Abstract
Patients with chronic liver disease often reach a state of protein-energy malnutrition, which may influence patient outcomes following surgery and subsequent quality-of-life (QOL). Recent assessments of QOL integrate a biochemical health model with a social science model that is based on the patient’s subjective perception of functioning and wellbeing across a range of physical, mental and social aspects of life. Since most liver neoplasms occur in patients with chronic liver disease, hepatic resection could potentially reduce QOL in these patients by further compromising liver function. Advances in surgical technology and perioperative management have led to hepatic surgical procedures, including liver resection and radiofrequency ablation, being the mainstay of curative treatment for not only hepatocellular carcinoma (HCC), but also metastatic liver tumours. However, hepatic surgery is still associated with postoperative morbidities due to the inevitable deterioration of liver function following a reduction in functioning liver mass. In general, it is recommended that nutrition is individualized according to a patient’s nutritional status and monitored to ensure well being and nutritional adequacy. Based on the clinical assessment, dieticians should therefore educate patients and carers about sodium and fluid restriction, and appropriate food choices. In this context, branched-chain amino acid (BCAA) nutritional supplementation improves postoperative QOL over the long term after hepatic resection by restoring and maintaining nutritional status and whole-body kinetics. BCAA may also inhibit carcinogenesis in heavier patients with cirrhosis and play a key role in liver regeneration. Individualized intervention is thus recommended based on patient’s nutritional status
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Abbreviations
- QOL:
-
Quality-of-life
- HCC:
-
Hepatocellular carcinoma
- BCAA:
-
Branched chain amino acid
- PEM:
-
Protein-energy malnutrition
- LES:
-
Late-evening snack
- GLUT:
-
Glucose transporters
- BM:
-
Bone marrow
Key Points
-
Patients with chronic liver disease often reach a state of protein-energy malnutrition, which may influence patient outcomes following surgery and subsequent quality-of-life (QOL).
-
Recent assessments of QOL integrate a biochemical health model with a social science model that is based on the patient’s subjective perception of functioning and well-being across a range of physical, mental and social aspects of life.
-
Since most liver neoplasms occur in patients with chronic liver disease, hepatic resection could potentially reduce QOL in these patients by further compromising liver function.
-
Advances in surgical technology and perioperative management have led to hepatic surgical procedures, including liver resection and radiofrequency ablation, being the mainstay of curative treatment for not only hepatocellular carcinoma (HCC), but also metastatic liver tumours.
-
Hepatic surgery is still associated with postoperative morbidities due to the inevitable deterioration of liver function following a reduction in functioning liver mass.
-
In general, it is recommended that nutrition is individualized according to a patient’s nutritional status and monitored to ensure well being and nutritional adequacy.
-
Based on the clinical assessment, dieticians should therefore educate patients and carers about sodium and fluid restriction, and appropriate food choices.
-
Branched chain amino acid (BCAA) nutritional supplementation improves postoperative QOL over the long term after hepatic resection by restoring and maintaining nutritional status and whole-body kinetics.
-
BCAA may also inhibit carcinogenesis in heavier patients with cirrhosis and play a key role in liver regeneration.
-
Individualized intervention is thus recommended based on patient’s nutritional status.
Introduction
In recent years there has been an increasing coincidence between a patient’s point of view and the assessment of their health status. Traditional medical outcomes, which are important endpoints for clinicians, need to be integrated with patients’ survival rate after adequate management for various diseases. However, it would be of note that it has been considered patients’ opinions on health status, reflecting how they really feel, and how much their disease affects their way of living to be more important, because the treatment consensus over almost all disorders has been established to some extent.
Liver is the central organ for nutrient production and metabolism [1]. Patients with chronic liver disease often become severely malnourished, which can seriously damage their capacity for liver regeneration [2] and increase the risk of hepatocellular carcinoma (HCC). In particular, a state of protein-energy malnutrition (PEM) [3] affects patient outcomes following management as shown by quality-of-life (QOL) estimations [4]. Recent assessments of QOL integrate a biochemical health model with a social science model that is based on the patient’s subjective perception of their physical, mental and social functioning and well-being [5].
