Abstract
Carotenoids are the colorful pigments abundant in fruits and vegetables that constitute an important part of the human diet. Decades of research on carotenoids has improved our understanding of the role of these ubiquitous pigments, which have emerged as important players in the fight against chronic and infectious diseases. This chapter describes the many facets of carotenoids including their occurrence and main dietary sources, the efficiency of absorption, and conversion to vitamin A. The role of conversion enzymes, the carotenoid oxygenases (BCMO1 and BCDO2), is explained, as well as the importance of carotenoid metabolites as bioactive compounds in the regulation of retinoid actions in the body. Their vital role as a source of vitamin A is reviewed in the context of increased need to prevent vitamin A deficiency in pregnancy and infancy, especially in developing countries. Specific functions in the macula and lens of the eye, in the corpus luteum of the ovary, and in skin are discussed. A description of the associations of plasma and dietary carotenoids with the risk of various acute and chronic diseases and conditions (cognitive decline, cancer, cardiovascular disease, diabetes, obesity, macular degeneration) is provided, along with explanations for possible mechanisms of action. Specifically, their antioxidant function in conditions of oxidative stress and inflammation is examined. The possibility of toxic effects for some carotenoids under certain circumstances is considered. Our knowledge of carotenoids continues to evolve with emerging scientific evidence, and a better understanding of these important plant pigments may improve human health and well-being worldwide.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
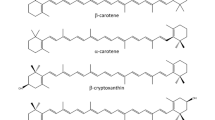
Although there are about 600 known carotenoids in nature, the human diet contains approximately 40, and only ~ 10 were found in human tissues and plasma [1]. Some abundant dietary carotenoids, like violaxanthin and antheraxanthin in green plant tissues, are poorly absorbed in the human intestinal tract. Nevertheless, humans and monkeys are able to absorb and utilize a remarkably wide variety of carotenoids, from the nonpolar hydrocarbons (carotenes) to polar hydroxycarotenoids (lutein, zeaxanthin) [2] . Carotenoids containing an unsubstituted β-ionone ring (β-carotene, α-carotene, γ-carotene, β-cryptoxanthin) are partially converted to vitamin A in the intestinal mucosa and other tissues. Other animals may absorb only carotenes (cows, felines) [3], or are practically unable to absorb any carotenoids unless fed with large pharmacological doses (carnivores) [4]. Some animals seem to absorb only provitamin A carotenoids and convert them so efficiently to vitamin A that none can be found in their blood or tissues (rats, mice). Birds utilize provitamin A carotenoids, as well as polar hydroxy- and keto-carotenoids, to produce pigments coloring their skin and feathers [5]. Because of these great differences in carotenoid utilization and metabolism , it is difficult to translate the data obtained from research with laboratory animals to human physiological response and dietary recommendations. However, genetic and proteomic investigations in animals and humans may reveal the causes of the differences between species and within human populations [6].
This chapter considers the role of carotenoids in various aspects of human physiology, possible benefits of dietary intake in preventing and treating disease, as well as the controversial issues of supplementation. Despite a very substantial body of existing literature, we are still at the beginning in the understanding of these complex problems.
Food Sources and Bioavailability
Plants are the main source of carotenoids in our diet, since the bright colors of many fruits and vegetables are due to the high content of these pigments . Carrots, sweet potato, pumpkin, squash, mango, and apricots are rich in β-carotene , which is also abundant in the dark-green leaves of spinach, turnip greens, and collards [7]. Carrots, pumpkin, and squash also contain considerable amounts of α-carotene, while oranges, tangerines, red peppers, yellow papaya, and persimmons are the main sources of β-cryptoxanthin. Lycopene is provided mostly by tomatoes and tomato products, but is also present in watermelon, guava, pink grapefruit, and red papaya. Lutein and zeaxanthin are delivered by dark-green leaves, broccoli, and corn (maize) .
Canned, cooked, or dried fruits and vegetables often contain more carotenoids per unit of weight than their fresh counterparts, due to dehydration, although some losses occur in processing at high temperatures. However, the bioavailability of carotenoids is greatly increased by processing (mincing, pureeing, cooking, canning) since it releases them from the food matrix. Thus, they may be easier to digest in the intestine, i.e., to incorporate into micelles of lipids and bile salts. Carotenoids are fat-soluble pigments, and therefore require the presence of fat in the same meal to be absorbed. Salad dressings should contain some fat to facilitate the absorption of carotenoids [8]. Mixed dishes containing carotenoids, like soups, stews, casseroles, and sauces, are better vehicles for carotenoid delivery than raw produce. For the same reason, animal sources of carotenoids are excellent providers of bioavailable β-carotene (milk, butter, cheese, and beef liver) or lutein and zeaxanthin (eggs, chicken fat, liver, and skin) .
Major contributors of individual carotenoids, as well as of total provitamin A carotenoids, in the diet of adult Americans are listed in Table 3.1 [9]. The average daily intake of carotenoids by adults > 19 years of age is close to 10 mg/day, including 2.63 mg provitamin A carotenoids (2.11 mg β-carotene , 0.39 mg α-carotene, 0.13 mg β-cryptoxanthin), 5.52 mg lycopene, and 1.50 mg lutein and zeaxanthin [10]. However, it was estimated from the National Health and Nutrition Examination Survey (NHANES) 2003–2006 data that only 5 % of adult males and 7 % of adult females meet dietary guidance recommendations for fruit and vegetable intake. Those individuals have a two- to threefold higher intake of carotenoids than the rest of the US population. Table 3.2 provides a list of mixed foods that contribute to carotenoid intake and are well accepted even by people who do not eat many fruits and vegetables .
Absorption of carotenoids in the intestine was long considered to occur passively together with dietary lipids [11]. After their release from plant tissues, they must be dissolved in fat, forming emulsions with the aid of bile acids. Carotenoid-containing lipid micelles are absorbed by the intestinal mucosal cells and incorporated in chylomicrons; however, this universal scheme does not explain considerable differences in carotenoid absorption among animal species. Recent cell culture and animal model studies indicate that carotenoid absorption is protein-mediated by a scavenger receptor class B type 1 (SR-B1), which also facilitates the uptake of α-tocopherol and cholesterol [6]. SR-B1 is regulated (attenuated) by the intestine-specific homeobox factor (ISX), which in turn is activated by retinoic acid . Further studies of the differences in activity and expression of these proteins may help to elucidate the diversity of carotenoid absorption among various species, as well as individual differences in the human population. It is worth mentioning that careful balance studies of carotenoids measured in the diet and feces provide some assessment of carotenoid absorption, although it may be overestimated due to bacterial degradation in the colon. The absorption of β-carotene from plant sources ranged from 5 to 65 % in adult humans [12] according to studies in the Netherlands, UK, and USA, a demonstration of the wide variation in carotenoid absorption within the human population .
Carotenoid Metabolism
The absorbed provitamin A carotenoids are centrally cleaved by β-carotene-15,15′-monooxygenase (BCMO1), producing retinal (vitamin A aldehyde) that is quickly reduced to retinol . Irreversible oxidation of retinol results in retinoic acid, which mediates the vitamin A functions in growth and development by binding to retinoic acid receptors (RARs). BCMO1, like the absorption facilitator SR-B1, is regulated (attenuated) by ISX, a transcription factor activated by RARs [6]. A heterozygotic mutation in BCMO1 causes elevated β-carotene and low retinol concentrations in blood [13]. Normally, about 40 % of absorbed β-carotene is not converted to vitamin A in the intestine, but incorporated in chylomicrons, entering circulation and taken up in various tissues by lipoprotein-specific receptors. Since BCMO1 is expressed in the liver and peripheral tissues (glandular cells of stomach, pancreas, colon, prostate, mammary tissue, steroidogenic cells of ovary, testis, and adrenals, as well as skin keratocytes, muscle cells, and retinal pigmented epithelium of the eyes), local production of retinoids may also occur .
The efficiency of provitamin A carotenoid conversion to vitamin A depends on bioavailability, absorption, and cleavage reactions that are obviously affected by many factors, like food preparation, health, and genetic variability in human subjects [12,14]. Low vitamin A status may increase the conversion in the absence of parasites, anemia, and other diseases. On the other hand, large dietary intakes of provitamin A carotenoids may decrease the efficiency of conversion due to the negative feedback regulation by retinoic acid. The equivalency ratio (weight of β-carotene in food: estimated weight of retinol formed) was better for fruits (12:1) than for vegetables (26:1) in most studies, but the best was found for biofortified Golden Rice (3.8:1), biofortified maize (6.5:1), spirulina (4.5:1), and red palm oil (RPO; 5.7:1). However, the reported ranges within studies were very wide, as shown in Table 3.3. In 2001, the US Institute of Medicine proposed a new unit, the retinol activity equivalent (1 µg RAE = 1 µg retinol), and advised that 1 µg RAE = 12 µg β-carotene or 24 µg of other provitamin A carotenoids for calculations of vitamin A equivalency for plant-derived food [15].
Besides the cytoplasmic enzyme BCMO1, there is also a mitochondrial carotenoid-degrading enzyme, β-carotene-9′,10′-dioxygenase 2 (BCDO2), that cleaves a wide range of carotenoids at position 9, 10 or 9′,10′, forming apocarotenals, β-ionone, or acyclic fragments, depending on the substrate [16]. BCDO2 degrades provitamin A carotenoids, xanthophylls (lutein and zeaxanthin), and lycopene cis isomers. Inhibition of BCDO2 gene expression may help to explain the accumulation of xanthophylls in birds (yellow skin of chickens) or an unusual yellow-fat phenotype in sheep. Thus, the apparent lack of carotenoids in blood or tissues of various animal species may be the result of active degradation by BCDO2. Apocarotenals seem to be quickly metabolized, since they do not accumulate in animal tissues. However, minute amounts of β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-12′-carotenal were found in mice and humans, derived from dietary apocarotenals or from β-carotene eccentric cleavage (enzymatic or nonenzymatic) [17]. Small amounts of β-apocarotenals (~1.5 % of β-carotene) were found in cantaloupe [18] and lycopenals (0.1 % of lycopene) were identified in tomatoes, tomato paste, watermelon, and red grapefruit [19].
Significance of Provitamin A Carotenoids in Developing Countries
Provitamin A carotenoid availability is of particular importance in developing countries where vitamin A deficiency (VAD) is a significant public health concern. The main underlying cause of VAD in low-income countries is a poor diet that is consistently insufficient in vitamin A, eventually leading to depleted stores that fail to achieve physiological needs [20, 21]. Persistent, severe deficiency can lead to xerophthalmia, a form of preventable, but irreversible, blindness in young children, and facilitates infectious diseases such as measles, diarrhea, and intestinal parasites, which increase infant mortality risks.
In such low-income populations, due to the poor availability of animal sources of preformed vitamin A, dietary carotenoids from plant sources, which need to be converted to vitamin A in the intestine, contribute to ~ 80 % of daily vitamin A intake and become highly necessary [12]. However, intestinal conversion of provitamin A to vitamin A is often compromised due to intestinal parasitic infections in young children, further exacerbating the deficiency condition. Some success has been observed with improving vitamin A status through supplementation with low-dose β-carotene (1.2 mg daily) coupled with deworming for roundworms, such as Ascaris lumbricoides, in young Bangladeshi children with subclinical VAD [22].
World Health Organization (WHO; [21]) has reported that about 250 million preschool children are affected by VAD, and an estimated 250,000–500,000 children go blind every year. WHO has outlined three main community-intervention strategies to combat and reduce VAD in affected populations: (1) fortification, (2) supplementation, and (3) dietary diversification. The first approach involves increasing dietary intake through fortification of a staple food with vitamin A. While this approach has been implemented and successful in Central and South America where sugar has been fortified for many years [23], and also in high-income populations where fats, oils, and cereal products are enriched, its success in low-income countries is still limited. As mentioned earlier, vitamin A equivalency ratios appear to be lower for biofortified foods that are staples in populations at risk for VAD in developing countries. However, since these ratios were estimated in healthy US adults, it remains to be seen whether these biofortified foods will be as favorable in these high-risk areas where nutritional deficiencies and intestinal parasites are highly prevalent [12].
In high-risk populations, periodic supplementation with 200,000 IU vitamin A in preschool children (< 5 years) has been shown to reduce the risk of xerophthalmia by 90 % and mortality by 23 % [20]. A recent meta-analysis evaluating the efficacy of vitamin A supplementation in reducing mortality and morbidity in children aged 6 months to 5 years found that out of the 43 trials included, 17 trials reported a 24 % reduction in the all-cause mortality rate. Seven trials reported a 28 % reduction in mortality associated with diarrhea, and vitamin A supplementation was associated with a reduced incidence of diarrhea and measles and a reduced prevalence of vision problems including night blindness and xerophthalmia [24]. Although effective, supplementation targets an immediate need, but is not regarded as a sustainable and economically viable approach.
