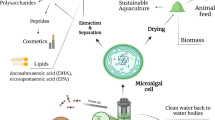
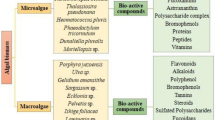
Abstract
Biomass derived from marine microalgae and macroalgae is globally recognized as a source of valuable chemical constituents with applications in the agri-horticultural sector (including animal feeds and health and plant stimulants), as human food and food ingredients as well as in the nutraceutical, cosmeceutical, and pharmaceutical industries. Algal biomass supply of sufficient quality and quantity however remains a concern with increasing environmental pressures conflicting with the growing demand. Recent attempts in supplying consistent, safe and environmentally acceptable biomass through cultivation of (macro- and micro-) algal biomass have concentrated on characterizing natural variability in bioactives, and optimizing cultivated materials through strain selection and hybridization, as well as breeding and, more recently, genetic improvements of biomass. Biotechnological tools including metabolomics, transcriptomics, and genomics have recently been extended to algae but, in comparison to microbial or plant biomass, still remain underdeveloped. Current progress in algal biotechnology is driven by an increased demand for new sources of biomass due to several global challenges, new discoveries and technologies available as well as an increased global awareness of the many applications of algae. Algal diversity and complexity provides significant potential provided that shortages in suitable and safe biomass can be met, and consumer demands are matched by commercial investment in product development.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Stengel DB, Connan S, Popper ZA (2011) Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol Adv 29(5):483–501. doi:10.1016/j.biotechadv.2011.05.016
Khan W, Rayir UP, Subramanian S et al (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Freitas AC, Rodrigues D, Rocha-Santos TA et al (2012) Marine biotechnology advances towards applications in new functional foods. Biotechnol Adv 30:1506–1515
Bedoux G, Hardouin K, Burlot AS et al (2014) Bioactive components from seaweeds: cosmetic applications and future development. Adv Bot Res 71:345–378
Ioannou E, Roussis V (2009) Natural products from seaweeds. In: Osbourn AE, Lanzotti V (eds) Plant-derived natural products: synthesis, function, and application. Springer, New York, pp 51–81
Stern RF, Horak A, Andrew RL et al (2010) Environmental barcoding reveals massive dinoflagellate diversity in marine environments. PLoS One 5:e13991. doi:10.1371/journal.pone.0013991
Evans FD, Critchley AT (2014) Seaweeds for animal production use. J Appl Phycol 26:891–899
Bodeau C, Deslandes E (2007) Composition cosmétique comprenant une association de floridoside et d’acide iséthionique particulièrement un extrait d’algue rouge. French Patent No. FR2888504. Paris, INPI
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
FAO (2014) The state of world fisheries and aquaculture 2014. Rome
Paradossi G, Cavalieri F, Chiessi E (2002) A conformational study on the algal polysaccharide ulvan. Macromolecules 35:6404–6411
Berteau O, Mulloy B (2003) Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 13:29R–40R
Mery AB, Joubert JM (2012) Laminarin (Vacciplant®) against apple scab (Venturia inaequalis) and gloeosporium on apple (Gloeosporium album et Perenans). In: AFPP (ed). Association Française de Protection des Plantes. 10ème Conférence Internationale sur les Maladies des Plantes, Tours, France, 3–5 December 2012. pp. 630–639
Harun R, Singh M, Forde GM et al (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Ren Sust Energ Rev 14:1037–1047
Plaza M, Herrero M, Cifuentes A et al (2009) Innovative natural functional ingredients from microalgae. J Agric Food Chem 57:7159–7170
Spolaore P, Joannis-Cassan C, Duran E et al (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Chacón-Lee TL, Gonzáles-Mariño GE (2010) Microalgae for ‘healthy’ foods—possibilities and challenges. Compr Rev Food Sci Food Safety 9:655–675
