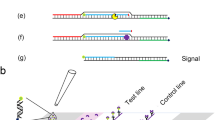
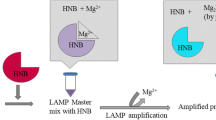
Abstract
Rapid detection of fish pathogens is mandatory for applying the crucial preventive and control measures to reduce fish losses and, consequently, minimize the economic impact of diseases on the fish farm owners. The currently used molecular diagnostic tools of fish infectious agents, such as PCR and RT-PCR, are sensitive and specific but still have some drawbacks. These tools are usually time consuming and laborious, need skilled persons, and require sophisticated devices to be performed. Therefore, next-generation tools for rapid diagnosis of fish infectious diseases were developed to conquer these shortages. One of these novel tools is the loop-mediated isothermal amplification (LAMP) technique. LAMP is considered a more advantageous tool than PCR because it needs only a heating block or a thermostatically controlled water bath as a source of constant temperature. It is considered to be more specific than the PCR assay as it uses 4–6 primers, which may diminish the occurrence of false-positive results. The time required for the amplification process by LAMP is ranging from 30 min to 1 h comparing to 3–5 h in the case of PCR. The visual detection methods coupled with the LAMP assay eliminates the post-run processing for detection of the amplification products. Its sensitivity is either comparable with the PCR or better than it. A variety of LAMP assays were developed for simple and rapid detection of a diversity of fish pathogens. Herein, we describe how to perform a LAMP assay and troubleshoot any potential problem arising during the process.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bernardet JF, Campbell AC, Buswell JA (1990) Flexibacter maritimus is the agent of ‘black patch necrosis’ in Dover sole in Scotland. Dis Aquat Org 8:233–237
Pazos F, Santos Y, Macías AR et al (1996) Evaluation of media for the successful culture of Flexibacter maritimus. J Fish Dis 19:193–197
Ambrosia RE, De Wall DT (1990) Diagnosis of parasitic disease. Rev Sci Tech 9:759–778
Craw P, Balachandran W (2012) Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip 12:2469–2486
Gill P, Ghaemi A (2008) Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids 27:224–243
Cunningham CO (2002) Molecular diagnosis of fish and shellfish diseases: present status and potential use in disease control. Aquaculture 206:19–55
Walker P, Subasinghe R (2000) DNA-based molecular diagnostic techniques: research needs for standardization and validation of the detection of aquatic animal pathogens and diseases. In: Report and proceedings of the joint FAO/NACA/CSIRO/ACIAR/DFID expert workshop, Bangkok, Thailand, 7–9 February 1999. FAO fisheries technical paper, no. 395. FAO, Rome, pp 93
Parida M, Sannarangaiah S, Dash PK et al (2008) Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol 18:407–421
Tomita N, Mori Y, Kanda H et al (2008) Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 3:877–882
Notomi T, Okayama H, Masubuchi H et al (2000) Loop mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63
Nagamine K, Watanabe K, Ohtsuka K et al (2001) Loop-mediated isothermal amplification reaction using a nondenatured template. Clin Chem 47:1742–1743
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop mediated isothermal amplification using loop primers. Mol Cell Probes 16:223–229
Soliman H, El-Matbouli M (2006) Reverse transcription loop-mediated isothermal amplification (RT-LAMP) for rapid detection of viral hemorrhagic septicaemia virus (VHS). Vet Microbiol 114:205–213
Soliman H, Midtlyng P, El-Matbouli M (2009) Sensitive and rapid detection of infectious pancreatic necrosis virus by reverse transcription loop mediated isothermal amplification. J Virol Methods 158:77–83
Mori Y, Notomi T (2009) Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 15:62–69
Soliman H, El-Matbouli M (2009) Immunocapture and direct binding loop mediated isothermal amplification simplify molecular diagnosis of Cyprinid herpesvirus-3. J Virol Methods 162:91–95
Mori Y, Nagamine K, Tomita N et al (2001) Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 289:150–154
Soliman H, El-Matbouli M (2010) Loop mediated isothermal amplification combined with nucleic acid lateral flow strip for diagnosis of cyprinid herpes virus-3. Mol Cell Probes 24:38–43
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
Soliman, H., Saleh, M., El-Matbouli, M. (2015). Detection of Fish Pathogens by Loop-Mediated Isothermal Amplification (LAMP) Technique. In: Cunha, M., Inácio, J. (eds) Veterinary Infection Biology: Molecular Diagnostics and High-Throughput Strategies. Methods in Molecular Biology, vol 1247. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-2004-4_12
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2004-4_12
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-2003-7
Online ISBN: 978-1-4939-2004-4
eBook Packages: Springer Protocols