Abstract
Obesity, the most common nutritional disorders, affects the majority of adults in Western society. It has become a leading health concern due to its link to insulin resistance, diabetes, and cardiovascular disease. Traditionally, prevention and treatment of obesity mainly depend on caloric restriction and increasing physical activity. Although short-term weight loss can be achieved by various dietary approaches, sustainability of weight loss seems to be difficult. Recently, several studies have shown that dietary manipulation of essential amino acids, including leucine, arginine, and glutamine, improves lipid and glucose metabolism. Specifically, dietary supplementation of leucine prevents HFD-induced obesity, mitochondrial dysfunction, and insulin resistance, suggesting the potential importance of dietary supplementation of leucine in the prevention of HFD-induced metabolic disorders. In this article, we review the metabolic roles of leucine and explore the underlying mechanisms by which leucine supplementation ameliorates HFD-induced metabolic disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Key Points
-
Excess consumption of a high fat diet is a major cause of obesity, which leads to lipid accumulation in fat tissue, skeletal muscle, and liver.
-
High fat diet-induced obesity is associated with chronic metabolic disorders, including mitochondrial dysfunction, insulin resistance, and type 2 diabetes.
-
Dietary leucine prevents high fat diet-induced obesity, mitochondrial dysfunction, and insulin resistance.
-
Leucine supplementation ameliorates high fat diet-induced metabolic disorders through coordinate activation of mammalian target of rapamycin, AMP-activated protein kinase, and sirtuin1 signaling pathways.
-
Dietary supplementation of leucine may provide an adjunct in the prevention and treatment of high fat diet-induced chronic metabolic disorders.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- BACC:
-
Branched chain amino acids
- 4EBP-1:
-
4E binding protein 1
- ER:
-
Endoplasmic reticulum
- FAO:
-
Fatty acid oxidation
- FoxO1:
-
Forkhead box O1
- HFD:
-
High fat diet
- IRS-1:
-
Insulin receptor substrate 1
- mTOR:
-
Mammalian target of rapamycin
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- PGC1α:
-
Peroxisome proliferator-activated receptor γ coactivator-1α
- ROS:
-
Reactive oxygen species
- Sirt1:
-
Sirtuin1
- TRB3:
-
Tribbles homolog 3
Introduction
Obesity, the most common nutritional disorders, affects the majority of adults in Western society. It has become a leading health concern due to its link to insulin resistance, diabetes, and cardiovascular disease [1]. Traditionally, prevention and treatment of obesity mainly depend on caloric restriction and increasing physical activity. Although short-term weight loss can be achieved by various dietary approaches, sustainability of weight loss seems to be difficult [2]. Recently, several studies have shown that dietary manipulation of essential amino acids, including leucine, arginine, and glutamine, improves lipid and glucose metabolism [3]. Specifically, dietary supplementation of leucine prevents HFD-induced obesity, mitochondrial dysfunction, and insulin resistance, suggesting the potential importance of dietary supplementation of leucine in the prevention of HFD-induced metabolic disorders [4]. In this article, we review the metabolic roles of leucine and explore the underlying mechanisms by which leucine supplementation ameliorates HFD-induced metabolic disorders.
High Fat Diet Induces Obesity
In industrial countries, excess consumption of a HFD plays an important role in the development of obesity because a HFD stimulates voluntary energy intake due to its high energy intensity and high palatability [5]. In addition, the excess consumption of diets rich in fatty acids does not stimulate FAO even when dietary fat is given in excess of energy expenditure. Thus, excess consumption of a HFD results in lipid accumulation in adipose tissue, muscle, and liver. Several studies have demonstrated that a HFD reliably produces obesity in rats, mice, dogs, and primates [6], while a low fat diet rarely induces obesity in animals, even when animals are maintained in small cages to limit physical activity. Moreover, switching rodents from a HFD to a low fat diet can ameliorate the HFD-induced obesity [7], suggesting a close correlation between the excess consumption of a HFD and the development of obesity. Observations in humans also suggest that a HFD promotes the development of obesity through increasing energy intake and reducing energy expenditure. In both lean and obese subjects, a HFD enhances fat intake much more than FAO, resulting in a positive fat balance. Furthermore, a HFD may disrupt the balance between the intake and oxidation of carbohydrate, making the obese individuals particularly prone to increase in body weight and fat mass. Thus, dietary fat plays an important role in the development of obesity.