Dietary diversification has been promoted in these regions in order to enhance the overall nutritional status of a community through nutrition education, and encouraging the consumption of vitamin A- and provitamin A-rich foods. The importance of home gardens to improve availability has also been emphasized. However, supplementation with plant sources of β-carotene in children and pregnant/lactating women have had variable effects [12]. In Mozambique, the promotion of production and consumption of orange-fleshed sweet potato (OSP) resulted in increased serum retinol concentrations in preschool children from the intervention area compared with children in the control area, following a 2-year period [25]. Similar results were obtained in Uganda, where introduction of OSP to farming households led to a decrease in the prevalence of inadequate vitamin A intake in both children and women, and a 9.5 % reduction in prevalence of serum retinol < 1.05 µmol/L in children [26]. In poor Bangladeshi women, however, 60 days of daily consumption of OSP increased plasma β-carotene concentrations from 0.1 ± 00 µmol/L to 0.18 ± 0.09 µmol/L (P < 0.0001), but the vitamin A body pool size was not significantly affected [27]. In Gambian children, the consumption of dried mango for 4 months increased serum retinol concentrations, compared to the control group who received a single placebo capsule of 40 mg α-tocopherol, followed by no intervention for 4 months. Dark-green leafy vegetables or orange-colored fruits, provided to Indonesian schoolchildren for 9 weeks, increased serum retinol concentrations compared to the control group that received a diet low in provitamin A. Filipino children also showed a positive response to a 9-week intervention with β-carotene-rich vegetables [12]. While these studies indicated that consumption of β-carotene through fruits and vegetables can increase the vitamin A status of at risk children, it is argued that the extent of vitamin A status improvement is difficult to quantify, because serum retinol is under homeostatic control and assessing these levels alone cannot provide information on the magnitude of change in vitamin A status [12].
Another food widely promoted for controlling VAD is RPO, the richest naturally occurring source of β-carotene, which generally contains a total of 500–800 mg of provitamin A carotenoids/kg oil [28] and is traditionally used for cooking in tropical rain forest regions of West Africa [29]. Rice and Burns [30] have presented a comprehensive review on the efficacy of RPO in preventing VAD. In the reviewed studies, RPO was provided mainly to preschool-aged children and pregnant or lactating women, either as a daily supplement, an in-home fortificant (such as mixing with breakfast, foods, and regular meals), or fortified into other foods (such as biscuits or cassava flour). In general, most studies showed an improvement in serum retinol concentrations in the targeted populations. Carotenoid levels increased in breast milk and maternal serum, but did not increase milk retinol concentrations in early lactation. The authors observed that the distinctive odor, color, and taste of RPO could be a potential barrier in achieving intervention success in cultures and countries where the oil is not traditionally used for cooking. However, if incorporated successfully, there is considerable evidence supporting the efficacy of RPO in ameliorating VAD.
In recent years, genetically modified (GM) crops have been advocated as a safe and super-efficient mode to increase the provitamin A levels in staple crops that otherwise contain negligible amounts of carotenoids, and Golden Rice (GR) has become a frontrunner in this regard. By genetically engineering the components of the β-carotene biosynthetic pathway into the endosperm of rice [31], scientists have been able to produce GR containing up to 37 µg total provitamin A carotenoids (~30 µg β-carotene) in 1.0 g milled and uncooked rice [32]. This technological advancement has provided an opportunity to improve the availability of provitamin A carotenoids to South Asian countries where rice is a main staple and VAD still prevalent. Tang et al. [33] compared the efficiency of isotope-labeled GR to spinach or pure β-carotene in oil in providing vitamin A to Chinese preschool children. The results indicated that GR was as effective as β-carotene in the oil capsule and better than spinach in contributing to the vitamin A intake in these children. Furthermore, the efficiency of conversion of β-carotene from GR to vitamin A was found to be better (2.3:1) in the Chinese children than that in US adults (3.8:1) [34]. If supplementation with GR produces similar outcomes in other high-risk countries, a considerable reduction in VAD can be expected due to the large consumption of this staple food. However, the economic cost of producing and supplying large amounts of GM crops to densely populated areas may be a challenge to consider. In the meantime, the consumption of carotenoids through homegrown vegetables and crops needs be continually encouraged in order to maintain a vitamin A status that would prevent fatal consequences.
Fertility and Reproductive Success
It is well known that carotenoids are crucial for the propagation of animal species, especially birds [35]. The bright colors of feathers or skin advertise gender (usually male), health and vigor, and the ability to procure a nutritious diet for offspring. Sexual attraction and choice of mate often depend, in birds, on the dietary supply of carotenoids. Carotenoids are accumulated in the egg yolk and provide a necessary supply of lutein and zeaxanthin for the retina of the developing young bird. Bird retina has more photoreceptors than human retina, and each cone cell contains an oil droplet with a high concentration of carotenoids that allows them to have excellent vision and to distinguish a great range of colors [36].
Recent simulation experiments indicated that subjects preferred carotenoid-related yellowish skin tones over suntan in potential mates, possibly because it advertises good health and may also signal robust fertility [37]. Dietary carotenoids normally found in the testes [38,39] and seminal plasma [40] were often at lower concentrations in men suffering from infertility. The relationship between carotenoids and fertility was investigated in 30 men with idiopathic sperm quality issues [41] who were given 2 mg lycopene twice a day for 3 months. Lycopene administration significantly improved sperm count, motility, and morphology. This short study resulted in six pregnancies.
Carotenoids also accumulate in ovaries, especially the corpus luteum , which develops in female mammals from the ovarian follicle after release of the egg, during the luteal phase of the estrous or menstrual cycle. Bovine corpus luteum is so rich in β-carotene that it is often referred to as corpus rubrum, and it increases from 14 to 175 µg/g during the estrous cycle [42]. The corpus luteum synthesizes progesterone to prepare the uterine endometrium for implantation of the fertilized egg, thus maintaining pregnancy. When studied in bovine luteal cell culture, this steroidogenesis was found to require the replenishing of high-density lipoprotein (HDL) cholesterol (substrate) and β-carotene [43]. It is possible that β-carotene fulfills a dual function in corpus luteum, as an antioxidant protecting the tissue from the action of free radicals released during the synthesis of progesterone [44] and as an endogenous source of vitamin A produced there by the enzyme BCMO1. Reproductive performance of cows seems to be enhanced by the intake of carotenoids from fresh hay and/or supplemental β-carotene [45].
Human corpus luteum is the primary source of progesterone for 4–5 weeks after implantation [46], until placental production takes over. It is quite obvious that the concentration of carotenoids in human corpus luteum is higher than in the rest of the ovary, but the quantitative data are missing due to the difficulty of obtaining specimens from different phases of the menstrual cycle and pregnancy. The ovaries contain all the dietary carotenoids found in human plasma (β-carotene, lutein, lycopene) [38, 39]. It is tempting to speculate that an increased intake of dietary carotenoids may improve conception rates and maintenance of early pregnancy in some women with unexplained infertility.
Maternal deficiency of vitamin A increases the risk of pregnancy-related mortality and impairs infant survival. Although not of concern in high-income populations, where preformed vitamin A-rich and fortified foods are readily available, in low-income, high-risk countries, supplemental vitamin A is recommended during pregnancy to prevent night blindness and postnatal infant mortality.
A randomized, double blind, placebo-controlled trial in Nepal administered 7 mg retinol, 42 mg β-carotene, or placebo to pregnant women on a weekly basis [47]. Both vitamin A and β-carotene significantly reduced maternal mortality during pregnancy and 12 weeks after birth. However, no such effect was seen in a similar trial conducted in rural Bangladesh, where VAD was much less prevalent and the diet included more protein and fat [48]. Infant mortality (till 12th week post partum) and the rate of stillbirth were not reduced by the weekly dose of retinol or β-carotene.
In a meta-analysis of 17 trials using vitamin A or β-carotene supplementation during pregnancy, no significant overall effect on birth weight, preterm birth, stillbirth, miscarriage, or fetal loss was found [49]. Supplementation was protective in HIV positive women against low birth weight (< 2.5 kg; RR = 0.79, CI = 0.64, 0.99) but not for preterm delivery or small-for-gestational-age infants. However, some evidence indicated that concurrent supplementation with vitamin A (5000 IU) and β-carotene (30 mg) was associated with an increase in HIV transmission from mother to child [50], thereby warranting caution against supplementation programs in high HIV prevalence areas. These authors concluded that the evidence in favor of vitamin A/β-carotene supplementation on maternal and infant mortality was lacking .
However, the WHO [51] has suggested a vitamin A supplementation scheme in areas with severe public health problems of night blindness and infant mortality. A daily dose of 10,000 IU or 25,000 IU weekly vitamin A in the form of an oral liquid or oil-based preparation of retinyl palmitate or retinyl acetate is recommended. Supplementation should be considered for a minimum of 12 weeks during pregnancy until delivery where prevalence of night blindness is ≥ 5 % in pregnant women and children aged 24–59 months. No recommendation for provitamin A carotenoids was given. Supplementation of provitamin A carotenoids or vitamin A during the last trimester of pregnancy may improve levels of retinol in breast milk [51], but a comprehensive study has not been performed. The initial postpartum breast secretion, colostrum, contains more carotenoids than later milk, especially in women who have lactated after earlier pregnancies [52]. The variability is great, but the carotenoids likely benefit the health of the newborn child. A multinational study of breast milk of healthy mothers revealed that the provitamin A carotenoids accounted for > 50 % of milk carotenoids and were highest in Japanese mothers [53]. US mothers had the lowest concentrations of total carotenoids.
Few studies have explored the associations between carotenoids and preeclampsia in pregnant women. Significantly lower plasma β-carotene levels were associated with the subsequent development of preeclampsia in pregnant women with diabetes [54]. Both placenta and plasma from preeclamptic women were significantly lower in β-carotene and lycopene compared to normal pregnant women [55]. Since oxidative stress characterizes the pathophysiology of preeclampsia [56], it is conceivable that consumption of carotenoids during pregnancy may favor an environment that minimizes oxidative damage. In any case, since pregnancy is a period of rapid growth and development, a carotenoid-rich diet may help to sustain a pregnancy free of complications and promote a healthy outcome.
Prevention of Oxidative Stress and Inflammation
The carotenes and xanthophylls are some of nature’s most efficient quenchers of singlet oxygen . Singlet oxygen arises from exposure of chromophores such as porphyrins, chlorophylls, and riboflavin to sunlight. These activated molecules can damage DNA, proteins, and lipids. Carotenoids absorb the excess energy of singlet oxygen and dissipate it as heat [57]. However, the idea that carotenoids are important classical antioxidants, which play a role in major diseases where oxidative stress and inflammation are causative factors, is less well established. Small amounts of carotenoids can be found in almost all the lipid membranes of the body. In the animal kingdom, they are often associated with specific proteins. The xanthophylls, such as lutein and zeaxanthin , having hydrophilic hydroxyl groups, orient themselves across membranes. The carotenes, such as β-carotene and lycopene, are oriented within the bilipid layers and can disturb the phospholipid structure to a small extent, allowing for greater penetration of small molecules [58]. Their location and orientation may play a role in their ability to act as classical antioxidants .
In vitro studies have shown that all the carotenoids accept the unpaired electron from a number of free radical species (sulfur-containing radicals, glutathione, nitric oxide, NO2, –ONOO–, and peroxyl radicals), as well as superoxide. The resulting carotenoid radical must be converted back to its original state to be considered an antioxidant . The residence time of the specific carotenoid radical, oxygen partial pressure, and the availability of other antioxidants determine whether a carotenoid acts as a prooxidant or an antioxidant [59]. For example, protection of lymphocytes from nitrogen radical attack increases almost tenfold in the presence of vitamins C and E and β-carotene compared to β-carotene alone [60]. Carotenoids can also accept electrons from each other, with lycopene being the ultimate acceptor from all other carotenoids [61].
Oxidative stress and inflammation processes occur together. Several human studies have found an association between low dietary carotenoids and/or blood carotenoid levels and increased markers of oxidative stress and inflammation [62–66]. Such associations may be more related to antioxidant- and anti-inflammation-promoting dietary patterns that include fruit and vegetable consumption than to carotenoid intake alone. Cell culture and animal studies, using models of oxidative stress, have found redox-based regulation of proinflammatory pathways by lutein, β-carotene, and lycopene [67–69].
Supplementation studies have been equivocal and their outcomes may depend upon the level of oxidative stress and the antioxidant status of participants during the study. Lutein supplementation of preterm infants [70] and healthy adults [71], mixed carotenoids in postmenopausal women [72], and numerous lycopene supplementation studies [73] have found decreases in markers of oxidative stress or inflammation. The two studies that used supplement combinations of β-carotene, vitamins C and E, and selenium found no change in markers of inflammation [74, 75].
