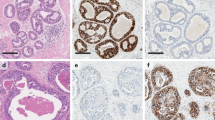
Abstract
Ductal carcinoma in situ (DCIS) of the breast consists of a group of heterogeneous and pre-invasive proliferation of neoplastic epithelial cells with the ductal phenotype. DCIS is one of the most frequently diagnosed pathologic entities of the breast, comprising approximately 25 % of all newly discovered breast carcinoma cases. The incidence of DCIS in United States has increased from 1.87 per 100,000 in 1973–1975 to 32.5 in 2004, reflecting in part the success of the widely adopted mammographic screening programs. With increased detection of DCIS, however, questions regarding appropriate risk assessment and therapeutic interventions have been raised, as only limited information on the natural biologic progression of untreated tumors exists. Few long-term follow-up studies available on untreated low-grade DCIS show the risk of developing invasive breast carcinoma ranges from 14 to 60 % after 10 years. Similar studies on high-grade (HG) DCIS are virtually nonexistent as most were excised at time of diagnosis, but it is reasonable to extrapolate that untreated HG-DCIS will be associated with even higher risks of invasive disease. Considering DCIS generally has an excellent prognosis after lumpectomy or mastectomy with 10-year breast cancer mortality rate at <2 %, the rationale for continuing the current standard of treatment certainly holds water. However, these statistics also demonstrate that not all DCIS invariably progress to invasive disease and as of yet, our current systems of risk stratification are inadequate in identifying those that may benefit from less or more aggressive forms of intervention. Concerns about unnecessary anxiety experienced by the patients and possible over-treatment of DCIS have precipitated a search for improved diagnostic and prognostic parameters, and has even led to proposals for reclassification of these tumors with less ominous terminology such as “intraepithelial neoplasia.” Recent developments in our understanding of the pathogenesis of invasive breast carcinoma has led to newly defined molecular subtypes with varying prognoses and has opened the door to more targeted therapies. Although the literature on DCIS is not as extensive, emerging data suggests a similar molecular classification system may be applicable to the in situ lesions as well. In this chapter, we review the current understanding of DCIS with emphasis on its molecular pathogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Ductal carcinoma in situ (DCIS) of the breast consists of a group of heterogeneous and pre-invasive proliferation of neoplastic epithelial cells with the ductal phenotype. DCIS is one of the most frequently diagnosed pathologic entities of the breast, comprising approximately 25 % of all newly discovered breast carcinoma cases [1]. The incidence of DCIS in United States has increased from 1.87 per 100,000 in 1973–1975 to 32.5 in 2004 [2], reflecting in part the success of the widely adopted mammographic screening programs. With increased detection of DCIS, however, questions regarding appropriate risk assessment and therapeutic interventions have been raised, as only limited information on the natural biologic progression of untreated tumors exists.
Few long-term follow-up studies available on untreated low-grade (LG) DCIS show the risk of developing invasive breast carcinoma ranges from 14 to 60 % [3–5] after 10 years. Similar studies on high-grade (HG) DCIS are virtually nonexistent as most were excised at time of diagnosis, but it is reasonable to extrapolate that untreated HG-DCIS will be associated with even higher risks of invasive disease. Considering DCIS generally has an excellent prognosis after lumpectomy or mastectomy with 10-year breast cancer mortality rate at <2 % [6], the rationale for continuing the current standard of treatment certainly holds water. However, these statistics also demonstrate that not all DCIS invariably progress to invasive disease and as of yet, our current systems of risk stratification are inadequate in identifying those that may benefit from less or more aggressive forms of intervention.
Concerns about unnecessary anxiety experienced by the patients and possible over-treatment of DCIS have precipitated a search for improved diagnostic and prognostic parameters, and has even led to proposals for reclassification of these tumors with less ominous terminology such as “intraepithelial neoplasia” [7–10]. Recent developments in our understanding of the pathogenesis of invasive breast carcinoma has led to newly defined molecular subtypes with varying prognoses and has opened the door to more targeted therapies [11]. Although the literature on DCIS is not as extensive, emerging data suggests a similar molecular classification system may be applicable to the in situ lesions as well. In this chapter, we review the current understanding of DCIS with emphasis on its molecular pathogenesis.
