Abstract
Autism spectrum disorders (ASD) are developmental disorders characterized by behavioral deficits in verbal and nonverbal communication as well as social interactions, and are accompanied by repetitive or stereotyped behaviors and interests. Numerous studies over the last forty years have recognized altered immune responses in individuals with ASD; concurrently basic research has highlighted the myriad of neuroimmune interactions and the cross talk that occurs between nervous and immune systems. Neuroinflammation, particularly in the cerebellum, has been found in post mortem brain tissues from individuals with ASD and is characterized by the presence of profound glia activation processes. This and altered gene expression profiles indicating perturbed immune suggest a contributing role for immunological systems in the pathology of ASD. Peripheral immune abnormalities have also been found; shifts in both direction of Th1 and Th2 skewing have been reported as well as autoantibody production, increased NK cell activation, T cell responses and monocyte cell function overwhelmingly suggesting the presence of immune dysfunction in individuals with ASD. Many of these findings are associated with worsening behavioral scores, suggesting treatment of immune function could be useful in alleviating symptoms associated with ASD. Immune activation in utero is also associated with an increased risk of the child for having a diagnosis of ASD, where increased cytokine production in the offspring is directly linked to changes in offspring behavior. In addition to peripheral changes, brain and CSF immune variations in ASD are reported as well as an increase in gastrointestinal/mucosal dysfunction which has led to an increased interest in exploring the gut-brain- immune connections and its role in ASD. Further research in neuroimmune interactions may bring further insight and elicit new therapeutic tools for ASD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adaptive immune system
- Autism spectrum disorders
- Behavior
- Cerebellum
- Cytokine
- Innate immune system
- Immunity
- Maternal immune
- activation
- Neuroimmunology
- Social interactions
- T cells
- Gastrointestinal
1 Immune-Brain Interactions
1.1 Cytokines and Neurodevelopment
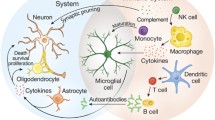
The immune system is involved in three main areas—surveillance of foreign antigen, fighting infections and tissue remodeling and participates in these functions in all types of tissue during development, health, disease and wound healing . The brain is no exception; many immune responses take part in shaping and maintaining normal central nervous system (CNS) function (Fig. 6.1). During the course of neurodevelopment and throughout adulthood, normal immunological processes take part in influencing appropriate neurological features and functions. Cytokines , the immune system’s signaling molecules have a significant role in both of these areas. Cytokines act as both chemoattractants, guiding direction of growth and migration as well as acting as neurotrophic factors that promote survival to developing neurons in the brain and spinal cord (Deverman and Patterson 2009). Interleukin (IL)-1β a prominent inflammatory cytokine of the immune system has been found to be involved in many different functions of the CNS. During development, IL-1β expression is observed in the embryonic spinal cord of both chickens (stage 17-HH) and rats (embryonic day 12) (de la Mano et al. 2007). Delivery of IL-1β on microbeads implanted near the spinal cord of chick embryos increased the number of proliferating (BrdU+) neuroepithelial cells in the dorsal spinal cord and led to a reduction in the ventral spinal cord. Blocking IL-1β through anti- IL-1β antibodies reduced BrdU incorporation in the dorsal spinal cord. (de la Mano et al. 2007). In the mammalian adult brain, cytokines including IL-1β can act on the neural and progenitor stem cells in response to injury, disease and stress influencing proliferation and neurogenesis. (Carpentier and Palmer 2009). IL-1β is also believed to be required for normal learning and memory processes in the hippocampus (Goshen et al. 2007). Gene expression of IL-1β has been found to be increased in the hippocampus 24 h after contextual learning and blocking IL-1β results in impairments in spatial memory and fear conditioning tests (Goshen et al. 2007). IL-1 receptor knockout mice were found to have a reduction in neuronal dendritic spine size which may contribute to the defects seen in memory in these mice (Goshen et al. 2009) .
a) Many immunological processes take part both in developing and maintaining the CNS. Microglia are the main contributor to many of these processes. As the resident phagocytic cell, microglia clear cellular debris and apoptotic neurons. They also further neural development by producing various growth factors and many cytokines that regulate neuronal survival and synaptogenesis. Microglia also respond to danger signals by producing reactive oxygen species and pro-inflammatory cytokines. Some of the cytokines that can affect the CNS include: IL-1β, TNF-α, IL-6 and TGF-β. These cytokines not only contribute to neuronal development but also play a role in learning and memory. b) Lymphocytes, part of the adaptive immune system have also been found to contribute to cognitive function. More specifically T cells have been demonstrated to contribute to visuospatial learning and enhance neurogenesis in the dentate gyrus. T cells important to cognitive function are believed to be found in the meningeal spaces where they produce cytokines; specifically IL-4 has been associated with visuospatial learning. c) Outside the CNS, neuro-immune interactions also take place. Many immune cells including macrophages express receptors for neurotransmistters. Some of these receptors include beta adrenergic, nicotine α-7, noradrenergic, metabotropic glutamate, neuropeptide and 5-hydroxytryptophan (5-HT) receptors. Signaling through these receptors can either enhance or suppress the immune response depending on the neurotransmitter and environmental conditions. Norepinephrine, for example, generally tends to have an inhibitory effect on pro-inflammatory cytokine production in macrophages but this can be reversed if other factors such as LPS are present. NGF nerve growth factor, BDNF brain derived neurotrophic factor, NT-3 neurotrophin-3, GDNF, Glial cell line-derived neurotrophic factor, IL interleukin, 5-HT 5-hydroxytryptophan
Other pro-inflammatory cytokines that are involved in spatial learning and memory include both IL-6 and Tumor necrosis factor (TNF)-α; however, these cytokines also have complex roles in shaping memory and learning and have reported beneficial and detrimental effects. A recent study showed that TNF-α signaling through the NFκB pathway lead to increased neural stem cell (NSC) proliferation. This proliferation was attributed to the activation of the IKK-β and NFκB pathway leading to up regulation of cyclin D1 (Widera et al. 2006). How cytokines affect the CNS is context dependent and environments surrounding the release of these cytokines play an equally important role in determining the ultimate effect than any single cytokine will play (Yirmiya and Goshen 2011). Transforming growth factor (TGF)-β, an important immune regulatory cytokine, is involved in signaling required for mouse mesencephalic progenitors to differentiate into tyrosine hydroxylase (TH) + dopaminergic neurons in vitro and in vivo (Roussa et al. 2006). When TGFβ2/TGFβ3 double knockout mice were examined it was found they have reduced numbers of TH+ neurons in the ventral mesencephalon, however, in the locus coeruleus TH+ neurons were not significantly different from controls indicating that while TGFβ signaling may be important in the ventral mesencephalon it does not seem to contribute to ventral midbrain dopaminergic neuron development (Roussa et al. 2006) .
