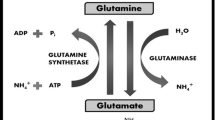
Abstract
Endotoxemia markedly modifies glutamine metabolism in several tissues with a decrease of intestinal glutamine uptake and metabolism, and decreased oxygen consumption. In liver, endotoxemia decreases glutamine content and mitochondrial oxygen consumption while in skeletal muscles, it increases glutamine synthesis and release resulting in decreased muscle glutamine content. In lungs, endotoxemia decreased glutamine uptake with increased glutamine synthesis and release. Mostly from endotoxemic animal model experiments (and much less frequently from clinical studies), it appears that supplementation with glutamine in its free or dipeptidic form moderates the increment of the intestinal permeability and bacterial translocation, decreases intestinal inflammation and increases the intestinal microcirculation. In the lung, glutamine supplementation attenuates inflammation and injury. It thus appears that the the rationale for oral glutamine supplementation is relatively strong, as long as the intestinal amino acid absorptive capacity is preserved.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Key Points
-
Endotoxemia markedly modifies glutamine metabolism in tissues with a decrease of intestinal glutamine uptake and metabolism.
-
In liver, endotoxemia increases glutamine uptake and utilization but decrease glutamine endogenous synthesis.
-
In skeletal muscles, endotoxemia increases synthesis and release of glutamine for other tissues, resulting in decrease of muscle glutamine content.
-
In lungs, endotoxemia results in a decrease of glutamine uptake with increased glutamine endogenous synthesis and release.
-
Supplementation with glutamine allows to mitigate in endotoxemic situation the increment of intestinal permeability and bacterial translocation, and, in intestine and lung, to decrease inflammation and injury.
Abbreviations
- ALI:
-
Acute lung Injury
- ARDS:
-
Adult Respiratory Distress Syndrome
- BCAA:
-
Branched-Chain Amino Acid
- GS:
-
Glutamine Synthetase
- GSH:
-
Glutathione
- HSP:
-
Heat Shock Protein
- IL:
-
Interleukin LPS: Lipopolysaccharide
- MAPK:
-
Mitogen-Activated Protein Kinase
- MPEC:
-
Microvascular Pulmonary Endothelial Cells
- NO:
-
Nitric Oxide
- PAEC:
-
Pulmonary Artery Endothelial Cells
- TLR:
-
Toll-Like Receptor
- TNF:
-
Tumor Necrosis Factor
Introduction
Endotoxemia is defined as the presence of endotoxin in blood and may result from a transfer of a pathological amount of endotoxin (also currently called bacterial lipopolysaccharide LPS) from the intestinal lumen to the bloodstream due to impaired gut selective barrier function. Major endotoxemia can lead to sepsis defined as a medical condition characterized by a whole-body inflammatory state (also called systemic inflammatory response syndrome or SIRS) [1]. Septic shock is another medical condition which results from severe infection and sepsis. Sepsis may begin with a localized infection which can reach the bloodstream. Septic shock can lead to the multiple organ dysfunction syndrome. Patients suffering from septic shock are taken in charge in intensive care units with a mortality rate being as high as 20–50 % [2, 3]. Although the pathogenesis of septic shock is much complicated and not entirely understood, it involves events including the release of cytokines, the activation of macrophages, neutrophils, and microvascular endothelial cells, as well as coagulation disorders and severe metabolic alterations [1, 4]. In critically ill patients, the gastrointestinal tract is believed to play a central role in the pathogenesis of septic shock [5, 6]. Indeed, increased gut permeability and bacterial translocation play an active role in multiple organ failure by inducing a vicious cycle of increased intestinal permeability, leading to increased transfer of luminal compounds to the bloodstream. In this way, the gut can be seen as both an instigator and a victim of post-injury multiple organ failure [5]. This idea was already present in the pioneering work of Deitch et al. [7] showing that intraperitoneal injection of endotoxin to mice was able to promote the translocation of bacteria from the gut.
In this general context, the aim of this chapter is to present an overview on (1) the effect of endotoxemia and sepsis on glutamine metabolism in tissues and (2) the effects of glutamine supplementation in endotoxemia and sepsis.
Endotoxemia and Glutamine Metabolism in Intestine
It is well known that one of the major roles of the small intestine is to absorb the nutrients (including amino acids) from the intestinal lumen to the bloodstream. However, a significant part of luminal amino acids are metabolized by enterocytes in the process of absorption. This is particularly true for several amino acids including glutamine which are very largely metabolized by the enterocytes during their transcellular journey [8]. Glutamine metabolism corresponds to energy supply in a context of rapid renewal of the epithelium (and thus intense ATP-consuming anabolic metabolism) and to sodium extrusion at the basolateral membrane through the action of the Na/K ATPase. As noted previously, glutamine also provides nitrogen for nucleotide synthesis, and is a precursor for several amino acids produced in enterocytes. Lastly, glutamine has been shown to be able to preserve protein synthesis in Caco-2 cells submitted to “luminal fasting.” [9]
Sepsis considerably alters the intestinal barrier functions, which in turn modifies the absorption and bioavailability of nutrients [10]. In septic patients, sodium-dependent glutamine transport is decreased in both jejunum and ileon when compared to control surgical healthy patients [11]. In addition, gut glutamine and oxygen consumption are markedly diminished in these patients [12]. In rats treated by intraperitoneal injection of LPS, transport data indicated decreases in both sodium-dependent jejunal glutamine uptake and glutaminase activity [11–13] suggesting that less glutamine is available for enterocyte metabolism. In the model of sepsis induced by cecal ligation and puncture, the capacity of enterocytes for glutamine oxidation and the intestinal mucosa glutaminase activity are decreased [14, 15] with a concomitant negative nitrogen balance. In contrast, in vivo, LPS treatment increases the activity of small intestine glutamine synthetase (GS) by 250 % [16]. However, experimental works by another group using the cecal ligation and puncture model of sepsis have shown that the effects of sepsis on glutamine transport may be biphasic with an initial increase followed by a decrease [17]. Simultaneously, intestinal extraction of glutamine from the bloodstream fell by 56 %. This latter reduction in the uptake of circulating glutamine cannot be accounted for by a fall in the arterial concentration. Thus, immediately after endotoxemia, brush border glutamine uptake is increased while consumption of glutamine entering across the basolateral membrane is decreased.