The endoplasmic reticulum (ER) is a major organelle in maintaining lipid metabolic homeostasis. Excessive intake of nutrients may stimulate the ER stress response or the unfolded protein response through three ER membrane proteins; inositol-requiring enzyme-1, activating transcription factor, and protein kinase-like ER kinase. Deregulation of the ER stress response has been implicated in obesity [8]. In pancreatic β cells, the activation of protein kinase-like ER kinase and eukaryotic translation initiation factor 2 subunit alpha upregulates the expression of sterol-regulatory binding proteins, which are major regulators of cholesterol and fatty acid synthesis [9]. In mammary epithelial cells, the loss of protein kinase-like ER kinase reduces the sterol-regulatory binding protein activity and lipogenesis [10]. Moreover, in cultured hepatocellular carcinoma HepG2 cells, elevated palmitate levels, which mimics fatty acid overload conditions, increases ER stress markers, upregulates the proteins related to fatty acid synthesis, and induces lipid accumulation. Similarly, feeding a HFD to mice stimulates the ER stress response, induces lipid accumulation, and reduces insulin sensitivity in mice. Importantly, inhibition of the unfolded protein response by pharmacological and genetic means prevents HFD-induced ER stress response and lipid accumulation. Thus, excess consumption of a HFD may induce obesity through stimulating the ER stress response.
HFD-Induced Obesity Is Associated with Mitochondrial Dysfunction and Insulin Resistance
The primary function of the mitochondria is to produce energy from carbohydrate, fat, and protein. As a major site of FAO, the mitochondria contain the enzymes essential for lipid metabolism. Excessive intake of dietary fat leads to mitochondrial dysfunction with consequential impaired lipid and glucose metabolism. Exposure of C2C12 myotubes to saturated free fatty acid impairs mitochondrial function as evidenced by decreases in both mitochondrial hyperpolarization and ATP generation. Meanwhile saturated free fatty acids reduce insulin-enhanced glycogen synthesis, glucose oxidation, and lactate production. The inhibition of mitochondrial respiration reduces FAO and increases triglyceride accumulation in 3T3L1 preadipocytes. These data suggest a close correlation among the consumption of a HFD, lipid accumulation, and mitochondrial dysfunction.
The excessive intake of dietary fat can also impair mitochondrial function through the inhibition of mitochondrial biogenesis. The genes encoding proteins involved in oxidative phosphorylation and mitochondrial biogenesis are downregulated in young men who consumed a HFD [11]. Likewise, reductions in the genes related to oxidative phosphorylation and mitochondrial biogenesis are also found in mouse models of HFD-induced obesity and insulin resistance [11]. Furthermore, exposure of rats to a HFD significantly impaired mitochondrial function by rapidly reducing ATP synthesis. The defects in mitochondrial oxidative capacity may further promote the development of obesity because a deficiency in β-oxidation and a lower oxidative metabolism led to lipid accumulation in non-adipose tissues.
The accumulation of fatty acids and their metabolites, including acyl-CoAs, ceramides, and diacylglycerol in non-adipose tissues, particularly in the muscle and liver, is closely associated with insulin resistance, because these “toxic” lipids can serve as signaling molecules that activate protein kinases such as protein kinase C, c-Jun N-terminal kinases, and the inhibitor of nuclear factor-κB kinase-β. These kinases, in turn, can phosphorylate IRS-1, leading to degradation of IRS-1 and impaired PI3K signaling. In addition, a HFD may upregulate TRB3 protein and promote the association between Akt and TRB3, which inhibits insulin-stimulated Akt phosphorylation (Ser473), leading to high blood glucose, impaired insulin tolerance, and low glucose infusion rate during a clamp [4].
Mitochondrial dysfunction is considered a major factor contributing to the development of insulin resistance and type 2 diabetes. Compared to insulin-sensitive control subjects, type 2 diabetic patients and insulin-resistant individuals with impaired glucose tolerance have fewer mitochondria, lower levels of mitochondrial oxidative enzymes, and lower ATP synthesis in their muscles [12]. The expression of genes related to PGC1-α and various mitochondrial constituents [13] is also lower in muscles from these patients. In addition, the number of mitochondria and the expression of the genes that regulate mitochondrial biogenesis are significantly lower in adipocytes from type 2 diabetic patients and obese individuals. However, other groups reported that consumption of a HFD increases mitochondrial biogenesis and fatty acid oxidative capacity in skeletal muscle [14]. Recently, Hancock et al. [15] showed that insulin resistance can develop in animals maintained on a HFD, despite a significant increase in the mitochondrial contents. Thus, the role of mitochondrial metabolism in the etiology of insulin resistance warrants further investigation.
