Abstract
Heat stress is one of the major abiotic stresses that plants encounter. Heat stress causes billions of dollars in losses of agricultural crops worldwide. Here, we summarize the molecular and whole genome responses due to heat stress in plants. It has been reported that there are cascades of biochemical reactions that lead to heat stress. In most cases, heat stress is coupled with drought stress response. With the advancements in genomic tools, we have more information on genes, which are up- or down-regulated in plants due to heat stress. The heat-stressed plants may exhibit various physiological responses, including stomatal closure, suppressed photosynthesis, stunted growth, etc. Microarray and transcriptome sequencing gave us the tools to perform genome-wide expression profiling in heat-stressed plants. Understanding how gene expression in heat-stressed plants works will help us to discover novel heat stress-tolerant genes. These genes could be overexpressed in crop plants to make transgenic heat-tolerant agricultural crops. Climate change and global warming are major concerns for us and production of thermotolerant plants could address the issue of global crop loss due to heat and drought stresses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abiotic stress
- Adaptation
- Genetic transformations
- Heat shock proteins
- Heat stress response
- Membrane fluidity
- Metabolites
- Osmolytes
- ROS scavenging system
- Small non-coding RNA
- Thermotolerance
- Transcriptome
1 Introduction
Plants are sessile organisms, and they are constantly exposed to environmental changes. Any change in the nonliving factors that can adversely affect the growth and development of the plant is known as abiotic stress. Extreme temperature is one of the major detrimental abiotic factors for the plant, causing heat stress (Yeh et al. 2012; Źróbek-Sokolnik 2012). The effects of high temperature can manifest through modification of membrane properties, stability of the protein structures, the rate of metabolic reactions, and the liquid viscosity inside plant cell organelles (Źróbek-Sokolnik 2012).
Generally, living organisms can be classified into three groups according to their temperature preferences: psychrophilic organisms (psychrophiles), mesophilic organisms (mesophiles), and thermophilic organisms (thermophiles) (Fig. 5.1). Most higher plants are classified as mesophilic organisms preferring temperatures between 10 and 30 °C (Źróbek-Sokolnik 2012). Although optimal plant growth can occur in a certain temperature range, mesophilic plants encounter a wide range of temperature fluctuations. As temperature deviates from the optimal level, cellular and molecular changes occur within the plant in order to maintain growth and cellular homeostasis. Despite the ability to adjust to temperature fluctuation, prolonged plant exposure to temperature above the threshold level may cause irreversible damage to plant growth and productivity, which is defined as heat stress (Willits and Peet 1998). According to the scientific standards, temperatures higher than the optimal level of 10–15 °C are denoted as heat stress (Larkindale et al. 2007). Heat stress has varying effects on plants due to intensity, duration, and the rate of temperature change. The duration of the plant exposure to high temperature is the main determinant of the lethal temperature range (Fig. 5.1) (Sung et al. 2003; Źróbek-Sokolnik 2012).
Classification of the plants, based on their sensitivity to high temperature (partly adapted from Źróbek-Sokolnik 2012)
Plants need to adapt to any abiotic changes by exhibiting appropriate response; this is necessary in order for plants to survive. The ability of plants to grow and remain productive under high temperatures is defined as heat tolerance (Huang and Xu 2008). Plants can be classified into three categories according to their tolerance levels to high temperature: heat sensitive, relatively heat tolerant, and heat tolerant (Fig. 5.1) (Hasanuzzaman et al. 2013; Źróbek-Sokolnik 2012). The form and magnitude of the heat tolerance depends on the plant, tissue, and cell type (Sung et al. 2003).
An increase in ambient temperature could affect crop productivity. In recent decades, human activities have caused an exponential increase in greenhouse gas emission especially carbon dioxide. It has been reported by the Intergovernmental Panel on Climate Change (IPCC) that the global mean temperature will increase by 0.3 °C per decade (Wheeler and von Braun 2013). This temperature increase may also affect distribution pattern of plant species. There are already losses of billions of dollars in agricultural crops worldwide due to heat stress. In addition, human demand for food is growing along with the world population growth. Any negative environmental effect on crop productivity can directly affect food security (Saidi et al. 2011).
Numerous studies have been performed that aimed to understanding plant heat stress responses (HSRs) and to minimize the detrimental effects of heat stress on plant productivity. It is believed that better understanding of the molecular mechanisms activated by heat stress may help in the development of more efficient crop thermotolerance (Yeh et al. 2012). Microarray and transcriptome sequencing gave scientists the tools to perform genome-wide expression profiling in heat-stressed plants. Understanding the gene expression profile in heat-stressed plants will help researchers discover novel heat stress-tolerant genes. These genes could be overexpressed in crop plants to make transgenic heat-tolerant agricultural crops. This chapter elaborates upon the impact of high temperatures on plant growth and development, and it emphasizes on physiological and molecular response of plants to heat stress.
2 Plant Responses to Heat Stress
Heat stress causes a series of biochemical, morphological, physiological, and molecular changes that adversely affect plant development (Fig. 5.2). The growth and productivity of the plants rely on numerous different biochemical reactions that are powered with temperature-sensitive proteins. At high temperatures, the enzyme functions can be disrupted with irreversible denaturation of proteins (Howarth 2005). If the temperature rises to extreme levels, severe cellular injuries may occur followed by immediate cell death within a few minutes; however, at moderately high temperatures, only long-term exposure may cause injury or death (Howarth 2005; Schoffl et al. 1999). The increased fluidity of membrane lipids, and protein denaturation and aggregation are immediate injuries occurring after exposure to high temperature (Howarth 2005). Slower heat injuries include loss of membrane integrity, inhibition of protein synthesis, enzyme inactivation in chloroplasts and mitochondria, and protein degradation (Essemine et al. 2010; Howarth 2005). Heat stress also affects cell cycle and cell division through changing the microtubules organization, elongation of phragmoplast microtubules, and formation of microtubule asters in mitotic cells (Smertenko et al. 1997). All these injuries together ultimately cause starvation, growth inhibition, decreased ion flux, accumulation of toxic compounds and reactive oxygen species (ROS) (Howarth 2005; Schoffl et al. 1999).
2.1 Morphological and Phenological Responses
Temperature plays an important role in controlling the rate, timing, and pattern of plant development. Therefore, growth retardation is the most prominent effect of heat stress on plants. In higher plants, cell division and cell elongation rates are significantly impaired under heat stress, which, in turn, can affect leaf size and weight. Plants’ exposure to severe heat stress reduces the stem growth, causing lower plant height (Prasad et al. 2006; Źróbek-Sokolnik 2012). Exposure to high temperatures during sowing time negatively affects the plant height and number of tillers (Ahamed et al. 2010).
The impact of high temperatures on plants may vary depending upon the developmental stages of the plant. During the reproductive stage, even a short exposure to high temperatures can cause significant abortion of floral buds and opened flowers; however, during the vegetative stage, high day temperature can only affect leaf gas exchange processes (Young et al. 2004). Long-term exposure of developing seeds to high temperatures can delay germination and diminish vigor, resulting in lower emergence and seedling establishment. Inhibition of seed germination under heat stress is often induced by abscisic acid (ABA) (Essemine et al. 2010). In developing shoots, high temperatures cause severe declines in the first internode length, shoot dry mass, and relative growth rate, leading to premature death of plants or early senescence (Patel and Franklin 2009). High temperature during growth stage can cause elongated stems and leaf hyponasty in some plant species (Patel and Franklin 2009). Moreover, heat stress may alter the total phenological duration by reducing the plant lifetime (Zhang et al. 2006). The temperature effects on phenological stages may vary based on the species and genotype due to great genetic variations (Hasanuzzaman et al. 2013).
Other morphological injuries derived from heat stress include scorching of leaves and twigs, sunburn of stem, branches and leaves, growth inhibition of shoot and root, leaf senescence and abscission, fruit discoloration and damage, along with a reduction in yield and dry matter production (Guilioni et al. 1997; Ismail and Hall 1999; Vollenweider and Günthardt-Goerg 2005). At the tissue and cellular level, water loss, stomata closure, cell size reduction, and increased number of xylem vessels in root and shoot are observed. The subcellular level damages include modifications in the thylakoid structure and swelling or loss of grana stacking (Karim et al. 1997; Wahid et al. 2007).
In tropical and temperate regions, heat stress is one of the most important causes of yield loss. Reproductive processes are significantly sensitive to high temperatures in most plants. Heat stress may adversely affect meiosis in both male and female reproductive organs, pollen germination, pollen tube growth, ovule survival, fertilization and post-fertilization processes, and growth of the endosperm and embryo (Foolad 2005). High temperatures also affect fruit quality, causing reduction in the levels of growth regulators and carbohydrates concentrations, leading to poor fruit set (Foolad 2005).
2.2 Physiological Responses
2.2.1 Water Relations
The most important variable affected by heat stress is plant water status. Plants are able to maintain the tissue water status in stable levels in humid environment regardless of temperature (Tsukaguchi et al. 2003). Heat-induced elevation in transpiration and water transportation are necessary tools for plant survival under high temperatures. It has been shown that in Pinus ponderosa seedlings, water transport through stems helps to cool plant by heat transferring mechanisms (Kolb and Robberecht 1996). Rapid water flow through seedling stems reduced the stem temperature by 30 °C during peak sunlight hours (Kolb and Robberecht 1996). However, heat stress is often linked with reduced water availability. Water deficit is significantly higher under the combined heat and drought stress rather than each single condition (Rampino et al. 2012).
Water loss most likely to occur more during daytime than nighttime under high temperatures. During the daytime, intensive leaf transpiration leads to water loss and reduction in water potential (Tsukaguchi et al. 2003). Since water is essential for any metabolic reaction, water deficiency causes disturbance of many physiological processes in plants. Significant water loss due to high temperatures can negatively affect both growth and biomass production (Simões-Araújo et al. 2003).
2.2.2 Osmotic Adjustment
Different plant species may accumulate certain organic compounds, which are generally known as compatible osmolytes (Sakamoto and Murata 2002). Some examples of the compatible osmolytes are sugars, sugar alcohols (polyols), proline, tertiary and quaternary ammonium compounds, and tertiary sulfonium compounds (Sairam and Tyagi 2004). It is thought that the accumulation of such compounds may be associated with enhanced stress tolerance in plants. For example, glycinebetaine (GB), an amphoteric quaternary amine, acts as a compatible solute in some plant species under heat stress. It has been suggested that GB synthesis may protect cellular redox potential under heat stress (Li et al. 2011). Maize plants (Quan et al. 2004) and sugar cane (Wahid and Close 2007) are able to accumulate high levels of GB due to water deficiency or high temperature while rice or mustard naturally don’t produce GB. Nowadays, genetic engineering approaches have provided researchers with a tool to introduce GB-biosynthetic pathway into GB-deficit strains. Heat tolerance in plants can be improved through use of these tools, through enhancing the production of such compatible osmolyte (Li et al. 2011; Quan et al. 2004).
