adjust to pH 6.0 by addition of 1 N NaOH, and then add H2O for final volume of 1 l.
-
11.
10× TBS buffer (10× Tris-buffered saline): 60.6 g Tris, 87.6 g NaCl in 800 ml ultrapure water. Adjust pH to 7.5 with 1 M HCl, and add ultrapure water to final volume of 1 l.
-
12.
TBS-T (1× TBS + 0.01 % Tween-20).
-
13.
0.6 % H2O2 in TBS (freshly made): 1 ml of 30 % H2O2 in 49 ml of TBS.
-
14.
Blocking solution: 10 % goat serum in TBS-T, with 0.01 % thimerosal, store at 4 °C.
-
15.
Blocking solution: 2 % goat serum in TBS-T, with 0.01 % thimerosal, store at 4 °C.
-
16.
Plastic Coplin jars, at least four for antigen retrieval.
-
17.
Microwave oven with rotating tray.
-
18.
Wax pen.
-
19.
Belly dancer rocking platform.
-
20.
Cover slips.
2.2 Rapamycin Treatment
-
1.
Rapamycin (LC Laboratories).
-
2.
Sterile dimethyl sulfoxide (DMSO).
-
3.
Tween80.
-
4.
1 ml Syringe (BD).
3 Methods
3.1 Immunohistochemistry for Pten, p-Akt, and p-4EBP1 in FFPE Tissue
For cryosections, mice were anesthetized and perfused transcardially with PBS followed by 4 % paraformaldehyde (PFA) in PBS. Following dissection, tissues were post-fixed overnight in 4 % PFA in PBS at 4 °C, and then equilibrated in 25 % sucrose in PBS for an additional 24 h at 4 °C. Tissues were embedded in embedding media OCT (Triangle Biomedical Sciences) on dry ice and cut into 12 μm thick cryosections. Tissue slides were equilibrated at room temperature for 20 min and then washed three times in PBS prior to staining.
For paraffin sections, tissue was processed the same way as above except after dissection, tissue was post-fixed for 24 h in 4 % PFA in PBS at 4 °C, then processed and embedded in paraffin, and cut into 5 μm sections.
For optimal IHC, tissue should not be left in fixing solution for more than 4 days before being processed for paraffin-embedding or equilibrated in 25 % sucrose in PBS for cryoprotection followed by embedding in OCT for cryosections.
3.1.1 Deparaffinization (Only for Paraffin Sections)
Transfer slides through the following series of solutions (see Notes 1 and 2 ):
-
1.
Xylenes 5–10 min.
-
2.
Xylenes 3 min × 2.
-
3.
100 % EtOH, 2 min × 2.
-
4.
95 % EtOH, 2 min.
-
5.
70 % EtOH, 1 min.
-
6.
50 % EtOH, 1 min.
-
7.
20 % EtOH, 1 min.
-
8.
H2O, 2 min × 2.
3.1.2 Antigen Retrieval
Saturation: Place sections in plastic Coplin jar(s) filled with the antigen-retrieval solution completely to the top as below:
-
1.
For deparaffinized sections: antigen-retrieval solution 10 min.
-
2.
Microwave. Prepare a total of four plastic Coplin jars either with or without slides (jars without slides can be filled with H2O).
-
3.
Seal the jar(s) as tightly as possible and position them at the center of a microwave oven with rotating tray.
-
4.
After each microwave interval, refill Coplin jars if any solution has leaked out.
-
5.
2.5 min at power 100 %.
-
6.
2.5 min at power 50 %.
-
7.
2.5 min at power 50 %.
-
8.
2.5 min at power 50 %.
-
9.
Open the lid and cool the solution to room temperature (at least 30 min; see Note 3 ).
3.1.3 Blocking Endogenous Peroxidase
-
1.
Wash sections with TBS in Coplin jars (up to 13 slides/jar) on belly dancer, 10 min.
-
2.
Treat sections with freshly made 1 % H2O2 (in TBS) for 30 min at room temperature.
-
3.
Wash with TBS, 5 min × 2, and with TBS-T, 5 min × 1, in Coplin jars on belly dancer.
-
4.
After the second wash draw a boundary with a wax pen around the area of the slide to be stained (see Note 4 ).
3.1.4 Blocking and Primary Antibody
-
1.
