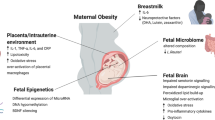
Abstract
It is perhaps not surprising that an inhospitable intrauterine environment can result in neurodevelopmental disorders, given the enormous changes in brain development that occur during gestation. Here we discuss: (1) Obesity is a state of low-grade inflammation and is thus a candidate for having an unfavorable impact on brain function in the offspring. (2) Maternal obesity has recently been associated with offspring attention deficit hyperactivity disorder and autism spectrum disorder. A recent study found differences in amniotic fluid mRNA for 20 genes in fetuses of obese versus lean women, and several of these genes impact on brain sculpting. (3) The balance between excitable and inhibitory neural function can be disturbed as a consequence of maternal obesity and can lead to hyperexcitability-linked cognitive decline later in life. (4) While most studies of brain development and function have focused on neurons, inflammation and oxidative stress have major effects on microglia and astrocytes, key cells in the sculpting of synapses, neural plasticity, and the formation of neural networks. (5) Animal models are, of necessity, widely used and the temporal trajectory of neurodevelopment to accommodate the requirements of the different species has recently been modeled. While detailed studies are essential for understanding mechanism, it is critical to test the outcomes of manipulating the system on behavior. In this regard considerable care is required to ensure that the most appropriate behavioral test and animal model are used. Thus, there is considerable scope for consolidating our understanding of the effects of maternal obesity on brain function in the offspring.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Fetal origins of neurodevelopmental disorders
- Hippocampal hyperexcitability
- Inflammation
- Astrocytes
- Microglia
- Excitatory/inhibitory balance
- Maternal obesity
1 Introduction
Optimal pregnancy outcomes rely on a supportive intrauterine environment. The population is becoming overweight and obese and this has resulted in an increase in obesity in pregnancy. Obesity is a low-grade inflammatory state, which constitutes an inhospitable environment not only for the pregnant woman but also for the fetus she is carrying. Maternal obesity contributes to complications of pregnancy, which include increased incidences of diabetes, hypertension, preeclampsia, prolonged pregnancy, and failure to progress in labor necessitating emergency caesarean delivery. A recent Australian report on 75,432 Queenslanders, over a period of just 12 years, found a correlation between maternal obesity and a twofold increase in morbidity needing neonatal intensive care and a threefold incidence in neonatal deaths [1]. In addition, offspring of obese mothers are further at increased risk of obesity in childhood and beyond, thus transmitting the problems of obesity to the next generation, condemning them to a vicious cycle. In adults, high BMI and metabolic syndrome are associated with a reduction in grey and white matter volumes [2], lower cognitive performance, and poor memory and executive function [3]. These problems are now emerging in the young (from mid-teens) [2–4]. Intellectual disability and neurodevelopmental abnormalities, e.g., reduced cognitive capacity, developmental delay, attention deficit hyperactivity disorder (ADHD), and autism spectrum disorders [5, 6] are a significant emotional burden on the individuals involved and their families, and to society in general.
2 Fetal Gene Regulation in Obese Pregnancies
For any maternal obesity effects that result in inappropriate brain architecture, neuron identity or density, then behavioral outcomes in the offspring might be anticipated. A recent study by Edlow, Bianchi, and colleagues used cell-free fetal RNA analysis of amniotic fluid during the second trimester of human pregnancy and reported significant changes in 20 genes in obese pregnancies compared with age-matched lean counterparts [7]. Genes for brain-specific apoD protein (involved in lipid metabolism and oxidative stress), presynaptic synaptotagmin, and carbonic anhydrase 11 (involved in blood–brain-barrier function and microglial activation) were significantly upregulated, whereas presynaptic cytomatrix protein was downregulated. In addition, genes involved in the control of apoptosis (e.g., anti-apoptotic Bcl 2/3) and inflammation (e.g., Bcl, canopy 3 homolog) were upregulated, while genes encoding serine/threonine kinase (STK) 24 and ATPase class VI (involved in metabolism) were downregulated compared with gene expression in the lean controls. During gestation, some neurons go through apoptosis, a process of programmed cell death, which is an important mechanism determining normal brain architecture and fetal neurodevelopment. Architectural disturbances and reduced pruning are disturbed in autism [8]. STK24 levels in pyramidal neurons are associated with epilepsy in humans and rats.