Recent advances in surgical technology and perioperative management have made hepatic surgical procedures, such as liver resection and radiofrequency ablation, the mainstay of curative treatment for both primary and metastatic liver tumours [6]. However, since most liver neoplasms occur in patients with chronic liver disease, hepatic resection could potentially reduce QOL in these patients by further compromising liver function. Hence, it is important to consider QOL among the more traditional treatment outcomes of operative mortality and long-term survival rates. Some recent studies implicated an important role for nutritional support using branched chain amino acids (BCAA) in the surgical management and postoperative QOL of patients undergoing hepatic resection for liver neoplasms [7, 8]. In this article, we revisit the basic concept of BCAA administration and review how BCAA supplementation affects long-term self-estimated QOL and health in these patients.
Quality-of-Life in Patients with Chronic Liver Diseases
Evaluation of QOL
A QOL assessment concept was developed in the mid-1990s by integrating biochemical and social science models of health assessment [9, 10]. According to the modern concepts of health-related QOL, the principle criteria guiding a patient’s acceptance of treatment are often subjective. In patients with hepatobiliary disease, this could include the patients’ feelings about their potential QOL following suggested surgical, medical or palliative interventions. Indeed, such perceptions could be more relevant to acceptance of treatment than predicted length of life, because patients are frequently more concerned about quality and disability than about longevity [11]. This is especially true with chronic diseases, where survival is not at risk for a long time, and the goal of interventions is to maintain symptom-free and community-living patients.
This conceptualisation is based on the World Health Organization definition of health as “a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity”. This definition is so broad that it includes elements that are beyond the traditional domain of medicine and healthcare systems. Opportunity, education, spiritual attitudes, social security, working satisfaction, social relationships and goods availability are elements of QOL that are independent of medicine. Various questionnaires, usually self-administered, have been developed to assess QOL. From the 1990s on, there has been a growing emphasis on assessing QOL in patients with cancer, and this assessment may be as important as the evaluation of long-term survival. The most widely used generic instruments to assess QOL for patients with cancer are the European Organization for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire (QLQ)—the EORTC QLQ-C30—and the Functional Assessment of Cancer Therapy—General (FACT-G) [12, 13]. More recently, a study about health-related QOL of chronic liver disease patients with and without hepatocellular carcinoma (HCC) reported that impaired QOL is not associated with the presence of cancer itself, but is dependent on the level of liver function, indicating the importance of preserving liver function [14]. In recent years, the Italian versions of the Medical Outcome Study Short Form-36 (SF-36) and the Nottingham Health Profile (NHP) questionnaires, two generic instruments assessing patients’ well-being, have been validated and used to compare the impacts of chronic diseases in a general population and to determine health policies and resource allocation (Table 16.1) [15].
Poor Health-Related Quality-of-Life of Patients with Cirrhosis
Globally, cirrhosis/chronic liver disease of varying aetiologies is responsible for major mortality and morbidity. Liver cirrhosis is one of the commonest causes of hospitalisation, and a number of studies over the past decade have convincingly demonstrated that health-related QOL is significantly impaired in patients with cirrhosis compared to the general population (Fig. 16.1) [16].
Hepatic Encephalopathy
Hepatic encephalopathy is the occurrence of confusion, an altered level of consciousness and coma as a result of liver failure. In the advanced stages it is called hepatic coma or coma hepaticum. The mildest form of hepatic encephalopathy presents as forgetfulness, mild confusion and irritability, and thus is difficult to diagnose clinically, but it may be demonstrated on neuropsychological testing. The progression of hepatic encephalopathy is characterised by an inverted sleep-wake pattern (sleeping by day, being awake at night), followed by lethargy and personality changes, then by worsened confusion, and finally, a progression to coma [17]. Hepatic encephalopathy thus has a significant impact on the patient’s health-related QOL. In addition, minimal hepatic encephalopathy is a part of the spectrum of overt hepatic encephalopathy, with a characteristic cognitive profile that cannot be diagnosed clinically [18]. On follow-up, patients with minimal hepatic encephalopathy are more likely to develop overt hepatic encephalopathy, as compared with cirrhotics without minimal hepatic encephalopathy [19]. Hepatic encephalopathy is associated with poor prognosis and is an independent predictor of survival [20].