Leucine Supplementation Prevents HFD-Induced Metabolic Disorders
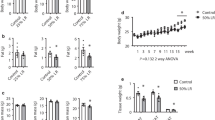
Leucine is an essential BCAA. In humans it cannot be produced by the body and has to be obtained from dietary source. In contrast to other essential amino acids that are mainly metabolized in the liver, leucine is primarily metabolized in peripheral tissues, such as muscle. Leucine serves not only as a building block for protein synthesis but also as a signal to activate mTOR kinase and its downstream targets [16] to regulate many cellular processes, including protein synthesis, cell growth, and metabolism. Like dietary protein, leucine supplementation has been implicated in the regulation of satiety, because leucine can directly stimulate mTOR signaling in the hypothalamus and induce the secretion of leptin [17], an important adipokine in regulating hunger and food consumption, leading to decreased food intake. Furthermore, increasing dietary intake of leucine has been shown to reduce body weight, plasma levels of cholesterol, and lipid accumulation in the mice subjected to a HFD [4, 18]. The beneficial effects of leucine supplementation are associated with the upregulation of uncoupling protein 3 in brown and white adipose tissues and skeletal muscle, which can increase resting energy expenditure.
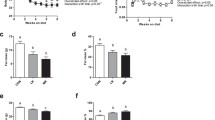
Dietary supplementation of leucine also increases mitochondrial biogenesis and improves mitochondrial function in the liver, brown adipose tissue, and muscle. Compared with normal chow diet-fed mice, HFD plus leucine-fed mice had greater expression of the genes related to mitochondrial biogenesis, such as PGC1-α, nuclear respiratory factor-1, mitochondrial DNA transcription factor A, and NADH dehydrogenase [ubiquinone] iron-sulfur protein 8. The high expression of mitochondrial biogenesis genes was associated with an increase in mitochondrial mass and an improvement of mitochondrial function, as determined by more citrate synthase activity and greater ATP content [4]. Moreover, addition of leucine to the HFD resulted in a significantly higher mRNA level of PPARα, an important enzyme controlling fatty acid metabolism. The functional relevance of this induction was validated by detecting more expression of two important genes regulating FAO, including carnitine palmitoyltransferase-1b (CPT-1b) and medium-chain acyl CoA dehydrogenase, which may stimulate FAO.
The mitochondria are essential organelles responsible for processing oxygen and converting substances from the foods into energy for essential cellular functions. Meanwhile, they are also a major source of ROS, which are associated with a wide variety of inflammatory and metabolic diseases [19]. The excessive intake of dietary fat may enhance the electron flow in the mitochondrial respiratory chain, which increases ROS generation, thereby inducing oxidative stress [20]. This may cause structural and functional damage to the liver, kidney, and heart. Thus, the improvement of mitochondrial biogenesis and function by leucine supplementation may also attenuate HFD-induced oxidative stress. As assessed by immunohistochemistry, the consumption of a HFD increased the formation of 3-nitrotyrosine, a footprint of oxidative stress. The increase was diminished by addition of leucine to the HFD [4].
Another beneficial effect of leucine supplementation is improvement of insulin sensitivity and glucose metabolism. In normal diet-fed mice, insulin stimulated Akt phosphorylation at Ser473, but in HFD-fed mice insulin failed to stimulate the Akt phosphorylation. The consumption of additional leucine with a HFD recovered the effect of insulin-stimulated Akt phosphorylation. Consistent with the improvement of insulin sensitivity, leucine supplementation normalized blood glucose levels, improved glucose tolerance, and enhanced glucose infusion rate during a hyperinsulinemic–euglycemic clamp study in HFD-fed mice.
In addition to improvement of glucose and lipid metabolism in HFD-induced obese mouse model, chronic leucine supplementation improves glucose-insulin homeostasis in other mouse models of obesity and diabetes with different etiologies. In RCS10 mice [21], a polygenic model predisposed to beta cell failure and type 2 diabetes, chronic supplementation of leucine prevents the development of overt diabetes through increasing insulin secretion. In yellow agouti mice, which carry a mutation of the agouti gene in chromosome 2 and exhibit phenotypes of mild hyperphagia, hyper-metabolism and insulin resistance, dietary leucine improves insulin sensitivity as estimated by lower insulin levels together with lower HbA1c and plasma glucose levels in leucine-treated yellow agouti mice [21]. Meanwhile, leucine also attenuates adipose tissue inflammation, increases resting metabolic rate, and upregulates the genes related to mitochondrial function and energy metabolism in these mice.