Although the mechanisms involved in the decreased glutamine metabolism in enterocytes recovered from septic animals remain unclear, interleukin-1 (IL-1) has been shown to act as a mediator of the alterations in gut glutamine metabolism in endotoxemia and sepsis [18, 19]. Karinch et al. [20] suggested that IGF-1 has a direct effect on the stimulation of the glutamine transport. However, the effect of endotoxemia and sepsis on glutamine metabolism in the intestinal mucosa appears rather unspecific since absorption of several other amino acids (including leucine, proline, glutamate, arginine) are also affected [11, 21–24]. Indeed, following endotoxemia, almost all circulating amino acids (including amino acids like citrulline and ornithine which are not incorporated in alimentary proteins but are derived from amino acid precursors) are markedly decreased suggesting a marked decrease of the intestinal functions [25]. In addition, endotoxemia affects the morphology of the intestinal mucosa [26, 27], and decreases the mucosal oxygen consumption [28, 29], reinforcing the view that endotoxemia affects the intestinal functions. The main alterations of glutamine metabolism in intestine due to endotoxemia are presented in Fig. 10.1.
Glutamine Supplementation and Intestinal Functions in Endotoxemia and Sepsis
Clinical and preclinical studies have suggested that glutamine supplementation may be beneficial to humans with altered intestinal functions. Using a gastrostomy-fed rat infant “pup-in-a-cup” model, the effects of glutamine supplementation on the proinflammatory response induced by LPS given before dietary supplementation via the gastrostomy tube were examined [30]. Using a 3.5 g/kg/day dose of glutamine, a decrease of the expression of the intestinal cytokine-induced neutrophil chemoattractant and a decrease of the myeloperoxidase activity (used as a parameter indicating neutrophil accumulation in intestinal mucosa), as well as a decrease of intestinal and plasma TNF-α concentrations occurred in endotoxemic animals when compared with control (untreated) animals. In a model of infant rats treated with intraperitoneal injection of LPS and receiving at the same time a supplementation of glutamine (2 mmol/g) by the same intraperitoneal way, glutamine supplementation was able to counteract the fast decrease of the circulating glutamine concentration and to partly prevent the increase of circulating TNF-α provoked by the LPS treatment. In rats receiving LPS by the subcutaneous way, supplementation with 2 % glutamine given in the drinking water before and following injection of LPS was able to prevent gut mucosal injury and to improve the mucosal recovery in terms of bowel weight and mucosal weight as well as villus height in the jejunum [31]. Ding and Li [32] found that prophylactic treatment with glutamine could minimize the increment of intestinal permeability and bacterial translocation caused by endotoxemia in rats treated with total parenteral nutrition. Uehara et al. [33] reported that glutamine pretreatment significantly ameliorated intestinal tissue injury and the survival of rats following LPS treatment.
When given before LPS administration, glutamine treatment significantly ameliorated LPS-induced mucosal injury, inflammation, and apoptotic cell death in the ileum and the colon, as judged by significant decreases in TNF-α gene expression and ameliorated histologic damage scores. In adult humans, glutamine pretreatment decreases the production of the proinflammatory cytokines IL-6 and IL-8 by the intestinal mucosa [34, 35]. Accordingly, glutamine deprivation enhanced IL-8 production by Caco-2 colonic human adenocarcinoma cells after LPS stimulation [36, 37]. In human fetal and adult intestinal epithelium, it has been shown that glutamine modulates LPS-induced IL-8 production through IκB/NFκB transduction pathways [38]. Zhou et al. [39] revealed that an oral preventive supply of combined glutamine and arginine before endotoxemia induction significantly decreases TNF-α and IL-1β mRNA abundance in both the jejunum and ileum, while they also significantly decrease anti-inflammatory IL-10 in the ileum. In the model of rats treated intravenously with LPS, supplementation with glutamine (0.75 g/kg) and with l-alanyl-l-glutamine dipeptide (same amount of glutamine than glutamine alone) before and after LPS injection prevented the LPS-induced decrease of the functional capillary density of the intestinal muscular and mucosal layers as well as the number of adherent leukocytes in the submucosal venules, indicating that glutamine supplementation is able to improve the intestinal microcirculation [40]. In the rat model injected with LPS via the intraperitoneal way and supplemented with glutamine in drinking water (2 %), it was reported that glutamine supplementation was able to downregulate the Toll-like receptor TLR-4 as well as the myeloid differentiation factor 88; and to ameliorate the intestinal mucosal injury caused by endotoxemia [41]. In the model of rats receiving LPS via intravenous administration and concomitantly glutamine at the dose of 0.75 g/kg by the same way, it was found that glutamine supplementation can efficiently attenuate the increase in circulating proinflammatory cytokines and protect against organ damage as assessed by histological examination, and decrease mortality from endotoxemia [42].
In rats receiving continuous intravenous injection of LPS, glutamine supplementation at a dose of 2 % was able to ameliorate the nitrogen balance and to increase the intestinal mucosal glutaminase activity [43]. In addition, the morphological aspect of the jejunal mucosa was ameliorated in glutamine-supplemented animals as judged from the villus height, crypt depth and wall thickness. Higashiguchi et al. [44] studied the effect of sepsis and the regulation by glutamine of protein synthesis in enterocytes isolated from the small intestine of rats. Sixteen hours after endotoxemia induction, protein synthesis was increased by 65 %, 89 %, and 137 %, respectively, in enterocytes from the tips and mid-portions of the villi and from the crypts. Addition of glutamine to incubated enterocytes stimulates protein synthesis in a dose-dependent manner. In the study by Okuma et al. [45], the authors induced sepsis in rats by continuous intraperitoneal administration of endotoxin, and supplemented the animals with 2 % l-alanyl-l-glutamine by total parenteral nutrition. Under such experimental conditions, they found that the supplementation with the dipeptide did not ameliorate neither the nitrogen balance nor bacterial translocation from the gut to the mesenteric lymph nodes. However, the supplementation with the dipeptide containing glutamine was able to increase the intestinal mucosal weight, and the villus height. In a study aiming at evaluating the effect of a supplementation with glutamine (0.75 g/kg, in its free form or in the form of the dipeptide l-alanyl-l-glutamine) on the microcirculation in the endotoxemic rat model, Sheibe et al. [46] found that glutamine supplementation before LPS challenge was able to reduce leukocyte adherence and mesenteric plasma extravasation, indicating that glutamine diminish the detrimental impact of endotoxemia on the mesenteric microcirculation. In endotoxemic rats treated with intraperitoneal injection of LPS followed by food deprivation for 24 h and then receiving glutamate supplementation (4 g/kg/d), glutamine concentration was increased in the jejunum and the intestinal villus height was higher in the jejunum of supplemented animals when compared to control [26].