Diagnosis, Classification, and Prognosis of DCIS
Diagnosis
Diagnosis of DCIS relies on several clinical and pathologic findings. Historically, DCIS was usually discovered when a tissue biopsy was performed for findings such as a palpable mass, skin retraction, or nipple discharge. Now, with the advent of the screening programs, the vast majority of DCIS are diagnosed with the mammographic discovery of clinically occult microcalcifications (76 %), soft-tissue densities (11 %), or both (13 %) [12]. Once diagnosed, the radiologically identified regions are excised with breast conserving surgery and the specimen is evaluated for extent of disease, concurrent invasive carcinoma, margins and hormone receptor status.
Microscopically, the diagnosis of DCIS is predicated on identification of clonal population of ductal epithelial cells confined within the boundary delineated by the myoepithelial cells and the basement membrane. Important diagnostic considerations for the pathologist include ruling out invasive breast carcinoma and differentiating DCIS from benign epithelial proliferations and other pre-malignant entities such as lobular carcinoma in situ (LCIS).
Compared to more benign lesions such as usual ductal hyperplasia (UDH), DCIS is comprised of a single, uniform epithelial population without the intermingling of spindled myoepithelial cells. Breast lesions with morphologic features suggestive of, but not diagnostic of DCIS are classified as atypical ductal hyperplasia (ADH). ADH essentially shares the cytologic features of DCIS, but importantly, lacks HG nuclear features and should not exceed 2 duct spaces or 2 mm. It is generally recognized that ADH and DCIS are both neoplastic proliferations with shared evolutionary pathway and the distinction between them can be quite subjective.
Classification
Several classification schemes to accurately stratify DCIS have been proposed over the years. Historically, DCIS was divided into architectural subtypes such as solid, cribriform, papillary, micropapillary, and clinging. These architectural features are still recognized and noted in pathologic reports; however, evidence has shown architectural subtyping to be of little clinical import and subject to considerable interobservor variability. Because of the insufficiencies of the architectural system, revised schemes (e.g., Holland classification [13]) focusing primarily on the cytonuclear features of the tumor cells were introduced, and found to be superior in reproducibility [14]. Van Nuys classification on the other hand, is a simplified system that categorizes DCIS based on the presence or absence of comedonecrosis and low- or HG nuclear features. Van Nuys, like the Holland classification, has demonstrated reproducibility [14] and has also been shown to be predictive of local recurrence rates and disease-free survival [15, 16]. The newly introduced concept of “ductal intraepithelial neoplasia,” proposed to alleviate patient anxiety and possibly reduce over zealous treatments, has yet to gain traction in general practice. Currently, most pathologists rely on a combination of features including nuclear grade, presence of comedonecrosis, and architectural pattern to evaluate and classify DCIS. Features characteristic of each histologic grade is summarized in Fig. 6.1.
It should be noted that while interobservor variability in the classification of DCIS has improved significantly since the adoption of the newer systems, it is far from being completely eradicated. Nuclear grading is still a subjective interpretation and even the exact definition of comedonecrosis is under debate.
Prognosis
Despite the lack of extensive data on the natural progression of untreated DCIS, several large randomized clinical trials and cohort studies have identified few independent clinical and pathologic features associated with risk of disease recurrence or progression. Some of the factors associated with higher rates of local recurrence were younger age (≤40 years old), older age group (≥50 years old), symptomatic detection of DCIS, higher nuclear grade, solid or cribriform growth pattern, comedonecrosis, uncertain or involved margins or treatment with local excision alone [17–19].
Treatment modality has been shown to have significant influence on the recurrence rate, if not the overall survival. Until recently, mastectomy was the conventional treatment of DCIS [20]; however, with the success of breast conserving surgery/lumpectomy in invasive cancer, this conservative approach has been extended to DCIS as well. No randomized clinical studies comparing the efficacy of these two surgical options are currently available. On the other hand, radiotherapy (RT) has been shown to significantly decrease the rate of disease recurrence in clinical trials [17, 18, 21–26]. After lumpectomy alone, the risk of contralateral or ipsilateral disease recurrence ranges from 14 to 32 %, which is reduced by 40–50 % when paired with RT [17, 18, 21–26]. However, because RT does not seem to influence the overall survival rate, there is still a lack of consensus on the appropriate use of adjunct RT.