1.2 Microglia
Microglia, the brain’s resident phagocytic cells, play a central role in CNS development and maintenance through regulating developmental neuronal death and the clearing of apoptotic neurons (Wakselman et al. 2008; Takahashi et al. 2005).They also phagocytose cellular debris and respond to ‘danger’ signals through production of reactive oxygen species (ROS) and inflammatory cytokines (Neumann et al. 2009; Ron-Harel et al. 2011). The significance of this function is illustrated through the depletion of microglia from murine neonatal cerebellar slice cultures. This specific elimination of microglia leads to increased Purkinje cell survival due to the reduction of phagocytosis of caspase-3-expressing Purkinje cells (Marin-Teva et al. 2004). More recently in vivo studies have shown that microglia regulate neurogenesis in the cerebral cortex of primates, rodents and human fetal tissues (Cunningham et al. 2013). Cunningham et al. (2013) show microglia enter and colonize the cortical proliferative zones near the end of neurogenesis and phagocytose neural precursor cells. Manipulation of microglia in rats either by suppressing microglia using doxycycline or activating them using injection of LPS resulted in increased and decreased numbers of neural precursors cells, respectively, further demonstrating the critical role microglia and the immune system play in regulating neuronal numbers in early brain development.
In general, microglia activation increases expression of inflammatory cytokines and can have toxic effects on the surrounding cells; however, they are also vital for the down regulation of immune responses through autocrine feedback loops and production of anti-inflammatory cytokines (Garden and Moller 2006). Embryonic microglia are known producers of TNF-α and are key regulators of developmental apoptosis and synaptogenesis. Disrupting TNF-α signaling through use of anti-TNF-α antibodies or soluble TNF-α receptor (TNFR1) results in the decrease of AMPA-type glutamate receptors on hippocampal neurons and thereby modulate synaptic strength in these neurons (Beattie et al. 2002). TNF-α has been shown to upregulate expression of β3 integrins that help increase synaptic strength through stabilization of AMPARs (Cingolani et al. 2008). TNF-α is therefore suggested to have a central role for the homeostatic potentiation of synaptic strength during developmental synaptic refinement. Glial cells, in response to levels of activity in hippocampal cultures, have been shown to regulate TNF-α levels (Deverman and Patterson 2009).
Macrophage colony-stimulating factor (M-CSF) a growth factor for macrophages and microglia is necessary for proper development of certain areas of the brain. M-CSF mutant mice containing a null mutation in the M-CSF gene were shown to have auditory and visual processing impairment with failure of the newborn pups to respond to external cues and electrophysiologic abnormalities detected by intracortical recordings of brainstem auditory evoked potentials and visual evoked potentials (Michaelson et al. 1996). The effects of M-CSF are thought to be indirectly regulated by cytokine secreting microglia (Deverman and Patterson 2009). In addition to cytokines and chemokines, microglia produce many other regulatory and trophic factors promoting neuronal survival including: nerve growth factor (NGF) , brain-derived neurotrophic factor (BDNF) , neurotrophin (NT)-3, basic fibroblast growth factor, and glial-derived neurotrophic factor (GDNF) (Garden and Moller 2006). Microglia not only influence neuronal cells but can also be influenced by neuronal activity through neurotransmitter receptors such as glutamate receptors and by astrocytes through purinergic receptors such as P2X4, P2X7, P2Y2, P2Y6 and P2Y12 (Biber et al. 2007; Hung et al. 2010; Ferrari et al. 2006).
1.3 Cytokines, Immune Cells and Cognitive Function
Cytokines and immune cells are supportive in brain function including neurogenesis and cognitive functioning. Lymphocytes, for example, have been found to have a supportive role in cognitive functioning (Kipnis et al. 2012) . Experiments with severe combined immune deficient (SCID) mice, that do not have any T or B cells, display impairments in hippocampal dependent spatial learning and memory through analysis of the Morris water maze behavioral test (MWM) (Kipnis et al. 2004). Furthermore, nude mice (lacking only mature T cells) display similar impairments assessed by MWM which could be partially rescued with replenishment of T cells from wild-type mice, demonstrating the role of T cells more specifically in areas of visuospatial learning (Kipnis et al. 2004; Ron-Harel et al. 2008; Brynskikh et al. 2008). To provide further support of the role of T cells in learning and memory another study looked at rats that were either raised under normal environmental conditions or under enriched conditions in which neurogenesis was enhanced in the dentate gyrus. To confirm the role of T cells, mice were used in which monospecific T cells to either myelin basic protein (auto specific) (Tmbp) or ovalbumin (OVA) (non-CNS specific) (Tova) were used. Tmbp mice were found to have higher amounts of proliferating neurons compared with controls and preformed better in the MWM, while Tova had less proliferating neurons than controls (Ziv et al. 2006). To emphasize the importance of T cell/microglia interactions in neurogenesis the Tmbp mice were treated with a microglia blocking drug, minocycline, which significantly decreased neurogenesis in the dentate gyrus (Ziv et al. 2006). These findings support the role of T cells mediating neurogenesis and spatial learning through possible interaction with microglia .
Although T cells are not normally found in the CNS parenchyma under normal conditions, T-cell based support of behavioral plasticity is thought to take place in the meningeal spaces (Derecki et al. 2010; Schwartz and Shechter 2010). Depletion of T cells from the meningeal spaces results in impairments of learning and memory based on MWM results. Of the meningeal T cell population, CD4+ IL-4+ T cells were found to be the most important for spatial learning and memory as IL-4 deficient mice showed defects in MWM (Derecki et al. 2010). Other studies have also used T cell manipulations to demonstrate improved learning and memory (Ron-Harel and Schwartz 2009; Ron-Harel et al. 2008). Manipulation of T cells in aged mice through bone marrow transplantation improved spatial memory in these animals compared to young animals and was increased when compared with non treated aged animals (Ron-Harel et al. 2008). It has been suggested that assessing T cell immunity in old age could be used as a predictor of potential future memory loss and enhancing T-cell immunity could benefit age associated memory loss (Ron-Harel and Schwartz 2009) .
Major histocompatibility complex (MHC) molecules are important cell surface molecules that interact with T cells involved in host immunity. It was long thought that the neural cells were among the small list of cells that did not express this family of surface proteins; however, in the late 1990’s it was found that not only are these important immune molecules present on neurons but they actually influence synapse plasticity (Elmer and McAllister 2012). Mice with deficient signaling of class I MHC displayed impaired synapse plasticity (Huh et al. 2000). Peptides from within the cell are presented in MHC molecules and MHC I: peptide complexes, in an immunological context are scanned by cytotoxic T cells to detect abnormal conditions such as the presence of a viral infection or tumor. In the CNS it is thought that similar roles of MHC molecules are employed in presenting peptides in order to regulate normal developmental elimination of inappropriate synaptic connections, although the mechanism remains elusive (Boulanger 2009).
Communication between the immune system and CNS is not one way. Signaling is multidirectional and information can also pass from the CNS to the immune system. Norepinephrine (NE) released from sympathetic nerve terminals can signal to macrophages through beta adrenergic receptors (Kin and Sanders 2006). In general NE seems to have inhibitory effects on pro-inflammatory cytokine production such as TNF-α, IL-1β, and sometimes IL-6, based on data from splenic macrophages (Meltzer et al. 2004; Ignatowski et al. 1996; Nance and Sanders 2007). IL-6 production has been found to be both increased or decreased in response to NE depending on other signals and stimuli such as the presence of LPS (Nance and Sanders 2007). Other neuro-based receptors found on immune cells include noradrenergic receptors, nicotinic α-7 receptors, receptors for neuropeptides and hormones, metabotropic glutamate receptors (mGluRs) and receptors for monoamines serotonin and dopamine (Nance and Sanders 2007; Tracey 2002; Besedovsky and Rey 2007; Friedman and Irwin 1997; Pacheco et al. 2004). Signaling through these receptors can regulate and modulate immune function and may be important in response to stress and or in neuro-psychiatric disorders were imbalances in neuromodulators have been observed .