2.2.3 Cellular Membranes
Cellular membranes play an important role in both photosynthesis and transpiration processes. High temperatures increase the membrane fluidity through either denaturation of proteins or through intensifying unsaturated fatty acids (Savchenko et al. 2002). Heat stress alters the tertiary and quaternary structures of the proteins within the membranes, enhancing their membrane permeability. Increased electrolyte leakage through membranes indicates a reduction in cell membrane thermostability (CMT) (Wahid et al. 2007). Different factors can affect solute leakage, including type of organ, degree of hardening, developmental stage, plant age, growing season, and plant species (Karim et al. 1997, 1999). It has been shown that adverse effects of heat stress are more severe on the mature leaves than the developing ones due to an enhanced number of unsaturated fatty acids (Karim et al. 1997, 1999). It has been suggested that alteration in membrane fluidity triggered by low or high temperatures influences temperature perception and gene expression (Saidi et al. 2009, 2010).
2.2.4 Photosynthesis
One of the most heat-sensitive physiological processes in green plants is photosynthesis (Crafts-Brandner and Salvucci 2002). Although there is a positive correlation between temperature changes and photosynthesis in the normal growing range of plants (15–45 °C), high temperatures disrupt the functionality of photosynthetic enzymes (Larkindale et al. 2008). The impact of high temperature on photosynthetic functionality is more enhanced in the C3 plants than in C4 plants. Any injuries on the photosynthetic apparatus that are inflicted by heat stress directly restrict plant growth. High temperatures affect photosynthetic capacity through disruption of enzyme activity in the electron transport chain, carbon metabolism, and oxygen-evolving complex (OEC) of PSII (Salvucci and Crafts-Brandner 2004). The photochemical reactions in thylakoid lamellae and carbon metabolism in the stroma of chloroplast are the most sensitive sites to injury under high temperatures (Wise et al. 2004). An increase in temperature can induce phase change in lipids incorporated in the thylakoid structure, which can eventually lead to lipid separation (Krumova et al. 2008).
Photosystem II (PSII), which is located in the thylakoid lamellae, is particularly sensitive to high temperatures; this causes its activity to be significantly decreased or even partially stopped. This is mainly due to the properties of the PSII location on the thylakoid membranes (Camejo et al. 2005). The damage to PSII is usually irreversible and it can cause a disassociation of the OEC (Sharkey and Zhang 2010). OEC is a water-oxidizing enzyme, and its dysfunctionality under heat stress causes an imbalance between an electron donor from OEC toward an electron acceptor of PSII. On the other hand, PSI stromal enzymes are thermostable in which PSI can drive cyclic electron pathway and associate with the thylakoid proton gradient (De Ronde et al. 2004).
The effect of heat stress on photosynthesis is also exhibited through a decline in soluble proteins, RuBisCO binding proteins (RBP), large subunits (LS), and small subunits (SS) of RuBisCO in darkness, and enhancement of them in light (Demirevska-Kepova et al. 2005). As temperature increases, photosynthesis declines due to increases in photorespiration that are faster than the subsequent increases in photosynthesis. The low affinity of RuBisCO, and its dual nature as an oxygenase and a carboxylase, is the main reason that limits the enhancement of net photosynthesis at higher temperatures. RuBisCO deactivation is the primary constraint for photosynthesis since it occurs at temperatures well below those that damage PSII (Salvucci and Crafts-Brandner 2004; Sharkey and Zhang 2010).
Another consequence of heat stress is degradation of chlorophyll a and b, which tends to be more significant in the mature leaves compared to the developing one (Karim et al. 1999). Such effects on chlorophyll may be associated with the production of active oxygen species (Camejo et al. 2005). Altered structural organization of thylakoids, grana swelling, and loss of grana stacking are among major alterations that occur in chloroplasts under heat stress (Djanaguiraman et al. 2010; Marchand et al. 2005). High temperatures also decrease the amount of photosynthetic pigments as a result of the lipid peroxidation of both chloroplast and thylakoid membranes (Djanaguiraman et al. 2010; Marchand et al. 2005).
Furthermore, high temperatures can greatly impact starch and sucrose synthesis by reducing the activity of sucrose phosphate synthase, ADP glucose pyrophosphorylase, and invertase (Chaitanya et al. 2001; Vu et al. 2001).
Photosynthetic responses of the thylakoid to heat stress significantly correlates with CO2 concentration (Sharkey and Zhang 2010). In addition, plant ability to sustain leaf gas exchange and CO2 assimilation rates under heat stress is directly associated with heat tolerance. High temperatures can significantly reduce the leaf CO2 assimilation rates during the vegetative stage (Hall 1992). Stomata closure under heat stress is the main reason of decline in the intercellular CO2 concentration, which can lead to impaired photosynthesis (Hasanuzzaman et al. 2013). Limitation of RuBisCO availability is another factor involved in the low CO2 assimilation rate (Sharkey and Zhang 2010).
2.2.5 Plant Hormones
Following heat stress, the level of selected phytohormones, such as ethylene, ABA, and salicylic acid (SA), is rapidly increased, while others, such as cytokine, auxin, and gibberellin, will be decreased (Larkindale and Huang 2005). The alterations in the level of these phytohormones accelerate plant aging (Larkindale and Huang 2005). Different abiotic stresses, including heat and drought stresses, result in increased levels of ABA (Larkindale and Huang 2005). ABA is involved in the biochemical pathways that are necessary for survival under heat-induced desiccation stress, and it is also responsible for stomatal closure under osmotic stress. Furthermore, it mediates the adaptation of plants to desiccation through modification of the expression status of numerous genes. It is also related to ROS generation in guard cells through Rboh regulation (Miller et al. 2008). It may also play a role in induction of several heat shock proteins (HSPs) to confer thermotolerance to plants (Miller et al. 2008; Wahid et al. 2007). Ethylene is a gaseous hormone, which is involved in regulation of plant growth and development by controlling seed germination, flowering, fruiting, and stress tolerance (Munné-Bosch et al. 2002). The effect of heat stress on ethylene production may vary in different plant species (Arshad and Frankenberger 2002). For example, in wheat leaves, the ethylene production is inhibited when temperature reaches to 40 °C, but in soybean plants, the ethylene production in hypocotyls is increased by increasing temperature up to 40 °C (Tan et al. 1988; Wahid et al. 2007). SA is also involved in HSRs since it functions in plant growth and development. SA prevents oxidative damage to membranes through detoxification of superoxide radicals. SA correlates in signaling pathways in response to hypersensitive response (HR) and systemic acquired resistance (SAR). SA can induce long-term thermotolerance in plants through association with the expression of HSP genes, \( {\mathrm{Ca}}^{2+} \) homeostasis, antioxidant mechanisms, improved fertility, and increased yield (Larkindale and Knight 2002; Wang and Li 2006). Among other hormones, gibberellins and cytokinins have been suggested to be involved in heat tolerance, where their effects are opposite to those of ABA. Under high temperature, the concentrations of these hormones begin to decrease, which correlates with the decline in root and shoot growth and dry matter production (Liu and Huang 2005). Also, it has been reported that the amount of endogenous auxin is reduced under heat stress, particularly in developing anthers (Teale et al. 2006).
2.3 Molecular Responses
2.3.1 Oxidative Responses
Heat stress can alter the function of many enzymes involved in different metabolic pathways. These alterations in enzymatic activity may lead to the accumulation of harmful ROS including singlet oxygen, superoxide radical \( \left({\mathrm{O}}^{2-}\right) \), hydrogen peroxide (H2O2), and hydroxyl radical (\( {\mathrm{OH}}^{-} \)) (Liu and Huang 2000). High temperatures induce generation of ROS, leading to oxidative stress, which ultimately can cause cellular injury. ROS disrupts membrane semi-permeability function by enhancing autocatalytic peroxidation of membrane lipids and pigments (Xu et al. 2006). The ROS generation can occur in peroxisomes, mitochondria, and chloroplasts, particularly in reaction centers of PSI and PSII (Apel and Hirt 2004; Sharma et al. 2012). Heat stress can dramatically increase the level of ROS, which may disturb cell homeostasis (Mittler et al. 2004). Disruption of the homeostasis balance may occur either through enhancement of ROS production or decline in antioxidant activity in the cell (Bowler et al. 1992).
Oxygen radicals are constantly produced in chloroplast and mitochondria. Singlet oxygen, which is formed during photoinhibition, and PSII electron transfer reactions, can directly oxidize DNA, proteins, and polyunsaturated fatty acids (Karuppanapandian et al. 2011). Superoxide radicals are formed in many photo-oxidation reactions in chloroplasts, electron transport chain reactions in mitochondria, and other reactions in the plasma membrane (Halliwell 2006). The function of superoxide dismutase (SOD) is to scavenge the superoxide to hydrogen peroxide, which is removed by ascorbate peroxidase (APX) or catalase (CAT) (free radical scavenging enzymes). In the presence of \( {\mathrm{Fe}}^{2+} \) and \( {\mathrm{Fe}}^{3+} \), the reaction of superoxide with hydrogen peroxide can form hydroxyl radicals (Haber–Weiss reaction). The toxicity of \( {\mathrm{OH}}^{-} \) is significantly higher than \( {\mathrm{O}}^{2-} \) and H2O2, because it can damage DNA, proteins, lipids, and other essential macromolecules. Plants are able to protect their metabolism and growth from the harmful impacts of ROS through detoxification systems (Sairam and Tyagi 2004). For example, induction of SOD or APX expression and activation is associated with the appearance of physiological injuries in plants under heat stress (Mazorra et al. 2002). However, in most plant species, extreme conditions induce higher production of ROS, which overwhelms the scavenging activity of the antioxidant system, leading to severe cellular injury (Fadzillah et al. 1996). It has been shown that under heat stress, plants accumulate higher amounts of non-enzymatic antioxidant and up-regulate the expression of antioxidant enzymes (Almeselmani et al. 2009). However in many plant species, these increased activities are not sufficient for development of stress tolerance (Almeselmani et al. 2009). Under stress conditions, protection against oxidative stress is an important factor in the determination of plant stress tolerance (Xu et al. 2006).