Photoprotection and Skin Health
Sunlight is an environmental hazard over the life of human skin. Not only UV-A and UV-B but also visible and infrared light are responsible for singlet oxygen and radical production, especially in the presence of natural photosensitizers such a porphyrins and riboflavin . This can result in photoaging (roughened or patchy skin, wrinkles), UV-induced erythema (sunburn), and skin cancer [76]. Given the role that carotenoids play in nature as photoprotectors, do they play the same role in human skin? A variety of carotenoids accumulate in various dermal layers of the skin. In fact, their presence in skin due to higher consumption of fruits and vegetables or carotenoid supplements can be visibly detected by ordinary observers [77, 78]. As noted before, the resulting facial skin tones are favored and are considered a sign of greater health and vigor [79, 80]. Various case reports document carotenodermia in individuals who have gone overboard in consuming carrot or tomato juice. The extreme yellow-to-orange pigmentation that concentrates in the palms of the hands disappears harmlessly with the termination of intake [81, 82]. Canthaxanthin was sold as tanning capsules in European countries in the 1980s until it was discovered that golden crystals formed in the paramacular region of the eye [83].
The first medical use of β-carotene was developed by Micheline Mathews-Roth and her collaborators. People suffering from erythropoietic protoporphyria (EPP) accumulate large quantities of protoporphyrin IX, a precursor of hemoglobin synthesis. Protoporphyrin is a photosensitizer in the visible light range, so sunscreens developed against UV light do not prevent the burning sensation and edema that occur when sufferers are exposed to even a small amount of sunlight. Mathews-Roth made the connection between carotenoid protection for chlorophyll in plants in the wavelength range of 380–560 nm and the similar structure of protoporphyrin. Clinical trials were very effective, with doses of 180 mg/day of β-carotene providing 84 % of patients a threefold greater ability to tolerate sunlight, especially for children who could now play outside. In 1975, the US Food and Drug Administration approved the use of β-carotene for the treatment of EPP [84]. The availability of β-carotene for human use motivated its employment in subsequent trials for cancer prevention .
A number of studies have evaluated dietary supplementation with β-carotene, lycopene, or mixed carotenoids, as protection from UV-radiation caused erythema. More positive results were observed for tomato extracts compared to lycopene alone. Phytoene and phytofluene (found in tomatoes) are precursors in the lycopene synthetic pathway and, unlike lycopene, absorb UV radiation. Greater efficacy was found in trials lasting ≥ 7 weeks with carotenoid doses ≥ 12 mg/day, possibly in combination with vitamin E [85, 86]. A meta-analysis of β-carotene supplementation trials found a protective effect regardless of dose, but increased in effectiveness with time ≥ 10 weeks. The sun protection factor (SPF) was 4 compared to sunscreens with SPFs between 10 and 40 [87]. Topical application of β-carotene in lotion may have benefits, providing protection from infrared light that has also been shown to produce radicals from distressed mitochondria [88].
Skin ages in light-exposed areas (extrinsic or photoaging) as well as covered areas (intrinsic aging). Reactive oxygen species (ROS) formation via mutations in mitochondrial DNA are important for both processes, but UVA-light exposure increases the mutation rate by 40 %. UV light accounts for 80 % of facial skin aging, with visible wrinkles and rough skin explained by the breakdown of collagen, degradation of elastin fibers, and 50 % slower renewal of the epidermis by the age of 80 years [89]. There is a general impression that a nonsmoking, healthy lifestyle leads to a more youthful appearance, but only a few studies have explored the association of carotenoid status to fewer wrinkles. A cross-sectional study of people > 70 years in a range of countries found that back-of-the-hand skin wrinkling was negatively associated with higher intakes of vegetables, olive oil, and legumes, with healthy diet explaining 34 % of the variance [90]. Forehead skin roughness (an early stage of wrinkling) was associated with dermal lycopene (R 2 = 0.7) concentration (measured by resonance Raman spectroscopy) but not with aging in a small study with a narrow age range (40–56 years) [91]. Photoaging nude mice models have shown Anti-wrinkling effects for β-carotene and lutein + zeaxanthin . A variety of carotenoids are associated with a lower incidence of skin cancer [92].
Vision and Diseases of the Eye
Aside from the conversion of some of the carotenoids to vitamin A compounds, the strongest evidence of the human need for carotenoids comes from the study of the eye and its diseases associated with aging. The human macula lutea, an indented area located roughly in the center of the retina, occupying a diameter of 5–6 mm and accounting for the central 15°–16° of vision, accumulates lutein and zeaxanthin at roughly 1000-fold the concentrations found in plasma [93]. The result is a visibly yellow hue referred to as macular pigment. A binding or transport protein that might explain this accumulation has not yet been unequivocally identified. These xanthophylls are organized with zeaxanthin predominating in the central region to about 2.5 mm from the center, with lutein exceeding zeaxanthin 2:1 at the periphery, and meso-zeaxanthin (thought to be an intermediate product) appearing throughout [94]. The relative location of lutein to zeaxanthin in the macula varies greatly from person to person with some people having concentric rings of greater pigmentation [95]. Lutein and zeaxanthin are localized in the Henle fiber layer that covers the photoreceptors, so light must be filtered through these pigments. An arrangement perpendicular to the Henle fibers is consistent with maximal absorption of blue-wavelength light, but their specific organization (protein-bound or not) is not known. The orientation of zeaxanthin’s β-ionone rings provides a more orderly arrangement compared to lutein [96].
The obvious function of macular pigment is to filter blue light (the most energetic in the light spectrum), thus protecting the photoreceptors, retinal pigment epithelium (RPE), and the underlying choriocapillaries during 70 + years of light exposure. The reduction in blue light intensity can range between 40 and 90 %, which is sufficient to account for the observed reduction in risk for age-related macular degeneration (AMD) in some epidemiological studies [96]. In addition, a role as in situ antioxidants has been proposed. Zeaxanthin and, to a lesser extent, lutein are effective quenchers of singlet oxygen [1O2] coming from UV exposure [97, 98]. This quenching is a physical process with the excess energy dissipated as heat and does not destroy the xanthophylls; so macular pigment levels are maintained long after xanthophyll intake has ceased [99]. They may also act as classical antioxidants because they are present in the perifoveal and peripheral regions of the retina that are exposed to high oxygen tensions and have high rates of metabolism . Several carotenoid derivatives are found in the retina including meso-zeaxanthin, (3 S,3′S)-zeaxanthin, and epilutein that may have been formed by redox reactions [96].
Whether there is an association between lutein and zeaxanthin status and the risk of AMD would seem to be a logical question. Late-stage AMD is the leading cause of legal blindness in people >65 years old in industrialized countries, affecting more than 10 million people in the USA and about 200,000 in the UK [100]. Non-Hispanic black persons have a lower prevalence [101]. Early-stage AMD starts in the macula with soft drusen (yellowish deposits containing no xanthophylls) and other pigmentation abnormalities in the RPE. It continues on to later stages characterized by atrophy of the photoreceptors and the RPE (dry AMD), often progressing further to choroidal neovascularization, retinal hemorrhage (wet AMD), detachment of the RPE, and retinal scarring [102]. The nature of this progressive disease complicates the interpretation of epidemiological studies and clinical trials. A recent meta-analysis of six longitudinal cohort studies found that the pooled relative risk (RR) of early- stage AMD was not related to baseline dietary intake of lutein and zeaxanthin, but late-stage AMD (RR = 0.74) and neovascular AMD (RR = 0.68) were risks for those in the lowest intake category [100].
Since dietary lutein and zeaxanthin are related to fruit, vegetable, and egg intake, does lutein or zeaxanthin supplementation increase macular pigment optical density (MPOD) in people with healthy sight and those suffering from various stages of AMD? Is increased MPOD related to visual function and the development or reversal of AMD? Lutein, lutein esters, zeaxanthin plus lutein, or these xanthophylls plus various antioxidant mixes do increase MPOD in college students [103], older men with healthy sight [104, 105], those with early AMD [106], and men with mild to moderately advanced AMD [107, 108]. There appeared to be few differences between these formulations (10–20 mg/day) in their effect on MPOD over the 6–12 month period in these studies. Furthermore, the ring structures of MPOD seen in some individuals, and thought to be protective, were neither attenuated nor generated de novo with xanthophylls supplementation [95]. In addition, those with lower baseline MPOD tended to have the greater increase in MPOD with xanthophyll supplementation. Lutein or zeaxanthin supplementation also improved visual acuity, foveal shape discrimination, subjective glare recovery, and contrast sensitivity. These improvements were correlated with the increases seen in MPOD in several of the studies cited above. A small study, using 6 mg/day of lutein plus a vitamin/mineral mixture for 9 or 18 months, failed to find improvements in the visual performance parameters listed above [105].
The Age-Related Eye Disease Study 1 (AREDS1), a national study using a daily supplement containing vitamins C and E, β-carotene (15 mg/d), and the minerals zinc and copper, reduced the 5-year risk of developing advanced AMD by 25 % in eyes with intermediate AMD, but had no effect on the development or risk of cataract [109, 110]. A recent Cochrane review evaluating four high-quality randomized placebo-controlled studies that included 62,520 people, using vitamin E and β-carotene in their supplement formulations, failed to prevent the onset of AMD [111]. The AREDS1 study was included in this review. At the time these studies commenced, neither lutein nor zeaxanthin were yet available.
The goal of AREDS2 was to determine whether lutein (10 mg/d) plus zeaxanthin (2 mg/d), or the omega-3 long-chain fatty acids (eicosapentaenoic acid (EPA) 650 mg/d plus docosahexanoic acid (DHA), 350 mg/d) in addition to the original AREDS1 formulation, could reduce the 5-year risk of progression to advanced AMD in those with intermediate AMD [112]. More than 4000 men and women were randomized to one of four treatments. A secondary randomization of the underlying AREDS formulation was made to answer the concern for the safety of β-carotene or high-dose zinc supplementation. There were no differences in the progression to advanced AMD for any of the primary treatments, although lutein + zeaxanthin appeared to have some efficacy for the reduction of neovascular AMD (wet AMD; HR = 0.89, P = 0.05) compared to geographic atrophy (dry AMD). Further subgroup analysis showed that those in the lowest quintile of lutein + zeaxanthin intake (0.1–1.4 mg/d) had a lower risk of AMD progression (HR = 0.74, P = 0.01) when supplemented compared to low-intake groups not receiving the lutein + zeaxanthin supplement, whereas there was no difference among the higher quintiles of intake. Smokers and recent quitters had a higher risk of lung cancer with β-carotene supplementation and there was evidence for competition for absorption between β-carotene and lutein + zeaxanthin. The authors were hesitant to make any conclusions based on these subgroup analyses but suggested that substitution of lutein + zeaxanthin in the AREDS1 formulation might be a safer choice for smokers.
Due to the macular location of lutein and zeaxanthin, and given their unique optical properties, they may enhance visual performance for everyone. Abundant macular pigment may (1) enhance visual acuity by reducing chromic aberration (like filters on a camera), (2) reduce visual discomfort by attenuating glare and dazzle, and (3) facilitate enhancement of details and visual contrast by the absorption of “blue haze,” (e.g., how mountains appear blue in the distance) [113, 114]. Studies enrolling younger, normal-sighted individuals have found positive relationships between macular pigment and contrast sensitivity, visual acuity, glare reduction, photo-stress recovery, and time discrimination of changing light [115–118]. Clinical trials using lutein or zeaxanthin supplements (10–20 mg/day) for 6–12 months found improved visual acuity, contrast sensitivity, glare attenuation, and light/dark adaptation [119–122]. Another supplementation study (6 mg/day lutein plus vitamins and minerals for 9 months) found no effect on visual performance parameters [123]. All of these studies had small numbers of subjects and none found a correlation between macular pigment density and improved visual function. Since a number of subjects may have already achieved optimal macular pigment before supplementation, any association with visual performance may have been attenuated.