1.4 Neuroinflammation
While there are many beneficial roles of the immune system in CNS function, too much inflammation can be detrimental. Exposure to pathogens which activate immune responses to protect against infections can result in increased production of pro-inflammatory cytokines that contribute to sickness behavior, while both anxiety and depression have also been associated with inflammation (Irwin and Miller 2007). Pro-inflammatory cytokines including IL-1β, TNF-α and IL-6 can act on the brain causing sickness behaviors ranging from loss of appetite, lethargy to irritability (Dantzer et al. 2008). In addition to inflammation or as a result of infection, events causing stress, injury and ageing can also induce these same inflammatory mediators (Yirmiya and Goshen 2011). In experiments where IL-1β was injected intracerbroventricularly (i.c.v.) into the right lateral cerebral ventricle either 24 h or 1 h before training in the MWM, those rats injected for 1 h but not those injected 24 h before training showed impaired performance in spatial memory the next day (Oitzl et al. 1993) suggesting changes may be fast acting but also dose-dependent and transient. Furthermore, increased peripheral levels of IL-1β following infection with Legionella pneumophila or by direct administration of IL-1β daily also showed impaired spatial memory and learning in mice (Gibertini et al. 1995) suggesting there may be a conditioning effect with repeated prolonged exposure to cytokines. Transgenic mice that over express IL-1β show impairments in spatial memory that are particularly restricted to hippocampal dependent memory (Hein et al. 2010; Moore et al. 2009). Introduction of LPS also increases hippocampal IL-1 levels and induce similar impairments to special learning and memory (Nguyen et al. 1998). However, other study designs with different regimens of IL-1β administration did not show memory or learning impairments suggesting that the conditions and environmental factors contribute to memory and learning (Yirmiya and Goshen 2011). Additionally IL-1β associated neuroinflammation is linked in ageing and may play a role in age associated memory loss (Krabbe et al. 2004). Caspase-1 inhibitors when administered to aged mice over time reduced hippocampal IL-1β and helped to improve contextual memory (Gemma et al. 2005; Krabbe et al. 2004). In Alzheimer’s disease increased levels of TNF-α, IL-6 and IL-1β have been detected in the serum and cerebral spinal fluid (Akiyama et al. 2000; Shaftel et al. 2008). Activation of microglia have been found in Alzheimer and other neurological diseases such as Parkinson’s disease, multiple sclerosis and acquired immune deficiency syndrome dementia complex (Kim and de Vellis 2005). As stated above, the immune system orchestrates a vital and delicate balancing act necessary for the proper development and maintenance of the CNS. When there is imbalance in either direction, increased or decreased, appropriate functions of the CNS can become impaired.
1.5 Neuroinflammation in ASD
Recent studies have suggested that neuroinflammation occurs in individuals with ASD . Inflammation in post mortem brain specimens of a wide range of individuals with ASD age 4–45 years old have been observed, specifically, the cerebellum , anterior cingular gyrus and the midfrontal regions of the brain (Vargas et al. 2005). Neuroglial activation and presence of increased levels of inflammatory cytokines such as IFN-γ, IL-1β, IL-6, TNF-α and chemokines CCL-2 were found in brain tissue and CSF (Li et al. 2009; Morgan et al. 2010; Vargas et al. 2005). Additionally postmortem brain samples of patients with ASD were also found to have increased levels of glial fibrillary acidic protein (GFAP) in the frontal, parietal and cerebellar cortices (Laurence and Fatemi 2005). GFAP is expressed in activated astrocytes and is also a sign of inflammation. The cerebellum in particular showed the most prominent histological changes and microglial activation in individuals with ASD. In addition, some of the cerebellar tissues from individuals with ASD, but none of the control tissues had accumulation of perivascular macrophages and monocytes and deposition of complement membrane attack complexes which suggest that the neuroinflammation seen may be primarily driven by innate immune responses (Vargas et al. 2005) . Furthermore, researchers found increases in Th1 with no differences in Th2 cytokines suggesting that ASD patients have increased neuroinflammatory immune response through the Th1 pathway (Li et al. 2009). Increases in Th1 cytokines such as IFNγ were not compensated by increases in IL-10 also suggesting a failure in immune regulation (Li et al. 2009). In addition to increases in cytokines, post-mortem temporal cortex samples from ASD and general population controls were assessed for transcriptome differences and increases in expression of immune related genes were found in the ASD population (Garbett et al. 2008). In particular cytokine signaling and immune regulatory genes were altered, which included genes from the NFκB, IL-1r, Toll, IL-6, Caspase, Th1/Th2 and FAS pathways. Interestingly, the ASD samples had higher variability in transcriptome differences when compared to controls (Garbett et al. 2008) . Furthermore, activation of microglial cells and perivascular macrophages measured by increased MHC II expression was seen in the cortical regions, white matter and most prominently in the cerebellum of patients with autism. This microglial and astroglial activation in the cerebellum was associated with degenerating purkinje cells, granule cells, and axons (Vargas et al. 2005). Altered microglial profiles found in post mortem brain samples of ASD patients showed an increase in average microglial somal volume and increase in microglial density in white and grey matter respectively and activation ranged from severe to mild in ASD brain specimens (Morgan et al. 2010). The data also suggested that microglial activation maybe particularly prominent in younger individuals, though more samples are needed to verify this (Morgan et al. 2010) .
The specific inducer of microglia activation in ASD is unknown and whether dysfunction in immune pathways leads to neuroinflammation or if CNS impairments in ASD lead to immune dysregulation, or in fact an interplay between the two systems, is yet to be fully elucidated. Both environmental and genetic risk factors are thought to play a role in ASD. Genetic contributions to ASD were first suggested in the 1980’s after investigation of co-occurrences of rare syndromes and chromosomal disorders were observed with ASD (Blomquist et al. 1985). Moreover the increased occurrences of ASD in families shown in twin and sibling studies further provided evidence for a genetic component to ASD (Kates et al. 2004; Bailey et al. 1995; Constantino and Todd 2000; Steffenburg et al. 1989; Jorde et al. 1991). Candidate gene association studies and whole-genome linkage studies have been used to identify loci of interest and assess copy number variation. Even with a long list of putative contributing genetic mutations and syndromes associated with ASD, these only account for 10–20 % of cases (Abrahams and Geschwind 2008). Genetic risk factors for ASD include genes that affect both CNS and immune pathways. Immune related genes associated with ASD include: phosphoinisitide–3 kinase (PI3K) pathway proteins such as MET, PTEN, TSC1 and 2, as well as MHC II, complement 4B, and macrophage inhibitory factor (MIF) (Onore et al. 2012) .