3 The Hypothalamus
The hypothalamus is the major brain region involved in the regulation of feeding. Activity is influenced by hormones, important among which are those released by peripheral organs involved in metabolic regulation, e.g., leptin produced by white adipocytes, insulin secreted by β-pancreatic cells, ghrelin released from the stomach. How the levels of these hormones change in the fetus and the effects of maternal obesity are not always clear. Also, our understanding of the timing of the development of feeding circuits remains incompletely understood. Neurons of the arcuate nucleus in the hypothalamus are critically involved in feeding circuits, and these neurons form important networks within other hypothalamic nuclei and within limbic nuclei. Leptin, transported across the blood–brain-barrier into the brain, acts on arcuate neurons to suppress feeding. This hormone also has a stimulatory effect on arcuate neuron axon growth and networking and, in adults, this effect is reversible. During a critical period of development (within the first 1–2 weeks of life in rodents), the stimulatory effects of leptin are not reversible and hence establish feeding patterns that persist for the long term [9]. Rodents have a spurt of leptin production in the first week of life, which has an irreversible effect on feeding circuitry, and this spurt is exaggerated in pups of obese dams [10]. Insulin receptors are present in hypothalamus, amygdala, cerebellum and hippocampus in fetal rodents [11]. Although the origins of insulin, peripheral or brain, remains a matter of debate, mRNA has been detected in the hypothalamus and hippocampus [12]. Insulin enhances neurite outgrowth and presynaptic activity in cerebellum and hippocampus [13]. Ghrelin stimulates neuron and astrocyte proliferation in the late-gestation hypothalamus [14]. Sculpting of neural circuits and networking involves cell death to balance proliferation and axonal growth. Thus, of importance is the report that failure of apoptosis in mice results in disruption of arcuate nucleus networking and metabolic regulation [15], and hence the changes in apoptotic mechanisms in the offspring of obese women, mentioned above, is of concern. While feeding circuits are not established in rodents until weeks 1–2 of life, they are established before birth in humans. Studies focussing on individuals that had been gestated during the Dutch Famine (1944–1945) have revealed critical windows for nutrient availability in humans. Famine in early gestation resulted in obesity in adulthood, while late gestation famine was associated with low adult fat mass and reduced insulin sensitivity.
While the central importance of the hypothalamus in the regulation of feeding control is recognized and the role of obesity during development on this system has been reviewed very recently [16], obesity and its consequences, elevated circulating glucose, lipid, hormones, e.g., leptin, insulin, ghrelin, and inflammation have effects in the brain that extend well beyond the hypothalamus. These effects include reduced motoneurone excitability in vagal circuits involved in gastrointestinal signaling of satiety, a result of a high fat diet during the perinatal period [17]. Overall, these widespread effects are hardly surprising since the control of feeding is so important to survival that it also incorporates hedonic mechanisms. The importance of the reward system and the involvement of dopaminergic mechanism in the control of feeding was demonstrated experimentally four decades ago [18] Reward systems are widespread throughout the neocortex, including prefrontal cortex, amygdala, basal ganglia, and hippocampus. The remainder of this review will focus on the effects of maternal obesity on these brain regions.
4 Hippocampus
The hippocampus is a major brain center involved in memory, learning, and decision making, and it also provides important input into emotional regulation, addiction and reward. Evidence indicates that obesity can exacerbate brain aging and thereby promote the development of cognitive alterations including dementia [19]. Hippocampal volume is negatively associated with BMI [20] and hippocampal dysfunction is likely to be a key contributor to age-associated memory impairment [21].
4.1 Hippocampal Oscillations
A striking feature of the hippocampus is its ability to generate strong oscillations in electrical activity due to powerful recurrent collateral connections, particularly in the CA3 region. Oscillations are critical for hippocampal function, with those in the gamma (~20–80 Hz) and theta (~4–10 Hz) bands being particularly important for learning and memory. Hypoactivity of the hippocampus occurs in subjects with well-established Alzheimer’s disease (AD) and is associated with impaired memory. Conversely, excessive activity can result in uncontrolled, runaway oscillations that can result in epileptic seizures. Thus the hippocampus is finely tuned by a balance between excitatory and inhibitory mechanisms.
4.2 Effects of Obesity on Seizures and Epileptic Activity
Interaction between diet and epileptic seizures has long been appreciated, and Hippocrates used fasting 2500 years ago to inhibit epileptic seizures. However, very few studies have addressed the issue of the effects of obesity on seizure activity. One of the difficulties is that changes due to obesity may be imperceptibly slow over many years or may not manifest until decades after the onset of obesity in susceptible individuals, making it difficult to link neuropathologic features with obesity [22]. Nevertheless, various studies have shown increased rates of obesity in patients with seizure disorders. Many of these associations were presumed to be secondary to the seizures due to lifestyle issues including less exercise, and the effects of antiepileptic drugs which can affect metabolism [22]. Thus, the issue of causality has been difficult to assess. For nearly a century the ketogenic diet (high fat, low carbohydrate, adequate protein) has been used for refractory epilepsy, with various ketogenic diets proven clinically effective by randomized or blinded trials [22]. Since decreased calorie intake can be beneficial for brain function, does the converse apply? Is a high calorie intake detrimental to the brain?
Significantly, in a study of children, those with newly diagnosed epilepsy had higher BMIs than controls [23]. Importantly, these children were not using antiepileptic drugs, thus ruling out an involvement of such drugs in contributing to the BMI. In adults, the incidence of seizures in overweight or obese individuals was more than double that expected for a normally distributed population [23]. A study of UK adults found a trend for a higher rate of seizures in the obese [24]. In Icelandic adults socioeconomic status (SES) was a risk factor for epilepsy, with no evidence of downward social drift [25]. Other studies indicate that the incidence of epilepsy is now higher in the elderly relative to pediatric populations, concordant with the rise of chronic diseases such as obesity, diabetes, and cerebrovascular disease [22]. Laboratory studies show that obesity is associated with enhanced kainic acid-induced seizures [22]. Thus the small number of studies supports the idea that obesity exacerbates the occurrence of seizures.
