Abstract
Autism is a heterogeneous neurodevelopmental disorder. The etiology of autism remains unknown although both genetic and environmental factors are likely to be involved. These factors disrupt the course of normal brain development from the cellular to the gross anatomical levels. The Reelin, gamma-aminobutyric acid (GABA), and fragile X mental retardation protein (FMRP)—metabotropic glutamate receptor 5 (mGluR5) signaling systems play important roles during the development of the nervous system. Disruption of these pathways is likely to lead to altered synaptic transmission and, ultimately, the cognitive and behavioral deficits associated with autism. This chapter describes each of these signaling systems and summarizes the current evidence that link them to autism. Therapies that target molecules in these signaling systems may provide new means of treating the core symptoms of autism.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Autism is a pervasive, debilitating, neurodevelopmental disorder characterized by three core symptoms: (1) abnormal social interaction; (2) impaired verbal and nonverbal communication; and (3) the presence of restrictive, repetitive or stereotyped behaviors (APA 2013). Currently, the prevalence of autism is rising in the United States with a rate of 14.7 per 1000 (1 in 68) for children aged 8 years old (CDC 2014). Individuals with autism often display a number of comorbidities including seizure disorder and intellectual impairment (Canitano 2007; Chakrabarti and Fombonne 2005). This chapter reviews the current evidence for dysfunction of the Reelin, GABAergic, and the FMRP -mGluR5 signaling pathways in autism. During development, the Reelin signaling system is instrumental in proper neuronal migration and brain lamination (Frotscher 1998; D’Arcangelo et al. 1995). In adults, Reelin is expressed in GABAergic interneurons (Pesold et al. 1998) and is involved in synapse formation and plasticity (reviewed by Stranahan et al. 2013). GABA is the most common inhibitory neurotransmitter in the brain. Disruption of the excitatory/inhibitory balance in the brain resulting from perturbations to this system may lead to the presence of seizures, abnormal information processing, and cognitive dysfunction associated with autism. Fragile X syndrome (FXS) is one of the most common forms of mental retardation and is often comorbid with autism. Behavioral deficits such as social anxiety, gaze avoideance, hyperarousal to sensory stimuli are common to both autism and FXS. In this chapter, we review current data from gene association and postmortem studies which implicate these three signaling systems in the pathology of autism.
1 Reelin and Autism
Reelin is a secreted extracellular matrix glycoprotein (DeBergeyck et al. 1998) with multiple functions during brain development including mediating proper brain lamination (Boyle et al. 2011; Hamburgh 1963; Tissir and Goffinet 2003) and facilitation of neuronal cell migration (D’Arcangelo et al. 1995; Hadj-Sahraoui et al. 1996). In adults, Reelin signaling is involved in synapse formation and modulation of synaptic transmission (Beffert et al. 2005; Chen et al. 2005; Groc et al. 2007; Herz and Chen 2006; Qiu and Weeber 2007; Ventruti et al. 2011; Weeber et al. 2002). Full length Reelin has a molecular weight of 410 kDa, while several cleavage products including Reelin 330 kDa, Reelin 180 kDa, and other fragments including a 220 kDa as well as 100 kDa C-terminal entity can be identified using SDS-PAGE (Jossin 2008; Fatemi et al. 2005a,b; Ignatova et al. 2004; Lambert de Rouvroit et al. 1999; Smalheiser et al. 2000). Reeler mice, which lack Reelin expression, display deficits in long term potentiation (Marrone et al. 2006) as do heterozygous Reeler mice (HRM) which are characterized by an approximately 50 % reduction in normal levels of Reelin (Tueting et al. 2006; Qiu and Weeber, 2007). These mouse strains also display behavioral deficits relevant to autism including impaired executive function, increased anxiety and motor impulsivity, impaired fear-conditioned learning, and deficits in sensorimotor gating behavior as measured by prepulse inhibition (PPI) (Ammassari-Teule et al. 2009; Barr et al. 2008; Ognibene et al. 2007; Qiu and Weeber, 2007; Tueting et al. 1999). It should be noted, however, that other groups have found no difference in PPI between HRM and wild type mice (Barr et al. 2008; Podhorna and Didriksen 2004; Teixeira et al. 2011) .
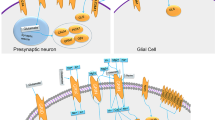
Reelin binds to three receptors: very low density lipoprotein receptor (VLDLR), apolipoprotein E receptor 2 (APOER2) (D’Arcangelo et al. 1999) and α3β1 integrin . Experiments involving Vldlr and Apoer2 knockout (KO) mice have demonstrated differing roles for each receptor with regard to neuronal migration with APOER2 enabling migration while Reelin binding to VLDLR may cease neuronal migration (Hack et al. 2007). Associated with APOER2 and VLDLR are ephrin B proteins (EFNBs) (Sentürk et al. 2011; Bouché et al. 2013). Reelin binding results in clustering of APOER2, VLDLR, and EFNBs and activation of FYN tyrosine kinase (Hiesberger et al. 1999; Strasser et al. 2004) and promoting the phosphorylation of disabled 1 (DAB1), a cytoplasmic adaptor protein (Hiesberger et al. 1999; Sentürk et al. 2011). Loss of function of EFNBs results in reduced phosphorylation of DAB1 and impaired neuronal migration (Sentürk et al. 2011). Phosphorylation of DAB1 activates a kinase cascade including phosphatidylinositol-3-kinase (PI3K) and protein kinase B (PKB/AKT) ultimately leading to the phosphorylation and inhibition of glycogen synthase kinase 3-beta (GSK3β) (Beffert et al. 2002; Hiesberger et al. 1999). Mouse embryos that express a nonphosphorylated Dab1 mutation display deficits in migration of sympathetic preganglionic neurons that is similar to what is observed in the Reeler mouse (Yip et al. 2007a). DAB1 phosphorylation also leads to the recruitment of the lissencephaly 1 (LIS1) complex which is important in both neuronal migration and proper cortical lamination (Assadi et al. 2003). GSK3β phosphorylates tau, a microtubule stabilizing protein (Hiesberger et al. 1999; Kwok et al. 2005). Inhibition of GSK3β leads to reduced phosphorylation of tau and allows for altered microtubule dynamics promoting neuronal cell migration. Figure 16.1 summarizes the Reelin signaling system .
The Reelin signaling cascade. Reelin binds to its extracellular receptors APOER2, VLDLR and α3β1 integrin resulting in clustering of the receptors and ephrin B proteins (ENFBs). This clustering recruits DAB1 proteins and activation of FYN kinase which phosphorylates DAB1. Phosphorylation of DAB1 leads to: (1) recruitment and activation of a kinase cascade involving PI3K and PKB/AKT1 which ultimately inhibits GSK3β; and (2) recruitment of LIS1 which promotes proper brain lamination. Inhibition of GSK3β results in dephosphorylation of tau which in turn destabilizes microtubule dynamics promoting neuronal migration. The binding of Reelin to its receptors and subsequent activation of FYN leads to phosphorylation of NMDA receptor subunits NR2A and NR2B. As a result, there is an inflow of calcium which induces long term potentiation (LTP) and synaptic plasticity. (Reprinted from Folsom and Fatemi 2013 Copyright (2013) with permission from Elsevier)
Reelin contributes to synapse formation and modulation of synaptic transmission via regulation of Ca2+ entry through N-methyl-d-aspartate (NMDA) receptors (Fig. 16.1) (Beffert et al. 2005; Chen et al. 2005; Groc et al. 2007; Herz and Chen 2006; Qiu and Weeber 2007; Ventruti et al. 2011; Weeber et al. 2002). Synaptic transmission in rodent models is impaired by mutations involving Apoer2 (Beffert et al. 2005, 2006) or loss of Dab1 (Trotter et al. 2013). Moreover, loss or transient downregulation of Dab1 results in impairments of associative learning, memory deficits, and impaired sensory motor gating as measured by prepulse inhibition, all of which are relevant to the pathology of autism (Teixeira et al. 2014; Trotter et al. 2013) .