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Cho Y-S, Kim S-K (2011) Pharmaceutical compositions containing fucoidan for stimulating and activating osteogenesis. U.S. Patent No. US20110301119 A1 Washington, DC, U.S. Patent and Trademark Office
Eriksen NT (2008) Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
Moigne JY (1998) Use of algae extract as antibacterial and/or antifungal agent and composition containing same. World Patent No. WO1998010656 A1Washington, DC, U.S. Patent and Trademark Office
Kim S-K, Kim Y-M, Lee M-S, et al (2013) Antibiotic composition including phlorotannin compound derived from Eisenia bicyclis as effective component. U.S. Patent No. US20130338217 A1 Washington, DC, U.S. Patent and Trademark Office
Singh S, Kate BN, Banerjee UC (2005) Bioactive compounds from Cyanobacteria and microalgae: an overview. Crit Rev Biotechnol 25:73–95
Chisti Y (2006) Microalgae as sustainable cell factories. Environ Eng Manag J 5:261–274
Colla LM, Bertolin TE, Vieira Costa JA (2004) Fatty acids profile of Spirulina platensis grown under different temperatures and nitrogen concentrations. Z Naturforsch 59c: 55–59
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210. doi:10.1016/j.biotechadv.2006.11.002
Fabregas J, Herrero C (1990) Vitamin content of four marine microalgae. Potential use as source of vitamin in nutrition. J Ind Microbiol 5:259–263
Gouveia L, Batista AP, Sousa I et al (2009) Microalgae in novel food products. In: Hagen KN (ed) Algae: nutrition, pollution control and energy sources. Nova Science Publishers, New York, pp 265–300
Chandini SK, Ganesan P, Suresh PV et al (2008) Seaweeds as a source of nutritionally beneficial compounds—a review. J Food Sci Technol 45:1–13
Kumari P, Kumar M, Gupta V et al (2010) Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem 120:749–757
Kumar M, Kumari P, Trivedi N et al (2011) Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol 23:797–810. doi:10.1007/s10811-010-9578-7
Schmid M, Guihéneuf F, Stengel DB (2014) Fatty acid contents and profiles of 16 macroalgae collected from the Irish coast at two seasons. J Appl Phycol 26:451–463. doi:10.1007/s10811-013-0132-2
Schmid M, Stengel DB (2015) Intra-thallus differentiation of fatty acid and pigment profiles in some temperate Fucales and Laminariales. J Phycol (in press)
Nishino H, Murakoshi M, Ii T et al (2002) Carotenoids in cancer chemoprevention. Cancer Metast Rev 21:257–264
Prasanna R, Sood A, Suresh A et al (2007) Potentials and applications of algal pigments in biology and industry. Acta Bot Hung 49:131–156. doi:10.1556/ABot. 49.2007.1-2.14
de la Coba F, Aguilera J, Figueroa FL et al (2009) Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J Appl Phycol 21:161–169
Nitschke U, Stengel DB (2014) A new HPLC method for the detection of iodine applied to natural samples of edible seaweeds and commercial seaweed food products. Food Chem 172:326–334
Müssig M (2009) Iodine-induced toxic effects due to seaweed consumption. In: Preedy VR, Burrow GN, Watson R (eds) Comprehensive handbook of iodine. Elsevier Academic Press, Oxford, pp 897–908
Görs M, Schumann R, Hepperle D et al (2010) Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J Appl Phycol 22:265–276
Borowitzka MA (1997) Microalgae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Mao Y, Yang H, Zhou Y et al (2009) Potential of the seaweed Gracilaria lemaneiformis for integrated multi-trophic aquaculture with scallop Chlamys farreri in North China. J Appl Phycol 21:649–656
Borowitzka MA, Huisman JM, Osborn A (1991) Culture of the astaxanthin-producing green alga Haematococcus pluvialis 1. Effects of nutrients on growth and cell type. J Appl Phycol 3:295–304
Belay A, Kato T, Ota Y (1996) Spirulina (Arthrospira): potential application as an animal feed supplement. J Appl Phycol 8:303–311