The use of improved DCIS classification, along with the identification of these risk factors has led to the development of prognostic systems such as the Van Nuys prognostic index (VNPI). The updated USC/VNPI stratifies DCIS patients according to age, size of the lesion, nuclear grade, and margin status and suggests differential treatment options according to the VPNI score [27, 28]. Although VPNI has been shown to be useful in a number of retrospective studies, it is yet to be validated in a prospective trial.
The current treatment protocol according to NCCN guidelines suggests lumpectomy ± radiation or mastectomy ± sentinel node biopsy. It was revised in 2008 to include lumpectomy alone as an option for those individuals with “low” risk, but does not specifically define that subset of patients. The guideline also recommends post-surgical treatment with tamoxifen in ER-positive DCIS, but does recognize that tamoxifen, like RT, reduces the risk of recurrence without improvement in overall survival rate [21, 25, 29]. The current guidelines demonstrate that despite the identification of several risk factors that are associated with higher disease recurrence, no systematically applied differential treatment protocols are currently in place for DCIS subtypes.
Tumorigenesis of DCIS
Several tumorigenesis pathways for DCIS have been proposed over the years. One model, first described by Wellings and colleagues in the 1970s, suggested flat epithelial atypia (FEA), ADH and DCIS as non-obligate precursor lesions to invasive ductal tumors [30–32]. Wellings further proposed that these ductal lesions, as well as lobular pre-malignant lesions, share a common progenitor in the terminal duct-lobular units (TDLUs) of the breast. Epidemiological, morphological, immunohistochemical and now molecular studies support this theory of evolutionary continuum between FEA, ADH, DCIS, and invasive ductal carcinoma (IDC)s, which is further detailed in the following section.
An alternative theory integrated benign epithelial proliferations such as UDH into this scheme, proposing progressive de-differentiation of UDH into malignancy [33]. Recent immunohistochemical and molecular studies however, have failed to demonstrate a clear relationship between UDH and other premalignant lesions. Rather, UDH appears to be more closely related to normal, non-proliferative breast epithelium and likely represents a distinct clinical entity unrelated to the pre-malignant lesions of the breast [34, 35].
The prevailing model of breast cancer progression has further refined Wellings’ original theory and now recognizes divergent pathways for low-and HG DCIS. First discovered in IDCs, it is now recognized that the same recurrent but differential molecular changes are largely recapitulated in the in situ lesions as well. For example, loss of 16q, the hallmark chromosomal abnormality of low-grade invasive carcinoma, is also observed in greater than 70 % of LG DCIS. In contrast, 16q loss is observed in only 30 % of HG DCIS. In addition, it has been recognized that low-grade DCIS are largely ER positive, whereas only a subset of the HG lesions express the hormone receptor. Furthermore, those HG DCIS that are ER positive tend to harbor the same chromosomal abnormalities typically associated with low-grade lesions. These findings, among others, suggest that while at least two distinct carcinogenetic pathways may exist, a subset of HG DCIS may indeed represent low-grade lesions that have progressively de-differentiated and possible points of intersection can be observed among the several breast cancer pathways (Fig. 6.2).
Divergent pathways of low and high-grade breast cancer. LG pathway is characterized by positivity for ER/PR, Bcl-2 and low Ki-67 index. Chromosomes tend to be diploid or near-diploid with recurrent changes such as loss of 16q or gains of 1q or 16p. FEA and ADH are thought to be precursor lesions of the low-grade pathway and share similar expression of biomarkers and chromosomal abnormalities. Luminal A DCIS is the predominant molecular subtype seen in the low-grade pathway. HG pathway is characterized by negativity for ER/PR, positivity for p53 and high Ki-67 index, producing tumors with TN/basal-like or HER 2+ phenotype. These tumors also are frequently aneuploid and/or exhibit complex karyotype. MGA has been proposed as a possible precursor lesion for high-grade lesions with TN/basal-like phenotype. Overlap also exists between the LG and HG pathways. Some IG and HG DCIS show molecular features of both low and high-grade lesions, and may represent de-differentiated lesions of the LG pathway. (Figure adapted with permission from [36])
ER Estrogen receptor; PR progesterone receptor; LG low-grade; IG intermediate grade; HG high-grade; FEA flat epithelial atypia; ADH atypical ductal hyperplasia; TN triple negative; MGA microglandular adenosis; DCIS ductal carcinoma in situ; IDC invasive ductal carcinoma
FEA and ADH, in keeping with the theory of a common evolutionary pathway, share many of the immunohistochemical and molecular signatures of low-grade DCIS. Like the low-grade DCIS, FEA and ADH are generally positive for ER and PR but negative for HER 2 and basal cell markers. They have also been shown to share many of the recurrent genetic imbalances (e.g., loss of 16q) and are often found in coexistence with low-grade DCIS and invasive carcinomas. These immunophenotypic, molecular, and epidemiologic evidence demonstrates the close developmental relationship among these low-grade lesions and provide strong evidence that FEA and ADH are non-obligate, neoplastic precursors of the low-grade cancerous lesions of the breast.