2.3.2 Stress-Related Proteins
Plants’ response to heat stress is composed of several integrated circuits including multiple pathways, specific cellular organelles, special cofactors, and signaling molecules coordinating an appropriate feedback. The high temperature signal is first perceived with the specific heat receptors on the membrane of the plant cells. The stimulus information is then transduced downstream leading to the activation of various heat stress-responsive genes. The products of these genes eventually result in the heat tolerance response or plant adaptation to survive during the harsh conditions (Iba 2002). The HSPs are known as the most important products of these genes for heat stress adaptation. It is believed that the HSPs are universal heat-protective agents against heat stress that maintain homeostasis in organisms. This is mainly based on the fact that the primary protein structure for HSPs is well conserved among prokaryotes and eukaryotes including higher plants. The heat shock response occurs after exposure of the plant tissue or cells to sudden high temperature stress, resulting in transient expression of the HSPs. The molecular masses of HSPs can vary but often ranges from about 10 to 200 kDa. Despite their different sizes and weights, they all have the ability of binding to structurally unstable proteins, having the chaperon-like function, and being involved in signal transduction during heat stress (Al-Whaibi 2011; Schoffl et al. 1998). The heat shock domain is the main characteristic of all HSPs, which is recognized by the presence of a conserved carboxylic terminal (Helm et al. 1993). HSPs are expressed in different locations, including the cytoplasm, ribosomes, endoplasmic reticulum, chloroplasts, mitochondria, and membranes (Vierling 1991). Certain HSPs can also be expressed in the absence of environmental stress during some stages of plant development, such as the development of pollen grains, embryogenesis, germination, and fruit ripening (Sun et al. 2002).
In plants, HSPs can be classified into five classes according to their molecular weight, amino acid sequence homologies, and functions: HSP100, HSP90, HSP70, HSP60, and the small heat shock proteins (sHSPs) (with molecular weight between 15 and 30 kDa) (Gupta et al. 2010; Kotak et al. 2007; Schoffl et al. 1998). HSPs production in plants can vary greatly according to the expression level and their type. Higher plants often contain about 20 types of sHSPs, whereas some species can express about 40 types of these proteins. These sHSPs show unusual abundance and diversity, in which it is suggested that these proteins can proffer adaptive tolerance to plants under heat stress (Korotaeva et al. 2001). In a study, wheat, rye, and maize seedling were subjected to 42 °C; the results show expression of five sHSPs in maize mitochondria while only one of them was expressed in rye and wheat (Korotaeva et al. 2001). These results suggest that higher expression of sHSPs is the reason for higher heat tolerance in maize than in wheat and rye (Korotaeva et al. 2001). In plants, six nuclear gene families encode all sHSPs, where each gene family is related to proteins present in distinct cellular compartments (Waters et al. 1996).
Under heat stress, HSPs aggregate and assemble into heat shock granules (HSGs), usually in the cytoplasm. It seems that HSGs function in protecting the protein synthesis machinery, which is critical for survival of plant cells under continuous heat stress. The aggregation state of HSPs can be essential for their role in prevention of protein denaturation caused by high temperatures (Miroshnichenko et al. 2005). The ability of plants for HSPs production, the intensity and duration of synthesis, is significantly different depending upon species and tissue types. Fast accumulation of HSPs under the stress is another key factor for developing heat tolerance in plants (Nieto-Sotelo et al. 2002).
Accumulation of HSPs is regulated by heat shock transcription factors (HSFs), which bind specifically to cis-acting sequences known as heat shock elements (HSEs) (Akerfelt et al. 2010). HSFs are main components in heat stress signaling that are sensitive to changes in temperature. Like many other transcription factors, they have a modular structure composed of a highly conserved N-terminal DNA binding domain and an adjacent oligomerization domain (OD). The DNA binding domain is characterized by an HLH motif and the oligomerization domain with a hydrophobic heptad repeat pattern (Baniwal et al. 2004). These structural domains are important in heat stress-dependent activation that switch inactive HSF monomers to the active trimeric form, which specifically bind to HSE in the promoter of HSF-responsive genes (Scharf et al. 2012).
Plants and vertebrates contain multiple families of HSF genes while invertebrates including yeast and Drosophila have a single HSF (Akerfelt et al. 2010). For example, heat shock treatment induces about 21 HSFs in Arabidopsis thaliana (Swindell et al. 2007) and at least 15 HSFs in tomato (von Koskull-Döring et al. 2007). Based on the peculiarities of the oligomerization domains, plant HSFs are classified into three conserved evolutionary classes: A, B, and C (Scharf et al. 2012). Among these three groups, class A has shown to form a regulatory network during response to heat stress, while class B or C has not shown any evident activities of transcription activators of their own (Czarnecka-Verner et al. 2004; Kotak et al. 2004).
Although low- and high-molecular-weight HSPs are the most important stress proteins toward heat stress tolerance, there are a number of other proteins that are involved in the HSR. Among those ubiquitin, cytosolic Cu/Zn-SOD, Mn-POD, Pir proteins, and dehydrins are well known. These proteins play roles in minimizing dehydration and oxidative damages, chloroplast stability, and prevention of protein degradation (Iba 2002; Khanna-Chopra and Sabarinath 2004; Schoffl et al. 1999; Yun et al. 1997). They act as chaperones to fold and unfold cellular proteins in order to inhibit adverse effects of high temperatures on the functional sites. The expression of these proteins can be specified to organelles and tissues (Wahid et al. 2007). A main function of these proteins is protection of cellular and subcellular structures against dehydration and oxidative damages (Schoffl et al. 1999).
2.3.3 Heat Stress Signaling
There are multiple signaling pathways involved in the HSR, in which some of them control expression and synthesis of HSPs, whereas others regulate the production or activation of different effector constituents (Fig. 5.3). Due to the complexity of multigenic traits, the molecular pathways involved in the HSR in plants are not fully understood. Generally, several signal transduction cascades are triggered by high temperature perception, which all together contribute in the activation of several transcription factors, including HSFs, that bind to the HSE and induce expression of HSPs, regulatory proteins, and proteins involved in metabolism and redox homeostasis (Hu et al. 2009).
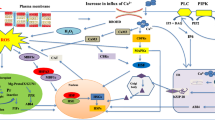
Overview of signaling pathways and factors involved in heat shock response. High temperatures affect PM and change membrane stability, which in turn causes activation of both lipid signaling and calcium channel located in PM. These activations result in an influx of calcium into the cytosol. Calcium can bind to calmodulin and activate multiple kinases and transcriptional factors, such as CBK, HSFs, and WRKY. Calcium can also lead to phosphorylation of CDPK, which activates the MAPKs signaling pathway. Another protein activated by calcium is ROS-generating NADPH oxidase (Respiratory burst oxidase homolog D-RBOHD) located in the PM (middle right). RBOHD-derived ROS triggers the ROS/redox signaling system, which in turn activates MBF1c and HSFs. The most-characterized part of the network contains heat stress transcription factors (HSFs) that regulate genes encoding heat stress proteins (HSPs), which act as molecular chaperones and repair damaged proteins (left) (Mittler et al. 2012; Qu et al. 2013; Sung et al. 2003)
A primary signaling for induction of the HSR is the role of calcium-mediated signaling. Increases in temperature are sensed at the plasma membrane (PM) by a specific calcium channel that serves as one of the primary heat sensors in plants. Activation of this membrane protein, which triggers the influx of calcium into the cytoplasm, may be due to heat-induced increase in fluidity of the PM (Saidi et al. 2011). Under heat stress, changes in membrane fluidity trigger the activation of phospholipase D (PLD) and phosphatidylinositol-4-phosphate 5-kinase (PIPK), which results in the accumulation of different lipid signaling molecules, including phosphatidylinositol-4,5-biphosphate (PIP2), myo-inositol-1,4,5-trisphosphate (IP3), and phosphatidic acid (PA). A G protein associating with PM perceives the heat signal, and leads to accumulation of PIP2 and PA. PIP2 and PA act as key mediators in the lipid signaling pathway, cytoskeletal organization, and membrane dynamics (Mishkind et al. 2009; Zheng et al. 2012). IP3, a product of PIP2 hydrolysis, is rapidly converted to IP6, which is responsible for the opening of calcium channels and stimulates the \( {\mathrm{Ca}}^{2+} \) influx into the cell (Mishkind et al. 2009; Zheng et al. 2012).
The heat-induced influx of \( {\mathrm{Ca}}^{2+} \) can trigger multiple signaling pathways in plants (Larkindale and Knight 2002; Saidi et al. 2009). Under high temperatures, the cytosolic \( {\mathrm{Ca}}^{2+} \) is sharply increased, which ultimately leads to protein phosphorylation and activation of various heat stress transcriptional factors (Saidi et al. 2011). One of the targets that cytosolic \( {\mathrm{Ca}}^{2+} \) can bind to is calcium-dependent protein kinase (CDPK), which can in turn, activate mitogen-activated protein kinase (MAPK), or the ROS-producing enzyme NADPH oxidase (RBOH). MAPK signaling pathways are highly conserved module involved in many responses to external signals, such as different abiotic stresses (Ichimura et al. 2000). CDPK can also lead to the activation of multiprotein-bridging factor 1c (MBF1c), a transcriptional regulator of the dehydration-responsive element-binding (DREB) transcription activator and several HSFs (Suzuki et al. 2011). High temperatures induce the production of DREB2A and DREB2B, which function during both heat and drought stresses. It has been shown that overexpression of DREB2A in Arabidopsis enhances thermotolerance (Qin et al. 2011). Calmodulin (CaM) is another calcium sensor protein that mediates \( {\mathrm{Ca}}^{2+} \) signal transduction. In Arabidopsis, AtCaM3 increases thermotolerance by activation of several transcription factors such as WRKY39 and HSFs. AtCaM3 also activates calmodulin-binding protein kinase (CBK), which phosphorylates HSFA1a (Liu et al. 2008; Zhang et al. 2009).
It has been shown that obstruction of \( {\mathrm{Ca}}^{2+} \) signaling by calcium channel blockers intensifies heat-induced oxidative damage in Arabidopsis (Larkindale and Knight 2002; Saidi et al. 2009). Heat stress is often accompanied by different degrees of oxidative stress, representing the presence of a cross-talk between heat and oxidative stress pathways. A short exposure to high temperatures can induce a significant increase in H2O2 levels due to NADPH oxidase activity. The histidine kinases sense the ROS signal and transduce it to HSFA4a, which in turn, activates downstream transcription factors by correlating with a MAPK signaling pathway. The transcription factors involved in the oxidative pathway are Zat, WRKY, MBF1c, and RBOH. Zat is necessary for the expression of APX and WRKY (Baniwal et al. 2007). MBF1c regulates the expression of SA and trehalose, which are important factors in the plant defense response (Suzuki et al. 2008). RBOH enhances the production of ROS signaling through the oxidation of NADPH (Miller et al. 2008). MAPK signaling pathways activate redox-sensitive transcription factors, which bind to the oxidative-sensitive cis elements in the gene promoter. These transcription factors enhance expression of antioxidants, including APX and catalase (CAT) that act as ROS scavengers under heat stress (Gill and Tuteja 2010).