Block nonspecific binding sites with 10 % goat serum (in TBS-T) for 30 min to 1 h, usually 300–500 μl for each slide in a humidified chamber at room temperature.
-
2.
Dilute primary antibody in 2 % goat serum (in TBS-T) (~300 μl/full slide) in a humidified chamber at room temperature. A. anti-Pten: 1:100 dilution. B. anti-p-Akt antibody 1:50 dilution. C. anti-p-4EBP1 1:500 dilution.
-
3.
Remove the blocking solution and incubate the sections with the primary antibody in a humidified chamber overnight at 4 °C.
3.1.5 Secondary Antibody and Signal Amplification
-
1.
Wash the sections with TBS-T in Coplin jars on belly dancer, 5 min × 3 (see Note 5 ). Incubate the sections with biotinylated anti-rabbit IgG antibody diluted to 1:200 in 2 % goat serum (in TBS-T) for 1 h in a humidified chamber at room temperature.
-
2.
Prepare Elite ABC solution at least 30 min before use: To 2.5 ml of TBS-T, add two drops of solution A. Mix by vortexing and add two drops of solution B.
-
3.
Mix by vortexing and leave the solution at room temperature.
-
4.
Wash the sections with TBS-T, 5 min × 2, and 10 min × 1, in a humidified chamber at room temperature.
-
5.
Optional tyramide amplification of p-Akt signal: (1) Wash the sections with TBS-T, 5 min × 3. (2) Add the biotinylated-tyramide (1:175 dilution in TBS-T) and incubate for 10 min at room temperature (300–500 μl/slide). (3) Wash the sections with TBS-T, 5 min × 3 (see Note 6 ).
-
6.
Add the ABC solution and incubate for 1 h in a humidified chamber at room temperature (300–500 μl/slide), or for 30 min if tyramide amplification was used (see Notes 7 and 8 ).
3.1.6 Color Development
IHC signals are visualized by incubating with NovaRED or diaminobenzidine (DAB), HRP substrates that produce a red or dark brown reaction product, respectively. NovaRED is generally more sensitive than DAB. The optimal substrate depends on the level of signal associated with the phenotype.
-
1.
Wash with TBS-T, 5 min × 2, and 10 min × 1.
-
2.
During the last wash prepare NovaRED solution (or DAB solution). To 5 ml of water, add three drops of reagent 1 and mix well by vortexing. Add two drops of reagent 2 and mix by vortexing. Add two drops of reagent 3 and mix by vortexing. Add two drops of H2O2 reagent and mix by vortexing.
-
3.
Apply the solution onto washed sections. Incubate until signals become distinguishable between sample and control. Pten usually takes approximately 7–10 min. p-Akt takes approximately 10–15 min without tyramide amplification and 4 min with tyramide amplification. p-4EBP1 takes approximately 10 min.
-
4.
Optimal incubation time will vary with tissue preparation. Also, if detecting pathological activation of the pathway, the optimal detection time will depend on the level of pathway activation. Observe under the microscope to select the optimal time to stop the development reaction. Representative immunohistochemistry images are shown in Fig. 1.
Fig. 1 Representative immunohistochemistry for Pten, p-Akt, p-S6, and p-4EBP1 from Pten cKO mice. (a–f): Nestin-creER; Pten flox/flox with cre activity induced by tamoxifen administration at postnatal days 0 and 1, with tissue collected at day 30. (a–c): Pten wt mouse; (d–f): Pten ko. (g–h): Nestin-creER; Pten flox/flox; Tp53 flox/flox mouse medulloblastoma. (i): GFAP-creER; Pten flox/flox; Tp53 flox/flox mouse astrocytoma. (a and d): Pten IHC: mouse dentate gyrus, Pten wild-type cells are red while Pten-null cells in the inner layer of the dentate gyrus stain only with hematoxylin counter stain. (b and e): p-Akt IHC,: Pten-null dentate gyrus neurons are strongly positive (red) for p-Akt. (c and f): p-S6 IHC: Pten-null dentate gyrus cells are more strongly positive (red) for p-S6 than the baseline p-S6 level in wild-type mice. (g) Pten IHC, mouse medulloblastoma: Pten-null tumor cells stain only with hematoxylin counter stain while wild-type blood vessel endothelial cells are brown. (h) p-Akt IHC, mouse medulloblastoma, and Pten-null tumor cells are strongly positive (red) while the adjacent normal brain is negative for p-Akt. (i) p-4ebp1 IHC, GFAP-creER; Pten; Tp53 double-knockout mouse astrocytoma are strongly positive for p-4ebp1. Scale bar = 50 μm. Substrate: Nova red, counter stain, hematoxylin
-
5.