In the early weaning piglet model, supplementation with glycyl-glutamine dipeptide (0.15 %) in LPS-treated animals is able to partly alleviate the suppression of growth and immune functions [47]. In the study by Dugan and McBurney [48], a segment of distal ileum was isolated from anesthetized piglets and perfused without or with 2 % glutamine before treatment with endotoxin. By using such an experimental design, the authors found that endotoxin-induced permeability changes (as measured using the plasma-to-lumen clearances of Cr-EDTA) can be partly prevented by supplying luminal glutamine. In the study of Haynes et al. [49], enterocytes from neonatal pigs were cultured in the presence of LPS with or without millimolar concentrations of glutamine in its free form or as the dipeptide l-alanyl-l-glutamine. The results obtained indicate that glutamine is able to reduce the LPS-induced cell death. In the same study, the authors gave an oral supplementation of glutamine or l-alanyl-l-glutamine to 7-day-old piglets before intraperitoneal administration of LPS, and found that the dietary supplementation with glutamine is able to ameliorate the LPS-induced intestinal injury, to enhance growth performance, and to reduce the intestinal expression of the LPS Toll-like receptor-4, of the active caspase 3, and of the transcription factor NF-κB. The main effects of glutamine supplementation on intestinal functions in endotoxemia are presented in Fig. 10.2.
Endotoxemia and Glutamine Metabolism in Liver
Endotoxin treatment in rats results in a tenfold increase of hepatic glutamine uptake owing to both an increase in hepatic blood flow and in glutamine extraction from the bloodstream [18]. This marked increase in metabolic activity is concomitant with signs of hepatocellular injury. Thus in this experimental model, the liver becomes the major organ for glutamine consumption [18]. In the study by Pacitti et al. [50], hepatic glutamine consumption is accordingly increased 11-fold after LPS-induced endotoxemia in rats. Glutamine uptake by the liver is markedly accelerated during endotoxemia, and this is due to an increase in hepatocyte plasma membrane transport activity. System N transporters in hepatocytes play a major role in hepatic glutamine transport. Studies in endotoxemic rodents have shown net hepatic glutamine uptake to be markedly increased, a result which is due partially to a three- to fourfold increase in hepatocyte transport System N activity [20]. LPS administration resulted in a time- and dose-dependent two- to threefold increase in Na+-dependent glutamine transport activity [51, 52] secondary to an increase in the transport maximal velocity (V max), consistent with the appearance of an increased numbers of corresponding transporter proteins in the hepatocyte plasma membrane vesicles. Maximal increases in transport was observed 4 h after exposure to endotoxin. This increase in transport activity represents an important mechanism regulating the accelerated hepatic glutamine uptake that occurs during severe infection. Wang et al. [53] investigated the changes of hepatic transporters in early endotoxemic rats provoked by intraperitoneal injection of LPS. They showed that both the mRNA and protein expression of SNAT3 and SNAT5 are enhanced by LPS in a time- and dose-dependent manner. Fischer et al. [54] showed that LPS treatment administered intraperitoneally in fed and fasted (48 h) rats increased transport activity 2.6- and 6-fold, respectively. This effect in endotoxemic starved rats is mediated by both an increase in System N V max and the induction of a high affinity System A amino acid carrier which transports glutamine. In this latter study, starvation and endotoxemia appear to regulate hepatocyte glutamine transport independently and synergistically. The authors propose that the hepatic response allows to provide glutamine to support key metabolic pathways in the liver during critical illness. However, the effect of endotoxemia on glutamine transport is not restricted to glutamine since rats treated with a single injection of endotoxin are characterized by a stimulation of arginine transport [55]. Vejchapipat et al. [56] showed that early stages of endotoxemia induced by LPS injection significantly decrease neonatal hepatic level of glutamine. The increase in liver glutamine utilization is associated with increases in parenchymal DNA and glutathione levels, as well as glutathione and urea release into the systemic circulation. This accelerated utilization provides carbons for energy and gluconeogenesis, and substrate for nucleotide and glutathione biosynthesis in order to support cell repair [18].
Glutamine synthetase (GS) in the liver is restricted to a small perivenous hepatocyte population and plays an important role in the scavenging of ammonia that has escaped the periportal urea-synthesizing compartment. Görg et al. [57] showed that LPS single intraperitoneal injection impairs hepatic ammonia detoxification by both downregulation of GS and its inactivation through tyrosine nitration. GS protein expression 24 h after LPS injection was decreased by approximately 20 %, whereas the corresponding enzymatic activity was lowered by 40–50 %. In line with GS inactivation, glutamine synthesis from ammonia in perfused livers obtained from LPS-treated rats is decreased by approximately 50 %. The resulting defect of the perivenous scavenger cell function with regard to ammonia elimination may partly contribute to sepsis-induced development of hyperammonemia. In rats treated with LPS, glutaminase activity and flux through glutaminase in intact mitochondria are markedly increased by the endotoxin treatment. The effect is associated with an increase in the sensitivity of glutaminase flux to the enzyme activator phosphate [58]. In the liver, glutamine plays an important role in ammonia detoxication and the regulation of pH homeostasis (“intercellular glutamine-glutamate cycle”). In addition, glutamine regulates liver metabolism by mechanisms that cannot be attributed to its metabolism. Examples include the stimulation of protein and glycogen synthesis, bile acid secretion, and inhibition of proteolysis in liver. A major trigger for such effects is an increased hepatocyte hydration due to the cumulative uptake of glutamine into the cells, which activates osmosignaling pathways involving mitogen-activated protein kinases (MAPK). Glutamine- and hypoosmolarity-induced cell swelling activates extracellular signal-regulated kinases (ERK) and p38 (MAPK). Also, the antiproteolytic effect of glutamine is largely due to glutamine-induced cell swelling, which activates osmosignaling pathways. The glutamine-induced p38 (MAPK) activation mediates the inhibition of autophagic proteolysis at the level of autophagosome formation [59].