Patil V, Källqvist T, Olsen E et al (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult Int 15:1–9
Archer GS, Friend TH, Caldwell D et al (2008) Impacts of feeding several components of the seaweed Ascophyllum nodosum on transported lambs. Animal Feed Sci Technol 140:258–271
O’Sullivan L, Murphy B, McLoughlin P et al (2010) Prebiotics from marine macroalgae for human and animal health applications. Mar Drugs 8:2038–2064
Ördög V, Stirk WA, Lenobel R et al (2004) Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J Appl Phycol 16:309–314
Craigie JS, MacKinnon SL, Walter JA (2008) Liquid seaweed extracts identified using 1H NMR profiles. J Appl Phycol 20:665–671
MacKinnon SL, Hiltz D, Ugarte R et al (2010) Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J Appl Phycol 22:489–494
Rayorath P, Jithesh MN, Farid A et al (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol 20:423–429
de Nys R, Steinberg PD (2002) Linking marine biology and biotechnology. Curr Opin Biotechnol 13:244–248
Häder DP, Kumar HD, Smith RC et al (1998) Effects on aquatic ecosystems. J Photochem Photobiol B 46:53–68
Zubia M, Payri C, Deslandes E (2008) Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J Appl Phycol 20:1033–1043
Cornish ML, Garbary DJ (2010) Antioxidants from macroalgae: potential applications in human health and nutrition. Algae 25:155–171
Blunt JW, Copp BR, Keyzers RA et al (2014) Marine natural products. Nat Prod Rep 31:160–258. doi:10.1039/c3np70117d
Plaza M, Santoyo S, Jaime L et al (2010) Screening for bioactive compounds from algae. J Pharmaceut Biomed 51:450–455
de la Mare JA, Sterrenberg JN, Sukhthankar MG et al (2013) Assessment of potential anti-cancer stem cell activity of marine algal compounds using an in vitro mammosphere assay. Cancer Cell Int 13:39. doi:10.1186/1475-2867-13-39
Rastogi RP, Sinha RS (2009) Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv 27:521–539
Zubia M, Fabre MS, Kerjean V et al (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116:693–701
Felício-Fernandes G, Laranjeira M (2000) Calcium phosphate biomaterials from marine algae. Hydrothermal synthesis and characterisation. Quím Nova 23:441–446. doi:10.1590/S0100-40422000000400002
Dimartino S, Lir I, Haber M et al (2013) Characterization of biomimetic adhesives from the red alga Gracilaria conferta for biomedical applications. In: Flammang P, Santos R, Aldred N, Gorb S (eds) Biological and biomimetic adhesives: challenges and opportunities. Royal Society of Chemistry, UK, pp 117–131
Rinaudo M (2007) Seaweed polysaccharides. In: Kalmerling JP (ed) Comprehensive glycoscience from chemistry to systems biology, vol 2. Elsevier, London, pp 691–735
Nakayasu S, Soegima R, Yamaguchi K et al (2009) Biological activities of fucose-containing polysaccharide ascophyllan isolated from the brown alga Ascophyllum nodosum. Biosci Biotechnol Biochem 73:961–964
Harnedy PA, FitzGerald RJ (2011) Bioactive protein, peptides and amino acids from macroalgae. J Phycol 47:218–232
Cabrita MT, Vale C, Rauter AP (2010) Halogenated compounds from marine algae. Mar Drugs 8:2301–2317
Plouguerné E, Hellio C, Cesconetto C et al (2010) Antifouling activity as a function of population variation in Sargassum vulgare from the littoral of Rio de Janeiro (Brazil). J Appl Phycol 22:717–724. doi:10.1007/s10811-010-9511-0
Davoult D, Engel CR, Arzel P et al (2011) Environmental factors and commercial harvesting: exploring possible links behind the decline of the kelp Laminaria digitata in Brittany, France. Cah Biol Mar 52:429–435
Sivertsen K (1997) Geographic and environmental factors affecting the distribution of kelp beds and barren grounds and changes in biota associated with kelp reduction at sites along the Norwegian coast. Can J Fish Aquat Sci 54:2872–2887
Christie H, Fredriksen S, Rinde E (1998) Regrowth of kelp and colonization of epiphyte and fauna community after kelp trawling at the coast of Norway. In: Baden S, Phil L, Rosenberg R et al (eds) Recruitment, colonization and physical-chemical forcing in marine biological systems. Springer, Netherlands, pp 49–58