The lens is clear, and along with the cornea, focuses a sharp image onto the retina. It is composed of tightly ordered fibrous cells that lose their nuclei, so their major proteins cannot be regenerated. Over our lifetime, new cells accumulate at the outer surface of the lens like the layers of an onion. The central tightly packed denucleated cells are subject to the so-called nuclear cataract formation. Cortical cataracts are formed in outer cells laid down after birth. The prevalence of age-related cataract varies widely throughout the world in people > 70 years old, with cortical cataract being more prevalent in some populations (30–45 %) and nuclear cataract more prevalent in others (10–80 %). Cataracts are observed as opacities of the lens that range from white to yellow to dark brown. The crystalline proteins (especially β) are chopped and cross-linked, forming large aggregates. This process is accompanied by the loss of reduced glutathione (the major antioxidant in the lens) and the enzyme, glutathione reductase, that regenerates it. There is also a loss of sulfhydryl groups in sulfur-containing amino acids of the lens proteins. Therefore, oxidative stress is thought to be a major factor in cataract formation [124, 125]. Do dietary antioxidants such as vitamins C and E and the carotenoids reduce the risk of cataract? The human lens contains substantial amounts of lutein plus zeaxanthin and vitamins A and E, but no hydrocarbon carotenoids, such as β-carotene and lycopene. Lutein and zeaxanthin are more concentrated in the cortical regions of the lens [126] and can be substantially increased throughout the lens with long-term supplementation [127]. A number of large population studies have found reduced risk for nuclear cataract (less so for cortical cataract) with higher intakes of lutein and zeaxanthin or higher plasma levels [128]. These associative studies may also be related to other substances in fruits and vegetables, and other lifestyle factors associated with healthy diets. For example, the Healthy Eating Index score was the strongest modifiable predictor for low prevalence of nuclear cataract (reduced by 37 %) in the Women’s Health Initiative Observational Study [129]. Furthermore, a number of population studies and clinical trials have found significant risk reductions for nuclear cataract with long-term use of Centrum and other multivitamin–mineral supplements [130]. Centrum has included small amounts of lutein (0.25 mg/day) for several years. Xanthophyll supplementation studies have focused on slowing visual decline in those with developing cataract, and visual performance was improved in two trials [131, 132]. Despite a plausible physiological rationale and largely consistent circumstantial evidence, the lack of xanthophyll-based, placebo-controlled clinical trials for the prevention of cataract or macular degeneration prompted the US Food and Drug Administration to determine that no credible evidence existed for a health claim about the intake of lutein or zeaxanthin and the risk of AMD or cataracts in 2006 [133].
Retinitis pigmentosa is an inherited disease affecting 1.5 million people worldwide. It starts with night vision problems in adolescence, progressing through the loss of peripheral vision, with final loss of central vision after the age of 60 years. The cause is the progressive loss of rod and cone photoreceptors [134]. The authors of a major 4-year clinical trial of 12 mg/day of lutein plus vitamin A (15,000 IU/day) versus vitamin A alone, in 225 nonsmoking retinitis pigmentosa patients, concluded that the lutein supplement slowed the loss of midperipheral visual field, but only in those achieving the highest serum level of lutein or those with the highest MPOD as a result of treatment. There was no difference in the primary endpoints [135]. This invoked a letter from their data safety- monitoring committee indicating that despite sound study design, the committee disapproved the use of subgroup analysis as the basis of the authors’ main conclusion [136]. Two small clinical trials have also found positive results using lutein supplementation [137, 138], but one 6-month study (20 mg lutein /day) found that only 50 % of their participants had any increase in MPOD resulting in no detectable change in central vision function during the intervention [139]. Whether lutein supplementation is beneficial for those suffering from retinitis pigmentosa is likely to be complicated by variable accumulation of lutein in the retina. MPOD increase with lutein supplementation was inversely proportional to serum total cholesterol levels, and MPOD became higher in those with brown irises and those who had retained more photoreceptors [140].
The possible anti-inflammatory, antiapoptotic effect of lutein on other retinal structures is a new area of inquiry, especially in acute retinal ischemic/reperfusions that are experienced during operations for retinal reattachment and arterial blockades. Preliminary studies on animals hold some promise [141–143].
Cognitive Decline and Alzheimer’s Disease
More than 16 carotenoids have been found in human brain tissue, with the xanthophylls (lutein and zeaxanthin) accounting for 66–77 %. Their distribution is not homogeneous, with the frontal cortex being particularly rich [144]. Tissue concentrations in some sections of the brain appear to be correlated with macular pigment density. A study of xanthophyll concentrations in postmortem brain tissue of subjects enrolled in the Georgia Centenarian study found positive correlations with better age-adjusted performance on various cognitive function tests, especially retention, with higher tissue levels [145].
Cognitive impairment refers to the subclinical complaint concerning memory functioning in the elderly and is so common that it has come to be thought of as an inevitable feature of the aging process. Cognitive decline is evaluated in longitudinal studies by changes in performance over time, in one or two domains of a series of cognitive tests (memory, orientation, language, executive function, or praxis). Using functional tests, cognitive impairment is defined as: (1) at least one standard deviation below the mean for young adults on one or more tests or (2) greater-than-expected decline in score for a person’s age and education level. Impairment is considered a risk factor for the development of Alzheimer’s disease (AD) [146].
AD is a degenerative disorder of the brain causing memory loss, progressing to the inability to perform activities of daily living, followed by death. It accounts for 60–80 % of cases of dementia and its current US prevalence is estimated at 5.1 million. Mild cognitive impairment occurs at much higher rates. This disability constitutes a large share of the ballooning health-care costs. Postmortem, it is characterized by senile amyloid-β protein plaques and neurofibrillary tangles composed of phosphorylated tau proteins that first accumulate in cortical tissue. The most vulnerable cortical tissue is that which was laid down during the later stages of fetal and newborn development. It then spreads to the hippocampus. This pathology can be found also in those with mild cognitive impairment, which makes diagnosis difficult in prevention and treatment studies. Furthermore, coexistent Parkinson disease and the evidence of infarcts or hemorrhage have been found in 20–25 % of AD brains . Aging, genetics (ApoE4—a genetic variant of a cholesterol reverse transport protein) and oxidative stress (tobacco use, diabetes) have been identified as causative factors. Progress is being made with imaging techniques such as magnetic resonance imaging (MRI) and position emission tomography (PET) correlated with postmortem pathology, so that more precise endpoints can be identified for future clinical trials [147–149].
A number of longitudinal studies of middle-aged populations have explored the relationship between plasma or dietary levels of β-carotene and cognitive decline. These studies have been mixed, but are largely negative. A number of clinical trials, designed to evaluate supplements containing vitamins C and E and β-carotene for heart disease and/or cancer endpoints, have added tests for cognitive decline. The results for all cognitive endpoints have been disappointing. A systematic review of both study types found no evidence that any of these antioxidants prevented cognitive decline in later life [150]. This systematic review was used as the basis for a National Institutes of Health (NIH) State-of-the-Science Conference Statement that explored modifiable factors that might reduce the risk of AD and cognitive decline in older adults. The major conclusion was that there was currently insufficient evidence to identify any modifiable factors that might reduce risk for either. This was due to the limitations of studies currently available [151, 152],including short follow-up time, starting interventions too late in the disease process, and lack of baseline measurements in the clinical trials allowing no subgroup analysis of those subjects who had low baseline intakes (a group more likely to benefit from intervention) [153]. None of these studies evaluated the xanthophylls, which have a more stabilizing effect on neuronal membranes.
A small preliminary study, supplementing DHA (an omega-3 fatty acid) or lutein (12 mg/day) for only 4 months to mentally unimpaired elderly women found improvements in memory scores and rates of learning with either or both supplements [154]. Cerebral ischemia with reperfusion (I/R) is often used in animal studies to simulate the effects of a stroke. Lutein supplementation, given to mice shortly before or subsequent to reperfusion, produced better survival rates, better neurological scores, and smaller areas of infarct [155]. Lutein also appears to protect neurons in models of retinal I/R [156].
It would be unfortunate to discard the possibility that the long-term intake of a fruit and vegetable-rich diet, that includes the xanthophylls and other carotenoids, might slow the progression of age-related cognitive decline before there are sufficient data.
Cancer Prevention and Treatment
Cancer is an umbrella term encompassing a number of tissue diseases and a variety of mechanistic antecedents . The retinoids have potent anticancer effects against a wide variety of experimental cancers, but their toxic side effects may be inseparable from their mode of action [157]. When the first population studies were published, showing a reduced risk for cancer with higher blood levels of retinol and β-carotene [158, 159], the search was on to find associations with other cancers in various populations. Some of these studies found risk reductions for greater intakes or higher blood levels of β-carotene or lycopene and occasionally the xanthophylls , but many did not [160]. Such studies may rather indicate beneficial dietary patterns (including greater intake of fruits and vegetables) and lifestyles associated with such patterns [161–163]. With high hopes, several clinical trials supplemented their subjects with large doses of β-carotene, combined with vitamins A or E, and followed cancer incidence. Surprisingly, two studies found an 18–24 % increase in lung cancer and 8–17 % increase in mortality over a 4–8 year follow-up, especially in those who received only β-carotene [α-tocopherol, β-carotene (ATBC) study] [164, 165]. A follow-up to one of these (ATBC study) found that the excess risk for any cancer, including lung cancer, was no longer evident 4–6 years after the ending of the intervention [166]. The early excess risk due to β-carotene supplementation in smokers and asbestos workers in these trials was widely hypothesized to be due to components of cigarette smoke in the presence of relatively high oxygen partial pressure in the lung [167]. The other clinical trials using β-carotene found neither reduced nor increased risk for any type of cancer [168, 169]. A study using a population of vitamin- and mineral-deficient Chinese men and women found that a mixture of vitamin E, β-carotene, and selenium decreased total cancers by 13 % and deaths by 9 % during a 5-year follow-up [170].
A search for mechanism ensued with numerous cell culture studies using cancer cell lines and animal cancer models. Carotenoids, especially β-carotene or lycopene, were found to exhibit functions consistent with blocking the development or growth of various cancers through the following mechanisms : (1) modulation of nuclear receptor superfamilies, (2) decreasing angiogenesis, (3) increasing apoptosis, (4) restoring gap junction communication, (5) prooxidant and antioxidant effects, especially protection from DNA damage, (6) inhibition of cell proliferation, and (7) modulation of phase I and phase II enzymes [160, 171, 172]. Cell culture experiments often used concentrations that were higher than physiologically feasible, with long incubations at high temperatures and oxygen partial pressures that could produce oxidation products. Indeed, under these conditions, oxidation products have been identified for lycopene, but not for β-carotene [173]. Oxidation products or metabolites of lycopene have also been identified in human plasma and tissue. These lycopenoids, mostly lycopenals, have similar structures to 9-cis retinoic acid (a powerful ligand for several nuclear receptors) and are present in comparable concentrations in human samples [174]. Beside β-carotene, lycopene is a substrate for the eccentric cleavage enzyme, BCDO2, which cleaves the 5-cis and 13-cis isomers for lycopene, but not the all-trans isomer. Lycopene cis isomers are prevalent in circulation after ingestion of tomato products or lycopene supplements , and the BCDO2-mediated conversion to apo-10′-lycopenal and then to apo-10′-lycopenoic acid which may be a substitute ligand for 9-cis retinoic acid. This hypothesis was investigated using the major retinoid receptors with only modest activity [173]. However, the lycopene degradation products have been shown to have a variety of anticarcinogenic activities and are often more potent than lycopene . Their actions include regulation of the cell cycle, apoptosis of cancer cells, and the induction of two important systems. The first is the electrophile response element/antioxidant response element (EpRE/ARE) system that mediates the induction of detoxifying and antioxidant enzymes responsible for inhibiting the mutagenic effects of carcinogens and oxygen radicals. The second is the nuclear factor kappa B (NF-κB) system which is responsible for the normal functioning of the immune system, but is crucial for the deleterious inflammatory response that often is associated with cancer risk [173, 174].
Several epidemiological studies have identified risk reduction with lycopene exposure while finding no other carotenoid associations. The data are especially strong for cancers of the prostate [175]. A systematic review of eight clinical trials using 15–30 mg/day lycopene supplements found reductions in serum prostate-specific antigen (PSA), but only a modest reduction in benign prostate hyperplasia (BPH), which was not statistically significant [176].
Cancer patients may be tempted to add various carotenoids as dietary supplements to their standard care which may include chemotherapy. As chemotherapeutic agents generate oxidative stress, there has been concern that antioxidant therapy may blunt the effects of these agents. However, numerous studies have demonstrated that antioxidants do not inhibit but actually enhance the cytotoxic effect of antineoplastic drugs on cancer cells. Several β-carotene and lycopene cell culture and animals studies using various agents have found an enhancement of their effects [177, 178]. A systematic review of 19 trials of antioxidant plus chemotherapy (only one of which had β-carotene as part of a vitamin mixture) found no detrimental effects, while several reported increased survival times and tumor responses as well as fewer toxicities compared to controls [179]. In a small trial of 50 patients with high-grade gliomas treated with radiation and paclitaxel, there was a modestly improved response in the lycopene-treated group [180].
The early promise of a variety carotenoids, as benign agents, for lowering the risk of various cancers is somewhat tarnished, but the intriguing biological activities of these carotenoids and their metabolites raise hope that they may yet be found to be useful adjuncts in the disruption of various carcinogenic processes .
Metabolic Syndrome, Obesity, Cardiovascular Disease, and Diabetes
Metabolic syndrome (MetS) is a clustering of many conditions including abdominal obesity, high blood pressure (HBP), hyperglycemia, elevated fasting triglycerides, and low levels of HDL cholesterol [181]. These metabolic abnormalities increase the risk for cardiovascular disease and diabetes mellitus, as also does obesity which is correlated with cardiovascular mortality through hypothesized instigating factors, such as an increase in chronic inflammation and oxidative stress [182]. Over the last decade, a consistent rise in the rates of obesity and MetS has been observed in the USA. As reported by the Centers for Disease Control and Prevention (CDC), 35.7 % of adults are obese, and it is expected that by 2030 more than half of US adults will be obese. More disconcerting is the increase in childhood obesity (16.9 %) and, consequently, of preventable diseases in this age group.