2 Maternal Immune Activation and ASD
2.1 Infection During Pregnancy
In addition to genetic contributions, environmental factors are also thought to play a role in ASD. Maternal immune activation (MIA) during pregnancy is one potential environmental factor that may increase the risk for developing ASD (Patterson 2009). Studies investigating viral and bacterial infections during pregnancy have shown associations with maternal infection and increases in ASD, including in 1964 when a rubella outbreak was connected with increased cases of autism (Chess et al. 1978). Other viruses that have been linked to congenital infection and associated with ASD include the herpes viruses: herpes simplex virus, cytomegalovirus, varicella and the paramyxovirus mumps (Libbey et al. 2005). The study of data from the Danish Medical Birth Register investigated 10,133 ASD diagnoses from children born from 1980 to 2005 looking at mothers who were hospitalized during pregnancy and found evidence to support association of viral infection during the first trimester and bacterial infection during the second trimester with increased risk of the child developing ASD (Atladottir et al. 2009). It is possible that genetically predisposed or susceptible individuals who encounter a prenatal infection may develop ASD due to high levels of cytokines or initiation of autoimmune processes resulting in increased maternal inflammation which could affect the developing fetal brain (Libbey et al. 2005). Additional evidence to infer maternal immune involvement in autism is data showing increased rates of autoimmunity in families with ASD (Croen et al. 2005; Atladottir et al. 2009). In support of a role of MIA , one study showed that mid-gestational findings of increased IFNγ, IL-4 and IL-5 in maternal serum significantly increased the risk of ASD (Goines et al. 2011).
2.2 Other Inflammatory Processes
In addition to increased frequencies of autoimmunity among families with individuals with ASD some reports have also identified fetal specific autoantibodies in the mothers of children with autism (Braunschweig et al. 2008; Croen et al. 2008). IgG maternal antibodies can cross the placenta and persist for up to 6 months after birth (Heininger et al. 2006). Antibodies with autoreactivity to fetal brain proteins were found at 37 kDA and 73 kDa molecular weights in approximately 12 % of mothers with an autistic child but no mothers of typically developing children or children with developmental delays other than ASD (Braunschweig et al. 2008); later another band with a molecular weight of 39 kDa was also found to be associated with ASD (Croen et al. 2008). Maternal antibodies are present in detectable levels at 18 weeks in the developing fetus and reach levels comparable to the mothers by 38 weeks of gestation (Croen et al. 2008). To further test the role these autoantibodies are playing in ASD, several studies have injected serum or purified IgG from mothers of children with ASD and mothers of controls into various animal models mid gestation. In one study pregnant mouse dams were intraperitoneally injected with purified IgG from mothers of children with autism disorders (MCAD) or from mothers of typically developing children. Injections were given daily during embryonic days 13–18, resulting in adolescent offspring from MCAD injected dams which displayed long-term behavioral differences compared with controls (Singer et al. 2009). In another study non-human primate, rhesus macaques were injected with purified IgG from mothers of children with ASD and from those of typically developed children. Animals were found to have higher amounts of stereotypical behaviors and increased motor activity than controls (Martin et al. 2008). These data suggest that dysfunction of the maternal immune system may play an active role in the pathology of some children who develop ASD.
2.3 Rodent Models of MIA
Other models that investigate the role of MIA include rodent models of immune activation of pregnant dams. IL-6 is an important cytokine involved in maternal immune influence of fetal development (Hsiao and Patterson 2011) . Injecting IL-6 in the absence of other immune stimulus at embryonic day 12.5 is sufficient to cause behavioral changes in the offspring, particularly in measurements of prepulse inhibition of adult offspring (Smith et al. 2007). Other pro-inflammatory cytokines such as IL-1β, TNF-α and IFN-γ did not cause any changes in behaviors. Likewise, injection of neutralizing anti IL-6 antibodies administered when MIA was induced prevented development of behavioral abnormalities. IL-6 knockout mice also exhibited resistance to in utero MIA induced behavioral changes (Smith et al. 2007). Pregnant mice infected with the human influenza virus on embryonic day 9.5 had offspring who as adults displayed behavioral defects in prepulse inhibition and acoustic startle response (Shi et al. 2003) . Additionally when polyinosinic –polycytidylic acid (poly I:C), a viral mimic, was injected into dams at embryonic day 12.5 the offspring had similar behavioral defects as the influenza infected offspring suggesting that the behavioral abnormalities are indeed due to activation of the maternal immune system not the virus itself (Shi et al. 2003). Recent studies with poly IC induced MIA in mice show behavioral changes in three areas relevant to those seen in ASD which include impairments in communication, social interactions and repetitive behaviors (Malkova et al. 2012; Schwartzer et al. 2013). Male offspring of MIA mice were found to produce less ultrasonic vocalizations (a murine form of communication) in different social situations compared with controls. In addition, the offspring were also found to spend less time with novel mice and more time with a novel object when compared to saline controls indicating a difference in social interactions and finally the MIA offspring displayed more repetitive behaviors as measured by time spent self grooming and time spent burying marbles compared with controls (Malkova et al. 2012) . In addition to poly I:C models, the use of the bacterial component lipopolysaccaride (LPS) as a mid-gestational activator of the maternal immune system has been tested and results in behavioral changes in offspring similar to those observed using poly I:C (Patterson 2009). In the latter model neuroglial activation and increased cytokine production has been shown that likely results in permanent elevation of cytokines in the brain that affect postnatal behaviors (Patterson 2009) . These models together with epidemiological data of human infection during pregnancy demonstrate that the immune status of the mother is important for the developing fetus (Patterson 2009). Since not all mothers who are infected with a pathogen have offspring with ASD it is likely that genetic background acts as a factor to enhance ASD risk. Gene—environment interactions are thought to play a major role in ASD. One study examined these interactions by testing MIA in mice heterozygous for the tuberous sclerosis 2 (Tsc2) gene. Offspring of dams injected with poly I:C exhibited increased asocial behavioral abnormalities more than MIA alone suggesting a double hit of genetic and environmental factors results in severe behavioral defects (Ehninger et al. 2012). In addition to alteration in fetal neurodevelopment it is also possible that MIA alters peripheral immune responses as well. In one study of MIA, a Th17 skewing of T cells were seen in poly I:C maternally exposed mice compared with controls (Mandal et al. 2011) .
3 Systemic Immune Activation in ASD
3.1 Peripheral Cytokines and Chemokines in ASD
Immune abnormities in ASD have been reported since 1977 (Stubbs and Crawford 1977) . Since that initial report there have been a number of immune related problems described with some conflicting findings likely reflecting the heterogeneity of ASD . Elevated pro-inflammatory cytokines have been found in plasma of children with ASD aged 2–5 years old including IL-1β, IL-6, IL-8 and IL-12p40 (Ashwood et al. 2011b). Elevated amounts of chemokines MCP-1, RANTES and eotaxin were also found in children with ASD (Ashwood et al. 2011d). In both studies these elevated inflammatory mediators were associated with more impaired or aberrant behaviors. Other reports of inflammatory cytokines found elevations of IFNγ (Singh 1996), MIF (Grigorenko et al. 2008) and platelet derived growth factor BB (PDGF-BB) in plasma of children with ASD (Kajizuka et al. 2010). Both MIF and PDGF correlated with behavioral scores as well. In addition to increases in pro-inflammatory cytokines, decreases in TGFβ, a regulatory cytokine, were also found in children with ASD which were associated with worsening behavioral scores (Ashwood et al. 2008). In addition to plasma cytokine differences, there have also been reports of differences in immunoglobulin levels. One study reported that children with autism have reduced levels of plasma IgG and IgM which also correlated with increased behavioral severity (Heuer et al. 2011). Other studies have reported increases in serum proteins attributed mostly to increases in albumin; however, IgG, specifically IgG2 and IgG4 were also seen elevated in individuals with ASD and these increases in immunoglobulin correlated with behavioral abnormalities (Croonenberghs et al. 2002; Enstrom et al. 2009a). Autoantibodies to various and diverse targets have been reported in children with autism and could point to cellular damage that may be involved in increasing inflammation , revealing antigens otherwise hidden and/or epitope spreading (Onore et al. 2012) .