Moreover, production of H2O2 stimulates the expression of heat shock response genes through activation of HSFs. Ultimately, HSPs’ production can achieve heat stress tolerance by acting as chaperons to protect protein synthesis (Kotak et al. 2007). Studies on H2O2 pretreatment, NADPH oxidase (Larkindale et al. 2005), and mitochondrial respiratory mutants have confirmed the relationship between ROS generation and induction of HSP synthesis; however, ROS roles in regulating HSPs expression under heat stress is poorly understood (Kotak et al. 2007; Kuzmin et al. 2004).
2.3.4 The Molecular and Genomic Responses to Heat Stress
Genetic studies on heat-tolerant crop plants have shown that tolerance to heat stress is a multigenic feature. Different sets of genes are involved in control of different heat tolerance components in various tissues at diverse developmental stages (Howarth 2005). Due to this complexity, many biochemical reactions involved in the molecular pathways toward heat tolerance are not yet understood completely. However, newly developed techniques in biotechnology have bestowed a better understanding of molecular and genetic basis of heat tolerance. Microarray technology, one of recent techniques, provides the possibility of simultaneous analysis of numerous genes. This approach has been widely used for identification of genes encoding Hsps and Hsfs in some model plants. For example, in Arabidopsis, 18,7, 8, 27, and 21 genes have been discovered for Hsp70, Hsp90, Hsp100, sHsp, and Hsf, respectively (Guo et al. 2008; Hu et al. 2009).
Whole-genome microarray studies on heat-stressed plants have revealed fast and global effect of heat stress on the transcriptome. In Arabidopsis, approximately 11 % of the screened genes show differential expression after 1 h of exposure to high temperatures (Busch et al. 2005). The hallmark of HSR is up-regulation of well-characterized HSPs such as Hsp70, Hsp101, and sHSPs as well as HSF (Larkindale and Vierling 2008). Additional genes have also been identified, and their expression is elevated under heat stress and are linked with stress-protective responses; these include cell respiration and oxidative stress response (Table 5.1). For example, elevated expression of genes encoding mitochondrial proteins, such as cytochrome c oxidase and subunits of NADH dehydrogenase, is associated with enhanced respiratory activity and was detected in heat-stressed plants along with elevated expression of APX, a defense enzyme regulated by HSFs, correlates with cell protection against reactive oxygen intermediate (Rizhsky et al. 2004). In other genes, their expression increases dramatically with high temperatures, and include galactinol synthase, peptidyl prolyl isomerases, enzymes in the raffinose oligosaccharide pathway and energy metabolism particularly in glycolysis, and members of DREB family of transcription factors. In addition, cluster analysis of gene expression has shown up-regulation of many genes with HSE (GAAnnTTC) (Larkindale and Vierling 2008). The HSEs located in the promoter of heat shock genes play a key role in the expression of a group of heat-inducible genes through interaction with HSFs (Hua 2009). Other common promoter motifs associated with up-regulated genes are site II motif, DRE, and ABRE (ABA response element, ACGTG). The oxidative stress-related genes, endoglucan transferase and xyloglucan endotransglycolsylases are among up-regulated genes containing ABRE sequence.
During heat stress, expression of many genes is also down-regulated. The disease resistance genes, expansions, cytochrome P450s, auxin-induced genes, and genes involved in cell detoxification (mainly glutathione S-transferases) are some of the genes whose expressions are greatly reduced under high temperature (Larkindale and Vierling 2008). The cluster analysis shows the presence of W-box and TATATA motifs in the promoter of down-regulated genes indicating their similar patterns of regulation (Molina and Grotewold 2005; Raffaele et al. 2006).
Expression data suggest that HSFs play important roles in regulating HSR, although the function of each one of these components is not yet fully understood (Guo et al. 2008). Previous studies have shown that the expression of certain HSF genes in plants is induced by environmental stresses, while others display constitutive expression. Among all 21 HSF genes in Arabidopsis, only six of them show significant increases in expression level under heat stress. Furthermore, it has been demonstrated that HSFs control the expression of HSPs, genes involved in protective environmental stress response as well as other HSFs (Busch et al. 2005; Rizhsky et al. 2004). Genome-wide transcriptome analysis of different HSF knock-out mutants has been used to elaborate the details of HSF functions. Such studies on Arabidopsis suggest that HSFA1a and HSFA1b play key roles in the initial phase of HSR (Busch et al. 2005) while HSFA2 functions in the later recovery phase and under prolonged heat stress condition (Schramm et al. 2006). In tomato, HSFA1a gene is constitutively expressed and induces expression of HAFA2 and HSFB1 under heat stress (Mishra et al. 2002). Although in tomato HSFA1a acts as a nuclear retention factor and a coactivator of HSFA2 by forming HSFA1a–HAFA2 heteroligomeric complexes, in Arabidopsis the heat stress-induced expression of HSFA2 is not influenced by either HAFA1a or HSFA1b (Bharti et al. 2004; Busch et al. 2005). Moreover, it has been shown that under high light intensity, HSFA2 regulates the expression of ascorbate peroxidase 2 (APX2), which encodes an important enzyme in oxidative stress response (Sakuma et al. 2006). These findings indicate that HSFA2 plays different roles under diverse abiotic stresses. It has been shown that the expression of Arabidopsis HSFA3 under heat stress is directly regulated by DREB2A, a transcription factor that interacts with cis-acting dehydration-responsive element (DRE) in drought and salt stress responses (Sakuma et al. 2006). In addition, the presence of DRE in the promoter of a cluster of heat-inducible genes has been demonstrated (Larkindale and Vierling 2008).
There are numerous genes and key regulators involved in heat stress tolerance, which remain to be discovered. Several biotechnological techniques, including transcriptome analysis, proffer the capability for discovering novel genes involved in the stress responses. Through these studies, additional heat-inducible transcription factors have been found that are related to thermotolerance. One of them is NF-X1 (nuclear transcription factor x-box binding 1) gene that promotes tolerance to salt and heat stress and shows a similar induction pattern to genes with DREs in their promoter (Larkindale and Vierling 2008). Heat tolerance may also be accelerated with induction of bZIP28 gene (a putative membrane-tethered transcription factor), since Arabidopsis plants harboring mutations in this gene exhibit a heat-sensitive phenotype (Gao et al. 2008). The transcriptional coactivator MBF1c, which accumulates rapidly after heat stress, is involved in several stress responses. MBF1c promotes thermotolerance by regulating several signaling pathways including salicylic acid (SA), ethylene, and trehalose during heat stress (Suzuki et al. 2008).
Among environmental stresses, high temperatures and water deficits are two main factors causing severe yield loss. Furthermore, simultaneous occurrence of different stresses is common in the field, such as high temperatures and drought periods particularly in semi-arid and arid areas. In order to develop multiple stress-tolerant crops through genetic manipulation, it is necessary to understand the molecular mechanism underlying the response of crop plants to the combination of abiotic stresses. Studies on tobacco, Arabidopsis, and wheat suggest that the response to the combination of stresses is very different from each individual stress (Rampino et al. 2012; Rizhsky et al. 2002, 2004). For example, a comparison of the effects between heat, drought, and combined stress on wheat plants showed a higher number of up-regulated genes in combined stressed plants with respect to each individual. These results indicate that combined stress induces a separate set of genes, which are not activated by each individual stress (Rampino et al. 2012; Rizhsky et al. 2004). It has been suggested that combined stress induces the activation of a specific genetic program that is mediated by key regulators (Rizhsky et al. 2002).
In the last decade, results obtained from different microarray experiments have been gathered in several genome-wide microarray datasets. These resources can be utilized in analyzing the response of Hsf and Hsp expression under different abiotic stresses. Many studies have focused on such responses to unlock the relation between different genes activated by diverse abiotic stresses (Hu et al. 2009; Rampino et al. 2012; Rizhsky et al. 2004). The results have shown that, while there are extensive overlapping response of Hsp and Hsf under different stresses, some genes show specific response to distinct stresses. For example, in a study on rice, the number of genes expressed under heat, cold, salt and drought stresses were 1,054, 276, 1,200, and 2,742 while the number of overlapped genes between heat and each one of cold, salt and drought were 33, 127, and 240 (Hu et al. 2009). Although the expression pattern is different under all these stresses, the overlapping response of Hsfs and Hsps implies their importance in cross-talk of stress signal transduction networks. Due to the fact that there is an urgent need for developing multiple stress-resistant crops to combat adverse effects of global warming, it is important to detect co-regulators of these overlapped genes (Hu et al. 2009). These results indicate that activation of similar HSFs during different environmental stresses leads to induction of similar responses. Although this fact has formed the fundamentals of many trials for developing multiple stress-resistant plants, these attempts have not yet reached to produce fruitful results due to the complexity of signal transduction in different environmental stresses.
3 Plant Adaptation to Heat Stress
The adaptation to heat stress or “heat tolerance” is commonly defined as plant ability to grow and produce economic yields under high temperatures. During evolution, plants have adapted to harsh environmental conditions by developing different stress-tolerance mechanisms. These mechanisms can be divided into long-term changes, such as phenological and morphological adaptations, or short-term acclimation, such as transpiration cooling, changing membrane lipid compositions, or leaf orientation (Adams et al. 2001). For example, plants growing in hot climate avoid heat stress by changing the orientation of leaf blades away from light, completing the entire reproductive cycle during cooler months, or developing small hairs (tomentose), small leaves, and heat-resistant buds (Fitter and Hay 2001). In well-hydrated plants, intensive transpiration keeps leaf temperature below ambient level and prevent heat stress. Such phenological and morphological adaptations are commonly linked with biochemical adaptations, such as net photosynthesis (Fitter and Hay 2001). Heat tolerance can also be induced in plants by prior treatment with high temperatures. This heat acclimation also lead to activation of the heat responsive molecular mechanisms, particularly the accumulation of HSPs (Hua 2009; Kotak et al. 2007).