Valero M, Destombe C, Mauger S et al (2011) Using genetic tools for sustainable management of kelps: a literature review and the example of Laminaria digitata. Cah Biol Mar 52:467–483
Sharp G, Semple R, Wilson M et al (2008) A survey of the distribution and abundance of Irish Moss (Chondrus crispus) on the south shore of Nova Scotia. Port Medway, Shelburne Co. to Pennant Point, Halifax Co. Cano Manuser. Rep Fish Aquat Sci 2856:Iii+34
Chopin T, Sharp G, Belyea E et al (1999) Open-water aquaculture of the red alga Chondrus crispus in Prince Edward Island, Canada. Hydrobiologia 398:417–425
Stagnol D, Renaud M, Davoult D (2013) Effects of commercial harvesting of intertidal macroalgae on ecosystem biodiversity and functioning. Estuar Coast Shelf Sci 130:99–110
Hafting JT, Critchley AT, Cornish ML et al (2012) On-land cultivation of functional seaweed products for human usage. J Appl Phycol 24:385–392
Stengel DB, Dring MJ (2000) Copper and iron concentrations in Ascophyllum nodosum (Fucales, Phaeophyta) from different sites in Ireland and after culture experiments in relation to thallus age and epiphytism. J Exp Mar Biol Ecol 246:145–161
Gellenbeck KW (2012) Utilization of algal materials for nutraceutical and cosmeceutical applications—what do manufacturers need to know? J Appl Phycol 24:309–313
Jassby A (1988) Spirulina: a model for microalgae as human food. In: Lembi CA, Waaland JR (eds) Algae and human affairs. Cambridge University Press, Cambridge, pp 149–179
Daroch M, Geng S, Wang G (2013) Recent advances in liquid biofuel production from algal feedstocks. Appl Energ 102:1371–1381
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Liu D, Keesing JK, Xing Q et al (2009) World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar Poll Bull 58:888–895
Buschmann AH, Correa JA, Westermeier R (2001) Red algal farming in Chile: a review. Aquaculture 194:203–220
Buschmann AH, Hernández-González MDC, Varela D (2008) Seaweed future cultivation in Chile: perspectives and challenges. Int J Environ Pollut 33:432–456. doi:10.1504/IJEP.2008.020571
Longford SR, Tujula NA, Crocetti GR et al (2007) Comparisons of diversity of bacterial communities associated with three sessile marine eukaryotes. Aquat Microb Ecol 48:217–229
Tujula NA, Crocetti GR, Burke C et al (2010) Variability and abundance of the epiphytic bacterial community associated with a green marine Ulvacean alga. ISME J 4:301–311
Moody JW, McGinty CM, Quinn JC (2014) Global evaluation of biofuel potential from microalgae. Proc Natl Acad Sci U S A 111:8691–8696. doi:10.1073/pnas.1321652111
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Luque R (2010) Algal biofuels: the eternal promise? Energy Environ Sci 3:254–257
Ugwu CU, Ogbonna JC, Tanaka H (2005) Light/dark cyclic movement of algal culture (Synechocystis aquatilis) in outdoor inclined tubular photobioreactor equipped with static mixers for efficient production of biomass. Biotechnol Lett 27:75–78
Pulz O, Scheibenbogen K (1998) Photobioreactors: design and performance with respect to light energy input. Adv Biochem Eng/Biotechnol 59:123–152
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Ugwu CU, Ogbonna JC, Tanaka H (2002) Improvement of mass transfer characteristics and productivities of inclined tubular photobioreactors by installation of internal static mixers. Appl Microbiol Biotechnol 58:600–607
Del Campo JA, Garcia-Gonzáles M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Doucha J, Lívanský K (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a middle and southern European climate. J Appl Phycol 18:811–826
Doucha J, Lívanský K (2009) Outdoor open thin-layer microalgal photobioreactor: potential productivity. J Appl Phycol 21:111–117
Grobbelaar JU (2009) Factors governing algal growth in bioreactors. The ‘open’ versus ‘closed’ debate. J Appl Phycol 21:489–492