Based on its role in brain development and synaptic transmission, the gene that codes Reelin (RELN) has been investigated as an autism candidate gene. An initial discovery found that an increase in the number of GGC triplet repeats in the 5′ untranslated region immediately before the RELN initiation codon, conferred vulnerability to the development of autism (Persico et al. 2001). RELN alleles with at least 11 triplet repeats in this region were preferentially transmitted to subjects with autism (Lugli et al. 2003; Persico et al. 2001). Subsequent studies in various population samples have verified an association between this region and autism (Dutta et al. 2007; Kelemenova et al. 2010; Skaar et al. 2005; Zhang et al. 2002). However, several other studies found no such association (Bonora et al. 2003; Devlin et al. 2004; Krebs et al. 2002; Li et al. 2004) .
A number of variants and single nucleotide polymorphisms (SNPs) of RELN have also been associated with autism in various populations including g.504742G > A in a Han Chinese sample (Tian 2012); rs2073559 in a Caucasian population sample (Ashley-Koch et al. 2007); rs736707 from intron 59 and rs362691 from exon 22 in a Caucasian population sample (Serajee et al. 2006); rs736707 in a Han Chinese population sample (Li et al. 2008), a finding that was recently confirmed in a South African population sample (Sharma et al. 2013). However, other groups have found no association between SNPs of RELN, including the ones listed above, and autism. A study of six RELN SNPs (rs727531, rs2072403, rs2072402, rs362691, rs362719, rs736707) in an Indian population sample failed to find a significant association for any of the SNPs and autism (Dutta et al. 2008). In contrast to the finding by Li et al. (2008), a separate study failed to find an association between rs736707 and autism in a Han Chinese population sample and, moreover, found no association for rs2229864, rs362691, and rs2073559 and autism (He et al. 2011) .
Reelin levels have been shown to be reduced in sera and in brains of subjects with autism (Fatemi et al. 2001, 2002, 2005a; Keller et al. 2000; Lugli et al. 2003). In sera from subjects with autism, reductions of Reelin 410 kDa have been observed (Fatemi et al. 2002; Keller et al. 2000). Lugli et al (2003) found that Reelin 330 kDa was significantly reduced by 25 % in sera of subjects with 11 or more CGG triplet repeats, suggesting that transmission of long alleles resulted in reduced Reelin expression. In brain, Fatemi et al (2001) found reduced expression of Reelin 180 kDa in cerebellum of subjects with autism when compared with healthy controls. A follow up experiment found reduced expression of Reelin 410 kDa, Reelin 330 kDa, and Reelin 180 kDa in prefrontal cortex of subjects with autism and reduction of Reelin 410 kDa and Reelin 180 kDa in cerebellum of subjects with autism, while there were no significant differences in parietal cortex of subjects with autism (Fig. 16.2) (Fatemi et al. 2005a). RELN mRNA was similarly reduced in both BA9 and cerebellum of subjects with autism as was mRNA for DAB1, while mRNA for VLDLR was significantly upregulated in both areas (Fatemi et al. 2005a). In addition to autism, Reelin downregulation has been observed in subjects with schizophrenia and mood disorders (Eastwood and Harrison 2003, 2006; Fatemi et al. 2000, 2005b; Guidotti et al. 2000; Impagnatiello et al. 1998) .
The gel mobility of Reelin bands of 410, 330, and 180 kDa in BA9 (a), cerebellar (b), and BA40 (c) homogenates of representative control (left histogram bar) and autistic adults (right histogram bar). a In BA9, there were significant reductions of Reelin 180 kDa/β-actin (p< 0.005), Reelin 330 kDa/β-actin (p < 0.035) and Reelin 410 kDa/β-actin (p < 0.035) versus control subjects. b In cerebella of subjects with autism, significant reductions in expression of Reelin 180 kDa/β-actin (p < 0.008) and Reelin 410 kDa/β-actin (p < 0.016) were observed versus control subjects. c In BA40, non-significant reductions were observed for full length reelin and the 330 kDa and 180 kDa fragments were observed. (Reprinted in a modified form Fatemi et al. 2005a. Copyright (2005) with permission from Elsevier)
2 GABA and Autism
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the brain. GABA has many important roles in brain development including stimulating the proliferation of neural progenitor cells, migration and differentiation of neurites, and synapse formation (reviewed by Pizzarelli and Cherubini 2011). Approximately 20 % of all central nervous system (CNS) neurons are GABAergic (Somogyi et al. 1998).
GABA is synthesized from glutamate by the enzyme glutamic acid decarboxylase. GABA is then transported to synaptic vesicles by the GABA transporter vGAT. When an action potential arrives at the nerve terminal, GABA is released into the synaptic cleft where it binds to its postsynaptic receptors. GABA receptors are either ionotropic (GABAA) which are ligand-gated ion channels or metabotropic (GABAB) which are G-protein coupled receptors. GABAA receptors are responsible for mediating the fast inhibitory action of GABA (Brandon et al. 2000). GABAA receptors are also the sites for clinical action of a number of drugs including benzodiazepines, barbiturates, and anesthetics. Thus far, 19 GABAA receptor subunits have been characterized (α1–α6, β1–β3, γ1– γ3, δ, ε, π, θ, and ρ1–ρ3) (Ma et al. 2005; Brandon et al. 2000) which combine to form the heteropentameric GABAA receptors. The most common GABAA receptors consist of two α subunits, two β subunits, and one γ subunit. GABAB receptors are heterodimeric, formed from two subunits: GABAB receptor 1 (GABBR1) and GABAB receptor 2 (GABBR2) (Jones et al. 1998). GABAB receptors require one GABBR1 and one GABBR2 subunits in order to be functional. GABAB receptors contribute to synaptic events in the mammalian brain presynaptically by facilitating the release of neurotransmitters, including dopamine, serotonin, glutamate and GABA (Steiniger and Kretschmer 2003; Takahashi et al. 2010; Waldmeier et al. 2008). Postsynaptically, stimulation of GABAB receptors results in the generation of slow, long-lasting, inhibitory potentials (Bowery 2000; Kuriyama et al. 2000).
Recent studies have investigated levels of GABA in brain and plasma of subjects with autism (Gaetz et al. 2013; Rojas et al. 2013; Russo 2013). Plasma levels of GABA have been shown to be significantly increased in children with autism and that high levels of GABA correlated significantly with increased hyperactivity and impulsivity, tip toeing severity, light sensitivity, and tactile sensitivity (Russo 2013). Rojas et al (2013) found that ratios of GABA to creatine (Cr) were significantly reduced when compared to controls in the perisylvian region of the left hemisphere as visualized by single-voxel, point resolved spectroscopy. Interestingly, unaffected siblings also displayed reduced GABA/Cr ratios when compared with controls (Rojas et al. 2013). A second study, using the same procedure, found reduced GABA/Cr ratios in motor cortex and auditory cortex regions of interest (ROI) but not in the visual cortex ROI (Gaetz et al. 2013). The reduction of GABA levels in the brains of subjects with autism supports the hypothesis that the excitatory/inhibitory balance is disrupted in this population, which might help to explain the presence of seizure disorders (Tuchman and Rapin 2002) as well as cognitive and behavioral deficits associated with autism.