4.3 Limbic System
Hippocampal oscillations are not necessarily restricted to the hippocampus but can propagate to other components of the limbic system such as entorhinal cortex and amygdala, structures typically affected during temporal lobe epilepsy. These components of the limbic system play an important role, not only in memory but also in emotional behavior and reward, during which components interact and affect each other. Dysfunction in these systems can contribute to depression and anxiety illnesses [26, 27].
4.4 Dementia
A significant body of evidence has recently accumulated indicating that, somewhat surprisingly, hyperactivity of the hippocampus is associated with impaired learning and memory. This excessive activity occurs in the CA3/dentate gyrus region of the hippocampus [21, 28–30]. Aged rats with normal cognitive function have normal hippocampal activity, whereas aged rats with impaired learning and memory have hyperactive hippocampi [21]. Similarly, aged humans with mild cognitive impairment (MCI) also display hyperactive hippocampi [28, 31], as do hAPP mouse models of AD [32]. Indeed, it has been suggested that increased hippocampal activity may be an early indicator of AD [31]. This is consistent with observations that patients with MCI who have epilepsy, and patients with AD who have epilepsy or subclinical epileptiform activity, present with symptoms of cognitive decline ~7 years earlier than those without epilepsy or epileptiform activity [33]. In rodents and humans, suppression of hippocampal hyperactivity restored learning and memory [28, 30, 32–35]. These results indicate a critical role of hippocampal hyperactivity in underlying the deficits in learning and memory. Importantly, these results also demonstrate proof-of-principle that targeting hippocampal hyperactivity can have significant therapeutic value. Unfortunately, such approaches currently have a number of side effects with, for example, some antiepileptic medications affecting metabolism to increase obesity [22].
4.5 Mechanisms Underlying Hippocampal Hyperactivity
As discussed, hippocampal activity involves a fine balance between excitatory and inhibitory mechanisms. Thus, hyperactivity could arise from increased excitatory and/or decreased inhibitory mechanisms. Mechanisms impacting this balance can include changes in expression of ion channels and transporters involved in cellular excitability, synaptic transmission, and neurotransmitter and substrate uptake and release processes by neurons and astrocytes. Genetic mutations can underlie hippocampal hyperactivity in some epilepsies, but these are less likely to be important for obesity-induced hippocampal hyperactivity. Inhibitory interneurons are considered to be more vulnerable to dysfunction than excitatory neurons [36, 37] and to be “exquisitely sensitive” to even minor environmental perturbations [38], hence more recent studies have tended to focus on these cells. Using GAD67 as a marker of GABAergic neurons, Spiegel et al. [39] found no difference with age in CA1, but age-related decreases in CA3 and dentate gyrus. Aged rats with memory deficits and hippocampal hyperactivity had significantly reduced numbers of somatostatin-positive (SOM) interneurons in the hilar region of the dentate gyrus than young or aged unimpaired rats. There was no change in hilar NPY immunoreactivity. Loss of hilar SOM neurons in aged impaired rats is consistent with the loss of inhibition and excess dentate gyrus/CA3 activity commonly observed when memory loss occurs in aging. The same group also reported hippocampal hyperactivity and decreased expression of GABAA α5 receptor subunit in CA3 of aged rats [29]. As these receptors are involved in tonic inhibition, their loss is consistent with CA3 hyperactivity. Other changes include decreased expression of chaperone/protein folding genes, mitochondrion and oxidative phosphorylation groups, AMPAR subtypes, and the Kv4.2 channel and its interacting protein [29]. A decreased excitability of GABAergic interneurons in the hAPP mouse model of Alzheimer’s disease makes a critical contribution to abnormalities in network synchrony and memory deficiencies [35]. The defect was attributed to decreased expression of NaV1.1 sodium channels in parvalbumin interneurons.
Hippocampal synaptic plasticity and function have been comprehensively reviewed in Nature Neuroscience [26], including dorsal versus ventral hippocampus. While it is clear that excitatory neurons and inhibitory GABA interneurons are crucial for memory processing and behaviors, the relative importance of various locations, hippocampus, or nearby subiculum, amygdala, entorhinal, prefrontal, or sensory/motor cortices likely differ for different facets of memory and behavior.
4.6 Treatments That Suppress Hippocampal Hyperactivity
Treatments aimed at decreasing hippocampal hyperactivity have included the antiepileptic drugs. Levetiracetam and valproate are effective in reducing hippocampal hyperactivity and improving memory performance in aged rats [30], in the hAPP mouse model of AD [32], and in humans with mild cognitive impairment [28]. Positive allosteric modulators of GABAA α5 receptors [34], or overexpressing NPY13–36 have been successful in enhancing CA3 inhibitory activity in aged impaired rats [30]. However, many of these agents have significant side-effects and a greater understanding of mechanisms is needed to ameliorate or prevent obesity-induced hyperactivity in the brain.