Carotenoids, by virtue of their antioxidant properties, may help to prevent the progression of chronic conditions related to obesity and MetS, and thereby decrease related morbidity and mortality rates. Many previous observational studies have indicated an inverse association between serum/dietary carotenoid levels and MetS [183–186]. In a recent analysis of NHANES cross-sectional data, serum carotenoid concentrations were found to be consistently lower in US adults with MetS compared to those without MetS (0.057–0.863 vs. 1.62–10.114 µmol/L total carotenoids), with an inverse association observed with all MetS components [187]. Furthermore, adolescents with MetS also had lower serum carotenoid concentrations compared to their counterparts without MetS that were inversely related to the inflammatory C-reactive protein (CRP) in their serum [188]. In middle-aged and elderly men, higher dietary intakes of total carotenoids, β-carotene, α-carotene, and lycopene were associated with lower waist circumference and less visceral and subcutaneous fat, while lycopene intake alone was related to lower serum triglyceride concentrations [186]. Despite the associations observed in these studies, long-term supplementation with antioxidants including a combination of vitamins C and E, β-carotene, zinc, and selenium did not prevent the incidence of MetS in adults free of the condition at baseline, although baseline serum β-carotene concentrations were negatively associated with the risk of MetS [189]. Due to the scarcity of similar randomized clinical trials, it is still unclear whether long-term carotenoid supplementation would prevent progression to MetS in susceptible individuals. Short-term supplementation studies in obese and overweight individuals have presented mixed results. Four weeks of supplementation with 30 mg/day lycopene in the form of tomato-derived Lyc-O-Mato did not affect the markers of inflammation and oxidative products in severely obese individuals [190]. However, in moderately overweight middle-aged individuals, 70 mg lycopene/week enhanced HDL functionality [191], as determined by increases in the activity of the antioxidant enzyme paraoxonase-1 and decreases in the cholesteryl ester transfer protein (CETP). Tomato juice consumption in overweight and obese females was also found to reduce systemic inflammation through decreases in inflammatory biomarkers such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α [192]. Most of these investigations have examined the effect on obesity-induced inflammation, since this is considered the therapeutic strategy to retard the progression to cardiovascular disease and diabetes mellitus, and among the carotenoids, lycopene has consistently demonstrated anti-inflammatory properties in humans, as well as in animal and cell culture models [193–195].
Low levels of carotenoids found in MetS and obesity possibly point to an unbalanced diet, leading to a deficiency in these micronutrients and/or an increased requirement due to a heightened state of oxidative stress. It is of interest that intake of a low caloric diet that provided more than adequate micronutrients [exceeding the dietary reference intakes (DRI) requirements] was unable to correct deficiencies of many micronutrients, including lycopene, in obese subjects undergoing a 3-month weight loss regimen, suggesting a larger need for antioxidants in obese individuals, especially during weight loss-induced stress [196].
Associations between carotenoids and cardiovascular disease (CVD) have been examined in epidemiological studies through the measurement of dietary intake, serum, and adipose tissue levels. Some early prospective studies showed that higher β-carotene status was related to a reduced risk of myocardial infarct and a modest decrease in the risk of stroke in men and women [197]. In a recent study, a twofold increase in the risk of sudden cardiac death was observed in men with lower serum β-carotene levels [198]. Many recent studies have focused on lycopene as a primary player in the prevention of CVD, likely due to its potency as a singlet oxygen quencher. Increased serum concentrations of lycopene were associated with decreased arterial stiffness and lower oxidized low-density lipoprotein (LDL) in healthy women [199]. When supplemented with 15 mg/d of lycopene for 8 weeks, healthy men showed a significant improvement in endothelial function with decreases in CRP and systolic blood pressure and an increase in the antioxidant enzyme superoxide dismutase [200]. A prospective study of Finnish men at risk for ischemic heart disease found that men in the highest quartile of serum lycopene had a 59 % lower risk of ischemic stroke and a 55 % lower risk of all strokes [201]. Moreover, in patients with heart failure, higher lycopene intake was associated with a significantly longer cardiac event-free survival, compared to those with lower lycopene intake [202]. These findings from human studies are corroborated by experimental studies using animal models, which suggest that lycopene’s antiatherogenic effects occur through the reduction of atherosclerotic plaques, decreases in serum total cholesterol and LDL cholesterol, and increases in HDL cholesterol [203]. In elderly Finnish men, the concentrations of plasma β-cryptoxanthin, lycopene, and α-carotene were found to decrease linearly with increasing intima-media thickness of the common carotid artery [204]. Serum levels of lutein and zeaxanthin were also found to be lower in early atherosclerotic patients compared to healthy subjects [205].
Oxidative stress may contribute to the etiology of diabetes by inducing insulin resistance in the peripheral tissues and impairing secretion from the pancreatic cells [206], and the antioxidant capacity of carotenoids may protect against the development or progression of the disease. High levels of commonly measured metabolic parameters such as fasting blood glucose, 2-h glucose level in the glucose tolerance test, and glycosylated hemoglobin were inversely correlated with serum or dietary carotenoid levels in cross-sectional studies [207–209], but few prospective studies have investigated this association. It has been reported that higher dietary intake of β-cryptoxanthin, but not other carotenoids, significantly reduced the risk of type 2 diabetes [210]. Long-term β-carotene supplementation in a randomized controlled trial did not affect the incidence of type 2 diabetes [211]. Baseline plasma carotenoid concentrations failed to show an association with the risk of type 2 diabetes [212].
Since cardiovascular disease and diabetes mellitus are characterized by high levels of oxidative stress, it would be natural to assume that an increased consumption of antioxidants such as carotenoids through fruits, vegetables, and other sources in these chronic conditions would decrease further oxidative stress-related deterioration. Whether regular intake of carotenoids would prevent onset of disease is unclear from available evidence.
Toxicity of Carotenoids
Large doses of preformed vitamin A may cause acute poisoning, but high intake of provitamin A carotenoids does not result in hypervitaminosis . In patients with erythropoietic protoporphyria, therapeutic doses up to 150 mg β-carotene per day greatly increased tolerance to sunlight and did not have deleterious effects [213]. Similarly, the use of two richest sources of β-carotene, RPO [214] and gac fruit (Momordica cochinchinensis) [215] in cooking is not associated with vitamin A toxicity .
However, high doses of supplemental β-carotene are not advisable, especially in people under unusual oxidative stress. The previously noted randomized intervention trial of smokers in Finland found significantly increased incidence of lung cancer [216] on a daily dose of 20 mg β-carotene for 5–8 years. Similar results were found in the US asbestos workers and former smokers [165] who received 30 mg β-carotene per day for ~4 years. However, the US trial participants also received 25,000 IU retinyl palmitate, which is more than eightfold the recommended daily allowance (RDA) for men and 10.5-fold RDA for women. The excess risks were restricted primarily to women and former smokers. Another randomized trial followed patients after removal of colorectal adenoma who received placebo or 25 mg β-carotene/day for 4 years [217]. The risk of recurrent adenoma was doubled by taking β-carotene supplements, but only for those who smoked and also drank more than one alcoholic drink per day. The nonsmokers and nondrinkers actually had their risk of adenoma markedly decreased by β-carotene supplements [218].
It has been long suspected that carotenoids may have prooxidant properties when present in high concentration or at high oxygen pressure [219]. Medical literature has noted a few cases of very high concentration of carotenoids in human tissues. Habitual and very excessive intake of tomato products caused lycopene deposits in the liver, accompanied by liver enlargement and abdominal pain [220]. Golden yellow crystals were observed in the retina of people taking canthaxanthin “tanning pills,” as well as in macaque monkeys treated with the same supplement [221]. The pills were banned, although they did not seem to damage the vision of the subjects .
Smoke exposure with concurrent β-carotene supplementation was studied in ferrets, a useful animal model for carotenoid absorption. High amounts of administered β-carotene, equivalent to 30 mg/day in humans, caused precancerous lung lesions in the smoke-exposed animals [222]. The lung extracts from these ferrets had enhanced β-carotene breakdown into apocarotenals in vitro. The ferret lung and other tissues express BCDO2 enzyme, which cleaves β-carotene to β-apo-10′-carotenal, while other β-apocarotenals may be formed in enzymatic or nonenzymatic reactions, especially at high oxygen pressures and the free radical-rich environment of smoker lungs. Recently, all possible β-apocarotenoids were investigated for their biological activity on retinoid receptors [17] using molecular modeling assays. One of them, β-apo-13-carotenone, was found to be an antagonist of the 9-cis-retinoic acid activation of retinoid X receptor (RXRα), as well as of three all-trans-retinoic acid receptors (RARα, RARβ, RARγ). Considering the importance of these nuclear hormone receptors in cell differentiation, the excessive production of antagonists may help to explain the negative effects of supplemental β-carotene trials .
Recent studies with human cell cultures (liver carcinoma HepG2) and BCDO2-deficient mice found that an excess accumulation of various carotenoids (β-carotene, lutein, zeaxanthin , lycopene) in mitochondria may produce oxidative stress [16]. It is hypothesized that gene polymorphism in the carotenoid splitting genes BCMO1 and BCDO2 may alter carotenoid and vitamin A homeostasis in some individuals and make them more susceptible to toxic effects of excess carotenoid supplementation .
Summary
Plants have evolved a vast number of carotenoids as essential compounds for their development and survival. Animals and humans, as plant consumers, have also evolved to take advantage of the unique properties of carotenoids. The mechanisms for the absorption and metabolism of carotenoids are well established. Vitamin A toxicity is efficiently averted. However, the great variability in these processes in human omnivores, whether they adapted as predominate plant eaters or hunter gatherers, raises the likelihood that carotenoids have no obligate dietary requirement but rather act as dietary enhancements. Human carnivores survive quite well on the preformed vitamin A obtained from animal flesh and dairy products. On the other hand, vegans must pay attention to the daily consumption of provitamin A carotenoids (rich sources, like carrots, sweet potato, winter squash, pumpkin, or RPO) in order to meet their vitamin A requirement [15]. Increased fruit and vegetable consumption along with carotenoid-enhanced foods such as Golden Rice is an important strategy for the elimination of the devastating effects of VAD that still is too prevalent in several countries.
The accumulation of the xanthophylls in the macula of the eye points to the utility of these carotenoids as blue light filters and may be the strongest evidence of a specific human function. To support eye health, the AREDS1 formulation, with the substitution of 10 mg/d of lutein and 2 mg/d of zeaxanthin for β-carotene (especially for smokers), may be our best interim estimate for beneficial effects [112]. The inclusion of a one half cup serving of a cooked dark-green leafy vegetable and/or egg yolk per day would be necessary to provide a similar dose of lutein + zeaxanthin from foods sources [7]. The accumulation of carotenoids in the corpus luteum and skin presents an intriguing possibility of their importance in fertility and sexual attraction, as it was found in many animals. The presence of xanthophylls in specific sections of the human brain should encourage further research into their role in protection from age-related cognitive decline and AD.
Carotenoids accumulate in lipid droplets and the lipids of various cell membranes and likely occupy a different niche than other exogenous antioxidants, such as vitamins C and E, or the endogenous antioxidants, such as glutathione and the antioxidant enzymes. Therefore, they can act as players among the cast of actors against oxidative stress and inflammation. Their ability to act as prooxidants in situations of high oxygen partial pressure and oxidative stress that was evidenced in cancer trials among smokers and asbestos workers argues against high levels of supplementation [167]. The array of population studies evaluating various cancers, heart disease, and diabetes, that have found reduced risk with higher intakes of dietary carotenoids, may be more a product of health-promoting dietary patterns, including bountiful intakes of carotenoid-containing fruits and vegetables.
In one metabolic abnormality, erythropoietic protoporphyria, massive doses of β-carotene far beyond what could be provided by diet alone were found to have great clinical benefit [84]. Other metabolic disorders, where fat absorption is compromised, such as cystic fibrosis, jeopardize the absorption of all fat-soluble vitamins including the carotenoids [223]. There is an indication that obesity and other circumstances that produce mild oxidative stress and inflammation may benefit from the increased consumption of carotenoids, since many of these conditions are accompanied by lower plasma levels of various carotenoids. Given that few individuals in the US population meet dietary advice for the consumption of fruits and vegetables, many of these conditions could be ameliorated by actually consuming at least five servings for fruits and vegetables per day.
The carotenoids are remarkable pigments. They color our foods and provide pleasure to our eating experience. They contribute to our vitamin A requirement and the reduction of oxidative stress. They may even contribute to seeing those pigmented foods more clearly, even if it is no longer in the bush but rather in the supermarket.