Glutamic acid decarboxylase 65 and 67 kDa proteins (GAD65/67) are the rate limiting enzymes responsible for the conversion of glutamate to GABA. GAD65 is significantly downregulated in cerebellum of subjects with autism and GAD67 is significantly reduced in parietal cortex of subjects with autism (Fatemi et al. 2002). More recently, significant reductions in GAD65 mRNA in the cerebellar dentate nuclei and significant reductions in GAD67 mRNA in Purkinje cells in cerebella from subjects with autism have been observed (Yip et al. 2007b 2009). Reductions in GAD65/67 could result in excessive glutamatergic (excitatory) and reduced GABAergic (inhibitory) signaling in important brain circuits that connect the cerebellum with the frontal cortex. The major deficiencies in levels of GAD 65 and 67 kDa proteins in two important brain areas in autism may subserve deficiency in the availability of GABA , thus affecting important biological functions including learning and motor activity. Additionally, decreased levels of GAD 65 and 67 kDa proteins could impact negatively on normal processing of visual, somatic, motor, and memory information processing, and could also explain the observations of increased blood, platelet, brain, and CSF glutamate levels in autistic patients (Moreno-Fuenmayor et al. 1996; Moreno et al. 1992; Shimmua et al. 2011; Shinohe et al. 2006).
A number of studies have now demonstrated that GABA receptors are reduced in brains of subjects with autism (Blatt et al. 2001; Samaco et al. 2005; Guptill et al. 2007; Fatemi et al. 2009a, b, 2010a, 2014; Oblak et al. 2010, 2011). Blatt et al. (2001) demonstrated a significant decrease in GABAA receptor binding sites (3H-muscimol-labeled binding sites) and benzodiazepine receptor binding sites (3H-flunitrazepam-labeled binding sites) in hippocampus of subjects with autism. Guptill et al. (2007), expanded on these experiments to demonstrate that the decrease in 3H-flunitrazepam-labeled benzodiazepine binding sites was due to a decrease in binding site number (Bmax) rather than altered affinity to ligand binding (Kd). Reduced 3H-muscimol-labeled GABAA receptor binding sites have also been observed in the posterior cingulate cortex and fusiform gyrus (Oblak et al. 2011). Finally, 3H-CGP54626-labeled GABAB receptor binding sites have also been observed to be reduced in the anterior and posterior cingulate cortex and fusiform gyrus of subjects with autism (Oblak et al. 2010).
Consistent with the results of binding assays, GABAA and GABAB receptor subunit proteins have been shown to be reduced in brains of subjects with autism (Fatemi et al. 2009a, b, 2010a, 2014). A comprehensive series of experiments have examined protein expression for GABA receptor subunits (16 GABAA and two GABAB) in superior frontal cortex [Brodmann Area 9 (BA9)], parietal cortex (BA40), and cerebellum of subjects with autism vs. matched controls. In BA9, there were reductions in GABAA receptor alpha 1 (GABRα1), GABAA receptor alpha 4 (GABRα4), GABAA receptor alpha 5 (GABRα5), GABAA receptor alpha 6 (GABRα6), GABAA receptor beta 1 (GABRβ1), GABAA receptor beta 2 (GABRβ2), GABAA receptor delta (GABRδ), GABAA receptor epsilon (GABRε), GABAA receptor gamma 2 (GABRγ2), GABAA receptor rho 2 (GABRρ2), and GABAB receptor 1 (GABBR1) proteins in brains of subjects with autism (Fig. 16.3) (Fatemi et al. 2009a, b, 2010a, 2014). In BA40, we observed significant reductions in GABRα1, GABAA receptor alpha 2 (GABRα2), GABAA receptor alpha 3 (GABRα3), GABRα5, GABAA receptor beta 3 (GABRβ3), and GABBR1 proteins in brains of subjects with autism (Table 16.1) (Fig. 16.3) (Fatemi et al. 2009a, b, 2010a, 2014). GABRα1, GABRβ3, GABBR1, and GABAB receptor 2 (GABBR2) proteins were significantly decreased in cerebella obtained from subjects with autism vs. matched controls (Fig. 16.3) (Fatemi et al. 2009a, b) (Table 16.1) (Fig. 16.3). Consistent with these findings, a previous report has also demonstrated reduction in GABRβ3 in cerebella of subjects with autism when compared with controls (Samaco et al. 2005). More GABA receptor subunits were reduced in BA9 than in BA40 or cerebellum. Gene expression changes have previously been demonstrated to be more robust in cerebral cortex of subjects with autism than in cerebellum, which is consistent with our findings (Voineagu et al. 2011). Altered protein expression for GABAA and GABAB receptor subunits have also been observed in the brains of subjects with schizophrenia and mood disorders (Fatemi et al. 2011a, 2013a, b, 2014).
Representative samples of GABRα1 (51 kDa), GABRα2 (51 kDa), GABRα3 (55 kDa), GABRα4 (64 kDa), GABRα5 (52 kDa), GABRα6 (50 kDa), GABRβ1 (55 kDa), GABRβ2 (56 kDa and 52 kDa), GABRβ3 (56 kDa), GABRδ (51 kDa), GABRε (45 kDa), GABRγ2 (45 kDa), GABRγ3 (51 kDa), GABRπ (50 kDa), GABRθ (70 kDa), GABRρ2 (54 kDa), GABBR1 (108 kDa), GABBR2 (105 kDa), and β-Actin (42 kDa) in BA9, BA40, and cerebellum of subjects with autism (A) and matched controls (C). [Reprinted in a modified from Fatemi et al. 2009a Copyright (2009) with permission from Springer Science+Business Media; from Fatemi et al. 2011b. Copyright (2011) with permission from Springer Science+Business Media; and from Fatemi et al. (2010a). Copyright (2010) with permission from Springer Science+Business Media]
mRNA species for the same GABAA and GABAB receptors were measured via qRT-PCR in BA9, BA40, and cerebellum of subjects with autism vs. controls using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin as housekeeping genes (Fatemi et al. 2010a, 2014). Increased mRNA was observed in BA9 of subjects with autism for GABRα6 while there were reduced mRNA species for GABRα2, GABRα3, GABRα4, GABRα5, GABRβ1, GABRβ3, GABRγ3, and GABAA receptor theta (GABRθ) (Table 16.1) (Fatemi et al. 2010a, 2014). In BA40, qRT-PCR revealed increased mRNA expression of GABRγ3 and GABBR1 and reduced mRNA expression for GABRα3 in subjects with autism (Table 16.1) (Fatemi et al. 2010a, 2014). qRT-PCR of cerebella from subjects with autism revealed significant upregulation of mRNA species for GABRα2, GABRα3, GABRα4, GABRα5, GABRβ1, GABRβ3, GABRγ2, GABRγ3, and GABRθ (Table 16.1) (Fatemi et al. 2010a, 2014). In contrast, there were reduced mRNA species for GABRα6, GABRβ2, and GABBR1 in cerebella of subjects with autism (Fatemi et al. 2010a, 2014). While mRNA expression changes were variable in each brain region, protein expression was consistently downregulated.
Gene association studies for GABA receptor subunit genes and autism have been equivocal. An analysis of 12 GABAA and two GABAB receptor subunit genes found that GABRA4 (which codes for GABRα4), potentially through interaction with GABRB1 (which codes for GABRβ1) was associated with autism (Ma et al. 2005), a finding that was subsequently confirmed (Collins et al. 2006). Several studies have implicated the 15q11.2–q13 region, which includes genes for GABRβ3, GABRα5, and GABRγ3 (GABRB3, GABRA5, GABRG3) with autism (Hogart et al. 2007; Maddox et al. 1999; McCauley et al. 2004). GABRB3 has been associated with autism in a Korean population sample (Kim et al. 2006). SNPs of GABRA5 and GABRB3 have been shown to be nominally associated with autism (McCauley et al. 2004) as have SNPs of GABRG3 (Menold et al. 2001). However, a study using a Japanese population sample failed to find significant associations between GABRB3, GABRA5, or GABRG3 and autism (Tochigi et al. 2007). Moreover, others have similarly found no association for these genes and autism (Ma et al. 2005; Kelemenova et al. 2010). Recently, truncating mutations of the genes that code for GABRα3 and GABRθ (GABRA3 and GABRQ) have shown an association with autism spectrum disorders (Piton et al. 2013). Taken together, these results suggest that association between GABA receptor genes and autism may be population specific.