Surgical neonates are at risk of sepsis with associated liver dysfunction. Hydrogen peroxide (H2O2) and nitric oxide (NO) are important mediators of sepsis, which impair neonatal hepatic metabolism. Glutamine by itself or in the form of dipeptides, have been shown to have beneficial effects on the altered hepatocyte metabolism and liver damage during neonatal sepsis. The study from Babu et al. [60] found that glutamine and its dipeptides are indeed efficient in reversing the effects of septic mediators on hepatic oxidative metabolism in neonatal rats.
Markley et al. [61] determined the effects of glutamine on hepatocyte energy metabolism under conditions of neonatal endotoxemia, and concluded that glutamine reverses the inhibition of mitochondrial metabolism observed in endotoxemia, which is primarily at the level of ATP synthesis. The addition of glutamine to hepatocytes from endotoxemic rats restored intramitochondrial oxygen consumption to control levels. Although glutamine did not reverse the inhibition of the thermogenic proton leak observed in endotoxemia, it significantly increased oxygen consumption due to mitochondrial ATP synthesis. Glutamine significantly increases the hepatocyte ATP/ADP ratio compared with hepatocytes from endotoxemic rats. Electron microscopy reveals morphological damage to the mitochondria of hepatocytes from endotoxemic rats, and evidences a return to a normal appearance with the addition of glutamine. Intramitochondrial O2 consumption is inhibited in isolated hepatocytes from suckling septic rats and this impairment can be reversed by glutamine. Kim et al. [62] showed that glutamine significantly increased fatty acid oxidation in hepatocytes from control and endotoxemic animals, suggesting that it may promote substrate oxidation during endotoxemia. In the pig model, after endotoxin-induced sepsis, the net protein synthesis is rapidly increased in the liver [63]. In the endotoxemic dog model, the net hepatic glucose output is increased approximately twofold [64], with an increase in the net hepatic glycogenolysis accounting for the majority of the increased hepatic glucose production. The main alterations of glutamine metabolism in liver due to endotoxemia is presented in Fig. 10.3.
Endotoxemia and Glutamine Metabolism in Skeletal Muscle
Skeletal muscle, the major repository of glutamine, plays a crucial role in maintaining nitrogen homeostasis during health and critical illness by synthesizing and exporting glutamine. Glutamine is both synthesized and degraded in skeletal muscle, and the balance of this intracellular cycle determines the net synthesis and release of glutamine from the tissue [65]. Skeletal muscles exhibit a twofold increase in glutamine release during infection, which is associated with a significant increase in endogenous glutamine biosynthesis. Skeletal muscle glutaminase activity is unchanged by endotoxemia, but expression of glutamine synthetase (GS) mRNA and specific activity increase in a time-dependent fashion. Despite an increase in GS activity in skeletal muscle, the intracellular glutamine pool becomes depleted, indicating that release rates likely exceed synthesis rates [20]. As a consequence, the muscle glutamine concentration fell in the endotoxin-treated animals by 25–40 %, an event that is apparent as early as 2 h after endotoxin treatment [66] and remains still visible 2 days after LPS injection [25]. This glutamine depletion is caused by accelerated muscle glutamine release [67] rather than by an increase in intracellular degradation or a fall in intracellular biosynthesis. The adaptive increase in GS expression requires de novo RNA and protein synthesis and may be designed to prevent complete depletion of the intracellular glutamine pool [66]. Simultaneously, the circulating pool of glutamine does not increase, suggesting accelerated uptake by other organs [20].
Ardawi and Majzoub [15] found that there is an enhanced rate of production of glutamine from skeletal muscle of septic rats in the cecal ligation and puncture model, which may be due to changes in efflux and/or increased intracellular formation of glutamine. Sepsis results in decreases of the concentrations of skeletal muscle glutamine, glutamate, 2-oxoglutarate, and adenosine monophosphate. Hindlimb blood flow showed no marked change in response to sepsis, but was accompanied by an enhanced net release of glutamine and alanine. The maximal activity of GS was increased only in quadriceps muscles of septic rats, whereas that of glutaminase was decreased in all muscles studied.
The expression of GS is induced in rat skeletal muscle cells (L-6) in response to treatment with the inflammatory cytokine TNF-α. Chakrabarti [68] demonstrated that the rat GS gene is transcriptionally regulated by TNF-α and identifies a TNF-α-responsive region at the 5′ flanking sequence of the GS gene. Lukaszewicz et al. [69] reported that induction of GS expression in skeletal muscle after endotoxin administration is adrenal gland dependent. Treatment of normal rats with LPS results in a marked increase in GS mRNA that appears dose and time dependent, and precedes the increase in GS protein. The increase in muscle GS mRNA observed in normal rats in response to LPS is abrogated in adrenalectomized rats at 3 h after treatment with a high dose of LPS. These and other studies implicate glucocorticoid hormones as a key, but not exclusive, regulator of GS expression in skeletal muscle after a catabolic insult.
Suojaranta-Ylinen et al. [70] reported that glutamine cannot prevent endotoxemia during or after cardiac surgery. However, Meador and Huey [71] suggested that glutamine supplementation provides an effective, novel, clinically applicable means of preserving muscle force during acute inflammation. LPS treatment is associated with a 33 % reduction in maximal plantarflexor isometric force and elevated serum TNF-α and IL-6. Glutamine completely prevents this LPS-induced force decrement and reduces muscle heat shock protein HSP-70 and IL-6. The main alterations of glutamine metabolism in skeletal muscles in endotoxemia are presented in Fig. 10.4.