Since plants are sessile organisms, their behavioral responses to abiotic stresses are strongly dependent on cellular and physiological mechanisms of adaptation. The adaptation mechanisms may differ based on different environmental stresses, developmental stages, or tissue types (Queitsch et al. 2000). Changes in temperature stimulate downstream signal transduction pathways to activate stress tolerance mechanisms to restore homeostasis and repair damaged proteins and membranes (Bohnert et al. 2006b; Vinocur and Altman 2005). Some major adaptive mechanisms activated under heat stress include HSPs, free-radical scavengers, osmoprotectants, and ion transporters (Wang et al. 2004). Heat stress manifests its initial effects on plasma membrane by inducing more fluidity of lipid bilayer. This stimulates the induction of calcium influx and cytoskeletal rearrangement, leading to up-regulation of mitogen MAPKs and CDPK. These proteins activate other mediators in the pathway, resulting in activation of tolerance responses, including production of antioxidant to cope with ROS produced in the organelles (such as mitochondria and chloroplast), or osmotic adjustment by production of compatible osmolytes for cell water balance (Bohnert et al. 2006b; Saidi et al. 2011; Sung et al. 2003). The antioxidant defense mechanisms play an essential role in the heat stress adaptation, and its strength associates with acquisition of thermotolerance (Hasanuzzaman et al. 2013). Also, higher ascorbic acid content or activities of CAT and SOD correlate with the capacity to acquire thermotolerance (Ara et al. 2013; Sairam and Tyagi 2004).
Heat tolerance also associates with higher degree of membrane lipid saturation, which increases the lipid phase transition (melting) temperatures, and inhibits membrane’s liquidity due to heat stress (Kotak et al. 2007). Under heat stress, some plant species may accumulate different types of osmolytes, including proline, sugars, sugar alcohols (polyols), tertiary sulfonium compounds, and tertiary and quaternary ammonium compounds, which may enhance heat tolerance (Singh and Grover 2008). For example, proline may buffer the cellular redox potential under environmental stresses including heat stress (Wahid and Close 2007).
Another mechanism of thermotolerance is induction of HSPs and other heat-induced proteins, such as dehydrins, LEA, Pir proteins, and ubiquitin. These proteins are involved in protein degradation pathway, oxidative stress, and protection against adverse effects of dehydration (Arora et al. 1998; Goyal et al. 2005; Schoffl et al. 1999; Yun et al. 1997). HSPs act as molecular chaperones, which facilitate removing of misfolded proteins and refolding of denatured proteins. As main components in the network of chaperon machinery, they also interact with other stress-response mechanisms, such as production of antioxidant and osmolytes. They also play a role in stress signal transduction, gene activation, and regulation of cellular redox state. HSPs confer heat tolerance to plant by improving physiological mechanisms, such as membrane stability, efficiency of assimilate partitioning, photosynthesis, and water and nutrient utilization (Al-Whaibi 2011; Kotak et al. 2007).
Alteration of gene expression is an important factor in acquisition of thermotolerance. Heat stress rapidly alters the pattern of gene expression by induction of HSPs expression, and inhibition of non-heat-induced genes through destabilization of their mRNA. Induction of HSPs expression is regulated at the transcriptional level by HSFs, which specifically bind to the HSE existing in the promoter of HSP genes (Scharf et al. 2012). Although the mechanisms leading to preferential up-regulation of the stress-responsive genes, such as HSPs are still unclear, it has been suggested that HSFs are central component in heat stress signaling (Scharf et al. 2012).
4 Biotechnological Approaches in Developing Thermotolerant Plants
Due to gradual increase in atmospheric greenhouse gases, global warming is expected to have significant impact on ecological system in the coming years. A report on region and emission scenarios (B1, A1B, and A2) has speculated that the extreme annual daily maximum temperature will likely increase by about 1–3 °C by mid-twenty-first century and by about 2–5 °C by the late twenty-first century (IPCC 2012). This rising temperature will have adverse effect on plant growth and vegetation. The growing population and subsequent increase in food demand also creates pressing needs to develop crop varieties with higher yields.
Unlike animals, plants are static and are very susceptible to different environmental stresses like drought, temperature, salinity, etc. However, some plants growing in adverse environment had evolved several strategies to cope with it. For example, expression of HSPs and osmolytes, changes in lipid membrane permeability, and production of super radicals detoxifying enzymes (Hasanuzzaman et al. 2013; Wahid et al. 2007). Understanding the molecular mechanism of thermal tolerance in plants will help to identify key genes, proteins, and different metabolites to develop heat stress-tolerant crop plants (Hasanuzzaman et al. 2013).
HSR is a complex phenomenon and current advances in X-omics (genomics, transcriptomics, epigenomics, proteomics, and metabolomics) studies have shown the involvement of different genes, proteins, and metabolites in thermotolerance as reviewed in Bokszczanin et al. (2013). Interestingly, small non-coding RNA has also been found to regulate plant stress response (Ruiz-Ferrer and Voinnet 2009; Sunkar et al. 2007). The difference in quantity of small RNAs in A. thaliana in response to various abiotic stresses was also reported (Sunkar and Zhu 2004).
The negative impact of high temperature on agriculture has been already observed (Hatfield et al. 2011; Lobell et al. 2011) and further loss may be more to global warming trends. The knowledge of conventional breeding and biotechnological strategies like marker-assisted selection (MAS) and genetic transformation is crucial to generate thermotolerant plant today than before. Realizing the potential of heat-tolerant plants to sustain the food demand, great efforts are being made to develop heat resistance plants.
4.1 Genetic Transformations
Several genetic and biochemical studies have revealed proteins encoded by certain genes or biomolecules governing thermotolerance properties of plants in certain developmental stage or plant parts. With the advent of new genetic transformations techniques, production of transgenic plants with enhanced thermotolerance was made possible. Heat stress affects almost all system of plants including its morphology, anatomy, physiology, growth, and reproduction as reviewed before (Bokszczanin et al. 2013; Wahid et al. 2007). In a similar way to any other organism, plants do also have the ability to adapt to environmental heat stress (Larkindale et al. 2005). Plants have known to use different strategies to cope with this stress by producing different chaperones, osmolytes, and secondary metabolites as reviewed (Bokszczanin et al. 2013; Wahid et al. 2007). Knowledge of genes or metabolites involved in abiotic stress response or tolerance is very crucial to develop a stress tolerance plant. Systematic study of such correlations has been made possible through advancement in different techniques including all kind of omics and high-throughput next generation sequencing. A general overview of genetic analysis of HSR and its application to develop a thermotolerant crop plant is represented in Fig. 5.4.
In addition to genetic transformation, some other strategies to develop thermotolerant plants are also known. One of them is pretreatment of seeds and plants for heat tolerance. Pretreatment of seeds and plants is a simple and fast approach to induce heat tolerance in plants. Despite the fact that genetic approaches tend to increase heat tolerance in plants, it may also suffer from low product yield. Pre-exposure to heat stress or addition of osmolytes can be used to increase thermotolerance in already high-yielding plant cultivars (Wahid et al. 2007). While genetic engineering and cross-breeding approaches may take years to produce high yielding heat-tolerant cultivars, preconditioning of plants could address immediate need to increase thermotolerance. For example, pre-sowing heat treatment of black spruce seedlings resulted in increased thermotolerance (Colclough et al. 1990). Similarly, barley seeds pre-treated with glycinebetaine, a low-molecular-weight osmolyte, demonstrated reduced membrane damage and higher photosynthetic rate alleviating effect of heat stress compared to control seeds (Wahid and Shabbir 2005). Under abiotic stress, Ca2+ acts as antioxidant in plants. Thus exogenous application of CaCl2 prior to heat treatment has shown to increase activity of super-radical scavenging enzyme including catalase, SOD, guaiacol peroxidase, that help to induce heat tolerance in plants (Kolupaev et al. 2005). Exogenous application of some of the hormones and hormone precursors was also found to induce heat stress tolerance. So far, use of ABA, salicylic acid (SA), and ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) was reported to improve the thermotolerance of A. thaliana by providing protection against oxidative damage caused from heat stress (Larkindale and Knight 2002).
Conventional breeding and MAS are other commonly used techniques for developing thermotolerant plants. These techniques have their own advantages, however, they are limited only to similar or related plant species. Heat tolerance in plants is a multigenic trait involving synergistic effect of multiple gene and its products (Wahid et al. 2007). This multigenetic nature of the process is one of the main challenges to generate a thermotolerant plant by genetic engineering. Furthermore, a plant may be more susceptible to a particular developmental stage and a specific tissue may be more prone to damage due to heat stress. So, a tissue or developmental stage-specific thermotolerance strategy could play crucial role for plant thermotolerance (Bohnert et al. 2006a; Howarth 2005). Understanding genetic backgrounds conferring plant heat tolerance would be keys to develop heat-tolerant crop cultivars with horticulture or agronomic importance. MAS is a traditional breeding technique. This technique is considered as another valuable way to develop plant with enhanced thermotolerance (Foolad 2005). Some of the quantitative trait loci (QTL) associated with heat tolerance has been identified in different plant species (Maestri et al. 2002). Four QTLs related to thermotolerance were characterized in Arabidopsis by creating heat-sensitive mutant library (Hong and Vierling 2000).
Genetic transformation has its own advantages over simplicity of above methods. Genetic engineering is specific and wide open in terms of genetic resources to prokaryotic to eukaryotic organisms. Some of the successful genetic transformation to improve thermotolerance in plant is represented in Table 5.2. Some of the most used strategies in developing a thermotolerant plant are described below.
4.2 Genetic Transformation Using Genes for HSPs and Heat Shock Factors
Initiation of HSPs production is one of the first lines of response to heat stress in most organisms ranging from prokaryotes to eukaryotes. Both sudden or a gradual raise in temperature enhanced the production of HSPs in plants (Nakamoto and Hiyama 1999; Schoffl et al. 1999). HSP’s production and accumulation in plants and its thermotolerance are clearly evident from numerous research works as reviewed in Bokszczanin et al. (2013), Singh and Grover (2008), and Wahid et al. (2007). Similarly, transcription factors regulating the expression of different HSPs (heat shock factors, HSFs) are also directly involved in thermotolerance. To date, different genetic engineering work to increase the production of different HSFs and HSPs to improve thermotolerance in plant has been reported as reviewed in Bokszczanin et al. (2013), Singh and Grover (2008), and Wahid et al. (2007).
Genetic transformation of A. thaliana with different HSFs such as Athsf1, Athsf3, and AtHsfA2 under CaMV35S promoter was reported to improve thermotolerance (Lee et al. 1995; Li et al. 2005; Prändl et al. 1998). Similarly enhanced thermotolerance was reported in A. thaliana expressing transcription factor gene OsHSFA2e from O. sativa (Yokotani et al. 2008). Constitutive expression of hsp101 gene in A. thaliana was shown to respond better to sudden changes in high temperature than controls (Queitsch et al. 2000). Also, when Arabidopsis hsp101 gene was expressed in Oryza sativa under maize Ubi 1 promoter, improved thermotolerance was observed (Katiyar-Agarwal et al. 2003).