Reduction in GABAA and GABAB receptor subunits may help explain comorbid seizure disorder and cognitive deficits present in subjects with autism. Seizure disorder was present in many of the medical histories of subjects with autism used in our postmortem studies (Fatemi et al. 2009a, b, 2010a, 2014). However, when analyzed as a confound, we did not find an impact of seizure disorder on our results (Fatemi et al. 2009a, b, 2010a, 2014). Animal models of seizure disorder have shown reduced expression of GABBR1 and GABBR2 in brain (Han et al. 2006; Princivalle et al. 2003; Straessle et al. 2003). GABBR1 KO mice display seizure disorder and memory deficits (Prosser et al. 2001; Schuler et al. 2001). Eplieptiform activity interferes with cognition by causing disturbances of vigilance, shifting attention, and language difficulties (Binnie 1993) phenomena that often occur in children with autism and epilepsy. It has been hypothesized that the regression of language skills in children between the ages of two and three with autism may be due to epileptiform activity (Canitano 2007).
3 FMRP and Autism
Autism and fragile X syndrome (FXS) share many commonalities including intellectual disability, presence of seizures, learning difficulties, social deficits, anxiety, decreased attention, poor eye contact, delayed language acquisition and disorders of expression. Previous reports have shown the presence of autistic behavior in 15–47 % of patients with FXS (Bailey et al. 1998; Hatton et al. 2006; Kau et al. 2004; Kauffman et al. 2004). Individuals with diagnoses of both autism and FXS display greater severity of symptoms (Kau et al. 2004; Philofsky et al. 2004) and greater impairment in nonverbal cognition and expressive language (Philofsky et al. 2004). Boys with autism and FXS also show more cognitive impairment, abnormal behavior, and less adaptive behavior when compared to those with FXS alone (Kau et al. 2004). Interestingly, autistic symptoms have been shown to improve with age in subjects with FXS (McDuffie et al. 2010). In addition to phenotypic overlap, recently identified biological substrates have been proposed that create intriguing avenues of scientific interest.
The fragile X mental retardation 1 (FMR1) gene is located to the X chromosome. Mutations in this gene, resulting in loss of function, are almost entirely responsible for the development of FXS. Under normal circumstances there are anywhere from 5-55 5’ CGG repeats in the untranlated portion of the gene (Fu et al. 1991). Individuals with 56–200 repeats are often found in FXS families but do not display clinical symptoms of FXS and are considered to have the FMR1 premutation (Bardoni et al. 2001). When more than 200 5′ CGG repeats are present, there is extensive methylation of the 5′ region, including the promoter of FMR1 resulting in transcriptional silencing of FMR1 and the presence of clinical symptoms of FXS (Pieretti et al. 1991). FMRP binds to approximately 5 % of all mRNAs expressed in brain (Darnell and Klann 2013) potentially controlling a large number of important processes. It has been hypothesized that reduction in FMRP expression leads to unregulated protein synthesis, induced by group 1 metabotropic glutamate receptors (mGluRs), which in turn is responsible for the multiple physical and cognitive pathologies of FXS (Bear et al. 2004; Dölen and Bear 2008).
Boys who have the FMR1 premutation, especially if they present as clinical probands, are more likely to have a comorbid diagnosis of autism than nonprobands or control siblings who lack the premutation (Chonchaiya et al. 2012; Farzin et al. 2006). Additionally, these probands displayed increased rates of seizure disorder (Chonchaiya et al. 2012) and attention deficit/hyperactivity disorder (Farzin et al. 2006). Carriers of the FMR1 premutation have also been shown to have reduced amygdala volume, reduced activation of the right amygdala during an emotion matching task, and higher ratings of autism spectrum symptoms (Hessl et al. 2011). The authors found that while reduced FMRP and increased FMR1 mRNA were associated with reduced activation, reduced FMRP expression was identified statistically as the primary factor associated with reduced amygdala activation (Hessl et al. 2011).
The FMR1 gene may be a candidate gene for autism. The Xq27-q28 region, which includes FMR1 and methyl CpG binding protein 2 (MECP2), has shown some association with increased risk of autism (Vincent et al. 2005). A rare point mutation of FMR1 (A to C substitution at nucleotide 879 in exon 9) may contribute to autism and mental retardation in Japanese patients (Shinahara et al. 2004). An intronic variant of FMR1 (IVS10+14C-T) showed no evidence of increasing the risk of autism (Vincent et al. 2004). There may also be structural differences in the FMR1 gene in subjects with autism vs. controls. An investigation of CGG repeat length and AGG interruption of the FMR1 gene in subjects with infantile autism vs. healthy controls found that in the case of infantile autism, there were less AGG interruptions (Poon et al. 1998). This pattern is similar to what is found in subjects with FXS, although none of the study subjects had a comorbid diagnosis of FXS (Poon et al. 1998).
A recent series of experiments involving postmortem brain tissue have shown dysregulation of FMRP , mGluR5, and downstream targets of FMRP-mGluR5 signaling in cerebellar vermis and PFC in subjects with autism (Fatemi and Folsom 2011; Fatemi et al. 2011b, 2013c; Rustan et al. 2013). Levels of FMRP were reduced in PFC and cerebellar vermis of adults with autism while there were no differences in these brain regions in children with autism (Fig. 16.4) (Fatemi and Folsom 2011; Fatemi et al. 2011b). Phosphorylated FMRP (p-FMRP) is reduced in cerebellar vermis of adults and children with autism and in PFC of adults with autism (Rustan et al. 2013). Dephosphorylation of FMRP leads to its ubiquitination and subsequent degradation, a process that may be the result of overactive mGluR5 signaling (Nalavadi et al. 2012). Indeed, in the same tissues, increased expression of mGluR5 was observed in PFC and cerebellar vermis of children with autism (Fig. 16.4) (Fatemi and Folsom 2011; Fatemi et al. 2011b). Interestingly, none of the subjects with autism had a comorbid diagnosis of FXS (Fatemi and Folsom 2011; Fatemi et al. 2011b, 2013c). Moreover, similar studies performed in subjects with schizophrenia and mood disorders found reduced expression of FMRP and mGluR5 in lateral cerebellum of subjects with schizophrenia, bipolar disorder, and major depression and in BA9 of subjects with schizophrenia and bipolar disorder (Fatemi et al. 2010b, 2013a, b). These results indicate that dysfunction in FMRP and mGluR5 expression may be common to multiple psychiatric disorders.
Expression of FMRP and mGluR5 in brains from subjects with autism vs. matched controls. a) Ratio of FMRP/NSE in cerebellar vermis. b) Ratio of mGluR5/NSE in cerebellar vermis. c) Ratio of FMRP/NSE in BA9. d) Ratio of mGluR5/NSE in BA9. Ratios are expressed as mean ± standard error *, p < 0.05. [Reprinted in a modified form Fatemi et al. 2011b Copyright (2011) with permission from John Wiley and Sons and from Molecular Autism; Fatemi and Folsom 2011. Copyright (2011) with permission from BioMed Central]
FMRP is a negative regulator of mGluR5. It has been proposed that in the absence of FMRP, mGluR5-dependent protein synthesis goes unchecked, resulting in the anatomical and physical deficits associated with FXS (Bear 2005; Bear et al. 2004; Dölen and Bear 2008). Evidence from FMR1 KO mice support this theory including: (1) increased long-term depression (LTD) (Huber et al. 2002); (2) an increased number of immature dendritc spines (Grossman et al. 2006); and (3) increased epileptiform activity (Yan et al. 2005), all of which are present in individuals with FXS (reviewed by Garber et al. 2008). Importantly, use of allosteric inhibitors of mGluR5 such as such as 2-methyl-6-(phenylethynyl)-pyridine (MPEP), or lowering of the concentration of mGluR5 reverse structural and behavioral deficits in FMR1 KO mice including the number of dendritic spines, deficits in prepulse inhibition, and presence of seizure that are also present in autism (de Vrij et al. 2008; Dölen et al. 2007; Westmark et al. 2009; Yan et al. 2005; Yuskaitis et al. 2010). The use of allosteric modulators of mGluR5 as well as other treatments for FXS and autism has been an ongoing line of research (Berry-Kravis et al. 2011; Hagerman et al. 2012; Li et al. 2013).