Endotoxemia and Glutamine Metabolism in Lung
Severe infection alters lung glutamine metabolism. Insufficient glutamine for the lungs during sepsis may contribute to an impairment of lung function. Lung glutamine metabolism is supported by both blood glutamine uptake and de novo biosynthesis using circulating BCAA and glutamate as precursors. Pan et al. [72] isolated lung plasma membrane vesicles from control and LPS-treated rats and assayed glutamine transport activity in these vesicles. They showed that 80 % of glutamine uptake in lung vesicles is mediated via the high affinity Na+-dependent carrier system ASC, while 19 % occurred via the Na+-independent System ASC. Treatment of rats with LPS resulted in a decrease in System ASC and XAG- activity in lung plasma membrane vesicles, which may contribute to reduced glutamate uptake, reduced lung glutamine availability, and impaired cellular metabolism and function during septic states.
During sepsis, the lung responds by exporting increased amounts of glutamine. This response is accompanied by increased enzymatic activity of GS. Austgen et al. [73] studied the alterations in lung glutamine metabolism that occurs in the endotoxin-injured lung in rats and subsequently correlated with flux changes that occur in patients with the adult respiratory distress syndrome (ARDS). In healthy control rats, net amounts of glutamine are released by the lungs into the systemic circulation. The rate of glutamine release from the lung doubled 30 min after intravenous endotoxin. This accelerated fractional release of glutamine by the lungs is no longer detected 2 h after endotoxin treatment. By the 12-h time point, a more than twofold increase in GS activity is recorded. Simultaneously, lung weights are increased by 21 % and histologic examination shows an interstitial infiltrate and pulmonary edema. Similar observations were made in patients with “early” sepsis which exhibited a marked increase in lung glutamine release. During septic states, efflux of glutamine from the lung increases, a response thus sustained by an increase in GS activity. Abcouwer et al. [74] have used rat epithelial cell line of pulmonary origin (L2 cells) to study the effect of several hormones and cytokines (which mediate the septic shock response) on GS expression. They found that GS expression, as determined by measurement of mRNA and protein contents, increases rapidly and severalfold in response to physiologically relevant levels of the synthetic glucocorticoid dexamethasone (Dex). In contrast, GS expression is not markedly induced by LPS, cytokines, activated complement C5a, or prostaglandins. The results of this study are consistent with a regulation of lung GS expression via a direct glucocorticoid receptor-mediated response. In addition, GS mRNA decay in L2 cells seems to be regulated by two independent mechanisms, one being sensitive to the protein synthesis inhibitor cycloheximide, and the other being sensitive to actinomycin D. It is also known that GS expression in the rat lung can be induced by glucocorticoid hormones. Lukaszewicz et al. [75] characterized the induction of GS expression during LPS-induced endotoxemia in normal, neutropenic, and adrenalectomized rats, and showed that GS gene induction during sepsis is only partially mediated by adrenal-derived glucocorticoid hormones. Normal rats exhibited a time- and dose-dependent induction of GS mRNA levels after a single intraperitoneal dose of LPS. In response to endotoxemia, GS mRNA levels in the lung of adrenalectomized rats increased twofold compared with sixfold in sham-operated control rats.
Herskowitz et al. [76] incubated pulmonary artery endothelial cells (PAECs) with endotoxin (1 μg/ml) and showed a significant increase in System ASC-mediated glutamine transport which was maximal after 12 h of exposure. Kinetic studies indicated that the increase in carrier-mediated activity was not due to a change in K m, but rather to a 73 % increase in V max. The increase in glutamine uptake by PAECs was completely blocked by actinomycin D and cycloheximide, indicating that the accelerated glutamine transport was most probably due to an increase in transporter synthesis. During septic states, the lungs produce increased amounts of glutamine, an event that is likely mediated by both LPS and glucocorticoid hormones and is presumed to be due to accelerated intracellular glutamine biosynthesis. Because enhanced net glutamine release in vivo could also be due to a decrease in cellular uptake, Pan et al. [77] determined glutamine transport in cultured rat microvascular pulmonary endothelial cells (MPECs), and showed that dexamethasone (0.1 μmol/L) and LPS (1 μg/mL) inhibited glutamine uptake by decreasing the V max of system ASC transporter (which mediates more than 90 % of glutamine transport) in a time- and dose-dependent manner. There was no synergistic or additive effect when both compounds were added together.
Acute lung injury (ALI) is a critical syndrome associated with respiratory dysfunction. It has been reported that glutamine can attenuate ALI after sepsis. Nakamura et al. [78] showed that glutamine added to the solution for total parenteral nutrition improved endotoxin-induced acute lung injury in rats. The survival rate and the nitrogen balance were significantly improved at 48 h after endotoxin administration as a result of glutamine treatment; the arterial oxygen partial pressure being significantly increased.
Neutrophils are considered to be central for the pathogenesis of ALI. It is noteworthy that Zhang et al. [79] indicated that glutamine could prevent neutrophil recruitment and infiltration, protect the alveolar barrier, and attenuate inflammatory injury during sepsis. These results may be related to enhanced glutathione (GSH) synthesis. Indeed, glutamine supplementation reduced the total protein concentration and total cell and neutrophil counts in bronchoalveolar lavage fluid after LPS challenge. Glutamine enhanced GSH synthesis and attenuated IL-8 release and myeloperoxidase activity in lung tissues. Glutamine also decreased CD11b expression in blood neutrophils and prevented lung histologic changes. L-buthionine-(S,R)-sulfoximine (BSO, a blocker of GSH synthesis) abolished the effects of glutamine and attenuated its protection on ALI.
Septic shock leads to alterations of cellular metabolism. Glutamine can enhance lung HSP70 expression which can preserve cellular metabolism after lethal endotoxemia and other forms of cellular stress. Singleton et al. [80] reported that a single dose of glutamine can enhance HSP70 in pulmonary epithelial cells and macrophages, and attenuate lung metabolic dysfunction by improving the ratio of adenosine triphosphate to adenosine diphosphate in the lung after sublethal endotoxemia. This beneficial effect on lung tissue may be mediated in part by enhanced expression of HSP70. Furthermore, Zhang et al. [81] investigated the role of GSH synthesis in the regulation on nuclear factor NF-κ activity and TNF-α released by glutamine in LPS-stimulated alveolar type II (AT-II) epithelial cells of rat lungs, and showed that glutamine can prevent the NF-kappaB activation and attenuate the release of TNF-α in LPS-stimulated AT-II cells, an effect that may be mediated via GSH synthesis.