4.3 Genetic Transformation Using Genes Involved in ROS Scavenging System
ROS produced in response to heat stress mainly causes oxidative damage to cellular components. So, different ROS scavenging enzyme systems have been used to reduce ROS and to produce thermotolerant plant. For instance, transgenic potato (Solanum tuberosum) plants expressing APX and Cu/Zn superoxide dismutase under oxidative inducible promoter (SWPA2) showed increased heat tolerance (Tang et al. 2006). Similarly, expression of HvAPX1 gene encoding APX under constitutive CaMV35S promoter also resulted in significant heat tolerance in A. thaliana compared to control plants (Shi et al. 2001).
4.4 Genetic Transformation Using Genes Involved in Synthesis of Different Osmolytes
Plants accumulate compatible solutes, low-molecular-weight organic compounds, which includes amino acids, quaternary amines, sugars, and polyols under stress conditions, especially to osmotic stresses (Gepstein et al. 2005). These osmolytes have been also known to involve and improve thermotolerance in different ways in plants. Therefore, overproduction of such a molecule is another strategy to develop plants with improved thermotolerance. For instance, expression of a bacterial choline oxidase (CodA) gene in A. thaliana, which increase internal pool (biosynthesis) of glycinebetaine, a quaternary ammonium compound, was reported to have more thermotolerance than wild type plant (Alia et al. 1998). Betaine aldehyde dehydrogenase (BADH) is an enzyme involved in glycinebetaine biosynthesis. Tobacco plant with higher level of glycinebetaine after overexpression of BADH gene from spinach showed better thermotolerance during young seedlings growth (Yang et al. 2005).
But, the role of such molecules as protecting agent might represent just for one species-specific adaption and might not be similar for all (Bokszczanin et al. 2013). For instance, accumulation of proline in response to heat stress has been identified as a protective mechanism in some plant types but not all as reviewed by Bokszczanin et al. (2013). In soybean, heat stress and drought tolerance were improved using overexpression of A. thaliana pyrroline-5-carboxylate reductase (AtP5CR) gene. However, overexpression of the Δ(1)-pyrroline-5-carboxylate synthetase 1 (AtP5CS1) gene which improves the biosynthesis of proline was found to reduce the thermotolerance in case of A. thaliana (Lv et al. 2011).
4.5 Genetic Transformation Using Genes Involved in Membrane Fluidity
A major effect of heat stress in plants is change in membrane fluidity. Protein denaturation as well as elevated level of unsaturated fatty acids in lipid bilayer of biological system cause increase in membrane fluidity (Savchenko et al. 2002). Elevated level of enzyme fatty acid desaturases was found to involve in increasing membrane fluidity by catalyzing unsaturation of membrane lipid as reported before (Murata 1983) in response to low temperature. Similarly, membrane rigidity was reported to increase with increase in saturation of membrane lipids as observed in response to high temperature (Thomas et al. 1986). The information presented above shows the possibility of developing a thermotolerant plant by altering the lipid membrane saturation. Lowering of unsaturation by lowering the amount of trienoic fatty acids than dienoic fatty acids in chloroplast membrane of a transgenic tobacco resulted in better photosynthesis and growth under heat stress (Murakami et al. 2000). In the above study, they achieved the lower unsaturation of chloroplast by silencing gene for chloroplast omega-3 fatty acid desaturase.
Plants growth as well as its yield is directly correlated with rate of photosynthesis, which in turn is severely affected by heat stress. One of the causes of photosynthesis inhibition in plant is thermal instability of Rubisco Activase, a chaperone protein required for proper functioning of Rubisco. Silencing of Rubisco Activase gene in tobacco plants has shown to increase plants’ sensitivity to heat stress than in control plants (Sharkey et al. 2001). Thus, efforts can be made to develop heat-tolerant plant by improving the expression of Rubisco Activase.
5 Conclusion
Crop losses from heat stress have serious economic impacts. It has been estimated that heat stress may cause multi-billion dollar crop damage worldwide. Loss in agricultural productivity will have other negative consequences in farming sector including rise of food prices, and higher cost of livestock production. These effects are more pronounced in the developing countries. However, heat stress is inevitable and plants have already developed various molecular physiological strategies to combat heat stress. There are numerous genes and transcription factors that work in synchronized way to produce protective proteins. With the advent of climate change and global warming, research on heat stress will become more relevant. Understanding modes of heat tolerance in plants will help us to develop better thermotolerant food crops. Availability of next generation sequencing technologies will help us understand the genomic make up of heat-tolerant plants. High-throughput computing tools will help us to identify economically important thermotolerant genes. The next step would be genetic modification of plants with thermotolerant genes. Transgenic approach to produce thermotolerant plant may not be the answer to combat crop loss due to heat stress; however, it is surely one of the options for agricultural scientists.
References
Adams SR, Cockshull KE, Cave CRJ (2001) Effect of temperature on the growth and development of tomato fruits. Ann Bot 88(5):869–877. doi:10.1006/anbo.2001.1524
Ahamed KU, Nahar K, Fujita M, Hasanuzzaman M (2010) Variation in plant growth, tiller dynamics and yield components of wheat (Triticum aestivum L.) due to high temperature stress. Adv Agric Bot 2:213–224
Akerfelt M, Morimoto RI, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11(8):545–555. doi:10.1038/nrm2938
Alia, Hayashi H, Sakamoto A, Murata N (1998) Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J 16(2):155–161
Almeselmani M, Deshmukh P, Sairam R (2009) High temperature stress tolerance in wheat genotypes: role of antioxidant defence enzymes. Acta Agron Hung 57(1):1–14
Al-Whaibi MH (2011) Plant heat-shock proteins: a mini review. J King Saud Univ Sci 23(2):139–150
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55(1):373–399
Ara N, Nakkanong K, Lv W, Yang J, Hu Z, Zhang M (2013) Antioxidant enzymatic activities and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (“Cucurbita maxima” and “Cucurbita moschata”) and their interspecific inbred line “Maxchata”. Int J Mol Sci 14(12):24008–24028
Arora R, Pitchay DS, Bearce BC (1998) Water-stress-induced heat tolerance in geranium leaf tissues: a possible linkage through stress proteins? Physiol Plant 103(1):24–34
Arshad M, Frankenberger WTJ (2002) Ethylene, agricultural sources and applications. Kluwer Academic/Plenum, New York
Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, von Koskull-Döring P (2004) Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 29(4):471–487
Baniwal SK, Chan KY, Scharf KD, Nover L (2007) Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J Biol Chem 282(6):3605–3613. doi:10.1074/jbc.M609545200
Bharti K, Von Koskull-Döring P, Bharti S, Kumar P, Tintschl-Körbitzer A, Treuter E, Nover L (2004) Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell 16(6):1521–1535. doi:10.1105/tpc.019927
Bohnert HJ, Gong Q, Li P, Ma S (2006a) Unraveling abiotic stress tolerance mechanisms—getting genomics going. Genome studies and molecular genetics: Part 1: Model legumes/edited by Nevin D Young and Randy C Shoemaker; Part 2: Maize genomics/edited by Susan R Wessler. Plant biotechnology/edited by John Salmeron and Luis R Herrera-Estrella. Curr Opin Plant Biol 9(2):180–188
Bohnert HJ, Gong Q, Li P, Ma S (2006b) Unraveling abiotic stress tolerance mechanisms–getting genomics going. Curr Opin Plant Biol 9(2):180–188. doi:10.1016/j.pbi.2006.01.003
Bokszczanin KL, Solanaceae Pollen Thermotolerance Initial Training Network (SPOT-ITN) Consortium, Fragkostefanakis S (2013) Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front Plant Sci 4:315. doi:10.3389/fpls.2013.00315
Bowler C, Montagu MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43(1):83–116
Busch W, Wunderlich M, Schöffl F (2005) Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J 41(1):1–14
Camejo D, Rodríguez P, Morales MA, Dell’Amico JM, Torrecillas A, Alarcón JJ (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162(3):281–289
Chaitanya KV, Sundar D, Reddy AR (2001) Mulberry leaf metabolism under high temperature stress. Biol Plant 44(3):379–384
Colclough M, Blumwald E, Colombo SJ (1990) The induction of heat tolerance in black spruce seedlings. In: Paper presented at the annual meeting of the American Society of Plant Physiologists, Indianapolis, IN, USA
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129(4):1773–1780. doi:10.1104/pp. 002170
Czarnecka-Verner E, Pan S, Salem T, Gurley WB (2004) Plant class B HSFs inhibit transcription and exhibit affinity for TFIIB and TBP. Plant Mol Biol 56(1):57–75. doi:10.1007/s11103-004-2307-3
De Ronde JA, Cress WA, Krüger GH, Strasser RJ, Van Staden J (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol 161(11):1211–1224. doi:10.1016/j.jplph.2004.01.014
Demirevska-Kepova K, Holzer R, Simova-Stoilova L, Feller U (2005) Heat stress effects on ribulose-1,5-bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol Plant 49(4):521–525
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48(12):999–1007
El-Kereamy A, Bi YM, Ranathunge K, Beatty PH, Good AG, Rothstein SJ (2012) The rice R2R3-MYB transcription factor OsMYB55 is involved in the tolerance to high temperature and modulates amino acid metabolism. PLoS One 7(12):e52030. doi:10.1371/journal.pone.0052030
Essemine J, Ammar S, Bouzid S (2010) Impact of heat stress on germination and growth in higher plants: physiological biochemical and molecular repercussions and mechanisms of defence. J Biol Sci 10:565–572
Fadzillah NM, Gill V, Finch R, Burdon R (1996) Chilling, oxidative stress and antioxidant responses in shoot cultures of rice. Planta 199(4):552–556
Fitter AH, Hay RKM (2001) Environmental physiology of plants, 3rd edn. Academic Press, London, pp 367
Foolad MR (2005) Breeding for abiotic stress tolerances in tomato. In: Ashraf M, Harris PJC (eds) Abiotic stresses: plant resistance through breeding and molecular approaches. Haworth Press, New York, pp 613–684
Fouad WM, Rathinasabapathi B (2006) Expression of bacterial L-aspartate-alpha-decarboxylase in tobacco increases beta-alanine and pantothenate levels and improves thermotolerance. Plant Mol Biol 60(4):495–505. doi:10.1007/s11103-005-4844-9
