Abstract
Glycopolypeptides are prepared either by the polymerization of glycosylated amino acid N-carboxyanhydrides (NCAs) or by the post-polymerization functionalization of polypeptides with suitable functional groups. Here we present a method for the in-situ functionalization and (co-) polymerization of allylglycine N-carboxyanhydride in a facile one-pot procedure, combining radical thiol-ene photochemistry and nucleophilic ring-opening polymerization techniques, to yield well-defined heterofunctional glycopolypeptides.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Synthetic polypeptides, and especially glycopolypeptides, recently attracted increasing interest as promising materials for applications in biomedicine and biotechnology, e.g., tissue engineering, drug delivery, or as polymer therapeutics [1–4]. The synthesis, structural characteristics, self-assembly behavior, and ability of glycopolypeptides to recognize and selectively bind to proteins (lectins) have been investigated and highlighted in numerous articles, manifesting the increasing importance of this class of materials [2, 5–9]. Although significant progress has been made [8], the preparation of well-defined glycopolypeptides is still a challenging task for synthetic polymer chemists. Recent efforts include the polymerization of glycosylated NCAs as well as the post-polymerization functionalization of ready-made polypeptides carrying appropriate functional groups in the side chains. However, most of these approaches require multiple steps and sophisticated, tedious purification protocols. Especially the works with hydrolytically unstable NCAs require careful and skillful handling and are labor- and time-consuming processes.
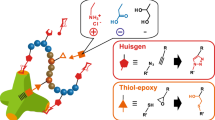
We suggested a very facilitated (and transition metal-free) synthesis of glycopolypeptides by in situ glycosylation and polymerization of AGly NCA, combining radical thiol-ene photochemistry and nucleophilic ROP (Fig. 1a) [10]. The so prepared glycopolypeptides contain a predetermined amount of sugar and remaining vinyl groups, which in a second step can be functionalized to yield heterofunctional glycopolypeptides with a variety of functionalities (exemplary here: carboxyl and glucosyl) (Fig. 1b). These additional functionalities (e.g., amine, ethylene glycols, other carbohydrates) could be used to introduce stimuli-responsiveness or trigger the folding of polypeptide chains into higher order structures.
(a) One-pot partial glycosylation and copolymerization of AGly NCA and (b) subsequent functionalization with thiol to yield heterofunctional glycopolypeptides. Reagents and conditions: a thiol-ene photoaddition: benzophenone, hν, THF, r.t., 45 min (y < x); b nucleophilic ROP: 1-hexylamine, THF/DMF, r.t., 7 days; c 3-mercaptopropionic acid, benzophenone, hν, THF, r.t., 16 h. Adapted from [10]
2 Materials
2.1 Chemicals
-
1.
dl-Allylglycine (>98 %) (BoaoPharma).
-
2.
Benzophenone (Sigma-Aldrich).
-
3.
N,N-Dimethylformamide (≥99.8 %, extra dry) (Sigma-Aldrich).
-
4.
Ethyl acetate (Th. Geyer GmbH & Co, KG), dried over CaH2 and distilled.
-
5.
Heptanes (99 %) n-heptane (99 %) (Roth).
-
6.
1-Hexylamine (>99.5 %) (Sigma-Aldrich).
-
7.
Isopropanol (tech.)
-
8.
3-Mercaptopropionic acid (99 %+) (Sigma-Aldrich).
-
9.
α-Pinene (Alfa Aesar).
-
10.
Silica-gel (Fluka), dried at 150 °C for 48 h
-
11.
Tetrahydrofuran (99.5 %, extra dry), 1,4-dioxane (Acros Organics).
-
12.
1-Thio-β-d-glucose-2,3,4,6-tetraacetate (97 %) (Sigma-Aldrich).
-
13.
Triphosgene (Merck).
2.2 UV Light Source
-
1.
Energy saving lamp Exo Terra ReptiGlo 5.0, 26W (Fig. 2) just below Subheading 2.2 UV light source.
Fig. 2 Energy saving lamp Exo Terra ReptiGlo 5.0, 26W (left) and UV/vis emission spectrum (right) (as provided by the manufacturer). Reprinted with permission from [11]. Copyright 2012, American Chemical Society
3 Methods
3.1 Monomer Synthesis [11]
-
1.