Lung epithelial cells are important barriers in the respiratory system that can prevent pathogens from invading the body after nuclear factor NF-κB activation. LPS is a common pathogen-associated stimulus that activates IκB kinase (IKK) to regulate NF-κB-mediated inflammation through modulating nuclear translocation and phosphorylation of NF-κB. Previously, it has been shown that Akt and mTOR are involved in the phosphorylation of IKK to activate NF-κB. Hou et al. [82] demonstrated that glutamine modulated LPS-induced activation of NF-κB through the Akt/mTOR/IKK pathway in BEAS-2B cells. Glutamine deprivation induces phosphorylation of Akt/mTOR/IKK signaling, increases the level of NF-κB nuclear translocation and phosphorylated NF-κB, and upregulates NF-κB-dependent transcriptional activity, which is suppressed by glutamine administration. These findings provide potential mechanisms for the modulation by glutamine of LPS-induced NF-κB activation in lung epithelial cells and strongly suggest that maintaining a physiological concentration of glutamine is essential in preventing LPS-induced lung inflammation. The main alterations of glutamine metabolism in lung due to endotoxemia is presented in Fig. 10.5; and the main effects of glutamine supplementation on lung functions in endotoxemia are summarized in Fig. 10.6.
Conclusions
Major endotoxemia can lead to sepsis, to septic shock and to multiple organ dysfunction syndrome. From experiments with animal models of endotoxemia, and (much less frequently) from clinical studies, it has been shown that endotoxemia markedly modifies glutamine metabolism in tissues with a decrease of intestinal glutamine uptake and metabolism, and decrease of oxygen consumption. However, this decrease is not specific for glutamine since numerous other amino acids are less absorbed in endotoxemia as a result of decreased intestinal functions. In liver, endotoxemia increases glutamine uptake and utilization while diminishing the glutamine synthetase activity, coinciding with decreased hepatic glutamine content and decreased mitochondrial oxygen consumption. In skeletal muscles, endotoxemia increases endogenous glutamine synthesis and release resulting in a decrease of the muscle glutamine content. In lungs, endotoxemia results in a decrease of glutamine uptake with increased glutamine endogenous synthesis and release. Supplementation with glutamine in its free form or in its dipeptide form allows to moderate in endotoxemic situation the increment of intestinal permeability and bacterial translocation, to decrease intestinal inflammation (and more generally of gut mucosal injury), and to increase the intestinal microcirculation and intestinal protein synthesis. In the lung, glutamine supplementation attenuates pulmonary inflammation and injury.
Mostly from experimental works with animal models, it thus appears that the rationale for glutamine supplementation in endotoxemia by the enteral way appears thus relatively strong, as long as a sufficient intestinal capacity for the absorption of this amino acid is preserved. Future works in experimental and clinical studies should consider, in endotoxemic/septic situation, supplementation with glutamine together with other conditionally essential amino acids to help in the nutritional management of this pathology.
References
Tsiotou AG, Sakorafas GH, Anagnostopoulos G, et al. Septic shock: current pathogenetic concepts from a clinical perspective. Med Sci Monit. 2005;11:RA76–85.
Nardi O, Polito A, Aboab J, et al. StO(2) guided early resuscitation in subjects with severe sepsis or septic shock: a pilot randomized trial. J Clin Monit Comput. 2013;27(3):215–21.
Venkatesh AK, Avula U, Bartimus H, et al. Time to antibiotics for septic shock: evaluating a proposed performance measure. Am J Emerg Med. 2013;31(4):680–3.
Pulido D, Nogues MV, Boix E, et al. Lipopolysaccharide neutralization by antimicrobial peptides: a gambit in the innate host defense strategy. J Innate Immun. 2012;4:327–36.
Hassoun HT, Kone BC, Mercer DW, et al. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10.
Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411–7.
Deitch EA, Berg R, Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987;122:185–90.
Blachier F, Boutry C, Bos C, et al. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr. 2009;90:814S–21.
Le Bacquer O, Laboisee C, Darmaun D. Glutamine preserves protein synthesis and paracellular permeability in Caco-2 cells submitted to « luminal fasting ». Am J Physiol. 2003;285:G128–36.
Elwafi F, Curis E, Zerrouk N, et al. Endotoxemia affects citrulline, arginine and glutamine bioavailability. Eur J Clin Invest. 2012;42:282–9.
Salloum RM, Copeland EM, Souba WW. Brush border transport of glutamine and other substrates during sepsis and endotoxemia. Ann Surg. 1991;213:401–9.
Souba WW, Herskowitz K, Klimberg VS. The effects of sepsis and endotoxemia on gut glutamine metabolism. Ann Surg. 1990;211:543–51.
Haque SM, Chen K, Usui N, et al. Effects of endotoxin on intestinal hemodynamics, glutamine metabolism, and function. Surg Today. 1997;27:500–5.
Ardawi MS, Jamal YS, Ashy AA, et al. Glucose and glutamine metabolism in the small intestine of septic rats. J Lab Clin Med. 1990;115:660–8.
Ardawi MS, Majzoub MF. Glutamine metabolism in skeletal muscle of septic rats. Metabolism. 1991;40:155–64.
Sarantos P, Abouhamze A, Chakrabarti R, et al. Glucocorticoids regulate intestinal glutamine synthetase gene expression in endotoxemia. Arch Surg. 1994;129:59–65.
Niu L, Qiao W, Li G, et al. Different alterations in rat intestinal glutamine transport during the progression of CLP- and LPS-induced sepsis. J Surg Res. 2011;169:284–91.
Austgen TR, Chen MK, Flynn TC, et al. The effects of endotoxin on the splanchnic metabolism of glutamine and related substrates. J Trauma. 1991;31:742–51.
Mester M, Tompkins RG, Gelfand JA, et al. Intestinal production of interleukin-1 alpha during endotoxemai in the mouse. J Surg Res. 1993;54:584–91.
Karinch AM, Pan M, Lin CM. Glutamine metabolism in sepsis and infection. J Nutr. 2001;131:2535S–8.
Abad B, Mesonero JE, Salvador MT, et al. Effect of lipopolysaccharide on small intestinal L-leucine transport in rabbit. Dig Dis Sci. 2001;46:1113–9.