Chen CY, Yeh KL, Aisyah R et al (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Gacheva GV, Gigova LG (2014) Biological activity of microalgae can be enhanced by manipulating the cultivation temperature and irradiance. Cent Eur J Biol 9:1168–1181
Chrismadha T, Borowitzka MA (1994) Effect of cell density and irradiance on growth, proximate composition and eicosapentaenoic acid production of Phaeodactylum tricornutum grown in a tubular photobioreactor. J Appl Phycol 6:67–74
Çelekli A, Dönmez G (2006) Effect of pH, light intensity, salt and nitrogen concentrations on growth and β-carotene accumulation by a new isolate of Dunaliella sp. World J Microbiol Biotechnol 22:183–189
Chaneva G, Furnadzhieva S, Minkova K et al (2007) Effect of light and temperature on the cyanobacterium Arthronema africanum - a prospective phycobiliprotein-producing strain. J Appl Phycol 9:37–544
Wang B, Li Y, Wu N et al (2008) CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol 79:707–718
Bondu S, Kervarec N, Deslandes E et al (2008) The use of HRMAS NMR spectroscopy to study the in vivo intra-cellular carbon/nitrogen ratio of Solieria chordalis (Rhodophyta). J Appl Phycol 20:673–679
Venugopal V, Prasanna R, Sood A et al (2006) Stimulation of pigment accumulation in Anabaena azollae strains: effects of light intensity and sugars. Folia Microbiol 51:50–56
Marxen K, Vanselow KH, Hintze R et al (2010) Comparison of two different modes of UV-B irradiation on synthesis of some cellular substances in the cyanobacterium Synechocystis sp. PCC6803. J Appl Phycol 22:677–690
Raja R, Hemaiswarya S, Rengasamy R (2007) Exploitation of Dunaliella for β-carotene production. Appl Microbiol Biotechnol 74:517–523
Ye ZW, Jiang JG, Wu GH (2008) Biosynthesis and regulation of carotenoids in Dunaliella: progresses and prospects. Biotechnol Adv 26:352–360
Bancroft BA, Baker NJ, Blaustein AR (2007) Effects of UVB radiation on marine and freshwater organisms: a synthesis through meta-analysis. Ecol Lett 10:332–345
Behrenfeld MJ, Falkowski PG (1997) Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol Oceanogr 42:1–20
Andersen RA (2005) Algal culturing techniques. Elsevier Academic Press, New York
Fabregas J, Herrero C, Abalde J et al (1986) The marine microalga Chlorella stigmatophora as a potential source of single cell protein: enhancement of the protein content in response to nutrient enrichment. J Ind Microbiol 1:251–257
Corsini M, Karydis M (1990) An algal medium based on fertilizers and its evaluation in mariculture. J Appl Phycol 2:333–339
Chen W, Zhang Q, Dai S (2009) Effects of nitrate on intracellular nitrite and growth of Microcystis aeruginosa. J Appl Phycol 21:701–706
Prasanna R, Sood A, Jaiswal P et al (2010) Rediscovering cyanobacteria as valuable sources of bioactive compounds (Review). Appl Biochem Microbiol 46:119–134
Fatma T (2009) Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull Environ Contam Toxicol 83:509–515
Nie ZY, Xia JL, Levert JM (2002) Fractionation and characterization of polysaccharides from cyanobacterium Spirulina (Arthrospira) maxima in nitrogen-limited batch culture. J Cent South Univ Technol 9:81–86
Colla LM, Reinehr CO, Reichert C et al (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98:1489–1493
Siron R, Giusti G, Berland B (1989) Changes in the fatty acid composition of Phaeodactylum tricornutum and Dunaliella tertiolecta during growth and under phosphorus deficiency. Mar Ecol Prog Ser 55:95–100
Piorreck M, Baasch KH, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 23:207–216
Ben-Amotz A (1987) Effect of irradiance and nutrient deficiency on the chemical composition of Dunaliella bardawil Ben-Amotz and Avron (Volvocales, Chlorophyta). J Plant Physiol 131:479–487
Roessler PG (1988) Effects of silicon deficiency on lipid composition and metabolism in the diatom Cyclotella cryptica. J Phycol 24:394–400
Durmaz Y, Monteiro M, Bandarra N et al (2007) The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J Appl Phycol 19:223–227