The role of dietary leucine in the regulation of energy metabolism remains controversial in the literature because some groups reported that dietary supplementation of leucine had no effect on lipid metabolism and did not alter susceptibility to diet-induced obesity in mice. In the in vitro experiments, incubation of extensor digitorum longus muscle with leucine reduces insulin-stimulated phosphorylation of Akt at Ser473 [22]. Moreover, supplemental BCAA worsens insulin resistance in HFD-fed rats even though the supplementation of BCAA reduces food intake and decreases body weight. Thus, further investigations are necessary for establishing the role of dietary leucine in energy metabolism.
Leucine and mTOR Signaling
The mammalian target of rapamycin, a coordinator between nutritional stress and cellular growth machinery, functions in an intracellular signaling pathway that senses the availability of amino acids. mTOR exists as two distinct protein complexes, mTOR complex1 and mTOR complex2 [23]. Activation of mTOR complex1 increased protein synthesis and ribosomal biogenesis, thereby playing a key role in coupling nutrients to protein synthesis [24].
In skeletal muscle, an increase in the leucine concentration stimulates the mTOR signaling pathway, which phosphorylates the inhibitory binding protein 4EBP-1, causing the binding protein to dissociate from the translational initiation factor, eukaryotic initiation factor-4E [25]. In addition, leucine-activated mTOR phosphorylates P70S6 kinase, leading to the phosphorylation of the S6 ribosomal protein [26]. P70S6 kinase and 4EBP-1 are two proteins essential in the regulation of protein synthesis. In adipose tissue, leucine-stimulated mTOR signaling regulates preadipocytes differentiation, adipose tissue morphogenesis, and leptin secretion [27]. For example, a high concentration of leucine in primary cultures of rat adipocytes activates mTOR signaling, leading to preadipocyte differentiation and adipogenesis [28].
Activation of the mTOR/S6K1 pathway increases IRS-1 phosphorylation at Ser1101 while it inhibits IRS-1 phosphorylation at tyr612, resulting in the degradation of IRS-1 and the impairment of PI3K signaling [29], critical events in the development of insulin resistance [30]. In one study, long-term supplementation of BCAA including leucine, isoleucine, and valine, to HFD-fed mice induced insulin resistance that was accompanied by higher phosphorylation of mTOR and IRS-1 on Ser307, all of which were attenuated by administration of rapamycin, an inhibitor of mTOR. In contrast, administration of leucine diminished the insulin resistance caused by consuming a HFD even though leucine increased insulin-stimulated phosphorylation of P70S6K in the muscle, liver, and brown adipose tissue [31]. Thus the role of leucine in insulin resistance needed to be further investigated.
Leucine and AMPK Signaling Pathway
AMPK is a heterotrimer comprising of α, β, and γ subunits [32]. The α subunit contains the catalytic domain. Increases in the ratio of AMP/ATP can activate AMPK through an allosteric effect and the inhibition of the dephosphorylation of Thr172 in the activation loop in the kinase domain [33]. To date, several upstream kinases have been identified to phosphorylate AMPK; these include the tumor suppressor kinase LKB1 [34, 35] and two calmodulin-dependent protein kinase kinases, CaMKK-α and CaMKK-β. AMPK is a fuel gauge that senses the intracellular energy status [36] and plays an important role in the regulation of glucose and lipid metabolism [37]. Activation of AMPK phosphorylates several target molecules, leading to the reduction of energy demands and an increase in energy supply [37]. In addition, activation of AMPK provides an important cellular protective response in various stress conditions, including hypoxia, oxidative stress, exercise, and starvation [38, 39].
Recent evidence suggests that the inhibition of AMPK may also actively participate in the regulation of many cellular processes. In OVE26 mice, a transgenic model of severe early-onset type 1 diabetes, and streptozotocin-induced diabetic mice, both the AMPK activity and the phosphorylation at Thr172 are significantly reduced in the diabetic hearts, which is accompanied by the suppression of autophagy and by impairment of cardiac structure and function [40, 41]. Feeding mice a HFD results in the dysregulation of AMPK, detected by both a reduction in AMPK protein expression and an inhibition of AMPK phosphorylation in skeletal muscle, heart, liver, aortic endothelium, and hypothalamus [42]. Incubation of endothelial cells in a medium containing palmitate to mimic fatty acid overload conditions results in an increase in ceramide production and the inhibition of the phosphorylation of AMPK and its downstream molecule acetyl-CoA carboxylase through activation of protein phosphatase 2 [43]. Similarly, feeding mice a HFD rich in palmitate also inhibits AMPK activity [43], suggesting that inhibition of AMPK activity may be an important mechanism underlying HFD-induced metabolic disorders. The consumption of additional leucine with a HFD restored AMKP phosphorylation, which was associated with a decrease in body weight and fat mass, as well as an improvement of insulin sensitivity and glucose metabolism. The activation of AMPK also stimulated the SIRT1 signaling pathway through increasing the expression of SIRT1 and NAMPT, a rate-limiting enzyme responsible for NAD+ biosynthesis [4]. These results suggest that dietary leucine can prevent HFD-induced metabolic disorders through coordinately regulating the AMPK and SIRT1 signaling pathways.