Gao H, Brandizzi F, Benning C, Larkin RM (2008) A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc Natl Acad Sci U S A 105(42):16398–16403. doi:10.1073/pnas.0808463105
Gepstein S, Grover A, Blumwald E (2005) Producing biopharmaceuticals in the desert: building an abiotic stress tolerance in plants for salt, heat and drought. In: Knablein J, Muller RH (eds) Modern biopharmaceuticals. Wiley, Weinhaum, pp 967–994
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. doi:10.1016/j.plaphy.2010.08.016
Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388(1):151–157
Guilioni L, Wery J, Tardieu F (1997) Heat stress-induced abortion of buds and flowers in pea: is sensitivity linked to organ age or to relations between reproductive organs? Ann Bot 80(2):159–168
Guo J, Wu J, Ji Q, Wang C, Luo L, Yuan Y, Wang J (2008) Genome-wide analysis of heat shock transcription factor families in rice and Arabidopsis. J Genet Genomics 35(2):105–118
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86(11–12):377–384
Hall AE (1992) Breeding for heat tolerance. In: Janick J (ed) Plant breeding reviews. Wiley, New York, pp 129–168
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684
Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D, Wolfe D (2011) Climate impacts on agriculture: implications for crop production. Agron J 103(2):351–370. doi:10.2134/agronj2010.0303
Helm KW, LaFayette PR, Nagao RT, Key JL, Vierling E (1993) Localization of small heat shock proteins to the higher plant endomembrane system. Mol Cell Biol 13(1):238–247
Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci U S A 97(8):4392–4397
Howarth CJ (2005) Genetic improvements of tolerance to high temperature. In: Ashraf M, Harris PJCE (eds) Abiotic stresses: plant resistance through breeding and molecular approaches. Howarth Press Inc., New York
Hu W, Hu G, Han B (2009) Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci 176(4):583–590
Hua J (2009) From freezing to scorching, transcriptional responses to temperature variations in plants. Curr Opin Plant Biol 12(5):568–573. doi:10.1016/j.pbi.2009.07.012
Huang B, Xu C (2008) Identification and characterization of proteins associated with plant tolerance to heat stress. J Integr Plant Biol 50(10):1230–1237
Huang J, Wang MM, Jiang Y, Bao YM, Huang X, Sun H, Zhang HS (2008) Expression analysis of rice A20/AN1-type zinc finger genes and characterization of ZFP177 that contributes to temperature stress tolerance. Gene 420(2):135–144. doi:10.1016/j.gene.2008.05.019
Iba K (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53:225–245. doi:10.1146/annurev.arplant.53.100201.160729
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24(5):655–665
IPCC (2012) Managing the risks of extreme events and disasters to advance climate change adaptation. In: Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL et al (eds) A special report of Working Groups I and II of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, p 522
Ismail AM, Hall AE (1999) Reproductive-stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop Sci 39(6):1762–1768
Jin XF, Xiong AS, Peng RH, Liu JG, Gao F, Chen JM, Yao QH (2010) OsAREB1, an ABRE-binding protein responding to ABA and glucose, has multiple functions in Arabidopsis. BMB Rep 43(1):34–39
Karim MA, Fracheboud Y, Stamp P (1997) Heat tolerance of maize with reference of some physiological characteristics. Ann Bangladesh Agric 7:27–33
Karim MDA, Fracheboud Y, Stamp P (1999) Photosynthetic activity of developing leaves of Zea mays is less affected by heat stress than that of developed leaves. Physiol Plant 105(4):685–693
Karuppanapandian T, Wang HW, Prabakaran N, Jeyalakshmi K, Kwon M, Manoharan K, Kim W (2011) 2,4-dichlorophenoxyacetic acid-induced leaf senescence in mung bean (Vigna radiata L. Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiol Biochem 49(2):168–177
Katiyar-Agarwal S, Agarwal M, Grover A (2003) Heat-tolerant basmati rice engineered by over-expression of hsp101. Plant Mol Biol 51(5):677–686
Khanna-Chopra R, Sabarinath S (2004) Heat-stable chloroplastic Cu/Zn superoxide dismutase in Chenopodium murale. Biochem Biophys Res Commun 320(4):1187–1192
Ko CB, Woo YM, Lee DJ, Lee MC, Kim CS (2007) Enhanced tolerance to heat stress in transgenic plants expressing the GASA4 gene. Plant Physiol Biochem 45(9):722–728. doi:10.1016/j.plaphy.2007.07.010
Kolb PF, Robberecht R (1996) High temperature and drought stress effects on survival of Pinus ponderosa seedlings. Tree Physiol 16(8):665–672
Kolupaev Y, Akinina G, Mokrousov A (2005) Induction of heat tolerance in wheat coleoptiles by calcium ions and its relation to oxidative stress. Russ J Plant Physiol 52:199–204
Korotaeva NE, Antipina AI, Grabelynch OI, Varakina NN, Borovski GB, Voinikov VK (2001) Mitochondrial low-molecular-weight heat shock proteins and tolerance of crop plant’s mitochondria to hyperthermia. Fizio Biokhim Kul’turn Rasten 29:271–276
Kotak S, Port M, Ganguli A, Bicker F, von Koskull-Döring P (2004) Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J 39(1):98–112. doi:10.1111/j.1365-313X.2004.02111.x
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf K-D (2007) Complexity of the heat stress response in plants. Physiology and metabolism Edited by Clint Chapple and Malcolm M Campbell. Curr Opin Plant Biol 10(3):310–316
Krumova SB, Dijkema C, de Waard P, Van As H, Garab G, van Amerongen H (2008) Phase behavior of phosphatidylglycerol in spinach thylakoid membranes as revealed by 31P-NMR. Biochim Biophys Acta 1778(4):997–1003. doi:10.1016/j.bbamem.2008.01.004
Kuzmin EV, Karpova OV, Elthon TE, Newton KJ (2004) Mitochondrial respiratory deficiencies signal up-regulation of genes for heat shock proteins. J Biol Chem 279(20):20672–20677. doi:10.1074/jbc.M400640200
Larkindale J, Huang B (2005) Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul 47(1):17–28
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128(2):682–695. doi:10.1104/pp. 010320
Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146(2):748–761. doi:10.1104/pp. 107.112060
Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138(2):882–897. doi:10.1104/pp. 105.062257
Larkindale J, Mishkind M, Vierling E (2007) Plant responses to high temperature. In: Jenks MA, Hasegawa PM (eds) Plant abiotic stress. Blackwell, Oxford, pp 100–144
Larkindale J, Mishkind M, Vierling E (2008) Plant responses to high temperature. In: Jenks MA, Hasegawa PM (eds) Plant abiotic stress. Blackwell, Oxford, pp 100–144
Lee JH, Hübel A, Schöffl F (1995) Derepression of the activity of genetically engineered heat shock factor causes constitutive synthesis of heat shock proteins and increased thermotolerance in transgenic Arabidopsis. Plant J 8(4):603–612
Li C, Chen Q, Gao X, Qi B, Chen N, Xu S, Wang X (2005) AtHsfA2 modulates expression of stress responsive genes and enhances tolerance to heat and oxidative stress in Arabidopsis. Sci China C Life Sci 48(6):540–550
Li S, Li F, Wang J, Zhang WEN, Meng Q, Chen THH, Yang X (2011) Glycinebetaine enhances the tolerance of tomato plants to high temperature during germination of seeds and growth of seedlings. Plant Cell Environ 34(11):1931–1943
Lim C, Yang K, Hong J, Choi J, Yun D-J, Hong J et al (2006) Gene expression profiles during heat acclimation in Arabidopsis thaliana suspension-culture cells. J Plant Res 119(4):373–383
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40(2):503–510
Liu X, Huang B (2005) Root physiological factors involved in cool-season grass response to high soil temperature. Environ Exp Bot 53(3):233–245
Liu HT, Gao F, Li GL, Han JL, Liu DL, Sun DY, Zhou RG (2008) The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J 55(5):760–773. doi:10.1111/j.1365-313X.2008.03544.x
Liu ZB, Wang JM, Yang FX, Yang L, Yue YF, Xiang JB, Yang Y (2014) A novel membrane-bound E3 ubiquitin ligase enhances the thermal resistance in plants. Plant Biotechnol J 12(1):93–104. doi:10.1111/pbi.12120
Lobell DB, Schlenker W, Costa-Roberts J (2011) Climate trends and global crop production since 1980. Science 333(6042):616–620. doi:10.1126/science.1204531
Lv WT, Lin B, Zhang M, Hua XJ (2011) Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol 156(4):1921–1933. doi:10.1104/pp. 111.175810
Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen H, Marmiroli N (2002) Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol Biol 48(5–6):667–681
Marchand FL, Mertens S, Kockelbergh F, Beyens L, Nijs I (2005) Performance of High Arctic tundra plants improved during but deteriorated after exposure to a simulated extreme temperature event. Glob Chang Biol 11(12):2078–2089
Mazorra LM, Núñez M, Hechavarria M, Coll F, Sánchez-Blanco MJ (2002) Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol Plant 45(4):593–596
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133(3):481–489. doi:10.1111/j.1399-3054.2008.01090.x
Miroshnichenko S, Tripp J, Nieden U, Neumann D, Conrad U, Manteuffel R (2005) Immunomodulation of function of small heat shock proteins prevents their assembly into heat stress granules and results in cell death at sublethal temperatures. Plant J 41(2):269–281
Mishkind M, Vermeer JE, Darwish E, Munnik T (2009) Heat stress activates phospholipase D and triggers PIP accumulation at the plasma membrane and nucleus. Plant J 60(1):10–21. doi:10.1111/j.1365-313X.2009.03933.x
Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD (2002) In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev 16(12):1555–1567. doi:10.1101/gad.228802
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37(3):118–125
Molina C, Grotewold E (2005) Genome wide analysis of Arabidopsis core promoters. BMC Genomics 6:25. doi:10.1186/1471-2164-6-25
Munné-Bosch S, López-Carbonell M, Alegre L, Van Onckelen HA (2002) Effect of drought and high solar radiation on 1-aminocyclopropane-1-carboxylic acid and abscisic acid concentrations in Rosmarinus officinalis plants. Physiol Plant 114(3):380–386
Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K (2000) Trienoic fatty acids and plant tolerance of high temperature. Science 287(5452):476–479
Murata N (1983) Molecular species composition of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Cell Physiol 24(1):81–86
Nakamoto H, Hiyama T (1999) Heat-shock proteins and temperature stress. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 399–416
Nieto-Sotelo J, Martínez LM, Ponce G, Cassab GI, Alagón A, Meeley RB, Yang R (2002) Maize HSP101 plays important roles in both induced and basal thermotolerance and primary root growth. Plant Cell 14(7):1621–1633
Ono K, Hibino T, Kohinata T, Suzuki S, Tanaka Y, Nakamura T, Takabe T (2001) Overexpression of DnaK from a halotolerant cyanobacterium Aphanothece halophytica enhances the high-temperature tolerance of tobacco during germination and early growth. Plant Sci 160(3):455–461
Park SM, Hong CB (2002) Class I small heat-shock protein gives thermotolerance in tobacco. J Plant Physiol 159(1):25–30