Xu XQ, Beardall J (1997) Effect of salinity on fatty acid composition of a green microalga from an antarctic hypersaline lake. Phytochemistry 45:655–658
Oren A (2005) A hundred years of Dunaliella research: 1905–2005. Saline Syst 1:1–14. doi:10.1186/1746-1448-1-2
Chen H, Jiang JG (2009) Osmotic responses of Dunaliella to the changes of salinity. J Cell Physiol 219:251–258
Rao AR, Dayananda C, Sarada R et al (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564
de Castro Araújo S, Garcia VMT (2005) Growth and biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246:405–412
Chu WL, Phang SM, Goh SH (1996) Environmental effects on growth and biochemical composition of Nitzschia inconspicua Grunow. J Appl Phycol 8:389–396
Huntley ME, Redalje DG (2007) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitig Adapt Strat GC 12:573–608
Papazi A, Makridis P, Divanach P et al (2008) Bioenergetic changes in the microalgal photosynthetic apparatus by extremely high CO2 concentrations induce an intense biomass production. Physiol Plant 132:338–349
Jeon H, Lee Y, Chang KS et al (2013) Enhanced production of biomass and lipids by supplying CO2 in marine microalga Dunaliella sp. J Microbiol 51:773–776
Guihéneuf F, Stengel DB (2013) Microalgae LC-PUFA enriched oil production: lipid and triacylglycerols containing n-3 LC-PUFA accumulation are triggered by nitrogen-limitation and inorganic carbon availability in the marine microalga Pavlova lutheri. Mar Drugs 11:4246–4266. doi:10.3390/md11114246
Marcoval MA, Villafane VE, Helbling EW (2007) Interactive effects of ultraviolet radiation and nutrient addition on growth and photosynthesis performance of four species of marine phytoplankton. J Photochem Photobiol B 89:78–87
Bermúdez J, Rosales N, Loreto C et al (2004) Exopolysaccharide, pigment and protein production by the marine microalga Chroomonas sp. in semicontinuous cultures. World J Microbiol Biotechnol 20:179–183
Halling C, Wikström S, Lilliesköld-Sjöö G et al (2013) Introduction of Asian strains and low genetic variation in farmed seaweeds: indications for new management practices. J Appl Phycol 25:89–95
Uppalapati SR, Fujita Y (2000) Red rot resistance in interspecific protoplast fusion product progeny of Porphyra yezoensis and P. tenuipedalis (Bangiales, Rhodophyta). Phycol Res 48:281–289
Xu P, Yang L, Zhu J et al (2011) Analysis of hybridization strains of Porphyra based on rbc L gene sequences. J Appl Phycol 23:235–241
Zhang QS, Tang XX, Cong YZ et al (2007) Breeding of an elite Laminaria variety 90-1 through inter-specific gametophyte crossing. J Appl Phycol 19:303–311
Weng M, Liu B, Jin D et al (2005) Identification of 27 Porphyra lines (Rhodophyta) by DNA fingerprinting and molecular markers. J Appl Phycol 17:91–97
Niwa K, Aruga Y (2006) Identification of currently cultivated Porphyra species by PCR-RFLP analysis. Fish Sci 72:143–148
Bi Y, Hu Y, Zhou Z (2011) Genetic variation of Laminaria japonica (Phaeophyta) populations in China as revealed by RAPD markers. Acta Oceanol Sin 30:103–112
Wu C, Guangheng L (1987) Progress in the genetics and breeding of economic seaweeds in China. Hydrobiologia 151:57–61
Lin H, Qin S (2014) Tipping points in seaweed genetic engineering: scaling up opportunities in the next decade. Mar Drugs 12:3025–3045. doi:10.3390/md12053025
Rasala BA, Chao SS, Pier M et al (2014) Enhanced genetic tools for engineering multigene traits into green algae. PLoS One 9:e94028. doi:10.1371/journal.pone.0094028
Radakovits R, Jinkerson RE, Darzins A et al (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Kim S, Lee YC, Cho DH et al (2014) A simple and non-invasive method for nuclear transformation of intact-walled Chlamydomonas reinhardtii. PLoS One 9:e101018. doi:10.1371/journal.pone.0101018
Poulsen N, Chesley PM, Kröger N (2006) Molecular genetic manipulation of the diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 42:1059–1065