It is yet unclear, however, what the precursor lesion of HG DCIS may be. The complex karyotype of HG DCIS intimates both the inherent genetic volatility of these lesions and the heterogeneity of its origin. A minority of the HG DCIS that harbor a similar genomic profile to the low-grade DCIS may represent de-differentiated lesions, while others may have arisen de novo. There exists, however, recent but limited evidence showing that a subset of microglandular adenosis (MGA) may be a precursor to triple negative (ER, PR, and HER2 negative) HG DCIS [36, 37]. MGA is a rare breast lesion composed of cytologically bland glands with an infiltrative growth pattern, largely considered to be a benign process. Its rarity however, in comparison to the incidence of HG DCIS, makes it an unlikely candidate as a common progenitor for HG lesions of the breast.
Chromosomal Aberrations of Low-and High-Grade DCIS
Low and HG DCIS, like their invasive counterparts, are characterized by distinct set of chromosomal aberrations. One of the hallmark chromosomal abnormalities seen in low-grade DCIS, as mentioned before, is the loss of 16q (70 %), as evidenced by multiple comparative genomic hybridization (CGH) and loss of heterozygosity (LOH) studies [38–40]. Other recurrent abnormalities associated with low-grade DCIS include loss of 17p and gain of 1q (>70 %) and 16p (>40 %). In addition, low-grade DCIS is characterized by diploid or near-diploid chromosome number and on average, have fewer total chromosomal abnormalities.
In contrast, HG lesions exhibit greater tendencies for aneuploidy, more complex karyotype and generally harbor multiple amplifications. Some of the specific and more frequently observed chromosomal abnormalities of HG DCIS include gains of 1q, 5p, 8q and losses of 8p, 11q, 13q, and 14q. The genomic profile of intermediate-grade DCIS, much like the nuclear and cytologic features that currently define the DCIS grading system, straddle the boundaries of the low-and HG lesions. Although intermediate-grade DCIS shared some of the distinct genetic signatures with the low-grade lesions, one study also found they had on average, higher number of genetic imbalances (5.5 vs. 2.5) compared to low-grade DCIS [38]. Table 6.1 is a detailed list of the recurrent genomic changes seen in low- and HG DCIS, as well as other proliferative breast lesions.
Immunophenotype of Low-and High-Grade DCIS
Immunohistochemical (IHC) studies of the transcriptomic profiles of DCIS also support the theory of divergent tumorigenesis. DCIS, like their invasive counterpart, can be divided into broad categories based on estrogen receptor (ER) positivity. ER is one of the most valuable and extensively studied biomarkers in the breast and is expressed in approximately 70 % of DCIS overall [41]. ER expression is strongly associated with low-grade in situ and invasive ductal lesions, with nearly 100 % of the low-grade DCIS expressing the hormone receptor. Molecular studies of IDC have also shown ER-positive and ER-negative tumors are intrinsically distinct entities with divergent pathologic and clinical features. ER expression, along with the presence of HER2 upregulation, is the major determinant in molecular classification of IDC.