5 Astroglia
Astrocytes contribute to normal neural electrical activity in a number of critical ways. They play a major role in neurotransmitter uptake, with GABA and glutamate being particularly relevant. It has been estimated that 80–90 % of extracellular glutamate is taken up by transporters located on astrocytes. In this way, astrocytes can influence extracellular levels of GABA and glutamate and thereby levels of tonic inhibition or excitation and excitotoxicity, respectively. Once taken up by astrocytes, GABA and glutamate are converted into glutamine by glutamine synthase within the glutamate–glutamine cycle and the glutamine is then transported out of the astrocytes and taken up by transporters on the glutamatergic and GABAergic neurons. The glutamine is converted into glutamate, and in GABAergic cells, on to GABA by glutamate decarboxylase (GAD). Astrocytes may also contribute to refueling the neurons by taking up glucose, converting it into lactate, and exporting the lactate to the neurons where it is used to fuel neuronal metabolic processes. These functions of astrocytes mean that any malfunctioning of astrocytes can have a major influence on neural activity in a number of ways as discussed above. Of relevance in this context, astrocytes are targets for leptin [40], insulin [41] and ghrelin [42] and these hormones have been reported to influence function of the astrocyte transporters discussed above, ion channels and to result in excessive stimulation.
5.1 Microglia
Microglia are very sensitive to perturbations by environmental challenges [43]. Microglia play an important role in learning and memory as a result of their interactions with the synapse [44], and may be involved in cognitive dysfunction associated with aging [45]. Some aspects of their function are likely to be due to their multiple fine processes that are dynamically extended and retracted, particularly in the region of synapses [45]. Microglial function is also mediated via the release of the neurotrophin BDNF (brain-derived neurotrophic factor). BDNF interacts with TrkB signaling in neurons to influence synaptic function and learning and memory [44]. Effects of microglial-derived BDNF can also include influences on inhibitory mechanisms [45, 46]. More traditionally, microglia are viewed as the primary immune responsive cells within the brain and the most likely source of pro-inflammatory cytokines [43, 45, 47]. As discussed below, an inflammatory state can have significant influences on cognitive function.
6 Inflammation
Obesity is a low-grade inflammatory state. While acute inflammation is essential for survival, chronic low-grade production of inflammatory cytokines plays a pivotal role in many diseases. Toll-like receptors (TLR) are plasma membrane receptors central to the initiation of inflammatory cascades. Nuclear factor κB (NF-κB) is disinhibited and enters the nucleus to activate production of inflammatory cytokines. NF-κB can be activated by intracellular stress (endoplasmic reticulum and oxidative stress). A number of inflammatory cytokines contribute to brain damage [48]. Cyclo-oxygenase is central to prostanoid production, important in neurovascular coupling. Nitric oxide synthase (NOS) results in NO production, toxic to glutamatergic neurons. Interleukins IL-1, IL-6 suppress neurogenesis, interfere with synaptic networking, resulting in abnormal brain electrical activity [49], and impair cerebral blood flow. Tumor necrosis factor α (TNF-α) has been linked to memory loss in adults [50]. Also, STK24, downregulated in second trimester amniotic fluid, is involved in immune defense. Inflammatory cytokines, oxidative stress, and protein nitrosylation via ongoing NO exposure have been linked to schizophrenia and depression in humans [51]. In adults, a high-fat diet is associated with inflammation in the brain and impairment of cognitive function [52, 53]. More recently, it has been concluded that obesity per se is not sufficient to precipitate neurological decline, but that it is due to pro-inflammatory mediators, particularly NOX2 (NADPH oxidase 2) [54]. Obesity and/or neuro-inflammation are associated with a range of effects on neural activity that can involve not only changes in synaptic plasticity, but also changes in the excitation/inhibitory balance that can result from effects on the astrocyte glutamate–glutamine cycle [55].
Astrocytes are sensitive to inflammation, with TLRs occurring in astrocytes [56] as well as in neurons [57]. While brief activation of TLRs can stimulate signaling that is protective, e.g., upregulation of ion channels, receptors, and calcium signaling [58], over-activation (during prolonged inflammation) stimulates NADPH oxidase and the production of reactive oxygen species (ROS) [59]. Increased ROS production [60] can also result in destabilization of the astrocyte/cerebrovascular relationship resulting in impairment of regional blood flow regulation and the blood–brain barrier. In astrocytes and neurons, ROS cause mitochondrial dysfunction, disruption of calcium homeostasis, altered function of redox-sensitive proteins, with disturbed signaling, DNA damage, and cell death (demyelination and neurodegeneration). Disruption of their function is associated with many pathologies [55]. Over-activation results in changes in astrocyte shape and rearrangement of processes servicing synapses. Thus, the support provided to neurons is reduced following activation of astrocytes [61].