Nakamura Y, Sasaki N, Kobayashi M et al (2013) The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis). PLoS One 8:e57122. doi:10.1371/journal.pone.0057122
Lü J, Sheahan C, Fu P (2011) Metabolic engineering of algae for fourth generation biofuels production. Energy Environ Sci 4:2451–2466. doi:10.1039/1754-5706/2008#Link
Cadoret JP, Garnier M, Saint-Jean B (2012) Microalgae, functional genomics and biotechnology. Adv Bot Res 64:285–341
Keeling PJ, Burki F, Wilcox HM et al (2014) The marine microbial eukaryote transcriptome sequencing project (MMETSP): illuminating the functional diversity of eukaryotic life in the oceans through transcriptome sequencing. PLoS Biol 12:e1001889. doi:10.1371/journal.pbio.1001889
Cock JM, Sterck L, Rouzé P et al (2010) The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465:617–621
Ritter A, Dittami SM, Goulitquer S et al (2014) Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol 14:116. doi:10.1186/1471-2229-14-116
Dittami SM, Scornet D, Petit JL et al (2009) Global expression analysis of the brown alga Ectocarpus siliculosus (Phaeophyceae) reveals large-scale reprogramming of the transcriptome in response to abiotic stress. Genome Biol 10:R66
Konotchick T, Dupont CL, Valas RE et al (2013) Transcriptomic analysis of metabolic function in the giant kelp, Macrocystis pyrifera, across depth and season. New Phytol 198:398–407
Leblanc C, Schaal G, Cosse A et al (2011) Trophic and biotic interactions in Laminaria digitata beds: which factors could influence the persistence of the marine kelp forests in northern Brittany? Cah Biol Mar 52:415–427
Cosse A, Leblanc C, Potin P (2007) Dynamic defense of marine macroalgae against pathogens: from early activated to gene‐regulated responses. Adv Bot Res 46:221–266
Kitade Y, Asamizu E, Fukuda S et al (2008) Identification of genes preferentially expressed during asexual sporulation in Porphyra yezoensis gametophytes (Bangiales, Rhodophyta). J Phycol 44:113–123
Wu S, Sun J, Chi S et al (2014) Transcriptome sequencing of essential marine brown and red algal species in China and its significance in algal biology and phylogeny. Acta Oceanol Sin 33:1–12
Georgianna DR, Mayfield SP (2012) Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 488:329–335
Goldstein DA, Thomas JA (2004) Biopharmaceuticals derived from genetically modified plants. Q J Med 97:705–716
Paul M, van Dolleweerd C, Drake PMW et al (2011) Molecular pharming—future targets and aspirations. Hum Vaccin 7:375–382
Geng D, Wang Y, Wang P et al (2003) Stable expression of Hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J Appl Phycol 15:451–456
Jones AC, Gu L, Sorrels CM et al (2009) New tricks from ancient algae: natural products biosynthesis in marine Cyanobacteria. Curr Opin Chem Biol 13:216–223
Hempel F, Lau J, Klingl A et al (2011) Algae as protein factories: expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PLoS One 6:e28424. doi:10.1371/journal.pone.0028424
Gerwick WH, Moore BS (2012) Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol 19:85–98
Saitoh T, Suzuki T, Sugimoto M et al (2003) Total synthesis of (+) −laurallene. Tetrahedr Lett 44:3175–3178
Nicolaou KC, Snyder SA (2005) Chasing molecules that were never there: misassigned natural products and the role of chemical synthesis in modern structure elucidation. Angew Chem Int Ed 44:1012–1044
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media, New York
About this protocol
Cite this protocol
Stengel, D.B., Connan, S. (2015). Marine Algae: a Source of Biomass for Biotechnological Applications. In: Stengel, D., Connan, S. (eds) Natural Products From Marine Algae. Methods in Molecular Biology, vol 1308. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-2684-8_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2684-8_1
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-2683-1
Online ISBN: 978-1-4939-2684-8
eBook Packages: Springer Protocols