Four downstream targets of FMRP-mGluR5 signaling have also been investigated: homer 1, amyloid beta A4 precursor protein (APP), ras-related C3 botulinum toxin substrate 1 (RAC1), and striatal-enriched protein tyrosine phosphatase (STEP) (Fatemi et al. 2013c). These proteins are involved in synapse formation and neural plasticity (APP); regulation of N-methyl-D-aspartate (NMDA) receptor function (STEP); synaptogenesis, receptor trafficking, and involvement in dopaminergic and glutamatergic signaling (homer 1); and modulation of dendritic spine morphology and density (RAC1), all of which are relevant to autism (Goebel-Goody et al. 2012; Nakayama et al. 2000; Priller et al. 2006; Szumlinski et al. 2006; Turner et al. 2003). Protein levels of RAC1, APP 120 kDa and APP 88 kDa were upregulated in BA9 of children with autism (Fatemi et al. 2013c). In BA9 of adults with autism, there was increased protein expression of RAC1 and STEP 46 kDa while there was reduced expression of homer 1 (Fatemi et al. 2013c). In cerebellar vermis of adults with autism there was significantly increased RAC1 protein expression, while there was significantly reduced expression of APP 120 kDa, STEP 66 kDa, STEP 27 kDa, and homer 1 (Fatemi et al. 2013c). In contrast, there were no changes observed in cerebellar vermis of children with autism (Fatemi et al. 2013c).
The reduced expression of FMRP in subjects with autism may help explain reduced expression of GABA receptor subunits and Reelin. In animal models of FXS, expression of GABAA receptor subunits have been shown to be reduced or eliminated by the loss of function of the FRM1 gene and consequent loss of FMRP (D’Hulst et al. 2006; El Idrissi et al. 2005, Gantois et al. 2006). El Idrissi et al. (2005) found reduced expression of the GABAA β subunit in cortex, hippocampus, brainstem, and diencephalon of fragile X (FraX) mice. Gantois et al (2006) found reduced GABRδ mRNA in hippocampus and neocortex of Fmr1 knockout (KO) mice. A separate study found reduced mRNA for GABRα1, GABRα3, GABRα4, GABRβ1, GABRβ2, GABRγ1, and GABRγ2 in cortex, but not cerebellum of Fmr1 KO mice (D’Hulst et al. 2006).
Reelin has also been identified as a downstream target of FMRP (Darnell et al. 2011). Altered expression of FMRP in subjects with autism may impact levels of Reelin as well.
4 Conclusions
Autism is a heterogeneous disorder in which multiple signaling systems are impacted. The Reelin, GABAergic, and FMRP-mGluR5 signaling systems separately, and perhaps synergistically, contribute to the pathology of autism. Dysfunction of the Reelin signaling system may result in abnormalities in brain morphology associated with autism as well as dysfunctional synaptic transmission. GABAergic system dysfunction could result in cognitive impairments as well as seizure disorder. Deficits in the FMRP-mGluR5 signaling system contribute to intellectual impairment, altered neuronal structure and seizure disorder. Reelin is known to regulate GABAergic and glutamatergic neurotransmission (Tissir and Goffinet 2003; Marrone et al. 2006). FMRP regulates GABAA receptor expression (D’Hulst et al. 2006; El Idrissi et al. 2005, Gantois et al. 2006) and targets Reelin (Darnell et al. 2011), potentially regulating its expression as well. Dysfunction in one system may lead to dysfunction in other systems. Interplay between these systems may result in multiple abnormal phenotypes associated with autism. These systems also identify targets for therapeutic intervention which may ameliorate multiple symptoms of autism.
References
American Psychiatric Association (APA) (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Washington, DC
Ammassari-Teule M, Sgobio C, Biamonte F, Marrone C, Mercuri NB, Keller F (2009) Reelin haploinsufficiency reduces the density of PV + neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl.) 204:511–521
Ashley-Koch AE, Jaworski J, Ma de Q, Mei H, Ritchie MD, Skaar DA, Robert Delong G, Worley G, Abramson RK, Wright HH, Cuccaro ML, Gilbert JR, Martin ER, Pericak-Vance MA (2007) Investigation of potential gene–gene interactions between APOE and RELN contributing to autism risk. Psychatr Genet 17:221–226
Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D’Arcangelo G, Clark GD (2003) Interaction of Reelin signaling and Lis1 in brain development. Nat Genet 35:270–276
Bailey DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L (1998) Autistic behavior in young boys with fragile x syndrome. J Autism Dev Disord 28:499–507
Bardoni B, Schenck A, Mandel J-L (2001) The fragile X mental retardation protein. Brain Res Bull 56:375–382
Barr AM, Fish KN, Markou A, Honer WG (2008) Heterozygous reeler mice exhibit alterations in sensorimotor gating but not presynaptic proteins. Eur J Neurosci 27:2568–2574
Bear MF (2005) Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav 4:393–398
Bear MF, Huber KM, Warren ST (2004) The mGluR theory of Fragile X mental retardation. Trends Neurosci 27:370–377
Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J (2002) Reelin-mediated signalling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J Biol Chem 51:49958–49964
Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J (2005). Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47:567–579
Beffert U, Nematollah Farsian F, Masiulis I, Hammer RE, Yoon SO, Giehl KM, Herz J (2006) ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biol 16:2446-2452
Berry-Kravis E, Knox A, Hervey C (2011) Targeted treatments for fragile X syndrome. J Neurodev Disord 3:193–210
Binnie CD (1993) Significance and management of transitory cognitive impairment due to subclinical EEG discharges in children. Brain Dev 15:23–30
Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML (2001) Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord 31:537–544
Bonora E, Beyer KS, Lamb JA, Parr JR, Klauck SM, Benner A, Paolucci M, Abbott A, Ragoussis I, Poustka A, Bailey AJ, Monaco AP (2003) International molecular genetic study of autism (IMGSAC). Analysis of reelin as a candidate gene for autism. Mol Psychiatry 10:885–892
Bouché E, Romero-Ortega MI, Henkemeyer M, Catchpole T, Leemhuis J, Frotscher M, May P, Herz J, Bock HH (2013) Reelin induces EphB activation. Cell Res 23:473–490
Bowery NG (2000) GABAB receptors structure and function. In: Martin DL, Olsen RW (eds) GABA in the nervous system: the view at fifty years. Lippincott, Williams and Wilkins, Philadelphia, pp 233–244
Boyle MP, Bernard A, Thompson CL, Ng L, Boe A, Mortrud M, Hawrylycz MJ, Jones AR, Hevner RF, Lein ES (2011) Cell-type-specific consequences of Reelin deficiency in the mouse neocortex, hippocampus, and amygdala. J Comp Neurol 519:2061–2089
Brandon NJ, Smart TG, Moss SJ (2000) Regulation of GABAA receptors by protein phosphorylation. In: Martin DL, Olsen RW (eds) GABA in the nervous system: the view at fifty years. Lippincott, Williams and Wilkins, Philadelphia, pp 191–206
Canitano R (2007) Epilepsy in autism spectrum disorders. Eur Child Adolesc Psychiatry 16:61–66
Centers for Disease Control and Prevention (CDC) (2014) Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States. MMWR Surveill Summ 63:1–21
Chakrabarti S, Fombonne E (2005) Pervasive development disorders in preschool children: confirmation of high prevalence. Am J Psychiatry 162:1133–1341
Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J (2005) Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci 25:8209–8216
Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, Mu Y, Tassone F, Nguyen DV, Hagerman RJ (2012). Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet 131:581–589
Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA (2006) Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics 7:167–174
D’Arcangelo G, Miao GG, Soares HD, Morgan JI, Curran T (1995) A protein related to extracellular matrix proteins detected in the mouse mutant reeler. Nature 374:719–723
D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24:471–479
D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF (2006) Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 1121:238–245