An important process that is impaired in reactive astrocytes is the glutamate–glutamine cycle. This cycle is relied upon by GABAergic signaling to a much greater extent than glutamatergic synapses, which may depend more on direct uptake of glutamate into neurons and/or have a higher basal cytoplasmic reserve of this amino acid than interneurons. Consequently, dysfunction of the glutamate–glutamine cycle has deleterious effects on inhibitory before excitatory synaptic responses [55, 62]. The inhibitory deficits associated with reactive astrocytosis can be sufficient to result in hyperexcitability of hippocampal networks [55]. Astrocytosis, inflammation, and significantly reduced levels of glutamine synthetase are often prominent features in some forms of epilepsy, including temporal lobe epilepsy (TLE) in humans [47, 62, 63]. Epilepsies are characterized by recurrent, unpredictable seizures that involve hyperexcitability and hypersynchronous neuronal activity. TLE is one of the most prevalent forms of localization-related epilepsies in humans and is characterized by spontaneous recurrent seizures that involve structures such as the hippocampus, amygdala, and entorhinal cortex [62]. It has recently been suggested that the glutamate–glutamine cycle plays such an important role in brain function that diminished glutamine synthetase can cause TLE [62].
Mitochondrial dysfunction occurs in obesity [54], epilepsy [64], and AD [65], and may be caused by oxidative stress. Mitochondria can also be a significant source of oxidative stress, though this is not necessarily detrimental to cells [66]. Mitochondrial dysfunction increases with age and may be increased by some antiepileptic drugs, e.g., valproic acid, phenobarbital, carbamazepine, phenytoin [67]. Diminished mitochondrial function predisposes to disrupted calcium handling with consequences for neurotransmission and excitability, and hence with the potential to influence hippocampal excitatory/inhibitory balance.
7 Gender
The brains of females and males are not the same, with differing expression of 1680 genes in the hippocampus alone! [68] Processes involved in synaptic plasticity feature significantly in sexual dimorphism, with some differences also occurring between “male” and “female” astrocytes [69]. Prenatal stress reduces the number of differentially expressed genes to just 191 genes in hippocampus [68]. Following decades of research in this area, McCarthy has recently written that “our understanding of the how, when, and why steroids direct brain development remains rudimentary” [70]. Sex steroids, synthesized in the brain, are differentially distributed between the sexes, brain regions, and during critical developmental periods in pregnancy and early life [71], and estradiol masculinizes the brain [70]. STK24 and docosahexaenoic acid, the only omega-3 fatty acid in brain, are influenced by estrogen. The extent of neuronal dendritic arborization is determined by estrogen in some neurons [72]. In cortical and hippocampal pyramidal cells, dendritic spine number is decreased by estrogen, and the estrogen receptor β (ERβ) has been implicated [73]. ERβ activation increases GAD, the enzyme responsible for synthesis of the inhibitory transmitter GABA, and enhances astrocyte projection density [73]. ERα reduces excitatory NMDA receptor levels. These observations could explain the gender bias in early life disadvantage [74], peripubertal anxiety [75], and some neurological disorders [4].
8 Stress
As with inflammation, acute stress is essential for survival, but chronic stress plays a major role in many diseases and, although cardiovascular disease is prominent, stress has also been implicated in cancers and neurological dysfunction such as depression, some epilepsies, and post traumatic stress disorder (PTSD). The major players are cortisol/corticosterone, which act on the genome via high-affinity mineralocorticoid receptors (MR) and low-affinity glucocorticoid receptors (GR). GR and MR are highly expressed in hippocampus but some aspects of the stress response are independent of these receptors. Putative membrane-bound MR/GR may be involved but a role for noradrenaline (NA) is a possibility. Normal NA levels in the prefrontal cortex (which interconnects intimately with the hippocampus) are critical for optimal working memory, whereas high levels of NA suppress working memory, effects achieved via glutamatergic transmission [76]. Corticotrophin-releasing hormone (CRH) can also influence neural structure and function, and is a potent convulsant in infant rats [77]. The stress and inflammatory systems interact. Glia produce IL-1β, IL-6, and TNF-α in response to stressors [47, 78]. Acute stress can suppress the immune system and anxiety responses to stress can be suppressed under conditions of chronic inflammation, likely involving reduced brain-derived neurotrophic factor (BDNF) [79, 80]. A recent behavioral study has demonstrated that just 1 week of fat feeding triggered anxiety-like behaviors and impaired learning and memory in young mice post weaning, associated with decreased hippocampal and cortical BDNF and nerve growth factor (NGF). The question remains as to the situation during early development, in utero, and the mechanisms involved.
9 Some Important Methodological Considerations
9.1 Recent Developments
The presence of fetal cell-free mRNA in the mother was first described in 2000 by Lo and colleagues [81]. This technique is very powerful as it permits monitoring of gene expression in the living fetus from around the 15th week of pregnancy. In addition to informing on fetal genetic abnormalities, analysis of cell-free mRNA may also provide information on developmental processes, as in the very recent study of high BMI pregnancies by Edlow [7]. In that study new information on events in the fetal brain was provided and thus approaches such as this have powerful potential.