3.2 Adaptive Responses in ASD
Adaptive immune responses in children with ASD also show increased cytokine production . Peripheral blood mononuclear cells (PBMC) isolated from the blood of children with ASD ages 2–5 were stimulated and compared to age matched controls. Unstimulated cells from children with ASD produced higher amounts of IL-8 when cultured overnight. After stimulation with phytohemagglutinin (PHA), cells from individuals with ASD produced larger quantities of GM-CSF, IL-13 and TNF-α (Ashwood et al. 2011c). A number of these increased cytokines also correlated to behavioral abnormalities. Increased production of TNF-α and IFN-γ were associated with more stereotyped behaviors. Increased impaired communications were associated with higher IFN-γ and IL-8 production. IL-12p40, a subunit of IL-12, correlated with worsening speech and increased hyperactivity (Ashwood et al. 2011c). This data suggest that perhaps an increased Th1 response may worsen behaviors. Both increases in IL-10 and IL-5 may help to improve behaviors—IL-10 increases were associated with better expressive language while increased IL-5 production correlated with improved fine motor skills. Besides increased production of cytokines, PBMC differences were also seen in T cell activation markers suggesting an altered activation of T cells which may contribute to the differences in cytokines produced (Ashwood et al. 2011c). Other studies have also looked at CD4 and CD8 T cells and have found a shift in Th1 and Th2 cytokines (Gupta et al. 1998) .
Adhesion molecules play an important role in leukocyte migration and are involved in modulating immune—CNS connections via passage of T cells through epithelial barriers. Soluble adhesion molecules such as sPECAM, sL-selectin, and sP-selectin were found in lower amounts in high functioning ASD individuals when compared to controls (Iwata et al. 2008; Tsuchiya et al. 2007). Reports of improved behaviors during febrile outbreaks in children with ASD have also been described; these changes in behavior are transient and may be attributed to increased up-regulation of adhesion molecules allowing for more T cell-CNS interactions (Onore et al. 2012; Curran et al. 2007) .
3.3 Innate Responses in ASD
Changes in innate immune responses have been described in children with ASD. Natural killer (NK) cells, normally involved in killing atypical host cells, have been found to have reduced ability to kill K562 target cells (an immortalized myelogenous leukemia cell line) in children with ASD (Warren et al. 1987; Enstrom et al. 2009b; Vojdani et al. 2008) . Factors that may contribute to decreased NK cell activity may be attributed to production of lower amounts of perforin, granzyme B and IFN-γ following stimulation conditions in children with ASD (Enstrom et al. 2009b). Increased numbers of circulating monocytes have also been reported in ASD (Sweeten et al. 2003). Moreover, increased expression of activation markers on these monocytes suggest that these cells are in an activated state (Ashwood et al. 2011a). Indeed these cells have been found to have released increased inflammatory cytokines such as IL-1β, TNF-α and IL-6 in response to TLR2 and TLR4 stimulus. Increased production of IL-6 and IL-1β correlated with increased impairment of social behaviors in children with ASD (Enstrom et al. 2010). Monocytes under certain conditions can give rise to other myeloid cells such as dendritic cells, tissue macrophages and microglia (Djukic et al. 2006; Geissmann et al. 2010). Altered activation and responses in myeloid cells, therefore, have many implications for inflammation in both peripheral and CNS systems .
4 Gastrointestinal Abnormalities in ASD
4.1 GI Symptoms and Frequency in ASD
Recent studies have suggested that many children with ASD suffer from gastrointestinal (GI) symptoms, dysfunction and inflammation . Associations between ASD and GI symptoms were first reported in the early 1970s (Goodwin et al. 1971). Goodwin looked at 15 autistic children and found seven of them had GI issues. Since then various other groups have reported the frequency of GI symptoms in the ASD population ranging from 17 to 86 % (Erickson et al. 2005). The differences in reported GI abnormalities in children with ASD are in part due to the design of these studies with many lacking proper controls or were based on referral biases that only include children with ASD who have GI complaints. Other factors contributing to the wide range of reports include the heavily skewed amount of retrospective studies that rely on either medical records which mainly look at confirmed diagnosis of GI disease and may not necessarily include GI symptoms experienced in all patients or via a parental survey . Conversely, purely parental based surveys have the disadvantage of often involving memory recall of past GI problems whilst additional impairments in language in non-verbal children make it more difficult for parents to perceive pain in children with ASD. Differences between studies can also be attributed to non standardized GI surveys which define GI symptoms differently among the various studies (Mannion et al. 2013). Common gastrointestinal symptoms that are reported include: diarrhea, constipation, foul smelling stools, gaseousness, abdominal pain, and food regurgitation/reflux .
4.2 Nutrition and GI Immunity in ASD
Many of the reports of gastrointestinal symptoms in children with ASD have led some researchers to look into the role that nutritional imbalance may be playing in some of the GI symptoms reported . Studies examining the relationship between nutritional input and its effect on ASD; however, have not supported this hypothesis (Levy et al. 2007). Core features of autism such as repetitive behaviors and resistance to change may impact feeding behaviors and nutrition in children with ASD (Erickson et al. 2005). Overall these studies show that while children with ASD tend to have increased food selectivity but that selectivity does not seem to cause malnutrition and overall, nutrient intake is adequate in children with ASD (Raiten and Massaro 1986; Shearer et al. 1982; Ahearn et al. 2001; Field et al. 2003). Some reports concerning increased rates of food allergies among ASD populations have been reported (Horvath and Perman 2002). One such study seeking to address these concerns found that children with ASD had more responses to food allergens as measured by positive pin prick reactions (Lucarelli et al. 1995). In another study addressing these same concerns, children with ASD were compared to normal siblings and children with known dietary protein intolerances; elevated levels of IFNγ and TNFα were found in PBMC response to dietary proteins in both the ASD group and the known dietary intolerances group (Jyonouchi et al. 2002) . Also of note in this same study a correlation between elevated IFNγ and TNFα responses with dietary proteins and elevated response to LPS was seen in children with ASD (Jyonouchi et al. 2002). This suggests that an imbalanced immune system may be playing a role in GI dysfunction .