Leucine Activates SIRT1 Signaling
SIRT1, an NAD+-dependent deacetylase, can enhance glucose utilization, increase mitochondrial FAO, and improve insulin sensitivity [44]. In the mouse liver, SIRT1 is required for the activation of PGC1α and induction of gluconeogenic genes in response to fasting signals [45]. Under starvation conditions, knockdown of SIRT1 by transfection of siRNA results in mild hypoglycemia, increased glucose tolerance, improved insulin sensitivity, and decreased hepatic glucose production [46]. In addition, knockdown of SIRT1 positively regulates the nuclear receptor LXR (liver X receptor) proteins, leading to the accumulation of free fatty acids and cholesterol in the liver, which can be reversed by SIRT1 overexpression [46].
The association between the low expression of SIRT1 protein and insulin resistance in skeletal muscle has been reported for obese and aged individuals and for type 2 diabetic patients [47]. Glucose restriction activates AMPK by transcriptional repression of the protein tyrosine phosphatase 1B [47], resulting in high expression of the NAMPT gene and activation of the SIRT1 signaling pathway, which in turn improves insulin sensitivity and glucose homeostasis. In addition, the activation of SIRT1 decreases acetylation of PGC1α and FoxO1, stimulating mitochondrial biogenesis, FAO [48], and gluconeogenesis [49]. Thus, activation of SIRT1 signaling is essential for the prevention of metabolic disorders.
Leucine supplementation in HFD-fed mice activated SIRT1 signaling through AMPK-mediated upregulation of SIRT1 and NAMPT. The activation of SIRT1 reduced acetylation of PGC1α and FoxO1 [50], which increased the expression of genes related to mitochondria biogenesis, leading to enhanced mitochondrial contents and improved mitochondrial function. In addition, the reduction in acetylation of PGC1α was associated with upregulation of the genes related to FAO and activate the signaling pathway that controls FAO, thereby normalizing plasma lipid profile, reducing subcutaneous and visceral fat mass, and preventing lipid accumulation [4].
FoxO1 was originally identified as a negative regulator of insulin signaling [51], but recent evidence suggests that it can improve hepatic insulin sensitivity through upregulation of TRB3. TRB3 is an endogenous inhibitor of Akt, which is a critical regulator in insulin signaling [52]. The expression of TRB3 has been implicated in the regulation of insulin signaling and glucose metabolism. For instance, the expression of TRB3 at the mRNA and protein levels is higher in livers from mice with diabetes than in mice without diabetes [53]. In cultured hepatocytes isolated from HFD-fed mice, overexpression of TRB3 disrupts insulin signaling by directly binding to Akt and prevents Akt phosphorylation, resulting in hyperglycemia and glucose intolerance in the mice [52]. Moreover, in the individuals susceptible to type 2 diabetes, TRB3 contributes to the development of insulin resistance by interfering with Akt activation [54]. These data suggested that upregulation of TRB3 suppresses insulin sensitivity via Akt inhibition. In support of this hypothesis, a recent study showed that the consumption of leucine to a HFD reduced the association of TRB3 and Akt and enhanced insulin-stimulated Akt phosphorylation, which was accompanied by lower blood glucose and increased glucose infusion rate during a clamp in HFD-fed mice. These studies suggest that leucine supplementation restores insulin sensitivity in HFD-fed mice through suppressing TRB3 expression and interfering with the interaction between TRB3 and Akt [4].