Patel D, Franklin KA (2009) Temperature-regulation of plant architecture. Plant Signal Behav 4(7):577–579
Prändl R, Hinderhofer K, Eggers-Schumacher G, Schöffl F (1998) HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol Gen Genet 258(3):269–278
Prasad PVV, Boote KJ, Allen LH Jr (2006) Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agr Forest Meteorol 139(3–4):237–251
Qi Y, Wang H, Zou Y, Liu C, Liu Y, Wang Y, Zhang W (2011) Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett 585(1):231–239. doi:10.1016/j.febslet.2010.11.051
Qin F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 52(9):1569–1582. doi:10.1093/pcp/pcr106
Qu A-L, Ding Y-F, Jiang Q, Zhu C (2013) Molecular mechanisms of the plant heat stress response. Biochem Biophys Res Commun 432(2):203–207
Quan R, Shang M, Zhang H, Zhao Y, Zhang J (2004) Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2(6):477–486. doi:10.1111/j.1467-7652.2004.00093.x
Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12(4):479–492
Raffaele S, Rivas S, Roby D (2006) An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Lett 580(14):3498–3504. doi:10.1016/j.febslet.2006.05.027
Rampino P, Mita G, Fasano P, Borrelli GM, Aprile A, Dalessandro G, Perrotta C (2012) Novel durum wheat genes up-regulated in response to a combination of heat and drought stress. Plant Physiol Biochem 56:72–78. doi:10.1016/j.plaphy.2012.04.006
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130(3):1143–1151. doi:10.1104/pp. 006858
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134(4):1683–1696. doi:10.1104/pp. 103.033431
Ruiz-Ferrer V, Voinnet O (2009) Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60:485–510. doi:10.1146/annurev.arplant.043008.092111
Saidi Y, Finka A, Muriset M, Bromberg Z, Weiss YG, Maathuis FJ, Goloubinoff P (2009) The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21(9):2829–2843. doi:10.1105/tpc.108.065318
Saidi Y, Peter M, Finka A, Cicekli C, Vigh L, Goloubinoff P (2010) Membrane lipid composition affects plant heat sensing and modulates Ca(2+)-dependent heat shock response. Plant Signal Behav 5(12):1530–1533
Saidi Y, Finka A, Goloubinoff P (2011) Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytol 190(3):556–565. doi:10.1111/j.1469-8137.2010.03571.x
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25(2):163–171
Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K (2006) Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci U S A 103(49):18822–18827. doi:10.1073/pnas.0605639103
Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120(2):179–186
Sanmiya K, Suzuki K, Egawa Y, Shono M (2004) Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. FEBS Lett 557(1–3):265–268
Savchenko GE, Klyuchareva EA, Abramchik LM, Serdyuchenko EV (2002) Effect of periodic heat shock on the inner membrane system of etioplasts. Russ J Plant Physiol 49(3):349–359
Scharf K-D, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta 1819(2):104–119
Schoffl F, Prandl R, Reindl A (1998) Regulation of the heat shock response. Plant Physiol 117:1135–1141
Schoffl F, Prandl R, Reindl A (1999) Molecular responses to heat stress. In: Shinozaki K, Yamaguchi-Shinozaki K (eds) Molecular responses to cold, drought, heat and salt stress in higher plants. R.G. Landes, Austin, pp 81–98
Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Döring P (2006) The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol Biol 60(5):759–772. doi:10.1007/s11103-005-5750-x
Sharkey TD, Zhang R (2010) High temperature effects on electron and proton circuits of photosynthesis. J Integr Plant Biol 52(8):712–722
Sharkey TD, Badger MR, von Caemmerer S, Andrews TJ (2001) Increased heat sensitivity of photosynthesis in tobacco plants with reduced Rubisco activase. Photosynth Res 67(1–2):147–156. doi:10.1023/A:1010633823747
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:26. doi:10.1155/2012/217037
Shi WM, Muramoto Y, Ueda A, Takabe T (2001) Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene 273(1):23–27
Simões-Araújo JL, Rumjanek NG, Margis-Pinheiro M (2003) Small heat shock proteins genes are differentially expressed in distinct varieties of common bean. Braz J Plant Physiol 15:33–41
Singh A, Grover A (2008) Genetic engineering for heat tolerance in plants. Physiol Mol Biol Plants 14(1–2):155–166
Smertenko A, DrÁBer P, ViklickÝ V, OpatrnÝ Z (1997) Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells. Plant Cell Environ 20(12):1534–1542
Sohn SO, Back K (2007) Transgenic rice tolerant to high temperature with elevated contents of dienoic fatty acids. Biol Plant 51(2):340–342
Sun W, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577(1):1–9
Sung DY, Kaplan F, Lee KJ, Guy CL (2003) Acquired tolerance to temperature extremes. Trends Plant Sci 8(4):179–187. doi:10.1016/S1360-1385(03)00047-5
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16(8):2001–2019. doi:10.1105/tpc.104.022830
Sunkar R, Chinnusamy V, Zhu J, Zhu J-K (2007) Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci 12(7):301–309. doi:10.1016/j.tplants.2007.05.001
Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R (2008) The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem 283(14):9269–9275. doi:10.1074/jbc.M709187200
Suzuki N, Sejima H, Tam R, Schlauch K, Mittler R (2011) Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. Plant J 66(5):844–851. doi:10.1111/j.1365-313X.2011.04550.x
Swindell W, Huebner M, Weber A (2007) Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics 8(1):1–15
Tan C, Yu ZW, Yang HD, Yu SW (1988) Effect of high temperature on ethylene production in two plant tissues. Acta Phytophysiol Sin 14:373–379
Tang L, Kwon SY, Kim SH, Kim JS, Choi JS, Cho KY, Lee HS (2006) Enhanced tolerance of transgenic potato plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against oxidative stress and high temperature. Plant Cell Rep 25(12):1380–1386. doi:10.1007/s00299-006-0199-1
Teale WD, Paponov IA, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7(11):847–859. doi:10.1038/nrm2020
Thomas PG, Dominy PJ, Vigh L, Mansourian AR, Quinn PJ, Williams WP (1986) Increased thermal stability of pigment-protein complexes of pea thylakoids following catalytic hydrogenation of membrane lipids. Biochim Biophys Acta 849(1):131–140
Tsukaguchi T, Kawamitsu Y, Takeda H, Suzuki K, Egawa Y (2003) Water status of flower buds and leaves as affected by high temperature in heat-tolerant and heat-sensitive cultivars of snap bean (Phaseolus vulgaris L.). Plant Prod Sci 6(1):24–27
Turóczy Z, Kis P, Török K, Cserháti M, Lendvai A, Dudits D, Horváth GV (2011) Overproduction of a rice aldo-keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol Biol 75(4–5):399–412. doi:10.1007/s11103-011-9735-7
Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42(1):579–620
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16(2):123–132
Vollenweider P, Günthardt-Goerg MS (2005) Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ Pollut 137(3):455–465
von Koskull-Döring P, Scharf K-D, Nover L (2007) The diversity of plant heat stress transcription factors. Trends Plant Sci 12(10):452–457
Vu JCV, Gesch RW, Pennanen AH, Allen Hartwell L Jr, Boote KJ, Bowes G (2001) Soybean photosynthesis, Rubisco, and carbohydrate enzymes function at supraoptimal temperatures in elevated CO2. J Plant Physiol 158(3):295–307
Wahid A, Close TJ (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51(1):104–109
Wahid A, Shabbir A (2005) Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycinebetaine. Plant Growth Regul 46(2):133–141
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61(3):199–223
Wang L-J, Li S-H (2006) Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci 170(4):685–694
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9(5):244–252
Waters ER, Lee GJ, Vierling E (1996) Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot 47(3):325–338. doi:10.1093/jxb/47.3.325
Wheeler T, von Braun J (2013) Climate change impacts on global food security. Science 341(6145):508–513. doi:10.1126/science.1239402
Willits DH, Peet MM (1998) The effect of night temperature on greenhouse grown tomato yields in warm climates. Agr Forest Meteorol 92(3):191–202. doi:10.1016/S0168-1923(98)00089-6
Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004) Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ 27(6):717–724
Wu Q, Lin J, Liu JZ, Wang X, Lim W, Oh M, Park S (2012) Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol J 10(8):945–955. doi:10.1111/j.1467-7652.2012.00723.x
Xu S, Li J, Zhang X, Wei H, Cui L (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56(3):274–285
Yang X, Liang Z, Lu C (2005) Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138(4):2299–2309. doi:10.1104/pp. 105.063164
Yeh CH, Kaplinsky NJ, Hu C, Charng YY (2012) Some like it hot, some like it warm: phenotyping to explore thermotolerance diversity. Plant Sci 195:10–23. doi:10.1016/j.plantsci.2012.06.004
Yokotani N, Ichikawa T, Kondou Y, Matsui M, Hirochika H, Iwabuchi M, Oda K (2008) Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta 227(5):957–967. doi:10.1007/s00425-007-0670-4
Young LW, Wilen RW, Bonham‐Smith PC (2004) High temperature stress of Brassica napus during flowering reduces micro‐ and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J Exp Bot 55(396):485–495. doi:10.1093/jxb/erh038
Yun D-J, Zhao Y, Pardo JM, Narasimhan ML, Damsz B, Lee H, Bressan RA (1997) Stress proteins on the yeast cell surface determine resistance to osmotin, a plant antifungal protein. Proc Natl Acad Sci U S A 94(13):7082–7087
Zhang Y, Mian MAR, Bouton JH (2006) Recent molecular and genomic studies on stress tolerance of forage and turf grasses. Crop Sci 46(2):497–511. doi:10.2135/cropsci2004.0572
Zhang W, Zhou RG, Gao YJ, Zheng SZ, Xu P, Zhang SQ, Sun DY (2009) Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol 149(4):1773–1784. doi:10.1104/pp. 108.133744
Zhao L, Wang Z, Lu Q, Wang P, Li Y, Lv Q, Li W (2013) Overexpression of a GmGBP1 ortholog of soybean enhances the responses to flowering, stem elongation and heat tolerance in transgenic tobaccos. Plant Mol Biol 82(3):279–299. doi:10.1007/s11103-013-0062-z
Zheng S-Z, Liu Y-L, Li B, Shang Z-L, Zhou R-G, Sun D-Y (2012) Phosphoinositide-specific phospholipase C9 is involved in the thermotolerance of Arabidopsis. Plant J 69(4):689–700
Źróbek-Sokolnik A (2012) Temperature stress and responses of plants. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, pp 113–134
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Hemmati, H., Gupta, D., Basu, C. (2015). Molecular Physiology of Heat Stress Responses in Plants. In: Pandey, G. (eds) Elucidation of Abiotic Stress Signaling in Plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2540-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2540-7_5
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2539-1
Online ISBN: 978-1-4939-2540-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)