Most studies of brain development and function have focused on neurons. However, it is well established that microglia and astrocytes provide considerable support in the adult brain [82]. Microglia defend the brain under attack and their processes continuously scan, removing debris and dead cells. They also sculpt synapses, augmenting or reducing both pre- and post-synaptic elements eg terminals, dendritic spines. Inflammation activates glia, and obesity is a pro-inflammatory state. It has recently been demonstrated that microglial progenitors present in the yolk sac invade the developing brain in a narrow time-window, via the meninges and ventricles at E6.5 and via the early establishment of the cerebral vasculature at E7.2-7.5 in mice [83]. In human fetuses, invasion via the meninges and ventricles occurs around week 5 [84] and intravascular influx occurs during week 10 [85]. Maternal obesity predisposes to autism spectrum disorders (ASD) in the offspring [5], and a greater density of activated microglia has been reported in ASD [86]. A mechanistic link requires investigation.
While the housekeeping role of astrocytes at synapses is well accepted, the possibility that astrocytes may also influence synaptic efficiency has been suggested [87], and even release of “neurotransmitter” has been mooted very recently [88, 89]. This possibility of a direct role for astrocytes in synaptic transmission is currently under vigorous debate [90, 91]. Calcium plays a major role in astrocyte function and approaches to recording changes in astrocyte calcium levels with a view to shedding greater light on this debate has been critically reviewed [92]. The role of astrocytes, or not, in the establishment of neural networks during development requires study, as is their potential involvement in attention deficit hyperactivity disorder and ASD.
9.2 Methodological Integration
Translation of knowledge to prevent or ameliorate human disease is a major aim of biomedical research and an important step in achieving this is to perform studies in animal models using approaches that can also be tested in human subjects. So, while in vitro slice electrophysiological techniques can be invaluable in providing insights into complex mechanisms at a significant level of detail, and the potential of this approach can be enhanced by the use of genetically modified animals, knock-down of signalling agents using transfection methods, the use of siRNA, and optogenetic technologies, integrating observations from these approaches with prior behavioural testing of the intact animal is critical for a more complete understanding of potential therapeutic strategies. Considerable care is required so that the most appropriate behavioural tests are used and considerations and pitfalls have been thoroughly reviewed and require careful attention [93, 94].
Combining detailed mechanistic studies with prior imaging technologies may also provide more tangible links towards translation. Magnetic resonance imaging (MRI) is widely used in clinical practice and has also been used in non-human studies. While humans remain fully conscious during MRI testing, laboratory species invariably require anaesthesia in order to prevent movement, which would invalidate measurements. MRI detects changes in blood oxygen (deoxyhaemoglobin levels) or changes in cerebral blood volume and all anaesthetic agents available influence basal blood flow and cerebral volume [95]. Furthermore, available anaesthetics alter the responsiveness and/or sensitivity to most neurotransmitters in the brain [95], confounding this method of enquiry. Attempts have been made to circumvent these problems with non-human MRI measurements. Restraint has been used but this introduces stress which, on its own, has repercussions on brain activity (see above). Habituation to restraint does not obviate this problem [96]. In a very elegant study, Scott and colleagues trained rats to place their heads voluntarily into a headport for 8 seconds which permitted quick clamping of the head in a precise location and recording of changes in intracellular free calcium of neurons in layers 2-5 of the cortex, using a two-photon laser-scanning microscopy [97]. Further development of this system will likely provide much-needed insights into cell signalling in intact, conscious experimental animals.
9.3 Animal Models
While epidemiological studies that report on the association between maternal obesity and outcomes in the offspring in human populations are critically important, more precise manipulation of the system to directly address cause-and-effect requires the use of non-human animal models. Brain development does not occur in a regular homogeneous progression. Rather, specific developmental events can occur rapidly or have specific requirements such as to give rise to sensitive, critical windows of vulnerability. Less than optimal conditions in the critical window can result in long-lasting dysfunction and may underpin neurobehavioral deficits after birth [98]. As a result, one of the first considerations when choosing a non-human animal model is the issue of its critical window compared with that in humans for the neurobehavioral condition of interest. Dobbing and Sands [99] provided a detailed description of the spurt in brain growth in six most commonly-used laboratory species. However, the relationship between this brain growth spurt and a critical window is not clear. Some light has been thrown on this issue in a very elegant recent study by Workman and colleagues [100]. They generated a model of neural development in 18 species, including human, macaque, several rodent species, sheep, cat and several marsupials. The model included brain stem, cerebellum, limbic system, thalamus, striatum, cortex, sensory peripheral system and retina, and these regions were further divided. Developmental events such as neurogenesis, axon extension, network formation, myelination, brain volume, behavioural milestones were also provided. This repository will be invaluable when choosing the most appropriate model when interrogating the mechanisms underpinning specific neurobehavioral problems and should result in faster translation from bench to bedside.
10 In Conclusion
Maternal obesity appears to have wide ranging effects across a broad range of brain regions and functions in the offspring. These effects can include increases in the propensity for the offspring to be obese through effects on circuitry controlling feeding and metabolism, thereby reinforcing the obesity epidemic. A range of neural disorders are likely to be exacerbated by maternal obesity, with long term sequelae for the individuals affected and those around them. Cognition is a major brain function that may be negatively impacted by maternal obesity and this may contribute to enhanced dementia in the affected individuals in later life. However, research into the effects of maternal obesity on brain function in the offspring, other than in the hypothalamus, is very much in its infancy. There is enormous scope for consolidating current concepts, and for studies to determine in greater depth and breadth the effects of maternal obesity and the underlying mechanisms in brain function of the offspring.