4.3 GI Immunity
The gastrointestinal tract is the immune system’s largest source of lymphoid tissue and is an important site of immune regulation (Turner and Goldsmith 2009) . It is therefore conceivable that mucosal immune dysfunction could be playing a role in children with ASD who have GI symptoms. Immunohistochemical findings of children with ASD and GI problems showed an increase in CD8 T cells in duodenal and colonic samples (Torrente et al. 2002; Furlano et al. 2001), with increases also seen in γδ T cells in transverse colon samples (Furlano et al. 2001). In addition, decreases in peripheral T cell numbers were reported in children with ASD who have GI symptoms (Ashwood et al. 2003) and may reflect numbers of T cells translocating to the GI mucosa in this subset of ASD individuals. Other immunohistochemical findings revealed deposition of IgG and complement C1q co-localized on the basolateral enterocyte membrane in ASD GI samples (Torrente et al. 2002; Ashwood et al. 2003) suggesting a possible autoimmune component to ASD GI dysfunction. Other studies have illustrated findings of increased number of paneth cells in children with autism and gastrointestinal symptoms (Horvath and Perman 2002; Torrente et al. 2002; Horvath et al. 1999) .
Common reports of children with ASD having “leaky gut” have been noted and one study in particular looked at 21 autistic children without known GI disease and found 9 with mucosal permeability; none of the 40 normal controls had permeability issues (D’Eufemia et al. 1996). Results of this study were confirmed when another group also found increased GI permeability in children with ASD and GI problems (Horvath 2000). In humans, disruption of mucosal barriers can occur in the absence of inflammation. Thus, increased mucosal permeability does not necessarily predict inflammation . Interestingly though, findings of atypical intestinal microbiota composition in ASD including increased findings of Clostridium species in stool samples of children with ASD, (Finegold et al. 2010; Finegold et al. 2004; Finegold et al. 2002) may elicit mucosal immune responses and GI dysfunction in some children with ASD and GI symptoms especially if bacteria are able to move across a more permeable intestinal barrier. Moreover, a small study developed on the reports of abnormal microbiota composition in children with ASD looked at 11 children with ASD, and treated with the antibiotic vancomycin, aiming to remove deleterious microbes with the antibiotic. Findings of this study include temporarily improved behavioral symptoms (Sandler et al. 2000). Another study addressed this issue by treating children with ASD and GI issues with oral human immunoglobulin (IG). The results showed that after administering oral IG for 8 weeks, 50 % of the patients had improvements in GI severity; however, benefits for the treatment were not maintained at the 30 day follow up. Behavioral improvements were also seen from baseline through the end of the 8 week treatment (Schneider et al. 2006). Other studies that assessed treatment for GI and behavioral symptoms include treatment with secretin. Erickson et al. (2005) reported findings from 11 double blind and 2 open label studies testing secretin treatments in the ASD population. While one of the open label studies reported behavioral improvements based on parental reporting, all double blind studies showed no differences between control (placebo) groups and secretin treatment groups (Erickson et al. 2005). These studies suggest that more research is needed to address the GI/immune/brain connection. There have been some studies that try to investigate the role GI inflammation has in altering behaviors and CNS function. One group of researchers infected AKR mice with the parasite trichurs muris and measured GI inflammation , brain biochemistry changes and behaviors related to anxiety. The authors found that chronic GI inflammation induces anxiety-like behavior in AKR mice (Bercik et al. 2010). Increased use and development of behavioral testing in mice now make it easier to measure behavioral changes associated with various induced and natural occurring conditions in mice.
4.4 Behavior and GI Dysfunction
Behavioral abnormalities associated with GI symptoms in autism include studies that have found children who had sleep abnormalities were also more likely to have GI problems (Maenner et al. 2012; Mannion et al. 2013; Ming et al. 2008) . Mazurek et al. (2012) looked at 2973 children enrolled in autism treatment network and found that children with ASD and GI problems had higher levels of anxiety and sensory overresponsivity. The authors also suggest that the relationship of these three symptoms could include the involvement of the hypothalamic-pituitary-adrenal (HPA) axis and amygdala based circuits. It is of note that the HPA axis also regulates immune function (Mazurek et al. 2012; Herman and Cullinan 1997). The likelihood of a connection between mucosal immune irregularities and behavior in ASD is intriguing. While it is possible that immune dysfunction in the GI tract interacts with an already abnormal brain to aggravate behavioral symptoms or whether GI problems are just another manifestation of systemic immune abnormalities are questions still to be explored. Many studies examining animal and human subjects reveal elevated peripheral cytokines are able to cause striking changes in behavior (Patterson 2009). It still remains to be seen if similar phenomenon occurs with mucosal inflammation .
5 Conclusion
ASD is a complex and heterogeneous spectrum of behavioral disorders made even more apparent by the wide array of genetic and environmental factors and influences that have been linked to it. This heterogeneity has also impacted studies of the immune system with many findings that are often confusing and sometimes conflicting. Overall one theme that has remained constant is that, inflammation is associated with ASD. Findings of neuroinflammation in the CSF and brain tissue, peripheral immune abnormalities on both the innate and adaptive responses and the increasing number of studies on GI dysfunction within ASD all come to a consensus that immune dysfunction can have persistent impact on behavior. More research is needed to further elucidate the mechanisms by which the immune system affects neurological and behavioral changes and to investigate immune modulating therapeutics for ASD.