Gardiner KR, Gardiner RE, Barbul A. Reduced intestinal absorption of arginine during sepsis. Crit Care Med. 1995;23:1227–32.
Gardiner KR, Ahrendt GM, Gardiner RE, et al. Failure of intestinal amino acid absorptive mechanisms in sepsis. J Am Coll Surg. 1995;181:431–6.
Gardiner K, Barbul A. The role of the imino transporter protein in sepsis-impaired intestinal proline absorption. JPEN J Parenter Enteral Nutr. 1993;17:507–12.
Boutry C, Matsumoto H, Bos C, et al. Decreased glutamate, glutamine and citrulline concentrations in plasma and muscle in endotoxemia cannot be reversed by glutamate or glutamine supplementation: a primary intestinal defect? Amino Acids. 2012;43:1485–98.
Chambon-Savanovitch C, Farges MC, Raul F, et al. Can a glutamate-enriched diet counteract glutamine depletion in endotoxemic rats? J Nutr Biochem. 1999;10:331–7.
Crouser ED, Julian MW, Weinstein DM, et al. Endotoxin-induced ileal mucosal injury and nitric oxide dysregulation are temporally dissociated. Am J Respir Crit Care Med. 2000;161:1705–12.
King CJ, Tytgat S, Delude RL, et al. Ileal mucosal oxygen consumption is decreased in endotoxemic rats but is restored toward normal by treatment with aminoguanidine. Crit Care Med. 1999;27:2518–24.
Crouser ED, Julian MW, Dorinski PM. Ileal VO(2)-O(2) alterations induced by endotoxin correlate with severity of mitochondrial injury. Am J Respir Crit Care Med. 1999;160:1347–53.
Li N, Liboni K, Fang MZ, et al. Glutamine decreases lipopolysaccharide-induced intestinal inflammation in infant rats. Am J Physiol. 2004;286:G914–21.
Sukhotnik I, Agam M, Shamir R, et al. Oral glutamine prevents gut mucosal injury and improves mucosal recovery following lipopolysaccharide endotoxemia in a rat. J Surg Res. 2007;143:379–84.
Ding LA, Li JS. Effects of glutamine on intestinal permeability and bacterial translocation in TPN-rats with endotoxemia. World J Gastroenterol. 2003;9:1327–32.
Uehara K, Takahashi T, Fujii H, et al. The lower intestinal tract-specific induction of heme oxygenase-1 by glutamine protects against endotoxemic intestinal injury. Crit Care Med. 2005;33:381–90.
Coeffier M, Miralles-Barrachina O, Le Pessot F, et al. Influence of glutamine on cytokine production by human gut in vitro. Cytokine. 2001;13:148–54.
Coeffier M, Marion R, Leplingard A, et al. Glutamine decreased interleukin-8 and interleukin-6 but not nitric oxide and prostaglandins E2 production by human gut in vitro. Cytokine. 2002;18:92–7.
Huang Y, Li N, Liboni K, et al. Glutamine decreases lipopolysaccharide-induced IL-8 production in Caco-2 cells through a non-NFκB p50 mechanisms. Cytokine. 2003;22:77–83.
Liboni KC, Li N, Neu J. Mechanism of glutamine-mediated amelioration of lipopolysaccharide- induced IL-8 production in Caco-2 cells. Cytokine. 2004;26:57–65.
Liboni KC, Li N, Scumpia PO, et al. Glutamine modulates LPS-induced IL-8 production through IκB/NF-κB in human fetal and adult intestinal epithelium. J Nutr. 2005;135:245–51.
Zhou X, Wu X, Yin Y, et al. Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids. 2012;43:813–21.
Lehmann C, Pavlovic D, Zhou J, et al. Intravenous free and dipeptide-bound glutamine maintains intestinal microcirculation in experimental endotoxemia. Nutrition. 2012;28:588–93.
Kessel A, Toubi E, Pavlotzky E, et al. Treatment with glutamine is associated with down-regulation of Toll-like receptor-4 and myeloid differentiation factor 88 expression and decrease in intestinal mucosal injury caused by lipopolysaccharide endotoxaemia in a rat. Clin Exp Immunol. 2008;151:341–7.
Wischmeyer PE, Kahana M, Wolfson R, et al. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol. 2001;90:2403–10.
Chen K, Okuma T, Okamura K, et al. Glutamine-supplemented parenteral nutrition improves gut mucosa integrity and function in endotoxemic rats. JPEN J Parenter Enteral Nutr. 1994;18:167–71.
Higashiguchi T, Noguchi Y, Meyer T, et al. Protein synthesis in isolated enterocytes from septic or endotoxaemic rats: regulation by glutamine. Clin Sci (Lond). 1995;89:311–9.
Okuma T, Kaneko H, Chen K, et al. Total parenteral nutrition supplemented with L-alanyl-L-glutamine and gut structure and protein metabolism in septic rats. Nutrition. 1994;10:241–5.
Scheibe R, Schade M, Grundling M. Glutamine and alanyl-glutamine dipeptide reduce mesenteric plasma extravasation, leukocyte adhesion and tumor necrosis factor-α (TNF-α) release during experimental endotoxemia. J Physiol Pharmacol. 2009;60:S19–24.
Jiang ZY, Sun LH, Lin YC. Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J Anim Sci. 2009;87:4050–6.
Dugan ME, McBurney MI. Luminal glutamine perfusion alters endotoxin-related changes in ileal permeability of the piglet. JPEN J Parenter Enteral Nutr. 1995;19:83–7.
Haynes TE, Li P, Li XL. L-Glutamine or L-alanyl-L-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids. 2009;37:131–42.
Pacitti AJ, Austgen TR, Souba WW. Mechanisms of increased hepatic glutamine uptake in the endotoxin-treated rat. J Surg Res. 1992;53:298–305.
Inoue Y, Pacitti AJ, Souba WW. Endotoxin increases hepatic glutamine transport activity. J Surg Res. 1993;54:393–400.
Plumley DA, Watkins K, Bode BP, et al. Cyclo-oxygenase blockade abrogates the endotoxin-induced increase in Na(+)-dependent hepatic amino acid transport. JPEN J Parenter Enteral Nutr. 1995;19:9–14.