Discard the solution into a beaker and stop the development by washing sections with water 5 min × 2 (see Note 9 ).
3.1.7 Counter Staining with Hematoxylin
-
1.
Incubate the sections in hematoxylin QS solution for 15 s.
-
2.
Rinse the sections in a Coplin jar with running tap water until the rinse is colorless (5–10 min).
-
3.
Dip the slides in water ten times.
3.1.8 Dehydration and Mounting
-
1.
Dehydration: 20 % EtOH 1 min, 50 % 1 min, 70 % 1 min, 95 % 2 min, 100 % 2 min × 2, xylenes 5 min × 3.
-
2.
Coverslip with Permount in the fume hood.
-
3.
Leave mounted slides in the hood at least overnight to dry.
3.2 Rapamycin Treatment
The inhibitory effects of rapamycin on mTOR complexes are dose and time dependent [15, 16]. Low-dose and short-term rapamycin treatment selectively inhibits mTorc1 activity, while high-dose and long-term treatment inhibits both mTorc1 and mTorc2 activity. We use loss of p-S6 as a downstream indication of rapamycin effects on mTorc1 and use p-Akt pSer473 as an indication of rapamycin effects on mTorc2. Increased phosphorylation of pS6 is more strongly associated with hypertrophic effects of mTorc1, while hyperproliferation is more strongly associated with p-4ebp1. In our Pten conditional knockout models of hypertrophy versus brain tumor proliferation, rapamycin appears to have much greater efficacy in blocking downstream S6 phosphorylation, but not p-4ebp1. Given the fact that rapamycin inhibition of mTorc1 signaling in brain increases with time, we typically dose a minimum of 5 days to see maximal pathway inhibition [18]. Tissue is collected 2 h after the last dose is administered.
3.2.1 Reagent Preparation
-
1.
Dissolve rapamycin powder in sterile DMSO at 20 mg/ml, stored at −20 °C as stock solution.
-
2.
Prepare 5.2 % Tween80 in MilliQ (make sure Tween80 is completely dissolved).
-
3.
Rapamycin working solution is prepared by diluting stock solution in 5.2 % Tween80 to 10–20 μg/ml immediately before use.
-
4.
Vehicle control is prepared by dissolving the same volume of DMSO in 5.2 % Tween80 immediately before use (see Note 10 ).
3.2.2 Injection of Rapamycin
-
1.
To selectively inhibit mTorc1 in the subventricular zone and rostral migratory stream in mice from postnatal day 8 (P8) to P31, low-dose rapamycin (1.5 μg rapamycin/g body weight) is injected i.p. daily in Pten cKO or wild-type control mice from postnatal day 8 to 31.
-
2.
At higher doses of rapamycin (7.5 μg rapamycin/g body weight) from P8-P31, both mTorc1 and mTorc2 are inhibited. Representative IHC to show pathway inhibition following rapamycin treatment is shown in Fig. 2. Rapamycin toxicity varies with age. Young mice cannot tolerate the higher doses used in adults, even when adjusted for body weight. 7.5 μg/g is the highest dose that FVB mice from P8–P31 could tolerate.
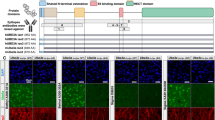
Fig. 2 Monitoring rapamycin inhibition of mTorc1 and mTorc2 activity by IHC. Representative Pten (a), p-Akt (b), and p-S6 (c) IHC images in the subventricular zone (SVZ) of vehicle- or rapamycin-treated Nestin-creER; Pten flox/flox mice induced to delete Pten by tamoxifen administration at P0, P1 [7]. Pten conditional loss in the SVZ causes an expanded SVZ with elevated levels of pAkt and pS6. High-dose rapamycin (7.5 μg/g) inhibits both p-Akt (S473) and p-S6 while low-dose (1.5 μg/g) inhibits p-S6 only. Both doses rescue the expanded size of the SVZ in Pten cKO mice.FF
-
3.