References
McIntyre HD et al (2012) Overweight and obesity in Australian mothers: epidemic or endemic? Med J Aust 196:184–188
Gunstad J et al (2008) Relationship between body mass index and brain volume in healthy adults. Int J Neurosci 118:1582–1593
Cohen RA (2010) Obesity-associated cognitive decline: excess weight affects more than the waistline. Neuroepidemiology 34:230–231
AP Association (2000) Diagnostic and statistical manual of mental disorders. APA, Washington, DC, Revised 4th edition
Krakowiak P et al (2012) Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129:e1121–e1128
Sullivan EL et al (2014) Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol Behav 123:236–242
Edlow AG et al (2014) Maternal obesity affects fetal neurodevelopmental and metabolic gene expression: a pilot study. PLoS One 9:e88661
Maximo JO et al (2014) The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev 24:16
Bouret SG et al (2004) Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110
Kirk SL et al (2009) Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One 4:e5870
Kar S et al (1993) Quantitative autoradiographic localization of [125I]insulin-like growth factor I, [125I]insulin-like growth factor II, and [125I]insulin receptor binding sites in developing and adult rat brain. J Comp Neurol 333:375–397
Blazquez E et al (2014) Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol 5:161
Heidenreich KA et al (1989) Insulin receptors mediate growth effects in cultured fetal neurons. II. Activation of a protein kinase that phosphorylates ribosomal protein S6. Endocrinology 125:1458–1463
Inoue Y et al (2010) Transitional change in rat fetal cell proliferation in response to ghrelin and des-acyl ghrelin during the last stage of pregnancy. Biochem Biophys Res Commun 393:455–460
Coupe B et al (2012) Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab 15:247–255
Bouret S et al (2015) Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev 95:47–82
Bhagat R et al (2015) Exposure to a high fat diet during the perinatal period alters vagal motoneurone excitability, even in the absence of obesity. J Physiol 593:285–303
Zis AP et al (1975) Neuroleptic-induced deficits in food and water regulation: similarities to the lateral hypothalamic syndrome. Psychopharmacologia 43:63–68
Uranga RM et al (2010) Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J Neurochem 114:344–361
Convit A et al (2003) Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A 100:2019–2022
Wilson IA et al (2006) Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci 29:662–670
Lee EB et al (2014) The neuropathology of obesity: insights from human disease. Acta Neuropathol 127:3–28
Daniels ZSB et al (2009) Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology 73:658–664
Gao S et al (2008) The incidence rate of seizures in relation to BMI in UK adults. Obesity 16:2126–2132
Hesdorffer DC et al (2005) Socioeconomic status is a risk factor for epilepsy in Icelandic adults but not in children. Epilepsia 46:1297–1303
Bannerman DM et al (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci 15:181–192
Stoop R et al (2000) Functional connections and epileptic spread between hippocampus, entorhinal cortex and amygdala in a modified horizontal slice preparation of the rat brain. Eur J Neurosci 12:3651–3663
Bakker A et al (2012) Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74:467–474
Haberman RP et al (2011) Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging 32:1678–1692
Koh MT et al (2010) Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology 35:1016–1025
Putcha D et al (2011) Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s Disease signature regions in non-demented elderly adults. J Neurosci 31:17680–17688
Sanchez PE et al (2012) Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A 109:E2895–E2903
Vossel KA et al (2013) Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70:1158–1166
Koh MT et al (2013) Selective GABAA a5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology 64:145–152
Verret L et al (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149:708–721
Luhmann HJ et al (1992) Hypoxia-induced functional alterations in adult rat neocortex. J Neurophysiol 67:798–811
Perez Velazquez JL et al (2007) Typical versus atypical absence seizures: network mechanisms of the spread of paroxysms. Epilepsia 48:1585–1593
Hsu F-C et al (2003) Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A 100:12213–12218
Spiegel AM et al (2013) Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol 521:3508–3523
Spanswick D et al (1997) Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390:521–525
Spanswick D et al (2000) Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3:757–758
Briggs DI et al (2014) Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology 155:2411–2422
Walker FR et al (2013) Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets 14:1262–1276
Parkhurst CN et al (2013) Microglia promote learning-dependent synapse formation through Brain-Derived Neurotrophic Factor. Cell 155:1596–1609
Wake H et al (2012) Physiological function of microglia. Neuron Glia Biol 7:1–3
Zheng K et al (2011) TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci U S A 108:17201–17206
Devinsky O et al (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174–184
Tornatore L et al (2012) The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol 22:557–566
Green HF et al (2012) Unlocking mechanisms in interleukin-1b-induced changes in hippocampal neurogenesis -a role for GSK-3b and TLX. Transl Psychiatr 2:e194
Ganz PA et al (2012) Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun 30(Suppl):S99–S108
Anderson G et al (2013) Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog Neuropsychopharmacol Biol Psychiatry 42:101
Beilharz JE et al (2014) Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun 37:134–141
Pistell PJ et al (2010) Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 219:25–32