Other biomarkers preferentially expressed in low-grade DCIS also include progesterone receptor (PR) and Bcl-2. PR, like ER, is a hormone receptor that is prognostic as well as predictive of response to hormone therapy [41]. Bcl-2 is an anti-apoptotic protein whose de-regulation has been associated with pathogenesis of breast cancer. The expressions of both proteins are positively associated with ER, and help define the immunoprofile of the low-grade ductal lesions of the breast.
Conversely, higher-grade lesions are negatively associated with ER, positively associated with [42–44] HER2 expression, p53 expression, and basal markers (CK5/6, EGFR) and display higher Ki-67 index. Somewhat paradoxically, HER2 amplification, which is typically associated with worse clinical outcome in invasive tumors, is seen with higher frequency in the in situ lesions (15–25 vs. 55–70 %). The reason for this disparity remains unclear, however. Some of the proposed mechanisms include: loss of HER2 expression as HER2-positive DCIS progresses to IDC; higher rates of disease progression in HER2-negative DCIS; and mammographic detection bias for HER2-positive DCIS due to their association with comedo necrosis and calcification, which may be more easily identified by imaging. Table 6.2 summarizes the expression rate of various biomarkers stratified by DCIS histologic grades.
Molecular Subtyping of DCIS
Microarray profiling of invasive breast carcinomas in the early 2000s introduced a novel classification method into at least four major intrinsic molecular subtypes with variable clinical outcomes: luminal A, luminal B, human epidermal growth factor receptor-2 (HER2) overexpressing and TN/basal-like (Table 6.3) [45]. Studies have shown the intrinsic molecular subtypes can be approximated with a panel of immunohistochemical markers, most commonly including ER, PR, HER2, CK5/6, and EGFR [46]. Similar categorization of DCIS has been explored in several studies [47–50], which were largely successful in recapitulating the molecular subtypes found in invasive tumors.
Several differences in the prevalence of the distinct molecular phenotypes between the in situ and invasive ductal lesions were noted in these studies. HER2 subtype was consistently shown to be more prevalent in DCIS (14–17 %) compared to IDC (3–6 %), as previously discussed. On the other hand, luminal type A was generally less common in DCIS (38–63 %) compared to IDC (58–75 %). Overall, no statistically significant difference was noted between the prevalence of luminal type B and TN/basal phenotypes, although the TN/basal phenotype was generally less common in DCIS.
Although the limited number of studies should preclude premature generalizations of the DCIS molecular subtypes, one study showed TN/basal-like phenotype to be associated with elevated risk of disease recurrence at 10 years [50], as well as being associated with other unfavorable prognostic variables such as high-grade nuclei, p53 expression, and elevated Ki-67 index [47, 48].
Molecular Features of DCIS Versus IDC
DCIS is generally recognized as a non-obligate precursor lesion to IDC due to a multitude of indirect but convincing evidence. Tissue resections of IDC nearly invariably show concurrent DCIS, usually of similar nuclear grade, helping demonstrate a close relationship between the two lesions and suggestive of a shared evolutionary pathway. However, contrary to expectations, global gene expression studies have shown no significant differences in molecular changes between invasive and in situ carcinomas [38], suggesting that the potential for invasiveness already resides in the mutations that first gave rise to the in situ neoplastic proliferations.
In light of this failure to find the specific genetic signatures that define invasiveness, several other mechanisms have been proposed. One theory suggests that epigenetic alterations regulating the expression of various genes may be contributory. Few studies have demonstrated stage-specific methylation of tumor suppressor genes in the tumor cells [51, 52], suggesting a possible role in the disease progression. On the other hand, others have shown that the changes in the microenvironment of the tumor may also be instrumental. Studies have shown that similar to the epithelial tumor cells that exhibit differential epigenetic gene regulation, the surrounding stromal cells and myoepithelial cells also show significant changes in gene expression during the transition from in situ to invasive carcinoma [53]. It has been suggested that these phenotypically aberrant stromal and myoepithelial cells, having lost their normal function, may facilitate invasion by creating a more permissive environment for the tumor cells.
It is likely that the progression of the in situ to IDC involves a complex set of changes including the intrinsic genetic abnormalities of the tumor, epigenetic de-regulation of the tumor/stromal/myoepithelial cells’ gene expression and other as-of-yet undefined deviations from the norm.