Darnell JC, Klann E (2013) The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci 16:1530–1536
Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146:247–261
de Vrij FM Levenga J van der Linde HC Koekkoek SK De Zeeuw CI Nelson DL Oostra BA Willemsen R (2008) Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis 31:127–132
DeBergeyck V, Naerhuyzen B, Goffinet AM, deRouvroit C (1998) A panel of monoclonal antibodies against Reelin, the extracellular matrix protein defective in reeler mutant mice. J Neurosci Meth 82:17–24
Devlin B, Bennett P, Dawson G, Figlewicz DA, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD, CPEA Genetics Network (2004) Alleles of a Reelin CGG repeat do not convey liability to autism in a sample from the CPEA network. Am J Med Genet 126B:46–50
Dölen G, Bear MF (2008) Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol 586:1503-1508
Dölen G, Osterweil E, Shankaranarayana Rao BS, Smith GB, Auerbach D, Chattarji S, Bear MF (2007) Correction of fragile X syndrome in mice. Neuron 56:955–962
Dutta S, Guhathakurta S, Sinha S, Chatterjee A, Ahmed S, Ghosh S, Gangopadhyay PK, Singh M, Usha R (2007) Reelin gene polymorphisms in the Indian population: a possible paternal 5’UTR-CGG-repeat-allele effect on autism. Am J Med Genet B Neuropsychiatr Genet 144B:106–112
Dutta S, Sinha S, Ghosh S, Chatterjee A, Ahmed S, Usha R (2008) Genetic analysis of Reelin gene (RELN) SNPs: no association with autism spectrum disorder in the Indian population. Neurosci Lett 441:56–60
Eastwood SL, Harrison PJ (2003) Interstitial white matter neurons express less Reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry 8:821–831
Eastwood SL, Harrison PJ (2006) Cellular basis for reduced cortical Reelin expression in schizophrenia. Am J Psychiatry 163:540–542
El Idrissi A Ding XH Scalia J Trenkner E Brown WT Dobkin C (2005) Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci Lett 377:141–146
Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R (2006) Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 27:S137–S144
Fatemi SH, Folsom TD (2011) Dysregulation of fragile X mental retardation protein and metabotropic glutamate receptor 5 in superior frontal cortex of subjects with autism: a postmortem brain study. Mol Autism 2:6
Fatemi SH, Folsom TD (2015) GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schiz Res, in press
Fatemi SH, Earle JA, McMenomy T (2000) Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder, and major depression. Mol Psychiatry 5:654–663
Fatemi SH, Stary JM, Halt AR, Realmuto G (2001) Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J Autism Dev Disord 6:529–535
Fatemi SH, Stary JM, Egan EA (2002) Reduced blood levels of Reelin as a vulnerability factor in pathophysiology of autistic disorder. Cell Mol Neurobiol 22:139–152
Fatemi SH, Snow AV, Stary JM, Araghi-Niknam M, Brooks AI, Pearce DA, Reutiman TJ, Lee S (2005a) Reelin signaling is impaired in autism. Biol Psychiatry 57:777–787
Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Egan E (2005b) GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res 72:109–122
Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD (2009a) GABA(A) Receptor downregulation in brains of subjects with autism. J Autism Dev Disord 39:223–230
Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD (2009b) Expression of GABA(B) receptors is altered in brains of Subjects with autism. Cerebellum 8:64–69
Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD (2010a) mRNA and protein levels for GABA(A) alpha 4, alpha 5, beta 1, and GABA(B)R1 receptors are altered in brains from subjects with autism. J Autism Dev Disord 40:743–750
Fatemi SH, Kneeland RE, Liesch SB, Folsom TD (2010b) Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr Res 124:246–247
Fatemi SH, Folsom TD, Thuras PD (2011a) Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr Res 128:37–43
Fatemi SH, Folsom TD, Kneeland RE, Liesch SB (2011b) Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec 294:1635–1645
Fatemi SH, Folsom TD, Rooney RJ, Thuras PD (2013a) mRNA and protein expression for novel GABAA receptors θ and ρ2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Transl Psychiatry 3:e271
Fatemi SH, Folsom TD, Rooney RJ, Thuras PD (2013b) Expression of GABAA α2-, β1- and ε-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl Psychiatry 3:e303
Fatemi SH, Folsom TD, Kneeland RE, Yousefi M, Liesch S, Thuras PD (2013c) Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cerebellar vermis and superior frontal cortex. Mol Autism 4:21
Fatemi SH, Reutiman TJ, Folsom TD, Rustan OG, Rooney RJ, Thuras PD (2014) Downregulation of GABAA receptor protein subunits α6, β2, ε, γ2, and ρ2 in superior frontal cortex of subjects with autism. J Autism Dev Disord 44(8):1833–1845
Folsom TD, Fatemi SH (2013) The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology 68:122–135
Frotscher M (1998) Cajal-Retzius cells, Reelin, and the formation of layers. Curr Opin Neurobiol 8:570–575
Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG Jr, Warren ST et al (1991) Variation in the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67:1047–1058
Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TP (2013) GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage 86:1–9
Gantois I, Vandescompele J, Speleman F, Reyniers E, D’Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF (2006) Expression profiling suggests underexpression of the GABAA receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis 21:346–357
Garber KB, Visootsak J, Warren ST, Oostra BA, Nelson DL, Caskey CT (2008) Fragile X syndrome. Eur J Hum Genet 16:666–672
Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ (2012) Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev 64:65–87
Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P (2007) NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci 27:10165–10175
Grossman AW, Elisseou NM, McKinney BC, Greenough WT (2006) Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 1084:158–164
Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E (2000) Decrease in Reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57:1061–1069
Guptill JT, Booker AB, Gibbs TT, Kemper TL, Bauman ML, Blatt GJ (2007) [3H]-flunitrazepam-labeled benzodiazepine binding sites in the hippocampal formation in autism: a multiple concentration autoradiographic study. J Autism Dev Disord 37:911–920
Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M (2007) Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development 134:3883–3891
Hadj-Sahraoui N, Frédéric F, Delhaye-Bouchaud N, Mariani J (1996) Gender effect on Purkinje cell loss in the cerebellum of the heterozygous reeler mouse. J Neurogenet 11:45–58
Hagerman R, Lauterborn J, Au J, Berry-Kravis E (2012) Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ 54:297–335
Hamburgh M (1963) Analysis of the postnatal developmental effects of “reeler,” a neurological mutation in mice. A study in developmental genetics. Dev Biol 19:165–185
Han Y, Qin J, Bu DF, Chang XZ, Yang ZX (2006) Successive alterations of hippocampal gamma-aminobutyric acid B receptor subunits in a rat model of febrile seizure. Life Sci 78:2944–2952
Hatton DD, Sideris J, Skinner M, Bailey DB Jr, Roberts J, Mirrett P (2006) Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A 140A:1804–1813
He Y, Xun G, Xia K, Hu Z, Lv L, Deng Z, Zhao J (2011) No significant association between RELN polymorphisms and autism in case-control and family-based association study in Chinese Han population. Psychiatry Res 187:462–464
Herz J, Chen Y (2006) Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci 7:850–859
Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F, Hagerman PJ, Rivera SM (2011) Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry 70:859–865
Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J (1999) Direct biding of Reelin to VLDL receptor and ApoE receptor 2 induces tyroene phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24:481–489
Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM (2007) 15q11–13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet 16:691–703
Huber KM, Gallagher SM, Warren ST, Bear MF (2002) Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A 99:7746–7750
Ignatova N, Sindic CJM, Goffinet AM (2004) Characterization of the various farms of the Reelin protein in the cerebrospinal fluid of normal subjects and in neurological diseases. Neurobiol Dis 15:326–330
Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting T, Sharma RP, Costa E (1998) A decrease of Reelin expression as a putative vulnerability factor in schizophrenia. Proc Nat Acad Sci U S A 95:15718–15723
Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C (1998) GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396:674–679
Jossin Y (2008) Chemistry of Reelin. In: Fatemi SH (ed) Reelin glycoprotein: structure, biology and roles in health and disease. Springer, New York, pp 37–48
Kau AS, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Gray RM, Cox C, Capone GT, Stanard P (2004) Social behavior profile in young males with fragile X syndrome: characteristics and specificity. Am J Med Genet A 126A:9–17
Kauffman WE, Cortell R, Kau A, bukelis I, Tierney E, Gray R, Cox C, Capone G, Stanard P (2004) Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet Part A 129A:225–234
Kelemenova S, Schmidtova E, Ficek A, Celec P, Kubranska A, Ostatnikova D (2010) Polymorphisms of candidate genes in Slovak autism patients. Psychiatr Genet 20:137–139
Keller F, Persico AM, Zelante L, Gasperial P, D’Agruma N, Maiorano N, Totaro A, Militerni R, Bravaccio C, Wassink TH, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo M, Pascucci T, Puglisi-Allegra S, Reichelt K-L, Conciatori M, Marino R, Baldi A, Quattrocchi CC (2000). Reelin gene alleles and haplotypes are associated with autistic disorder. Soc Neurosci 26:77(31.11)
Kim SA, Kim JH, Park M, Cho IH, Yoo HJ (2006) Association of GABRB3 polymorphisms with autism in Korean trios. Neuropsychobiology 54:160–165
Krebs MO, Betancur C, Leroy S, Bourdel MC, Gillberg C, Leboyer M (2002) Paris Autism Research International Sibpair (PARIS) study. Absence of association between a polymorphic GGC repeat in the 5’ untranslated region of the reelin gene and autism. Mol Psychiatry 7:801–804
Kuriyama K, Hirouchi M, Kimura H (2000) Neurochemical and molecular pharmacological aspects of the GABA(B) receptor. Neurochem Res 25:1233–1239
Kwok JB, Hallupp M, Loy CT, Chan DK, Woo J, Mellick GD, Buchanan DD, Silburn PA, Halliday GM, Schofield PR (2005) GSK3B polymorphisms alter transcription and splicing in Parkinson’s disease. Ann Neurol 58:829–839
Lambert de Rouvroit C de Bergeyck V Cortvrindt C Bar I Eeckhout Y Goffinet AM (1999) Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase. Exp Neurol 156:214–217
Li J, Nguyen L, Gleason C, Lotspeich L, Spiker D, Risch N, Myers RM (2004) Lack of evidence for an association between WNT2 and RELN polymorphisms and autism. Am J Med Genet 126B:51–57
Li H, Li Y, Shao J, Li R, Qin Y, Xie C, Zhao Z (2008) The association analysis of RELN and GRM8 genes with autistic spectrum disorder in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet 147B:194–200
Li G, Jørgensen M, Campbell BM (2013) Metabotropic glutamate receptor 5-negative allosteric modulators for the treatment of psychiatric and neurological disorders (2009–July 2013). Pharm Pat Anal 2:767–802
Lugli G, Krueger JM, Davis JM, Persico AM, Keller F, Smalheiser NR (2003) Methodological factors influencing measurement and processing of plasma reelin in humans. BMC Biochem 4:9
Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA (2005) Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet 77:377–388
Maddox LO, Menold MM, Bass MP, Rogala AR, Pericak-Vance MA, Vance JM, Gilbert JR (1999) Autistic disorder and chromosome 15q11-q13: construction and analysis of a BAC/PAC contig. Genomics 62:325–331
Marrone MC, Marinelli S, Biamonte F, Keller F, Sgobio CA, Ammassari-Teule M, Bernardi G, Mercuri NB (2006) Altered cortico-striatal synaptic plasticity and related behavioural impairments in reeler mice. Eur J Neurosci 24:2061–2070
McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, Jacobs MM, Folstein SE, Haines JL, Sutcliffe JS (2004) A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. Am J Med Genet B Neuropsychiatr Genet 131B:51–59
McDuffie A, Abbeduto L, Lewis P, Kover S, Kim JS, Weber A, Brown WT (2010) Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. Am J Intellect Dev Disabil 115:307–326
Menold MM, Shao Y, Wolpert CM, Donnelly SL, Raiford KL, Martin ER, Ravan SA, Abramson RK, Wright HH, Delong GR, Cuccaro ML, Pericak-Vance MA, Gilbert JR (2001) Association analysis of chromosome 15 GABAA receptor subunit genes in autistic disorder. J Neurogenet 15:245–259
Moreno H, Borjas L, Arrieta A, Salz L, Prassad A, Estevez J, Bonilla E (1992) Clinical heterogeneity of the autistic syndrome: a study of 60 families. Invest Clin 33:13–31
Moreno-Fuenmayor H, Borjas L, Arrieta A, Valera V, Socorro-Candanoza L (1996) Plasma excitatory amino acids in autism. Invest Clin 37:113–128
Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20:5329–5338
Nalavadi VC, Muddashetty RS, Gross C, Bassell GJ (2012) Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation. J Neurosci 32:2582–2587
Oblak AL, Gibbs TT, Blatt GJ (2010) Decreased GABA(B) receptors in the cingulate cortex and fusiform gyrus in autism. J Neurochem 114:1414–1423
Oblak AL, Gibbs TT, Blatt, GJ (2011) Reduced GABA(A) receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res 1380:218–228
Ognibene E, Adriani W, Granstrem O, Pieretti S, Laviola G (2007) Impulsivity-anxiety-related behavior and profiles of morphine-induced analgesia in heterozygous reeler mice. Brain Res 113:173–180
Persico AM, D’Agruma L, Maiorano N, Totaro A, Militerni R, Bravaccio C, Wassink TH, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo M, Pascucci T, Puglisi-Allegra S, Reichelt KL, Conciatori M, Marino R, Quattrocchi CC, Baldi A, Zelante L, Gasparini P, Keller F, Collaborative Linkage Study of Autism (2001) Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry 6:150–159
Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ (1998) Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Nat Acad Sci U S A 95:3221–3226
Philofsky A, Hepburn SL, Hayes A, Hagerman RJ, Rogers SJ (2004) Linguistic and cognitive functioning and autism symptoms in young children with fragile X syndrome. Am J Ment Retard 109:208–218
Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL (1991) Absence of expression of FMR-1 gene in fragile X syndrome. Cell 66:817–822
Piton A, Jouan L, Rochefort D, Dobrzeniecka S, Lachapelle K, Dion PA, Gauthier J, Rouleau GA (2013) Analysis of the effects of rare variants on splicing identifies alterations in GABA(A) receptor genes in autism spectrum disorder individuals. Eur J Hum Genet 21:749–756
Pizzarelli R, Cherubini E (2011) Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast 2011:297153
Podhorna J, Didriksen M (2004) The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res 153:43–54
Poon PM, chen QL, Lai KY, Wong CK, Pang CP (1998) CGG repeat interruptions in the FMR1 gene in patients with infantile autism. Clin Chem Lab Med 36:649–653
Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J (2006) Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 26:7212–7221
Princivalle AP, Richards DA, Duncan JS, Spreafico R, Bowery NG (2003) Modification of GABA(B1) and GABA(B2) receptor subunits in the somatosensory cerebral cortex and thalamus of rats with absence seizures (GAERS). Epilepsy Res 55:39–51
Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN (2001) Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci 17:1059–1070
Qiu S, Weeber SJ (2007) Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J Neurophysiol 97:2312–2321
Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS (2013) Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage 86:28–34