References
Abrahams BS, Geschwind DH (2008) Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 9:341–355
Ahearn WH, Castine T, Nault K, Green G (2001) An assessment of food acceptance in children with autism or pervasive developmental disorder-not otherwise specified. J Autism Dev Disord 31:505–511
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421
Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ (2003) Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J Clin Immunol 23:504–517
Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, Van de Water J (2008) Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol 204:149–153
Ashwood P, Corbett BA, Kantor A, Schulman H, Van de Water J, Amaral DG (2011a) In search of cellular immunophenotypes in the blood of children with autism. PLoS One 6:e19299
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J (2011b) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25:40–45
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J (2011c) Altered T cell responses in children with autism. Brain Behav Immun 25:840–849
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J (2011d) Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunology 232:196–199
Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET (2009) Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 124:687–694
Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25:63–77
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNFalpha. Science 295:2282–2285
Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM (2010) Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139:2102–2112
Besedovsky HO, Rey AD (2007) Physiology of psychoneuroimmunology: a personal view. Brain Behav Immun 21:34–44
Biber K, Neumann H, Inoue K, Boddeke HW (2007) Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci 30:596–602
Blomquist HK, Bohman M, Edvinsson SO, Gillberg C, Gustavson KH, Holmgren G, Wahlstrom J (1985) Frequency of the fragile X syndrome in infantile autism. A Swedish multicenter study. Clin Genet 27:113–117
Boulanger LM (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64:93–109
Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J (2008) Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology 29:226–231
Brynskikh A, Warren T, Zhu J, Kipnis J (2008) Adaptive immunity affects learning behavior in mice. Brain Behav Immun 22:861–869
Carpentier PA, Palmer TD (2009) Immune influence on adult neural stem cell regulation and function. Neuron 64:79–92
Chess S, Fernandez P, Korn S (1978) Behavioral consequences of congenital rubella. J Pediatrics 93:699–703
Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y (2008) Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron 58:749–762
Constantino JN, Todd RD (2000) Genetic structure of reciprocal social behavior. Am J Psychiary 157:2043–2045
Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J (2005) Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 159:151–157
Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharrazi M, Hansen RL, Ashwood P, Van de Water J (2008) Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry 64:583–588
Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, Bosmans E, Egyed B, Deboutte D, Maes M (2002) Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med 32:1457–1463
Cunningham CL, Martinez-Cerdeno V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33:4216–4233
Curran LK, Newschaffer CJ, Lee LC, Crawford SO, Johnston MV, Zimmerman AW (2007) Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 120:e1386–1392
D'Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M, Cardi E, Giardini O (1996) Abnormal intestinal permeability in children with autism. Acta Paediatrica 85:1076–1079
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
de la Mano A Gato A Alonso MI Carnicero E Martin C Moro JA (2007) Role of interleukin-1beta in the control of neuroepithelial proliferation and differentiation of the spinal cord during development. Cytokine 37:128–137
Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J (2010) Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 207:1067–1080
Deverman BE, Patterson PH (2009) Cytokines and CNS development. Neuron 64:61–78
Djukic M, Mildner A, Schmidt H, Czesnik D, Bruck W, Priller J, Nau R, Prinz M (2006) Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain 129:2394–2403
Ehninger D, Sano Y, de Vries PJ, Dies K, Franz D, Geschwind DH, Kaur M, Lee YS, Li W, Lowe JK, Nakagawa JA, Sahin M, Smith K, Whittemore V, Silva AJ (2012) Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol Psychiatry 17:62–70
Elmer BM, McAllister AK (2012) Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci 35:660–670
Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van de Water JA, Ashwood P (2009a) Increased IgG4 levels in children with autism disorder. Brain Behav Immun 23:389–395
Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen RL, Pessah IN, Hertz-Picciotto I, Van de Water JA, Sharp FR, Ashwood P (2009b) Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun 23:124–133
Enstrom AM, Onore CE, Van de Water JA, Ashwood P (2010) Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun 24:64–71
Erickson CA, Stigler KA, Corkins MR, Posey DJ, Fitzgerald JF, McDougle CJ (2005) Gastrointestinal factors in autistic disorder: a critical review. J Autism Dev Disord 35:713–727
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176:3877–3883
Field D, Garland M, Williams K (2003) Correlates of specific childhood feeding problems. J Pediatr Child Health 39:299–304
Friedman EM, Irwin MR (1997) Modulation of immune cell function by the autonomic nervous system. Pharmacol Ther 74:27–38
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, Collins MD, Lawson PA, Summanen P, Baysallar M, Tomzynski TJ, Read E, Johnson E, Rolfe R, Nasir P, Shah H, Haake DA, Manning P, Kaul A (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35:S6–S16
Finegold SM, Lawson PA, Vaisanen ML, Molitoris DR, Song Y, Liu C, Collins MD (2004) Anaerofustis stercorihominis gen. nov., sp. nov., from human feces. Anaerobe 10:41–45
Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA, 3rd (2010) Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16:444–453
Furlano RI, Anthony A, Day R, Brown A, McGarvey L, Thomson MA, Davies SE, Berelowitz M, Forbes A, Wakefield AJ, Walker-Smith JA, Murch SH (2001) Colonic CD8 and gamma delta T-cell infiltration with epithelial damage in children with autism. J Pediatrics 138:366–372
Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM (2008) Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis 30:303–311
Garden GA, Moller T (2006) Microglia biology in health and disease. J Neuroimmun Pharmacol 1:127–137
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Gemma C, Fister M, Hudson C, Bickford PC (2005) Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci 22:1751–1756
Gibertini M, Newton C, Friedman H, Klein TW (1995) Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun 9:113–128
Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J (2011) Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol Autism 2:13
Goodwin MS, Cowen MA, Goodwin TC (1971) Malabsorption and cerebral dysfunction: a multivariate and comparative study of autistic children. J Autism Child Schizophr 1(1):48–62
Goshen I, Avital A, Kreisel T, Licht T, Segal M, Yirmiya R (2009) Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neuroscience 29:3395–3403
Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R (2007) A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 32:1106–1115
Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, Mulder EJ, de Bildt A, Minderaa RB, Volkmar FR, Chang JT, Bucala R (2008) Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics 122:e438–445
Gupta S, Aggarwal S, Rashanravan B, Lee T (1998) Th1- and Th2-like cytokines in CD4 + and CD8 + T cells in autism. J Neuroimmunol 85:106–109
Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK (2010) Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun 24:243–253
Heininger U, Desgrandchamps D, Schaad UB (2006) Seroprevalence of Varicella-Zoster virus IgG antibodies in Swiss children during the first 16 months of age. Vaccine 24:3258–3260
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84
Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB (2011) Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Transl Psychiatry 1:e48
Horvath K (2000) Secretin treatment for autism. New Eng J Med 342:1216; author reply 1218
Horvath K, Perman JA (2002) Autism and gastrointestinal symptoms. Curr Gastroenterol Rep 4:251–258
Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT (1999) Gastrointestinal abnormalities in children with autistic disorder. J Pediatr 135:559–563
Hsiao EY, Patterson PH (2011) Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun 25:604–615
Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ (2000) Functional requirement for class I MHC in CNS development and plasticity. Science 290:2155–2159
Hung J, Chansard M, Ousman SS, Nguyen MD, Colicos MA (2010) Activation of microglia by neuronal activity: results from a new in vitro paradigm based on neuronal-silicon interfacing technology. Brain Behav Immun 24:31–40
Ignatowski TA, Gallant S, Spengler RN (1996) Temporal regulation by adrenergic receptor stimulation of macrophage (M phi)-derived tumor necrosis factor (TNF) production post-LPS challenge. J Neuroimmunol 65:107–117
Irwin MR, Miller AH (2007) Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 21:374–383