Future of Molecular Testing in DCIS
Recent advancements in molecular methodologies have allowed the emergence of multiple RNA-and DNA-based commercial tests to categorize breast carcinomas into prognostically significant subgroups. Tests such as Oncotype Dx® and Mammaprint® are RNA-or DNA-based assays used to evaluate the expression of key genes involved in cell proliferation, invasion, hormone receptors, HER-2, and other house keeping genes. Oncotype DX for example, is an assay performed using quantitative RT-PCR on formalin-fixed, paraffin-embedded samples to generate Recurrence Score® (RS) to categorize the tumors into three prognostic categories. For early stage invasive tumors, these molecular assays have become widely accepted as ancillary tests to help identify those patients that may benefit from adjuvant chemotherapy.
More recently in 2011, a validation study using ECOG E5194 dataset showed these assays might also be applicable to in situ tumors as well [54, 55]. Oncotype DX assays on DCIS showed that similar to invasive tumors, the risk of ipslateral breast event (IBE) was significantly increased in those with higher RS. Low, intermediate, and high-risk groups within this study had 10-year risk of IBE of 10.6, 26.7, and 25.9 % respectively, and 3.7, 12.3, and 19.2 % risk of invasive IBE (both log rank P ≤ 0.006) [55]. These results indicate that DCIS can be stratified into meaningful prognostic subgroups using this tool and we may be one step closer to identifying those patients in the “low-risk” category mentioned, but not specified, in the NCCN guidelines. Oncotype Dx® in DCIS has, however, not been universally accepted as is the case in invasive carcinoma and additional larger studies with long term follow-up may be needed to clearly define its role in planning adjuvant RT in DCIS. With accurate identification of risk groups, we can better individualize treatment for women with DCIS and reduce the incidence of morbidity that can often accompany aggressive therapy.
Key Points
-
DCIS is a heterogeneous group of breast lesions hitherto categorized into three grades based primarily on nuclear and cytologic features.
-
The prevailing model of breast cancer progression now recognizes divergent pathways for low-and high-grade DCIS.
-
FEA, ADH, and low-grade DCIS are now considered to be non-obligate precursors of low-grade invasive ductal breast carcinoma; the precursor lesions of HG DCIS and invasive carcinoma are yet unknown.
-
Low-grade DCIS, like its invasive counterpart, is characterized by loss of 16q and ER/PR positivity. HG DCIS is characterized by aneuploidy, p53 positivity, and HER2 amplification.
-
Molecular studies of DCIS have shown categorization of the in situ lesions into at least four intrinsic molecular subtypes is possible, albeit with some differences from their invasive counterpart (e.g., higher incidence of HER2 type).
-
Progression from in situ to invasive ductal lesions may be facilitated by epigenetic changes in the tumors’ gene expression, as well as changes in their microenvironment.
-
Commercial molecular testing for DCIS is now available and may play a role in directing adjuvant therapy for some patients. Caution should still be exercised in interpreting the results of these tests however, as the data supporting the validity of molecular testing for DCIS is not yet extensive.
References
Brinton LA, et al. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100(22):1643–8.
Virnig BA, et al. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170–8.
Betsill WL Jr, et al. Intraductal carcinoma. long-term follow-up after treatment by biopsy alone. JAMA. 1978;239(18):1863–7.
Eusebi V, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994;11(3):223–35.
Page DL, et al. Continued local recurrence of carcinoma 15-25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;76(7):1197–200.
Ernster VL, et al. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160(7):953–8.
Tavassoli FA. Ductal carcinoma in situ: introduction of the concept of ductal intraepithelial neoplasia. Mod Pathol. 1998;11(2):140–54.
Tavassoli FA. Breast pathology: rationale for adopting the ductal intraepithelial neoplasia (DIN) classification. Nat Clin Pract Oncol. 2005;2(3):116–7.
Veronesi U, et al. Rethinking TNM: breast cancer TNM classification for treatment decision-making and research. Breast. 2006;15(1):3–8.
Veronesi U, et al. Breast cancer classification: time for a change. J Clin Oncol. 2009;27(15):2427–8.
Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23.
Stomper PC, et al. Clinically occult ductal carcinoma in situ detected with mammography: analysis of 100 cases with radiologic-pathologic correlation. Radiology. 1989;172(1):235–41.