Wang W, Li Y, Zhang W, et al. Changes of plasma glutamine concentration and hepatocyte membrane system N transporters expression in early endotoxemia. J Surg Res. 2011;166:290–7.
Fischer CP, Bode BP, Souba WW. Starvation and endotoxin act independently and synergistically to coordinate hepatic glutamine transport. J Trauma. 1996;40:688–93.
Inoue Y, Bode BP, Souba WW. Hepatic Na(+)-independent amino acid transport in endotoxemic rats: evidence for selective stimulation of arginine transport. Shock. 1994;2:164–72.
Vejchapipat P, Eaton S, Fukumoto K, et al. Hepatic glutamine metabolism during endotoxemia in neonatal rats. Nutrition. 2002;18:293–7.
Görg B, Wettstein M, Metzger S, et al. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065–73.
Ewart HS, Qian D, Brosnan JT. Activation of hepatic glutaminase in the endotoxin-treated rat. J Surg Res. 1995;59:245–9.
Häussinger D, Graf D, Weiergräber OH. Glutamine and cell signaling in liver. J Nutr. 2001;131:S2509–14.
Babu R, Eaton S, Drake DP, et al. Glutamine and glutathione counteract the inhibitory effects of mediators of sepsis in neonatal hepatocytes. J Pediatr Surg. 2001;36:282–6.
Markley MA, Pierro A, Eaton S. Hepatocyte mitochondrial metabolism is inhibited in neonatal rat endotoxaemia: effects of glutamine. Clin Sci. 2002;102:337–44.
Kim SC, Pierro A, Zamparelli M, Spitz L, Eaton S. Fatty acid oxidation in neonatal hepatocytes: effects of sepsis and glutamine. Nutrition. 2002;18:298–300.
Bruins MJ, Soeters PB, Deutz NE. Endotoxemia affects organ protein metabolism differently during prolonged feeding in pigs. J Nutr. 2000;130:3003–13.
Meinz H, Lacy DB, Ejiofor J, et al. Alterations in hepatic gluconeogenic amino acid uptake and gluconeogenesis in the endotoxin treated conscious dog. Shock. 1998;9:296–303.
Wu G, Thompson JR, Baracos VE. Glutamine metabolism in skeletal muscle from the broiler chick (Gallus domesticus) and the laboratory rat (Rattus norvegicus). Biochem J. 1991;274:769–74.
Austgen TR, Chakrabarti R, Chen MK, et al. Adaptive regulation in skeletal muscle glutamine metabolism in endotoxin-treated rats. J Trauma. 1992;32:600–6.
Vesali RF, Klaude M, Rooyackers O, et al. Amino acid metabolism in led muscle after an endotoxin injection in healthy volunteers. Am J Physiol. 2005;288:360–4.
Chakrabarti R. Transcriptional regulation of the rat glutamine synthetase gene by tumor necrosis factor-alpha. Eur J Biochem. 1998;254:70–4.
Lukaszewicz GC, Souba WW, Abcouwer SF. Induction of muscle glutamine synthetase gene expression during endotoxemia is adrenal gland dependent. Shock. 1997;332–338.
Suojaranta-Ylinen R, Ruokonen E, Pulkki K. Preoperative glutamine loading does not prevent endotoxemia in cardiac surgery. Acta Anaesthesiol Scand. 1997;41:385–91.
Meador BM, Huey KA. Glutamine preserves skeletal muscle force during an inflammatory insult. Muscle Nerve. 2009;40:1000–7.
Pan M, Fischer CP, Wasa M, et al. Characterization of glutamine and glutamate transport in rat lung plasma membrane vesicles. J Surg Res. 1997;69:418–24.
Austgen TR, Chen MK, Salloum RM, et al. Glutamine metabolism by the endotoxin- injured lung. J Trauma. 1991;31:1068–74.
Abcouwer SF, Lukaszewicz GC, Souba WW. Glucocorticoids regulate glutamine synthetase expression in lung epithelial cells. Am J Physiol. 1996;270:L141–51.
Lukaszewicz GC, Abcouwer SF, Labow BI, et al. Glutamine synthetase gene expression in the lungs of endotoxin-treated and adrenalectomized rats. Am J Physiol. 1997;273:L1182–90.
Herskowitz K, Bode BP, Block ER, et al. The effects of endotoxin on glutamine transport by pulmonary artery endothelial cells. J Surg Res. 1991;50:356–61.
Pan M, Wasa M, Ryan U, et al. Inhibition of pulmonary microvascular endothelial glutamine transport by glucocorticoids and endotoxin. JPEN J Parenter Enteral Nutr. 1995;19:477–81.
Nakamura T, Yamakawa M, Maeda J, et al. Effect of glutamine on acute lung injury in rats with endotoxemia. Clin Nutr. 1997;16:79–83.
Zhang F, Wang X, Pan L, et al. Glutamine attenuates lipopolysaccharide-induced acute lung injury. Nutrition. 2009;25:692–8.
Singleton KD, Serkova N, Banerjee A, et al. Glutamine attenuates endotoxin-induced lung metabolic dysfunction: potential role of enhanced heat shock protein 70. Nutrition. 2005;21:214–23.
Zhang F, Wang X, Wang W, et al. Glutamine reduces TNF-alpha by enhancing glutathione synthesis in lipopolysaccharide-stimulated alveolar epithelial cells of rats. Inflammation. 2008;31:344–50.
Hou YC, Chiu WC, Yeh CL, et al. Glutamine modulates lipopolysaccharide-induced activation of NF-κB via the Akt/mTOR pathway in lung epithelial cells. Am J Physiol. 2012;302:L174–83.
Acknowledgments
The authors wish to warmly thank INRA, AgroParisTech and the National Natural Science Foundation of China (#31110103909), the China Basic Research Program (#2013CB127301), the National Science and Technology Support Program Funding (#2012BAD39B03) and the University of Sao Paulo (Brazil, FAPESP grant 2012/07319-0) for their support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Blachier, F. et al. (2015). Endotoxemia and Glutamine. In: Rajendram, R., Preedy, V., Patel, V. (eds) Glutamine in Clinical Nutrition. Nutrition and Health. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1932-1_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1932-1_10
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1931-4
Online ISBN: 978-1-4939-1932-1
eBook Packages: MedicineMedicine (R0)