The dose of rapamycin required to block mTorc1 signaling varies between different brain regions. In adult mice, 25 μg rapamycin/g body weight was sufficient to block mTorc1 signaling in the dentate gyrus of Pten conditional knockout mice. Increasing the dose to 50 μg rapamycin/g body weight was necessary to block the downstream signaling in cerebellum [18].
4 Notes
-
1.
Prepare all solutions using ultrapure water (MilliQ, prepared by purifying deionized water to attain a sensitivity of 18 MΩ at 25 °C) and analytical grade reagents.
-
2.
Work with xylene in a fume hood.
-
3.
Labeling of microwave power output is not accurate, and does not necessarily translate from one microwave to the next, even if they are labeled as equivalent power output. The correct power output should be empirically determined by placing filled Coplin jars and monitoring time to boiling. The key point in this antigen retrieval is to boil the sections for at least the last 7.5 min with minimal air exposure. Therefore new conditions (power levels) may need to be set up for each microwave oven.
-
4.
The area inside the wax boundary can be adjusted based on the size of sections as long as the entire section is completely contained inside the boundary. The amount of primary and secondary antibody incubation solutions can be reduced accordingly based on the area encircled inside the boundary.
-
5.
To minimize cross-contamination among antibodies, keep slides with different primary antibodies in separate jars.
-
6.
The p-Akt signal can be significantly enhanced with tyramide amplification without increasing background. This step is optional depending on the intensity of the p-Akt stain associated with the phenotype under study.
-
7.
Elite ABC is a kit developed by Vector Laboratory, which utilizes the biotin-avidin complex to substantially amplify immunohistochemistry signals and increase detection sensitivity. Solution A contains avidin which complexes with biotinylated horseradish peroxidase (HRP) in Solution B. This complex binds with extremely high affinity to the biotinylated secondary antibody. HRP will react with substrate for colorimetric detection.
-
8.
Secondary Ab incubation and Elite ABC incubation time can be extended to total 2–3 h. In this case, wash 5 more minutes for each 30-min incubation extension.
-
9.
NovaRed and DAB (a known mutagen) are hazardous materials that are harmful if inhaled, ingested, or through direct contact with skin. Handle according to the materials safety data sheet, and collect and dispose as hazardous waste.
-
10.
Adjust rapamycin working solution concentration to make final injection volume around 0.5–1 ml/mouse.
References
Chalhoub N, Baker SJ (2009) PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 4:127–150
Alessi DR et al (1997) 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol 7(10):776–789
Sarbassov DD et al (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712):1098–1101
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40(2):310–322
Shah OJ, Hunter T (2006) Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol 26(17):6425–6434
Harrington LS et al (2004) The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166(2):213–223
Zhu G et al (2012) Pten deletion causes mTorc1-dependent ectopic neuroblast differentiation without causing uniform migration defects. Development 139(18):3422–3431
Chalhoub N et al (2009) Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev 23(14):1619–1624
Endersby R, Baker SJ (2008) PTEN signaling in brain: neuropathology and tumorigenesis. Oncogene 27(41):5416–5430
Fraser MM et al (2004) Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res 64(21):7773–7779
Kwon CH et al (2001) Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet 29(4):404–411
Groszer M et al (2001) Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294(5549):2186–2189
Backman SA et al (2001) Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet 29(4):396–403
Sperow M et al (2012) Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J Physiol 590(Pt 4):777–792
Huang S, Bjornsti MA, Houghton PJ (2003) Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther 2(3):222–232
Sarbassov DD et al (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22(2):159–168
O’Reilly KE et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66(3):1500–1508
Kwon CH et al (2003) mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A 100(22):12923–12928
Acknowledgments
We thank Sherri Rankin for the p-4ebp1 IHC image. SJB was supported in part by grants from the NIH (CA096832 and CA188516) and by the American Lebanese and Syrian Associated Charities of St Jude.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Zhu, G., Baker, S.J. (2016). Detecting PTEN and PI3K Signaling in Brain. In: Salmena, L., Stambolic, V. (eds) PTEN. Methods in Molecular Biology, vol 1388. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3299-3_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3299-3_5
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3297-9
Online ISBN: 978-1-4939-3299-3
eBook Packages: Springer Protocols