Add (suspend) 2.5 g of AGly (21.7 mmol, 1.0 equiv) in 100 mL of THF and heat to 50 °C.
-
2.
At this temperature, add 13.75 mL of α-pinene (86.8 mmol, 4.0 equiv) and 2.57 g of triphosgene (8.7 mmol, 0.4 equiv) and flush a constant stream of argon through the reaction mixture.
-
3.
A clear solution usually forms within 45 min; otherwise add additional triphosgene (0.05 equiv/30 min).
-
4.
After 3 h, concentrate the solution to 1/3 of the volume and precipitate in excess heptanes.
-
5.
Collect the white precipitate and remove residual heptanes under high vacuo (ca. 1 h).
-
6.
Redissolve the powder in minimum amount of ethyl acetate and filter through standard filter paper into tenfold volume of heptanes.
-
7.
Repeat steps 5 and 6 two times.
-
8.
Collect the white precipitate and remove residual solvent under high vacuo. Yield: 1.6 g (11.3 mmol, 52 %) (see Note 1 )
-
9.
Characterize the product by melting point (see Note 2 ) and 1H NMR (see Note 3 ). Melting point: 89–90 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.59 (s, 1H, NH), 5.74 (m, 1H, H2C = CH), 5.28 (m, 2H, H 2C = CH), 4.40 (dd, 3 J = 7.0 Hz, 4 J = 4.3 Hz, 1H, H2C-CH-NH), 2.53 (td, 3 J = 14.6 Hz, 3 J = 7.4 Hz, 1H diast., H 2C-CH-NH), 2.10 (td, 3 J = 14.6 Hz, 3 J = 7.4 Hz, 1H diast., H 2C-CH-NH).
3.2 One-Pot Glycosylation/Polymerization (Exemplary Procedure)
-
1.
Dissolve AGly NCA (1.0 equiv), benzophenone (0.2 eq), and the respective amount of AcGlcSH (0.8 equiv) in dry THF (0.15 M) under an argon atmosphere.
-
2.
Irradiate the reaction mixture with UV light from two energy saving lamps (Exo Terra ReptiGlo 5.0 26W) (distance UV lamp to reaction vessel: ca. 5 cm) for ~45 min (see Note 4 ).
-
3.
Remove vessel from the lamps and add dry DMF (overall concentration 5 wt%) and desired amount of a 0.1 M solution of freshly distilled 1-hexylamine ([NCA]0/[amine]0 = 30) in dry DMF
-
4.
Stir the reaction mixture for 7 days under reduced pressure (ca. 0.5 mbar) at room temperature (see Note 5 ).
-
5.
Quench the polymerization by precipitation into a tenfold volume of isopropanol.
-
6.
Collect the product by centrifugation and dry at 65 °C in high vacuum. Isolated yield: 80 %.
-
7.
Characterize the product by 1H NMR (see Note 3 ) and SEC (see Note 6 ) and 1H NMR (400 MHz, TFA-d): δ (ppm) = 5.6–5.8 (-HC = C-), 5.6–5.4 (S-CH-O), 5.4–5.3 (Glc), 5.3–5.2 (-HC = CH 2, Glc), 4.3–4.8 (C(=O)-CH-NH), 3.8–4.0 (Glc), 3.4 (CH2-CH 2-NH2), 2.9–2.4 (S-CH 2), 2.3–1.6 (S-CH2-CH2-CH 2, OAc), 1.3–1.2 (CH3-CH 2-CH 2-CH 2-CH 2-), 0.8 (CH 3). Composition (AGly)/(GlcAGly) = 0.32/0.68 (1H NMR), average number of Gly repeat units: 28 (1H NMR end group analysis), number-average molar mass: M n app = 8500 g/mol (SEC), dispersity: Đ = 1.22 (SEC).
3.3 Post-polymerization Functionalization (Exemplary Procedure)
-
1.
Dissolve partially glycosylated polypeptide ((AGyl)/(GlcAGly) = 0.32/0.68 (9/19 units)), benzophenone (0.1 equiv with respect to double bonds), and 3-mercaptopropionic acid (1.5 equiv with respect to double bonds) in THF (ca. 1.0 wt% with respect to AGly units) and put it under an inert argon atmosphere.
-
2.