Pepping JK et al (2013) NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am J Physiol Endocrinol Metab 304:E392–E404
Ortinski PI et al (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13:584–U93
Elovitz MA et al (2006) Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res 59:50–55
Tang SC et al (2007) Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A 104:13798–13803
Kushnir R et al (2011) Peripheral inflammation upregulates P2X receptor expression in satellite glial cells of mouse trigeminal ganglia: a calcium imaging study. Neuropharmacology 61:739–746
Block ML et al (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Ma D et al (2013) The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J Neuroimmunol 254:10–18
Steele ML et al (2012) Reactive astrocytes give neurons less support: implications for Alzheimer’s disease. Neurobiol Aging 33(423):e1–e13
Coulter DA et al (2012) Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 60:1215–1226
Wetherington J et al (2008) Astrocytes in the epileptic brain. Neuron 58:168–178
Khurana DS et al (2013) Mitochondrial dysfunction in epilepsy. Semin Pediatr Neurol 20:176–187
Wang X et al (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 1842:1240
Accardi MV et al (2014) Mitochondrial reactive oxygen species regulate the strength of inhibitory GABA-mediated synaptic transmission. Nat Commun 5(3168):1–12
Finsterer J et al (2012) Mitochondrial toxicity of antiepileptic drugs and their tolerability in mitochondrial disorders. Expert Opin Drug Metab Toxicol 8:71–79
Biala YN et al (2011) Prenatal stress diminishes gender differences in behavior and in expression of hippocampal synaptic genes and proteins in rats. Hippocampus 21:1114–1125
Liu M et al (2007) Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab 27:135–141
McCarthy MM (2013) Sexual differentiation of the brain in man and animals. Am J Med Genet C Semin Med Genet 163:3–15
Konkle AT et al (2011) Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152:223–235
Amateau SK et al (2002) A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci 22:8586–8596
Tan XJ et al (2012) Reduction of dendritic spines and elevation of GABAergic signaling in the brains of mice treated with an estrogen receptor beta ligand. Proc Natl Acad Sci U S A 109:1708–1712
Oomen CA et al (2009) Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One 4:e3675
Hayward C et al (2002) Puberty and the emergence of gender differences in psychopathology. J Adolesc Health 30:49–58
Zhang Z et al (2013) Norepinephrine drives persistent activity in prefrontal cortex via synergistic alpha1 and alpha2 adrenoceptors. PLoS One 8:e66122
Baram TZ et al (1991) Corticotropin-releasing hormone is a rapid and potent convulsant in the infant rat. Brain Res Dev Brain Res 61:97–101
Cowley TR et al (2012) Rosiglitazone attenuates the age-related changes in astrocytosis and the deficit in LTP. Neurobiol Aging 33:162–175
Clark SM et al (2014) Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain Behav Immun 38:192
Shiraev T et al (2009) Differential effects of restricted versus unlimited high-fat feeding in rats on fat mass, plasma hormones and brain appetite regulators. J Neuroendocrinol 21:602–609
Poon LL et al (2000) Presence of fetal RNA in maternal plasma. Clin Chem 46:1832–1834
Bilimoria PM et al (2014) Microglia function during brain development: new insights from animal models. Brain Res 1617:7–17
Ginhoux F et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845
Monier A et al (2007) Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol 66:372–382
Verney C et al (2010) Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat 217:436–448
Morgan JT et al (2010) Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 68:368–376
Tasker JG et al (2012) Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol 24:566–576
Rusakov DA (2012) Astroglial glutamate transporters trigger glutaminergic gliotransmission. J Physiol 590:2187–2188
Rusakov DA (2012) Depletion of extracellular Ca(2)(+) prompts astroglia to moderate synaptic network activity. Science signaling 5:pe4
Hamilton NB et al (2010) Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11:227–238
Smith K (2010) Neuroscience: settling the great glia debate. Nature 468:160–162
Khakh BS et al (2015) Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol 7(4):a020404
Alleva E et al (2000) Important hints in behavioural teratology of rodents. Curr Pharm Des 6:99–126
Ohl F et al (2003) Behavioural screening in mutagenised mice—in search for novel animal models of psychiatric disorders. Eur J Pharmacol 480:219–228
Haensel JX et al (2015) A systematic review of physiological methods in rodent pharmacological MRI studies. Psychopharmacology (Berl) 232:489–499
Reed MD et al (2013) Behavioral effects of acclimatization to restraint protocol used for awake animal imaging. J Neurosci Methods 217:63–66
Scott BB et al (2013) Cellular resolution functional imaging in behaving rats using voluntary head restraint. Neuron 80:371–384
Meredith RM (2014) Sensitive and critical periods during neurotypical and aberrant neurodevelopment: a framework for neurodevelopmental disorders. Neurosci Biobehav Rev 50:180–188
Dobbing J et al (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3:79–83
Workman AD et al (2013) Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33:7368–7383
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Coleman, H.A., Parkington, H.C. (2016). Maternal Obesity in Pregnancy: Consequences for Brain Function in the Offspring. In: Walker, D. (eds) Prenatal and Postnatal Determinants of Development. Neuromethods, vol 109. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3014-2_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3014-2_10
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3013-5
Online ISBN: 978-1-4939-3014-2
eBook Packages: Springer Protocols