Holland R, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol. 1994;11(3):167–80.
Bethwaite P, et al. Reproducibility of new classification schemes for the pathology of ductal carcinoma in situ of the breast. J Clin Pathol. 1998;51(6):450–4.
Silverstein MJ, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995;345(8958):1154–7.
Poller DN, et al. Ideas in pathology. Ductal carcinoma in situ of the breast: a proposal for a new simplified histological classification association between cellular proliferation and c-erbB-2 protein expression. Mod Pathol. 1994;7(2):257–62.
Bijker N, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of european organization for research and treatment of cancer trial 10853. J Clin Oncol. 2001;19(8):2263–71.
Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). The national surgical adjuvant breast and bowel project collaborating investigators. Cancer. 1995;75(6):1310–9.
Kerlikowske K, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95(22):1692–702.
Fonseca R, et al. Ductal carcinoma in situ of the breast. Ann Intern Med. 1997;127(11):1013–22.
Houghton J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102.
Julien JP, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC breast cancer cooperative group and EORTC radiotherapy group. Lancet. 2000;355(9203):528–33.
Fisher B, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328(22):1581–6.
Fisher B, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from national surgical adjuvant breast and bowel project B-17. J Clin Oncol. 1998;16(2):441–52.
Fisher B, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the national surgical adjuvant breast and bowel project experience. Semin Oncol. 2001;28(4):400–18.
Fisher ER, et al. Pathologic findings from the national surgical adjuvant breast project (NSABP) eight-year update of protocol B-17: intraductal carcinoma. Cancer. 1999;86(3):429–38.
Silverstein MJ. The university of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186(4):337–43.
Silverstein MJ, Buchanan C. Ductal carcinoma in situ: USC/Van Nuys prognostic index and the impact of margin status. Breast. 2003;12(6):457–71.
Fisher B, et al. Tamoxifen in treatment of intraductal breast cancer: national surgical adjuvant breast and bowel project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000.
Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55(2):231–73.
Wellings SR, Jensen HM. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973;50(5):1111–8.
Lerwill MF. Flat epithelial atypia of the breast. Arch Pathol Lab Med. 2008;132(4):615–21.
Xu S, et al. Evidence of chromosomal alterations in pure usual ductal hyperplasia as a breast carcinoma precursor. Oncol Rep. 2008;19(6):1469–75.
Boecker W, et al. Usual ductal hyperplasia of the breast is a committed stem (progenitor) cell lesion distinct from atypical ductal hyperplasia and ductal carcinoma in situ. J Pathol. 2002;198(4):458–67.
Otterbach F, et al. Cytokeratin 5/6 immunohistochemistry assists the differential diagnosis of atypical proliferations of the breast. Histopathology. 2000;37(3):232–40.
Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol. 2011;223(2):307–17.
Shin SJ, et al. Molecular evidence for progression of microglandular adenosis (MGA) to invasive carcinoma. Am J Surg Pathol. 2009;33(4):496–504.
Buerger H, et al. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol. 1999;187(4):396–402.
O’Connell P, et al. Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst. 1998;90(9):697–703.
Gao Y, et al. Genetic changes at specific stages of breast cancer progression detected by comparative genomic hybridization. J Mol Med (Berl). 2009;87(2):145–52.
Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–61.
Claus EB, et al. Pathobiologic findings in DCIS of the breast: morphologic features, angiogenesis, HER-2/neu and hormone receptors. Exp Mol Pathol. 2001;70(3):303–16.
Lebrecht A, et al. Histological category and expression of hormone receptors in ductal carcinoma in situ of the breast. Anticancer Res. 2002;22(3):1909–11.
Meijnen P, et al. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer. 2008;98(1):137–42.
Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Cheang MC, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–76.
Tamimi RM, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10(4):R67.
Livasy CA, et al. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol. 2007;38(2):197–204.
Dabbs DJ, et al. Basal phenotype of ductal carcinoma in situ: recognition and immunohistologic profile. Mod Pathol. 2006;19(11):1506–11.
Zhou W, et al. Molecular subtypes in ductal carcinoma in situ of the breast and their relation to prognosis: a population-based cohort study. BMC Cancer. 2013;13:512.