Seal the vessel and irradiate it with UV light for 16 h (see Note 7 ).
-
3.
Dilute the reaction mixture and extensively dialyze (RC 1000) against THF (see Note 8 ).
-
4.
Removal of THF and freeze-drying from 1,4-dioxane yield the final products as fluffy solids.
-
5.
1H NMR (400 MHz, TFA-d): Fig. 3. Quantitative conversion of AGly units (1H NMR), number-average molar mass: M n app = 10,280 g/mol (SEC), dispersity: Đ = 1.26 (SEC).
Fig. 3 1H-NMR spectrum (400 MHz, TFA-d) of carboxylated glycopolypeptide. Reprinted with permission from [10]. Copyright 2014, American Chemical Society
4 Notes
-
1.
The AGly NCA monomer needs to be stored in the freezer and is stable for up to 3 months.
-
2.
Melting points are determined using a MEL-TEMP® apparatus from Lab Devices INC, USA with a Fluke 51 thermometer.
-
3.
1H NMR measurements are conducted at room temperature using a Bruker DPX-400 spectrometer operating at 400 MHz. Deuterated chloroform and TFA are used as solvents (Sigma-Aldrich); 1H NMR signals are referenced to the signals of CDCl3 δ 7.26 ppm and TFA-d δ 11.52 ppm, respectively.
-
4.
Irradiation must start immediately after mixing of the reactants. The reaction can be accelerated by using more lamps.
-
5.
The impact of pressure on polymerization has not been investigated. Key is the removal of CO2 which is released during monomer addition.
-
6.
SEC with simultaneous UV and RI detection is performed with NMP (+0.5 wt% LiBr) as the eluent, flow rate: 0.8 ml/min, at 70 °C using a set of two 300 × 8 mm2 PSS-GRAM columns with average particle sizes of 7 μm and porosities of 100 and 1000 Å. Calibration was done using poly(methyl methacrylate) standards (PSS, Mainz, Germany).
-
7.
Full conversion of monomer is usually achieved within 3–5 h.
-
8.
Dialysis bags are becoming brittle in THF and should be handled with care (to avoid damage or rupture).
References
Deming TJ (2007) Synthetic polypeptides for biomedical applications. Prog Polym Sci 32:858–875
Huang J, Heise A (2013) Stimuli responsive synthetic polypeptides derived from N-carboxyanhydride (NCA) polymerisation. Chem Soc Rev 242:7373–7390
Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2:347–360
Ringsdorf H, Schlarb B, Venzmer J (1988) Molecular architecture and function of polymeric oriented systems: models for the study of organization, surface recognition, and dynamics of biomembranes. Angew Chem Int Ed Engl 27:113–158
Bonduelle C, Lecommandoux S (2013) Synthetic glycopolypeptides as biomimetic analogues of natural glycoproteins. Biomacromolecules 14:2973–2983
Quadir MA, Martin M, Hammond PT (2014) Clickable synthetic polypeptides: routes to new highly adaptive biomaterials. Chem Mater 26:461–476
Kricheldorf HR (2006) Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides. Angew Chem Int Ed 45:5752–5784
Kramer JR, Deming TJ (2014) Recent advances in glycopolypeptide synthesis. Polym Chem 5:671–682
Krannig K-S, Schlaad H (2014) Emerging bioinspired polymers: glycopolypeptides. Soft Matter 10:4228–4235
Krannig K-S, Doriti A, Schlaad H (2014) Facilitated synthesis of heterofunctional glycopolypeptides. Macromolecules 47:2536–2539
Krannig K-S, Schlaad H (2012) pH-responsive bioactive glycopolypeptides with enhanced helicity and solubility in aqueous solution. J Am Chem Soc 134:18542–18545
Acknowledgment
Financial support was given by the Max Planck Society and the German Research Foundation (within the IUPAC Transnational Pilot Call in Polymer Chemistry).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Krannig, KS., Schlaad, H. (2016). Heterofunctional Glycopolypeptides by Combination of Thiol-Ene Chemistry and NCA Polymerization. In: Sun, XL. (eds) Macro-Glycoligands. Methods in Molecular Biology, vol 1367. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3130-9_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3130-9_6
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3129-3
Online ISBN: 978-1-4939-3130-9
eBook Packages: Springer Protocols