References
Bendich A (1993) Biological functions of carotenoids. In: Canfield LM et al (eds) Carotenoids in human health. New York Academy of Sciences, New York, pp 61–67
Davies BH (1991) Carotenoid metabolism as a preparation for function. Pure Appl Chem 63:131–140
Slifka KA, Bowen PE, Stacewicz-Sapuntzakis M, Crissey SD (1999) A survey of serum and dietary carotenoids in captive wild animals. J Nutr 129:380–390
Korytko PJ, Rodvold KA, Crowell JA, Stacewicz-Sapuntzakis M, Diwadkar-Navsariwala V, Bowen PE, Schalch W, Levine BS (2003) Pharmacokinetics and tissue distribution of orally administered lycopene in male dogs. J Nutr 133:2788–2792
McGraw KJ (2006) Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ (eds) Bird coloration, vol 1, Mechanisms and measurements. Harvard University Press, Cambridge, pp 177–242
von Lintig J (2012) Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr 96(suppl):1234–1244
Holden JM, Eldridge AL, Beecher GR, Buzzard I, Bhagwat S, Davis CS, Douglass LW, Gebhardt S, Haytowitz D, Schakel S (1999) Carotenoid content of U.S. Foods: an update of database. J Food Comp Anal 12:169–196
Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS (2004) Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressing as measured by electrochemical detection. Am J Clin Nutr 80:396–403
Block G (1994) Nutrient sources of provitamin A carotenoids in the American diet. Am J Epidemiol 139:290–293
Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK (2012) Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet 122:222–229
Kopsell DA, Kopsell DE (2006) Accumulation and bioavailability of dietary carotenoids in vegetable crops. Trends Plant Sci 11:499–507
Haskell MJ (2012) The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion-evidence in humans. Am J Clin Nutr 96(suppl):1193S–203S
Lindqvist A, Sharvill J, Sharvill DE, Andersson S (2007) Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr 137:2346–2350
Tang G (2012) Techniques for measuring vitamin A activity from β-carotene. Am J Clin Nutr 96(suppl):1185S–1188S
Food and Nutrition Board, Institute of Medicine (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy Press, Washington, DC, pp 82–161
Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J (2011) A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 25: 948–959
Harrison EH, dela Sena C, Eroglu A, Fleshman MK (2012) The formation, occurrence, and function of β-apocarotenoids: β-carotene metabolites that may modulate nuclear receptor signaling. Am J Clin Nutr 96(suppl):1189S–1192S
Fleshman MK, Lester GE, Riedl KM, Kopec RE, Narayanasamy S, Curley RW, Schwartz SJ, Harrison EH (2011) Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: determinations of β-carotene bioaccessibility and bioavailability. J Agric Food Chem 59:4448–4454
Kopec RE, Riedl KM, Harrison EH, Curley RW Jr, Hruszkewycz DP, Clinton CK, Schwartz SJ (2010) Identification and quantification of apolycopenals in fruits, vegetables and human plasma. J Agric Food Chem 58:3290–3296
World Health Organization (WHO) (2009) Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO global database on vitamin A deficiency, pp 1–55. www.whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf
WHO (2012) http://www.who.int/nutrition/topics/vad/en/index.html. Accessed 27 Dec 2012
Haque R, Ahmed T, Wahed MA, Mondal D, Hamidur Rahman ASM, Albert MJ (2010) Low dose β-carotene supplementation and deworming improve serum vitamin A and β-carotene concentrations in preschool children of Bangladesh. J Health Popul Nutr 28(3):230–237
Arroyave G, Mejia LA (2010) Five decades of vitamin A studies in the region of Central America and Panama. Food Nutr Bull 31:118–129
Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA (2011) Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 343:d5094. doi:10.1136/bmj.d5094. Pubmed PMID: 21868478
Low JW, Arimond M, Osman N, Cunguara B, Zano F, Tschirley D (2007) A food-based approach introducing orange-fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. J Nutr 137:1320–1327
Hotz C, Loechl C, Lubowa A, Tumwine JK, Ndeezi G, Nandutu Masawi A, Baingana R, Carriquiry A, de Brauw A, Meenakshi JV, Gilligan DO (2012) Introduction of β-carotene-rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. J Nutr 142:1871–1880
Jamil KM, Brown KH, Jamil M, Peerson JM, Keenan AH, Newman JW, Haskell MJ (2012) Daily consumption of orange-fleshed sweet potato for 60 days increased plasma β-carotene concentration but did not increase total body vitamin A pool size in Bangladeshi women. J Nutr 142:1896–18902
Sundram K, Sambanthamurthi R, Tan YA (2003) Palm fruit chemistry and nutrition. Asia Pac J Clin Nutr 12:355–362
Atinmo T, Bakre AT (2003) Palm fruit in traditional African food culture. Asia Pac J Clin Nutr 12:350–354
Rice AL, Burns JB (2010) Moving from efficacy to effectiveness: red palm oil’s role in preventing vitamin A deficiency. J Am Coll Nutr 29:302S–313S
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A β-carotene biosynthetic pathway into (carotenoid free) rice endosperm. Science 287:303–305
Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of golden rice through increased provitamin A content. Nat Biotechnol 23:482–487
Tang G, Hu Y, Yin SA, Wang Y, Dallal GE, Grusak MA, Russell RM (2012) β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. Am J Clin Nutr 96:658–664
Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA (2009) Golden Rice is an effective source of vitamin A. Am J Clin Nutr 89:1776–1783
Blount JD, Houston DC, Sural PF, Pape Moller A (2004) Egg-laying capacity is limited by carotenoid pigment availability in wild gulls Larus fuscus. Proc R Soc Lond B 271:S79–S81
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromancy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Stephen ID, Coetzee V, Perrett DI (2011) Carotenoid and melanin pigment coloration affect perceived human health. Evol Human Behav 32:216–227
Kaplan LA, Lau JM, Stein EA (1990) Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem 8:1–10
Stahl W, Schwarz W, Sundquist AR, Sies H (1992) Cis-trans isomers of lycopene and β-carotene in human serum and tissues. Archiv Biochem Biophys 294:173–177
Palan P, Naz R (1996) Changes in various antioxidant levels in human seminal plasma related to immunofertility. Arch Androl 36:139–143
Gupta NP, Kumar R (2002) Lycopene therapy in idiopathic male infertility: a preliminary report. Int J Urol Nephrol 34:369–372
Schweigert FJ (2003) Research note: changes in the concentration of β-carotene, α-tocopherol and retinol in the bovine corpus luteum during the ovarian cycle. Arch Anim Nutr 57:307–310
O’Shaughnessy PJ, Wathes DC (1998) Bovine luteal cell activity in culture. Maintenance of steroidogenesis by high density lipoprotein containing high or low beta-carotene concentrations. Anim Reprod Sci 17:165–176
Rapoport R, Sklan D, Wolfeson D, Shaham-Albancy A, Hanukoglu I (1998) Antioxidant capacity is correlated with steroidogenic status of the corpus luteum during the bovine estrous cycle. Biochem Biophys Acta 1380:133–140
Folman Y, Rosenberg M, Ascarelli I, Kaim M, Herz Z (1983) The effect of dietary and climatic factors on fertility, and on plasma progesterone and oestradiol-17 beta levels in dairy cows. J Steroid Biochem 19:863–868
Csapo AI, Pulkkinen M (1978) Indispensability of the human corpus luteum in the maintenance of early pregnancy: luteectomy evidence. Obstet Gynecol Surv 33:69–81
West KP Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Connor PB, Dali SM, Christian P, Pokhrel RP, Sommer A (1999) Double blind, cluster randomized trial of low dose supplementation with vitamin A or β-carotene on mortality related to pregnancy in Nepal. BMJ 318:570–575
West KP Jr, Christian P, Labrique AB, Rashid M, Shamin AA, Klemm RDW, Massie AB, Mehra S, Schulze KJ, Ali H, Ullah B, Wu LSF, Katz J, Banu H, Akhter HH, Sommer A (2011) Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh. A cluster randomized trial. JAMA 305:1986–1995
Thorne-Lyman A, Fawzi WW (2012) Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatric Perinatal Epidemiol 26(Suppl 1):36–54
Villamor E, Koulinska IN, Aboud S, Murrin C, Bosch RJ, Manji KP, Fawzi WW (2010) Effect of vitamin supplements on HIV shedding in breast milk. Am J Clin Nutr 92:881–886
WHO (2011) Guideline: vitamin A supplementation in pregnant women. http://www.who.int/elena/titles/vitamina_pregnancy/en/. Accessed 28 Dec 2012
Patton S, Canfield LM, Huston GE, Ferris AM, Jensen RG (1990) Carotenoids of human colostrums. Lipids 25:159–165
Canfield LM, Clandinin MT, Davies DP, Fernandez MC, Jackson J, Hawkes J, Goldman WJ, Pramuk K, Reyes H, Sablan B, Sonobe T, Bo X (2003) Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr 42:133–141
Azar M, Basu A, Jenkins AJ, Nankervis AJ, Hanssen KF, Scholz H, Henriksen T, Garg SK, Hammad SM, Scardo JA, Aston CE, Lyons TJ (2011) Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: a longitudinal study. Diabetes Care 34:1258–1264
Palan PR, Mikhail MS, Romney SL (2001) Placental and serum levels of carotenoids in preeclampsia. Obstet Gynecol 98:459–462
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10:49. doi:10.1186/1477-7827-10-49. Pubmed PMID: 22748101
Bohm F, Edge R, Truscott G (2012) Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: potential effects for human health. Mol Nutr Food Res 56:205–216
Gruszecki W (2010) Carotenoids in lipid membranes. In: Landrum J (ed) Carotenoids: physical, chemical and biological functions and properties. CRC Press, Boca Raton, pp 19–30
Edge R, Truscott G (2010) Properties of carotenoid radicals and excited states and their potential role in biological systems. In: Landrum J (ed) Carotenoids: physical, chemical and biological functions and properties. CRC Press, Boca Raton, pp 283–308
Bohm F, Edge R, McGarvey D, Truscott T (1998) β-carotene with vitamins E and C offers synergistic cell protection against NO x . FEBS Lett 436:387–389
Bohm F, Edge R, Truscott G (2012) Interactions of dietary carotenoids with singlet oxygen (1O2) and free radicals: potential effects for human health. Acta Biochim Pol 59:27–30
Walston J, Xue Q, Semba R, Ferrucci L, Cappola A, Ricks M, Guraink J, Fried L (2006) Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol 163:18–26
Holt E, Steffen L, Moran A, Basu S, Steinberger J, Ross J, Hong C, Sinaiko A (2009) Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc 109:414–421
Thomson C, Stendell-Hollis N, Rock C, Cussler E, Flatt S, Pierce J (2007) Plasma and dietary carotenoids are associated with reduced oxidative stress in women previously treated for breast cancer. Cancer Epidemiol Biomarkers Prev 16:2006–2015
Hozawa A, Jacobs D, Steffes M, Gross M, Steffen L, Duk-Hee L (2007) Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: The Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clinic Chem 53:447–455
D’Adamo C, Miller R, Shardell M, Orwig D, Hochberg M, Ferrucci L, Semba R, Yu-Yahiro J, Magaziner J, Kicks G (2012) Higher serum concentrations of dietary antioxidants are associated with lower levels of inflammatory biomarkers during the year after hip fracture. Clin Nutr 31:659–665
Kim J-H, Na H-J, Kim C-K, Kim J-Y, Ha K-S, Lee H, Chung H-T, Kwon H, Kwaon Y-G, Kim Y-M (2008) The non-provitamin A carotenoid, lutein, inhibits NF-κB-inducing kinase pathways: role of H2O2 in NF-κB activation. Free Radic Biol Med 45(6):885–896
Izumi-Nagai K, Nagai N, Ohgami K, Satfuka S, Ozawa Y, Tsubota K, Umezawa K, Ohno S, Oike Y, Ishida S (2007) Macular pigment lutein is anti-inflammatory in preventing choroidal neovascularization. Aterioscler Thromb Vasc Biol 27:2555–2562
Di Tomo P, Canali R, Ciavardelli D, Di Silvestre S, De Marco A, Giardinelli A, Pipino C, Di Pietro N, Virgili F, Pandolfi A (2012) β-carotene and lycopene affect endothelial response to TNF-α reducing nitro-oxidative stress and interaction with monocytes. Mol Nutr Food Res 56:217–227
Rubin L, Chan G, Barrett-Reis B, Fulton A, Hansen R, Ashmeade T, Oliver R, Mackey A, Dimmit R, Hartmann E, Adamkin D (2012) Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol 32:418–424
Graydon R, Hogg RE, Chakravarthy U, Young I, Woodside J (2012) The effect of lutein- and zeaxanthin-rich foods vs supplements on macular pigment level and serological markers of endothelial activation, inflammation and oxidation: pilot studies of healthy volunteers. Br J Nutr 108:334–342
Zhao X, Aldini G, Johnson E, Rasmussen H, Kraemer K, Woolf H, Musaeus N, Krinsky N, Russell R, Yeum K (2006) Modification of lymphocyte DNA damage by carotenoid supplementation in postmenopausal women. Am J Clin Nutr 83:163–169
Basu A, Imrhan V (2007) Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. European J Clin Nutr 61:295–303
Dunstan J, Breckier L, Hale J, Lehmann H, Franklin P, Lyons G, Ching S, Mori T, Barden A, Prescott S (2007) Supplementation with vitamins C, E, beta-carotene and selenium has no effect on anti-oxidant status and immune responses in allergic adults: a randomized controlled trial. Clin Exp Allergy 37:180–187
Rytter E, Vessby B, Asgard R, Ersson C, Moussavian S, Sjodin A, Abramsson-Zetterberg L, Moller L, Basu S (2010) Supplementation with a combination of antioxidants does not affect glycaemic control, oxidative stress or inflammation in type 2 diabetes subjects. Free Radic Res 44:1445–1453
Meinke M, Friedrich A, Tscherch K, Haag S, Darvin M, Vollert H, Groth N, Lademann J, Rohn S (2013) Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharmaceut Biopharm Dec 13; 84:365–373
Mayne S, Cartmel B, Scarmo S, Lin H, Leffell D, Welch E, Ermakov I, Bhosale P, Bernstein P, Getterman W (2010) Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr 92:794–800
Alaluf S, Heinrich U, Stahl W, Tronnier H, Wiseman S (2002) Dietary carotenoids contribute to normal human skin color and UV photosensitivity. J Nutr 132:399–403
Stephen I, Smith M, Stirrat M, Perret D (2009) Facial skin coloration affects perceived health of human faces. Int J Primatol 30:845–857