Pasquali L, et al. Quantification of CpG island methylation in progressive breast lesions from normal to invasive carcinoma. Cancer Lett. 2007;257(1):136–44.
Ai L, et al. Epigenetic silencing of the tumor suppressor cystatin M occurs during breast cancer progression. Cancer Res. 2006;66(16):7899–909.
Hu M, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37(8):899–905.
Duggal S, Julian TB. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ. J Natl Cancer Inst. 2013;105(10):681–3.
Solin LJ, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–10.
Washington C, et al. Loss of heterozygosity in fibrocystic change of the breast: genetic relationship between benign proliferative lesions and associated carcinomas. Am J Pathol. 2000;157(1):323–9.
Lakhani SR, et al. Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab Invest. 1996;74(1):129–35.
Aubele MM, et al. Accumulation of chromosomal imbalances from intraductal proliferative lesions to adjacent in situ and invasive ductal breast cancer. Diagn Mol Pathol. 2000;9(1):14–9.
Boecker W, et al. Ductal epithelial proliferations of the breast: a biological continuum? Comparative genomic hybridization and high-molecular-weight cytokeratin expression patterns. J Pathol. 2001;195(4):415–21.
Gong G, et al. Genetic changes in paired atypical and usual ductal hyperplasia of the breast by comparative genomic hybridization. Clin Cancer Res. 2001;7(8):2410–4.
Jones C, et al. Molecular cytogenetic comparison of apocrine hyperplasia and apocrine carcinoma of the breast. Am J Pathol. 2001;158(1):207–14.
Moinfar F, et al. Genetic abnormalities in mammary ductal intraepithelial neoplasia-flat type (“clinging ductal carcinoma in situ”): a simulator of normal mammary epithelium. Cancer. 2000;88(9):2072–81.
Dabbs DJ, et al. Molecular alterations in columnar cell lesions of the breast. Mod Pathol. 2006;19(3):344–9.
Simpson PT, et al. Columnar cell lesions of the breast: the missing link in breast cancer progression? A morphological and molecular analysis. Am J Surg Pathol. 2005;29(6):734–46.
Lakhani SR, et al. Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol. 1995;48(7):611–5.
Amari M, et al. LOH analyses of premalignant and malignant lesions of human breast: frequent LOH in 8p, 16q, and 17q in atypical ductal hyperplasia. Oncol Rep. 1999;6(6):1277–80.
Larson PS, et al. Quantitative analysis of allele imbalance supports atypical ductal hyperplasia lesions as direct breast cancer precursors. J Pathol. 2006;209(3):307–16.
Bijker N, et al. Histological type and marker expression of the primary tumour compared with its local recurrence after breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2001;84(4):539–44.
Lebeau A, et al. EGFR, HER-2/neu, cyclin D1, p21 and p53 in correlation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2003;79(2):187–98.
Collins LC, Schnitt SJ. HER2 protein overexpression in estrogen receptor-positive ductal carcinoma in situ of the breast: frequency and implications for tamoxifen therapy. Mod Pathol. 2005;18(5):615–20.
Warnberg F, et al. Tumour markers in breast carcinoma correlate with grade rather than with invasiveness. Br J Cancer. 2001;85(6):869–74.
Clark SE, et al. Molecular subtyping of DCIS: heterogeneity of breast cancer reflected in pre-invasive disease. Br J Cancer. 2011;104(1):120–7.
Carey LA, et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295(21):2492–502.
Kurebayashi J, et al. The prevalence of intrinsic subtypes and prognosis in breast cancer patients of different races. Breast. 2007;16(Suppl 2):S72–7.
Rakha EA, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32.
Kwan ML, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31.
Lopez-Garcia MAl, Geyer FC, Lacroix-Triki M, Marchió C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. 2010;57:171–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Gong, Y., Kandil, D., Khan, A. (2015). Molecular Pathology of Pre-Invasive Ductal Carcinoma. In: Khan, A., Ellis, I., Hanby, A., Cosar, E., Rakha, E., Kandil, D. (eds) Precision Molecular Pathology of Breast Cancer. Molecular Pathology Library, vol 10. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2886-6_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2886-6_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2885-9
Online ISBN: 978-1-4939-2886-6
eBook Packages: MedicineMedicine (R0)