Conclusions
Leucine functions as a nutrient signal to coordinately regulate mTOR, AMPK, and SIRT1 signaling pathways in the liver, skeletal muscle, and adipose tissue. Dietary supplementation of leucine significantly ameliorates the deleterious effects of consumption of a HFD, including obesity, hepatic lipid accumulation, mitochondrial dysfunction, and insulin resistance. Therefore, leucine supplementation may be beneficial to obese individuals and type 2 diabetic patients. The metabolic benefits of leucine supplementation are associated with the upregulation of genes related to mitochondrial biogenesis and FAO, increases in metabolic rates, and suppression of inflammation in adipose tissue. Understanding the molecular mechanism by which dietary supplementation of leucine improves glucose and lipid metabolism may help to define novel nutritional and pharmacological approaches for the treatment of obesity, insulin resistance, and type 2 diabetes. Thus, further investigations are needed to clearly define the beneficial effects of dietary leucine on energy metabolism in obese individuals and diabetic patients.
References
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–76.
Katz DL. Competing dietary claims for weight loss: finding the forest through truculent trees. Annu Rev Public Health. 2005;26:61–88.
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135:714–21.
Li H, Xu M, Lee J, He C, Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am J Physiol Endocrinol Metab. 2012;303:E1234–44.
Miller WC, Lindeman AK, Wallace J, Niederpruem M. Diet composition, energy intake, and exercise in relation to body fat in men and women. Am J Clin Nutr. 1990;52:426–30.
Atkins CE, LeCompte PM, Chin HP, Hill JR, Ownby CL, Brownfield MS. Morphologic and immunocytochemical study of young dogs with diabetes mellitus associated with pancreatic islet hypoplasia. Am J Vet Res. 1988;49:1577–81.
Hill JO, Dorton J, Sykes MN, Digirolamo M. Reversal of dietary obesity is influenced by its duration and severity. Int J Obes. 1989;13:711–22.
Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803.
Colgan SM, Tang D, Werstuck GH, Austin RC. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biol. 2007;39:1843–51.
Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, Diehl JA. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:16314–9.
Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–33.
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–71.
Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:15439–50.
Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–92.
Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008;105:7815–20.
Li F, Yin Y, Tan B, Kong X, Wu G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41:1185–93.
Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am J Physiol Endocrinol Metab. 2006;291:E621–30.
Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–54.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61.
Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–23.
Guo K, Yu YH, Hou J, Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond). 2010;7:57.
Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426–34.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302.
Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–65.
Kimball SR, Jefferson LS. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care. 2001;4:39–43.
Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–82.
Lynch CJ. Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. J Nutr. 2001;131:861S–5.
Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–32.
Hartley D, Cooper GM. Role of mTOR in the degradation of IRS-1: regulation of PP2A activity. J Cell Biochem. 2002;85:304–14.
Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310.
Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine—an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187.
Hardie DG, Carling D, Halford N. Roles of the Snf1/Rkin1/AMP-activated protein kinase family in the response to environmental and nutritional stress. Semin Cell Biol. 1994;5:409–16.
Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345(Pt 3):437–43.
Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–62.
Xie Z, Dong Y, Zhang J, Scholz R, Neumann D, Zou MH. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol Cell Biol. 2009;29:3582–96.
Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–83.
Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24.
Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–75.
Taleux N, De Potter I, Deransart C, Lacraz G, Favier R, Leverve XM, Hue L, Guigas B. Lack of starvation-induced activation of AMP-activated protein kinase in the hypothalamus of the Lou/C rats resistant to obesity. Int J Obes (Lond). 2008;32:639–47.
Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–8.
He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270–81.
Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–95.
Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–88.
Jiang WJ. Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun. 2008;373:341–4.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–8.
Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–6.
Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–19.
Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–23.
Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–95.
Yu J, Auwerx J. Protein deacetylation by SIRT1: an emerging key post-translational modification in metabolic regulation. Pharmacol Res. 2010;62:35–41.
Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–9.
Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–7.
Matsushima R, Harada N, Webster NJ, Tsutsumi YM, Nakaya Y. Effect of TRB3 on insulin and nutrient-stimulated hepatic p70 S6 kinase activity. J Biol Chem. 2006;281:29719–29.
Prudente S, Hribal ML, Flex E, Turchi F, Morini E, De CS, Bacci S, Tassi V, Cardellini M, Lauro R, Sesti G, Dallapiccola B, Trischitta V. The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes. 2005;54:2807–11.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Xie, Y., Xie, Z. (2015). Experimental Models of High Fat Obesity and Leucine Supplementation. In: Rajendram, R., Preedy, V., Patel, V. (eds) Branched Chain Amino Acids in Clinical Nutrition. Nutrition and Health. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1923-9_18
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1923-9_18
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1922-2
Online ISBN: 978-1-4939-1923-9
eBook Packages: MedicineMedicine (R0)