Iwata Y, Tsuchiya KJ, Mikawa S, Nakamura K, Takai Y, Suda S, Sekine Y, Suzuki K, Kawai M, Sugihara G, Matsuzaki H, Hashimoto K, Tsujii M, Sugiyama T, Takei N, Mori N (2008) Serum levels of P-selectin in men with high-functioning autism. Br J Psychiatry 193:338–339
Jorde LB, Hasstedt SJ, Ritvo ER, Mason-Brothers A, Freeman BJ, Pingree C, McMahon WM, Petersen B, Jenson WR, Mo A (1991) Complex segregation analysis of autism. Am J Hum Genet 49:932–938
Jyonouchi H, Sun S, Itokazu N (2002) Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology 46:76–84
Kajizuka M, Miyachi T, Matsuzaki H, Iwata K, Shinmura C, Suzuki K, Suda S, Tsuchiya KJ, Matsumoto K, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Takei N, Mori N (2010) Serum levels of platelet-derived growth factor BB homodimers are increased in male children with autism. Prog Neuropsychopharmacol Biol Psychiatry 34:154–158
Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, Reiss AL, Pearlson GD (2004) Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry 161:539–546
Kim SU, de Vellis J (2005) Microglia in health and disease. J Neurosci Res 81:302–313
Kin NW, Sanders VM (2006) It takes nerve to tell T and B cells what to do. J Leukocyte Biol 79:1093–1104
Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M (2004) T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A 101:8180–8185
Kipnis J, Gadani S, Derecki NC (2012) Pro-cognitive properties of T cells. Nat Rev Immunol 12:663–669
Krabbe KS, Pedersen M, Bruunsgaard H (2004) Inflammatory mediators in the elderly. Exp Gerontol 39:687–699
Laurence JA, Fatemi SH (2005) Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum 4:206–210
Levy SE, Souders MC, Ittenbach RF, Giarelli E, Mulberg AE, Pinto-Martin JA (2007) Relationship of dietary intake to gastrointestinal symptoms in children with autistic spectrum disorders. Biol Psychiatry 61:492–497
Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M (2009) Elevated immune response in the brain of autistic patients. J Neuroimmunol 207:111–116
Libbey JE, Sweeten TL, McMahon WM, Fujinami RS (2005) Autistic disorder and viral infections. J Neurovirol 11:1–10
Lucarelli S, Frediani T, Zingoni AM, Ferruzzi F, Giardini O, Quintieri F, Barbato M, D'Eufemia P, Cardi E (1995) Food allergy and infantile autism. Panminerva Medica 37(3):137–141
Maenner MJ, Arneson CL, Levy SE, Kirby RS, Nicholas JS, Durkin MS (2012) Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. J Autism Dev Disord 42:1520–1525
Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH (2012) Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 26:607–616
Mandal M, Marzouk AC, Donnelly R, Ponzio NM (2011) Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun 25:863–871
Mannion A, Leader G, Healy O (2013) An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with autism spectrum disorder. Res Autism Spect Disord 7:35–42
Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M (2004) Microglia promote the death of developing Purkinje cells. Neuron 41:535–547
Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG (2008) Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun 22:806–816
Mazurek MO, Shattuck PT, Wagner M, Cooper BP (2012) Prevalence and correlates of screen-based media use among youths with autism spectrum disorders. J Autism Dev Disord 42:1757–1767
Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, Nance DM (2004) Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun 18:262–273
Michaelson MD, Bieri PL, Mehler MF, Xu H, Arezzo JC, Pollard JW, Kessler JA (1996) CSF-1 deficiency in mice results in abnormal brain development. Development 122:2661–2672
Ming X, Brimacombe M, Chaaban J, Zimmerman-Bier B, Wagner GC (2008) Autism spectrum disorders: concurrent clinical disorders. J Child Neurol 23:6–13
Moore AH, Wu M, Shaftel SS, Graham KA, O'Banion MK (2009) Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience 164:1484–1495
Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP (2010) Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 68:368–376
Nance DM, Sanders VM (2007) Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21:736–745
Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132:288–295
Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF (1998) Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neuroscience 18:2239–2246
Oitzl MS, van Oers H, Schobitz B, de Kloet ER (1993) Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res 613:160–163
Onore C, Careaga M, Ashwood P (2012) The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun 26:383–392
Pacheco R, Ciruela F, Casado V, Mallol J, Gallart T, Lluis C, Franco R (2004) Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J Biol Chem 279:33352–33358
Patterson PH (2009) Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204:313–321
Raiten DJ, Massaro T (1986) Perspectives on the nutritional ecology of autistic children. J Autism Dev Disord 16:133–143
Ron-Harel N, Schwartz M (2009) Immune senescence and brain aging: can rejuvenation of immunity reverse memory loss? Trends Neurosci 32:367–375
Ron-Harel N, Segev Y, Lewitus GM, Cardon M, Ziv Y, Netanely D, Jacob-Hirsch J, Amariglio N, Rechavi G, Domany E, Schwartz M (2008) Age-dependent spatial memory loss can be partially restored by immune activation. Rejuv Res 11:903–913
Ron-Harel N, Cardon M, Schwartz M (2011) Brain homeostasis is maintained by “danger” signals stimulating a supportive immune response within the brain’s borders. Brain Behav Immun 25:1036–1043
Roussa E, Wiehle M, Dunker N, Becker-Katins S, Oehlke O, Krieglstein K (2006) Transforming growth factor beta is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: ectopic induction in dorsal mesencephalon. Stem Cells 24:2120–2129
Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, Nelson MN, Wexler HM (2000) Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 15:429–435
Schneider CK, Melmed RD, Barstow LE, Enriquez FJ, Ranger-Moore J, Ostrem JA (2006) Oral human immunoglobulin for children with autism and gastrointestinal dysfunction: a prospective, open-label study. J Autism Dev Disord 36:1053–1064
Schwartz M, Shechter R (2010) Protective autoimmunity functions by intracranial immunosurveillance to support the mind: the missing link between health and disease. Mol Psychiatry 15:342–354
Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P (2013) Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry 3:e240
Shaftel SS, Griffin WS, O'Banion MK (2008) The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation 5:7
Shearer TR, Larson K, Neuschwander J, Gedney B (1982) Minerals in the hair and nutrient intake of autistic children. J Autism Dev Disord 12:25–34
Shi L, Fatemi SH, Sidwell RW, Patterson PH (2003) Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23:297–302
Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M (2009) Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol 211:39–48
Singh VK (1996) Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol 66:143–145
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27:10695–10702
Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M (1989) A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry 30:405–416
Stubbs EG, Crawford ML (1977) Depressed lymphocyte responsiveness in autistic children. J Autism Child Schizophr 7:49–55
Sweeten TL, Posey DJ, McDougle CJ (2003) High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry 160:1691–1693
Takahashi K, Rochford CD, Neumann H (2005) Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201:647–657
Torrente F, Ashwood P, Day R, Machado N, Furlano RI, Anthony A, Davies SE, Wakefield AJ, Thomson MA, Walker-Smith JA, Murch SH (2002) Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol Psychiatry 7:375–382, 334
Tracey KJ (2002) The inflammatory reflex. Nature 420:853–859
Tsuchiya KJ, Hashimoto K, Iwata Y, Tsujii M, Sekine Y, Sugihara G, Matsuzaki H, Suda S, Kawai M, Nakamura K, Minabe Y, Yagi A, Iyo M, Takei N, Mori N (2007) Decreased serum levels of platelet-endothelial adhesion molecule (PECAM-1) in subjects with high-functioning autism: a negative correlation with head circumference at birth. Biol Psychiatry 62:1056–1058
Turner MS, Goldsmith JD (2009) Best practices in diagnostic immunohistochemistry: spindle cell neoplasms of the gastrointestinal tract. Arch Path Lab Med 133:1370–1374
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57:67–81
Vojdani A, Mumper E, Granpeesheh D, Mielke L, Traver D, Bock K, Hirani K, Neubrander J, Woeller KN, O'Hara N, Usman A, Schneider C, Hebroni F, Berookhim J, McCandless J (2008) Low natural killer cell cytotoxic activity in autism: the role of glutathione, IL-2 and IL-15. J Neuroimmunol 205:148–154
Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A (2008) Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci 28:8138–8143
Warren RP, Foster A, Margaretten NC (1987) Reduced natural killer cell activity in autism. J Am Acad Child Adolesc Psychiatry 26:333–335
Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B (2006) Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci 7:64
Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25:181–213
Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M (2006) Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9:268–275
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Rose, D., Ashwood, P. (2015). Immunology of Autism. In: Fatemi, S. (eds) The Molecular Basis of Autism. Contemporary Clinical Neuroscience. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2190-4_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2190-4_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2189-8
Online ISBN: 978-1-4939-2190-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)