Whitehead R, Ozakinci G, Stephen I, Perrett D (2012) Appealing to vanity: could potential appearance improvement motivate fruit and vegetable consumption? Am J Pub Health 102:207–211
Karthik S, Campbell-Davis D, Isherwood D (2006) Carotenemia in infancy and is association with prevalent feeding practices. Ped Dermatol 23:571–573
Takita Y, Ichimiya M, Hamamoto Y, Muto M (2006) A case of carotenemia associated with ingestion of nutrient supplements. J Dermatol 2:132–134
Hueber A, Rosentreter A, Severin M (2011) Canthaxanthin retinopathy: long-term observations. Ophthalmic Res 46:103–106
Mathews-Roth M (1986) Beta-carotene therapy for erythropoetic protoporphyria and other photosensitivity diseases. Biochimie 68:875–884
Stahl W, Sies H (2012) β-carotene and other carotenoids in protection from sunlight. Am J Clin Nutr 96(suppl):1179–1184
Zussman J, Ahdout J, Kim J (2010) Vitamins and photoaging: do scientific data support their use? J Am Acad Dermatol 63:507–525
Kopcke W, Krutmann J (2008) Protection from sunburn with β-carotene: a meta-analysis. Photochem Photobiol 84:284–288
Darvin M, Fluhr J, Meinke M, Zastrow L, Sterry W, Lademann J (2011) Topical beta-carotene protects against infra-red-light-induced free radicals. Exper Dermatol 20:125–129
Kohl E, Steinbauer J, Landthaler M, Szeimies R (2011) Skin ageing. J Euro Acad of Dermatol Venereol (JEADV) 25:873–884
Purba M, Kouris-Blazos A, Wattanapenpalboon N, Lukito W, Rothenberg E, Steen B, Wahlqvist M (2001) Skin wrinkling: can food make a difference. J Am Coll Nutr 20:71–80
Darvin M, Patzelt A, Gehse S, Schanzer S, Benderoth C, Sterry W, Lademann J (2008) Cutaneous concentration of lycopene correlates significantly with the roughness of skin. Eur J Pharmaceut Biopharmaceut 69:943–947
Evans J, Johnson E (2010) The role of phytonutrients in skin health. Nutrients 2:903–928
Drasdo N, Fowler CW (1974) Non-linear projection of the retinal image in a wide-angle schematic eye. Br J Ophthalmol 58:709–714
Bone R, Landrum J, Fernandez L, Tarsis S (1988) Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci 29:843–949
Zeimer M, Dietzel M, Hense H, Helmes B, Austermann U, Pauleikhoff D (2012) Profiles of macular pigment optical density and their changes following supplemental lutein and zeaxanthin: new results from the LUNA study. Invest Ophthalmol Vis Sci 53:4852–4859
Krinsky N, Landrum J, Bone R (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Ann Rev Nutr 23:171–201
Edge R, McGarvey D, Truscott G (1997) The carotenoids as antioxidants: a review. J Photochem Photobiol B Biol 41:189–200
Ahmed S, Lott M, Marcus D (2005) The macular xanthophylls. Surv Ophthalmol 50:183–193
Bone R, Landrum J, Guerra L, Ruiz J (2003) Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr 133:992–998
Ma L, Dou H-L, Wu Y-Q, Huang Y-M, Huang Y-B, Xu X-R, Zou Z-Y, Lin X-M (2012) Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr 107:350–359
Klein R, Chou C-F, Klein B, Zhang X, Meuer S, Saddine J (2011) Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 129:75–80
Muni R, Altaweel M, Tennant M, Weaver B, Kertes P (2008) Agreement among Canadian retina specialists in the determination of treatment eligibility for photodynamic therapy in age-related macular degeneration. Retina 28:1421–1426
Bone R, Landrum J (2010) Dose-dependent response of serum lutein and macular pigment optical density to supplementation of lutein esters. Arch Biochem Biophys 504:50–55
Schalch W, Cohn W, Barker F, Kopcke W, Mellerio J, Bird A, Robson A, Fitzke F, van Kujk F (2007) Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin: the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys 458:128–135
Barlett H and Eperjesi F (2008) A randomized controlled trial investigating the effect of lutein and antioxidant dietary supplementation on visual function in healthy eyes. Clinical Nutr 27:218–227
Ma L, Yan S-F, Huang Y-M, Lu X-R, Qian F, Pang H-L, Xu X-R, Zou Z-Y, Dong P-C, Xiao X, Wang X, Sun T-T, Dou H-L, Lin X-M (2012) Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmol 119:2290–2297
Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J (2004) Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 75:216–230
Richer S, Stiles W, Graham-Hoffman K, Levin M, Ruskin D, Wrobel J, Park D-W, Thomas C (2011) Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration. Optometry 82:667–680
Age-Related Eye Disease Study Group (2001) A randomized, placebo-controls clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS report no. 8. Arch Ophthalmol 119:1417–1436
Age-Related Eye Disease Study Group (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamin C and E and beta-carotene for age-related cataract with vision loss: AREDS report no. 9. Arch Ophthalmol 119:1439–1452
Evans J, Lawrenson J (2012) Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration (Review) The Cochrane Collaboration Cochrane Library 2012, Issue 6 www.thecochranelibrary.com John Wiley
The Age-Related Eye Disease Study 2 (AREDS2) Research Group (2013) Lutein + Zeaxanthin and omega-3 fatty acids for age-related macular degeneration. The age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA 309:2005–2015
Loughman J, Davidson P, Molan J, Akkali M, Beatty S (2010) Macular pigment and its contribution to visual performance and experience. J Optometry 3:74–90
Hammond B, Fletcher L (2012) Influence of the dietary carotenoids lutein and zeaxanthin on visual performance: application to baseball. Am J Clin Nutr 96(Suppl):1207–1300
Stringham J, Hammond B (2007) The glare hypothesis of macular pigment function. Optometry Vis Sci 84:859–864
Stringham J, Garcia P, Smith P, McLin L, Foutch B (2011) Macular pigment and visual performance in glare: benefits for photostress recovery, disability glare and visual discomfort. Ophthalmol Vis Sci 52:7406–7415
Loughman J, Akkali M, Beatty S, Scanlon G, Davidson P, O’Dwyer V, Cantwell T, Major P, Stack J, Nolan J (2010) The relationship between macular pigment and visual performance. Vis Res 50:1249–1256
Renzi L, Hammond B (2010) The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthal Physiol Opt 30:351–357
Ma L, Lin X-M, Zou Z-Y, Xu X-R, Li Y, Xu R (2009) A 12-week lutein supplementation improved visual function in Chinese people with long-term computer display light exposure. Br J Nutr 102:186–190
Kvansakul J, Rodriguez-Camona M, Edgar D, Barker F, Kopcke W, Schalch W, Barbur J (2006) Supplmentation with the carotenoids lutein and zeaxanthin improves human visual performance. Ophthal Physiol Opt 26:362–371
Nolan J, Loughman J, Akkali M, Stack J, Scanlon G, Davidson P, Beatty S (2011) The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vis Res 51:459–469
Loughman J, Nolan J, Howard A, Connolly E, Meagher K, Beatty S (2012) The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Invest Ophthalmol Vis Sci 53:7871–7880
Barlett H, Eperjesi F (2007) Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: a randomized controlled trial. Eur J Clin Nutr 61:1121–1127
Michael R, Bron A (2011) The ageing lens and cataract: a model of normal and pathological ageing. Phil Trans R Soc B 366:1278–1292
Truscott R (2005) Age-related nuclear cataract: oxidation is the key. Exper Eye Res 80:709–725
Yeum K-J, Shang F, Schalch W, Russell R, Taylor A (1999) Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr Eye Res 19:502–505
Bhosale P, Zhao D-Y, Bernstein P (2007) HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Invest Ophthalmol Vis Sci 48:543–549
Barker F (2010) Dietary supplementation: effects on visual performance and occurrence of AMD and cataracts. Curr Med Res Opin 26:2011–2023
Mares J, Voland R, Adler R, Tinker L, Millen A, Moeller S, Blodi B, Gehrs K, Wallace R, Chappell R, Meuhouser M, Sarto G (2010) Healthy diets and the subsequent prevalence of nuclear cataract in women. Arch Ophthalmol 128:738–749
Milton R, Sperduto R, Clemons T, Ferris F (2006) Centrum use and progression of age-related cataract in the age-related eye disease study: a propensity score approach. AREDS report No. 21. Ophthalmology 113:1264–1270
Olmedilla B, Granado F, Blanca I, Vaquero M, Cajigal C (2001) Lutein in patients with cataracts and age-related macular degeneration: a long term supplementation study. J Sci Food Agric 81:904–909
Olmedilla B, Granado F, Blanco I, Vaquero M (2003) Lutein, but not alpha tocopherol supplementation improves visual function in patients with age-related cataracts: a two-year double-blind, placebo-controlled pilot study. Nutrition 19:21–24
Trumbo P, Ellwood K (2006) Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration’s evidence-based review system for health claims. Am J Clin Nutr 84:971–974
Berson E (1993) Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 34:1659–1676
Berson E, Rosner B, Sandberg M, Weigel-DeFranco C, Brockhurst R, Hayes C, Johnson E, Anderson E, Johnson C, Guadio A, Willet W, Schaefer E (2010) Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol 128:403–411
Wittes J, Gorin M, Mayne S, McCarthy C, Sternberg P, Wall M (2011) Letter from the DSMC regarding a clinical trial of lutein in patients with retinitis pigmentosa. Arch Ophthalmol 129:675
Dagnelie G, Zorge I, McDonald T (2000) Lutein improves visual function in some patients with retinal degeneration: a pilot study via the internet. Optometry 71:147–164
Bahrami H, Melia M, Dagnelie G (2006) Lutein supplementation in retinitis pigmentosia: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial. BMC Ophthalmol 6:23. www.biochemicalcontrol.com/1471-2415-6-23
Aleman T, Duncan J, Bieber M, de Castro E, Marks D, Gardner L et al (2001) Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci 42:1873–1881
Sandberg M, Johnson E, Berson E (2010) The relationship of macular pigment optical density to serum lutein in retinitis pigmentosa. Invest Ophthalmol Vis Sci 51:1086–1091
Li S-Y, Fung F, Zhong J-F, Wong D, Chan H, Lo A (2012) Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: in vivo and in vitro studies. Invest Opththalmol Vis Sci 53:5976–5984
Li S-Y, Fu Z-J, Ma H, Jang W-C, So K-F, Wong D, Lo A (2009) Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Invest Ophthalmol Vis Sci 50:836–843
Woo T, Li S-Y, Lai W, Wong D, Lo A (2013) Neuroprotective effects of lutein in a rat model of retinal detachment. Graefes Arch Clin Exp Ophthalmol 251:41–51
Craft N, Haitema T, Garnett K, Fitch K, Dorey C (2004) Carotenoid, tocopherol and retinol concentrations in elderly human brain. J Nutr Health Aging 8:156–162
Johnson E (2012) The possible role for lutein and zeaxanthin in cognitive function in the elderly. Am J Clin Nutr 96:1161S–1165S
Ritchie K, Touchon J (2000) Mild cognition impairment: conceptual basis and current neurological status. Lancet 355:225–228
Lahiri D, Greig N (2004) Lethal weapon: amyloid β-peptide, role in the oxidative stress and neurodegeneration of Alzheimer’s disease. Neurobiol Aging 25:581–587
Farooqui T, Farooqui A (2009) Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Aging Dev 130:203–215
Thompson P, Vinters H (2012) Pathologic lesions in neurodegenerative diseases. Prog Mol Biol Trans Sci. 107:1–40
Plasman B, Williams J, Burke J, Holsinger T, Benjamin S (2010). Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 153:182–193
Daviglus M, Bell C, Berrettini W, Bowen P, Connolly S, Cox N, Dunbar-Jacob J, Granieri E, Hunt G, McGarry K, Patel D, Potosky A, Sanders-Bush E, Silberbert D, Trevisan M (2010) National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer’s disease and cognitive decline. Ann Intern Med 153:176–181
Daviglus M, Plassman B, Pirzuda A, Bell C, Bowen P, Burke J, Connolly S, Dunbar-Jacob J, Granieri E, McGarry K, Patel D, Trevisan M, Williams J (2011) Risk factors and preventive interventions for Alzheimer’s disease. Arch Neurol 68:1185–1190
Yaffe K (2007) Antioxidants and prevention of cognitive decline: does duration of use matter? Arch Intern Med 167:2167–2168
Johnson E, McDonald K, Caldarella S, Chung H, Troen A, Snodderly D (2008) Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci 11:75–83
Li S-Y, Fu Z-J, Woo T, Wong D, Lo A (2012) Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiol Dis 45:624–632
Li S-Y, Fu Z-J, Lo A (2012) Hypoxia-induced oxidative stress in ischemic retinopathy. Oxid Med Cell Longev 2012:426769. doi:10:1155/2012/426769
Greenberg E (1993) Retinoids or carotenoids: is there another choice? Prev Med 22:723–727
Peto R, Doll R, Buckley J, Sporn M (1981) Can dietary beta-carotene materially reduce human cancer rates? Nature 290:201–208
Wald N (1987) Retinol, beta-carotene and cancer. Cancer Surv 6:635–651
Tanaka J, Shnimizu M, Moriwaki H (2012) Cancer prevention by carotenoids. Molecules 17:3202–3242
Vainio H, Weiderpass E (2006) Fruit and vegetables in cancer prevention. Nutr Cancer 54:111–142
Marques-Vidal P, Ravasco P, Camilo M (2006) Foodstuffs and colorectal cancer risk: a review. Clin Nutr 25:14–36
Flood A, Rasotogi T, Wirfalt E, Mitrou P, Reedy J, Subar A, Kipnis V, Mouw T, Hollenbeck A, Leitzmann M, Schatzkin A (2008) Dietary patterns as identified by factor analysis and colorectal cancer among middle-age Americans. Am J Clin Nutr 88:176–184
Heinonen O, Albanes D (1994) The effect of vitamin E and β-carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. N Engl J Med 330:1029–1035
Omenn G, Goodman G, Thornquist M, Balmes J, Cullen M, Glass A, Koegh J, Meyskens F, Valanis B, Williams H et al (1996) Effects of a combination of β-carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334:1150–1155
The ATBC Study Group (2003) Incidence of cancer and mortality following α-tocopherol and β-carotene supplementation. JAMA 290:476–485
Mayne S, Handelman G, Beecher G (1996) β-carotene and lung cancer promotion in heavy smokers: a plausible relationship. J Natl Cancer Inst 88:1513–1515
Hennekens C, Buring J, Manson J, Stampfer M, Rosner B, Cook N, Belanger C, LaMotte F, Gaziano J, Ridker P et al (1996) Effects of a combination of β-carotene and vitamin A on lung cancer and cardiovascular disease. N Eng J Med 334:1150–1155
Kelloff G, Boone C, Crowell J, Steele V, Lubet R, Doody L, Malone W, Hawk E, Sigman C (1996) New agents for cancer prevention. J Cell Biochem Suppl 26:1–28.