Russo AJ (2013) Correlation between hepatocyte growth factor (HGF) and Gamma-aminobutyric acid (GABA) plasma levels in autistic children. Biomark Insights 8:69–75
Rustan OG, Folsom TD, Yousefi MK, Fatemi SH (2013) Phosphorylated fragile X mental retardation protein at serine 499, is reduced in cerebellar vermis and superior frontal cortex of subjects with autism: implications for fragile X mental retardation protein -metabotropic glutamate receptor 5 signaling. Mol Autism 4:41
Samaco RC, Hogart A, LaSalle JM (2005) Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet 14:483–492
Schuler V, Lüscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Käslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B (2001) Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31:47–58
entürk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A (2011) Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature 472:356-360
Serajee FJ, Zhong H, Mahbubul Huq AH (2006) Association of Reelin gene polymorphisms with autism. Genomics 87:75–83
Sharma JR, Arieff Z, Gameeldien H, Davids M, Kaur M, van der Merwe L (2013) Association analysis of two single-nucleotide polymorphisms of the RELN gene with autism in the South African population. Genet Test Mol Biomarkers 17:93–98
Shimmua C, Suda S, Tsuchiya KJ, Hashimoto K, Ohno K, Matsuzaki H, Iwata K, Matsumoto K, Wakuda T, Kameno Y, Suzuki K, Tsujii M, Nakamura K, Takei N, Mori N (2011) Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One 6:e25340
Shinahara K, Saijo T, Mori K, Kuroda Y (2004) Single-strand conformation polymorphism analysis of the FMR1 gene in autistic and mentally retarded children in Japan. J Med Invest 51:52–58
Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Minabe Y, Sugiyama T, Kawai M, Iyo M, Takei N, Mori N (2006) Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry 30:1472–1477
Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, DeLong GR, Moore JH, McCauley JL, Sutcliffe JS, Ashley-Koch AE, Cuccaro ML, Folstein SE, Gilbert JR, Pericak-Vance MA (2005) Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry 10:563–571
Smalheiser NR, Costa E, Guidotti A, Impagnatiello F, Auta J, Lacor P, Kriho V, Pappas GD (2000) Expression of Reelin in adult mammalian blood, liver, pituitary pars intermedia, and adrenal chromaffin cells. Proc Nat Acad Sci USA 97:1281–1286
Somogyi P, Tamás G, Lujan R, Buhl EH (1998) Salient features of synaptic organization in the cerebral cortex. Brain Res Brain Res Rev 26:113–135
Steiniger B, Kretschmer BD (2003) Glutamate and GABA modulate dopamine in the pedunculopontine tegmental nucleus. Exp Brain Res 149:422–430
Straessle A, Loup F, Arabadzisz D, Ohning GV, Fritschy JM (2003) Rapid and long-term alterations of hippocampal GABAB receptors in a mouse model of temporal lobe epilepsy. Eur J Neurosci 18:2213–2226
Stranahan AM, Erion JR, Wosiski-Kuhn M (2013) Reelin signaling in development, maintenance, and plasticity of neural networks. Ageing Res Rev 12:815–822
Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J (2004) Receptor clustering is involved in Reelin signaling. Mol Cell Biol 24:1378–1386
Szumlinski KK, Kalivas PW, Worley PF (2006) Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol 16:251–257
Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA (2010) GABA(B) receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci 30:11771–11780
Teixeira CM, Martín ED, Sahún I, Masachs N, Pujadas L, Corvelo A, Bosch C, Rossi D, Martinez A, Maldonado R, Dierssen M, Soriano E (2011) Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology 36:2395–2405
Teixeira CM, Masachs N, Muhaisen A, Bosch C, Pérez-Martínez J, Howell B, Soriano E (2014) Transient downregulation of Dab1 protein levels during development leads to behavioral and structural deficits: relevance for psychiatric disorders. Neuropsychopharmacology 39:556–568
Tian P (2012) RELN gene polymorphisms and susceptibility to autism in Chinese Han population. Neurol India 60:581–584
Tissir F, Goffinet AM (2003) Reelin and brain development. Nature Rev Neurosci 4:496–505
Tochigi M, Kato C, Koishi S, Kawakubo Y, Yamamoto K, Matsumoto H, Hashimoto O, Kim SY, Watanabe K, Kano Y, Nanba E, Kato N, Sasaki T (2007) No evidence for significant association between GABA receptor genes in chromosome 15q11–q13 and autism in a Japanese population. J Hum Genet 52:985–989
Trotter J, Lee GH, Kazdoba TM, Crowell B, Domogauer J, Mahoney HM, Franco SJ, Müller U, Weeber EJ, D’Arcangelo G (2013) Dab1 is required for synaptic plasticity and associative learning. J Neurosci 33:15652–15668
Tuchman R, Rapin I (2002) Epilepsy in autism. Lancet Neurol 1:352–358
Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C (1999). The phenotypic characteristics of heterozygous reeler mouse. NeuroReport 10:1327–1334
Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E (2006) Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev 30:1065–1077
Turner PR, O’Connor K, Tate WP, Abraham WC (2003) Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 70:1–32
Ventruti A, Kazdoba TM, Niu S, D’Arcangelo G (2011) Reelin deficiency causes specific defects in the molecular composition of the synapses in the adult brain. Neuroscience 189:32–42
Vincent JB, Thevarkunnel S, Kolozsvari D, Paterson AD, Roberts W, Scherer SW (2004) Association and transmission analysis of the FMR1 IVS10 + 14C-T variant in autism. Am J Med Genet B Neuropsychiatr Genet 125B:54–56
Vincent JB, Melmer G, Bolton PF, Hodgkinson S, Holmes D, Curtis D, Gurling HM (2005) Genetic linkage analysis of the X chromosome in autism, with emphasis on the fragile X region. Psychiatr Genet 15:83–90
Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH (2011) Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474:380–384
Waldmeier PC, Kaupmann K, Urwyler S (2008) Roles of GABAB receptor subtypes in presynaptic and auto- and heteroreceptor function regulating GABA and glutamate release. J Neural Transm 115:1401–1411
Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J (2002) Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem 277:39944–39952
Westmark CJ, Westmark PR, Malter JS (2009) MPEP reduces seizure severity in Fmr-1 KO mice over expressing human Abeta. Int J Clin Exp Pathol 3:56–68
Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP (2005) Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49:1053–1066
Yip YP, Kronstadt-O’Brien P, Capriotti C, Cooper JA, Yip JW (2007a) Migration of sympathetic preganglionic neurons in the spinal cord is regulated by Reelin-dependent Dab1 tyrosine phosphorylation and CrkL. J Comp Neurol 502:635–643
Yip J, Soghomonian JJ, Blatt GJ (2007b) Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathologica 113:559–568
Yip J, Soghomonian JJ, Blatt GJ (2009) Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: an in situ hybridization study. Autism Res 2:50–59
Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS (2010) Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol 79:632–646
Zhang H, Liu X, Zhang C, Mundo E, Macciardi F, Grayson DR, Guidotti AR, Holden JJ (2002) Reelin gene alleles and susceptibility to autism spectrum disorders. Mol Psychiatry 7:1012–1017
Acknowledgments
Grant support from NICHD (R01HD052074), NIMH (R01MH086000), the Bernstein Endowed Chair in Adult Psychiatry, and the Ewald Bipolar Disease Research Fund to SHF is gratefully acknowledged. Dr. Fatemi currently holds United States patents for Reelin as a diagnostic marker of autism (7341844) and as a diagnostic marker for schizophrenia, bipolar disorder, and major depression (7682805).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Folsom, T., Fatemi, S. (2015). Reelin, GABA, FMRP, and Autism. In: Fatemi, S. (eds) The Molecular Basis of Autism. Contemporary Clinical Neuroscience. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2190-4_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2190-4_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2189-8
Online ISBN: 978-1-4939-2190-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)