Ziegler R, Mayne S, Swanson C (1996) Nutrition and lung cancer. Cancer Causes Control 7:157–177
Chatterjee M, Roy K, Janarthan M, Das S, Chatterjee M (2012) Biological activity of carotenoids: its implications in cancer risk and prevention. Curr Pharmaceut Biotech 13:180–190
Kelkel M, Schumacher M, Dicato M, Diederich M (2011) Antioxidant and anti-proliferative properties of lycopene. Free Rad Res 45:925–940
Sharoni Y, Linnewiel-Hermoni K, Khanin M, Salman H, Veprik A, Danilenko M, Levy J (2012) Carotenoids and apocarotenoids in cellular signaling related to cancer: a review. Mol Nutr Food Res 56:259–269
Ford N, Erdman J (2012) Are lycopene metabolites metabolically active? Acta Biochim Pol 59:1–4
Etminan M, Takkouche B, Caamano-Isoma F (2004) The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomark Prev 13:340–345
Ilic D, Misso M (2012) Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: a systematic review. Maturitas 72:269–276
Conklin K (2003) Dietary antioxidants during cancer therapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer 37:1–18
Sahin K, Sahin N, Kucuk O (2010) Lycopene and chemotherapy toxicity. Nutr Cancer 62:988–995
Block K, Koch A, Mead M, Tothy P, Newman R, Gyllenhaal C (2007) Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treatment Rev 33:407–418
Puri T, Goyal S, Julka K, S, Nair O, Sharma D, Roth G (2010) Lycopene in treatment of high-grade gliomas: a pilot study. Neurol India 58(1):20–23
Meigs JB (2002) Epidemiology of the metabolic syndrome, 2002. Am J Manag Care 8(11 Suppl):283S–292S
Roberts CK, Sindhu KK (2009) Oxidative stress and metabolic syndrome. Life Sci 84:705–712
Ford ES, Mokdad AH, Giles WH, Brown DW (2003) The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes 52:2346–2352
Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Matsumoto H, Ando F, Shimokata H, Yano M (2008) Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr 100:1297–1306
Coyne T, Ibiebele TI, Baade PD, McClintock CS, Shaw JE (2009) Metabolic syndrome and serum carotenoids: findings of a cross-sectional study in Queensland, Aust Br J Nutr 102:1668–1677
Sluijs I, Beulens JW, Grobbee DE, van der Schouw YT (2009) Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J Nutr 139:9879–9892
Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang W, Zonderman AB (2011) Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J Nutr 141:903–913
Beydoun MA, Canas AJ, Beydoun HA, Chen X, Shroff MR, Zonderman AB (2012) Serum antioxidant concentrations and metabolic syndrome are associated among U.S. adolescents in recent national surveys. J Nutr 142:1693–1704
Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, Faure H, Huxley R, Hercberg S, Ahluwalia N (2009) Effects of long-term antioxidant supplementation and association of serum antioxidant concentration with risk of metabolic syndrome in adults. Am J Clin Nutr 90:329–335
Markovits N, Amotz AB, Levy Y (2009) The effect of tomato derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. IMAJ 11:598–601
McEneny J, Wade L, Young IS, Masson L, Duthie G, McGinty A, McMaster C, Thies F (2013) Lycopene intervention reduced inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J Nutr Biochem 24:163–168. doi:10.1186/1475-2891-11-34
Ghavipour M, Saedisomeolia A, Djalali M, Sotoudeh G, Eshraghyan MR, Moghadam AM, Wood LG (2013) Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br J Nutr 109:2031-2035
Riso P, Visioli F, Grand S, Guarnieri S, Gardana C, Simonetti P, Porrini M (2006) Effect of tomato based drink on markers of inflammation, immunomodulation, and oxidative stress. J Agric Food Chem 54:2563–2566
Palozza P, Simone R, Catalano A, Monego G, Barini A, Mele MC, Parrone N, Trombino S, Picci N, Ranelletti FO (2011) Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-κB nuclear binding and increase in PPARγ expression. J Nutr Biochem 22(3):259–268
Gouranton E, Thabuis C, Riollet C, Malezet-Desmoulins C, El Yazidi C, Amiot MJ, Borel P, Landrier JF (2011) Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem 22:642–648
Damms-Machado A, Weser G, Bischoff S (2012) Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr J 11:34. doi:10.1186/1475-2891-11-34. Pubmed PMID: 22657586
Voutilainen S, Nurmi T, Mursu J, Rissanen TH (2006) Carotenoids and cardiovascular health. Am J Clin Nutr 83:1265–1271
Karppi J, Laukkanen JA, Makikallio TH, Ronkainen K, Kurl S (2013) Serum β-carotene and the risk of sudden cardiac death in men: a population-based follow-up study. Atherosclerosis 226:172–177
Kim OY, Yoe HY, Kim HJ, Park JY, Kim JY, Lee SH, Lee JH, Lee KP, Jang Y, Lee JH (2010) Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis 208:581–586
Kim JY, Paik JK, Kim OY, Park HW, Lee JH, Jang Y, Lee JH (2011) Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 215:189–195
Karppi J, Laukkanen JA, Sivenius J, Ronkainen K, Kurl S (2012) Serum lycopene decreases the risk of stroke in men: a population based follow-up study. Neurology 79:1540–1547
Biddle M, Moser D, Song EK, Heo S, Payne-Emerson H, Dunbar SB, Pressler S, Lennie T (2013) Higher dietary lycopene intake is associated with longer cardiac event-free survival in patients with heart failure. Eur J Cardiovasc Nurs 12:377–384
Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A (2012) Effect of lycopene and tomato products on cholesterol metabolism. Ann Nutr Metab 61:126–134
Karppi J, Kurl S, Laukkanen JA, Rissanen TH, Kauhanen J (2011) Plasma carotenoids are related to intima-media thickness of the carotid artery wall in men from eastern Finland. J Intern Med 270:478–485
Xu XR, Zou ZY, Huang YM, Xiao X, Ma L, Lin XM (2012) Serum carotenoids in relation to risk factors for development of artherosclerosis. Clin Biochem 45:1357–1361
Ceriello A, Motz E (2004) Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 24:816–823
Ford ES, Will JC, Bowman BA, Venkat Narayan KM (1999) Diabetes mellitus and serum carotenoids: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 149:168–176
Suzuki K, Ito Y, Nakamura S, Ochiai J, Aoki K (2002) Relationship between serum carotenoids and hyperglycemia: a population-based cross-sectional study. J Epidemiol 12(5):357–366
Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S, Leonard D, Shaw J (2005) Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr 82:685–693
Montonen J, Knekt P, Järvinen R, Reunanen A (2004) Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 27:362–366
Liu S, Ajani U, Chae C, Hennekens C, Buring JE, Manson JE (1999) Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA 282:1073–1075
Wang L, Liu S, Pradhan AD, Manson JE, Buring JE, Gaziano JM, Sesso HD (2006) Plasma lycopene, other carotenoids, and the risk of type 2 diabetes in women. Am J Epidemiol 164:576–585
Mathews-Roth MM (1993) Carotenoids in erythropoietic protoporphyria and other photosensitivity diseases. Annals NY Acad Sci 691:127–138
Lietz G, Henry CJK, Mulokozi G, Mugyabuso JKL, Ballart A, Ndossi GD, Lorri W, Tomkins A (2001) Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. Am J Clin Nutr 74:501–509
Vuong LT, Dueker SR, Murphy SP (2002) Plasma β-carotene and retinol concentrations of children increase after a 30-d supplementation with the fruit Momordica cochinchinensis (gac). Am J Clin Nutr 75: 872–879
Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa E-R, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK (1996) α-Tocopherol and β-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of baseline characteristics and study compliance. J Natl Cancer Inst 88:1560–1570
Baron JA, Cole BF, Mott L, Haile R, Grau M, Church TR, Beck GJ, Greenberg ER (2003) Neoplastic and antineoplastic effects of β-carotene on colorectal adenoma recurrence: results of a randomized trial. J Natl Cancer Inst 95:717–722
Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL, Omenn GS, Valanis B, Williams JH Jr (2004) The beta-carotene and retinol efficacy trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping β-carotene and retinol supplements. J Natl Cancer Inst 96:1743–1750
Palozza P (1998) Prooxidant actions of carotenoids in biologic systems. Nutr Rev 56:257–256
Reich P, Shwachman H, Craig JM (1960) Lycopenemia: a variant of carotenemia. New Eng J Med 262:263–269
Goralczyk R, Buser S, Baush J, Bee W, Zühlke U, Barker FM (1997) Occurrence of birefringent retinal inclusions in cynomolgus monkeys after high doses of canthaxanthin. Invest Opthalmol Vis Sci 38:741–752
Russell RM (2004) The enigma of β-carotene in carcinogenesis: what can be learned from animal studies. J Nutr 134:262S–268S
Back E, Frindt C, Nohr D, Frank J, Ziebach R, Stern M, Banke M, Biesalski H (2004) Antioxidant deficiency in cystic fibrosis: when is the right time to take action? Am J Clin Nutr 80:374–384
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Bowen, P., Stacewicz-Sapuntzakis, M., Diwadkar-Navsariwala, V. (2015). Carotenoids in Human Nutrition. In: Chen, C. (eds) Pigments in Fruits and Vegetables. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2356-4_3
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2356-4_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2355-7
Online ISBN: 978-1-4939-2356-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)