Hyperammonaemia
Hyperammonaemia is a condition characterised by raised serum ammonia levels. Mild and transient hyperammonaemia can often be asymptomatic and is usually triggered by protein loads and catabolic states. In symptomatic cases, the clinical features may be variable and episodic. Many cases present with acute mental status changes characterised by confusion, personality changes, irritability, ataxia, visual disturbance, lethargy and somnolence may also report nausea, vomiting and hyperventilation. More severe cases can lead to encephalopathy characterised by stupor and coma. The pathogenesis of hyperammonaemic encephalopathy remains unclear. Changes in mental status have been attributed to high levels of ammonia and the presence of other organic acids, with raised brain ammonia concentrations sometimes present even when the serum ammonia level is normal [21]. Hyperammonaemia can also cause encephalopathy via the inhibition of glutamate uptake by astrocytes [22]. The resulting astroglial processes surround the brain microvessels of the blood–brain barrier and swell in the presence of advanced hepatic encephalopathy. However, despite these significant astroglial changes, the barrier function remains intact, suggesting that cytotoxic rather than vasogenic mechanisms predominate in the pathogenesis of hepatic encephalopathy [23]. As with delirium, elderly patients may present with several concomitant predisposing factors for developing hyperammonaemia and encephalopathy.
Hepatitis Viral Infection and Health-Related QOL
Chronic viral hepatitis infection has been associated with a significant reduction in health-related QOL that is not related to the severity of liver disease. Possible pathophysiological mechanisms affecting QOL in such cases of HCV infection include alterations in mood (increased anxiety and depression) and cognition, together with changes in both the midbrain serotoninergic and striatal dopaminergic systems, irrespective of viraemia or state-ofliver function [24]. The existence of brain alterations directly caused by HCV is evidenced by reported deficits in attention, executive function and verbal learning, by electroencephalogram recordings slowing in the absence of liver cirrhosis and/or substance abuse disorder, and atypical changes on magnetic resonance spectroscopy in HCV-infected patients. It remains controversial whether QOL in patients with HBV-associated cirrhosis is reduced compared to that in patients with HCV-associated or HBV/HDV-associated cirrhosis. Another study showed less impairment of QOL in patients suffering from HBV-associated cirrhosis compared to other causes of cirrhosis such as HCV or cholestasis [25]. However, overall the number of chronic hepatitis B patients reporting reduced QOL compared to patients with hepatitis C was small. Hence, more studies are required to confirm the poor QOL in patients with hepatitis B viral infection.
Serum Sodium and Ascites
A previous study demonstrated the importance of serum sodium concentration for health-related QOL in cirrhosis, whereby patients with hyponatraemia reported a marked impairment in health-related QOL compared to patients with normal serum sodium concentration [26]. Hyponatraemia is common in patients with cirrhosis and ascites and its frequency increases with disease progression [27]. Hyponatraemia with concomitant hypo-osmolality is associated with an adaptive response of the central nervous system aimed at preventing the passage of fluid from the extracellular to the intracellular space and the development of cerebral edema. In patients with marked hyponatraemia, a low-grade cerebral edema exists despite this adaptive brain response and increased ammonia levels also play a role in low-grade cerebral edema [28]. The value of serum sodium concentration in the prediction of health-related QOL persisted after adjustment for possible confounding factors related to the severity of liver failure. Furthermore, serum sodium level was an independent predictive factor in both physical and mental summary scores of health-related QOL, and in six of the eight domains of the SF-36: role physical, physical functioning, general health, social functioning, vitality and mental health [26].
QOL in Patients Undergoing Liver Resection
Branched Chain Amino Acids (BCAA) Improved Both PEM and QOL
Protein-energy malnutrition (PEM) is a common finding in chronic liver disease and affects about 50 % of patients with liver cirrhosis [4]. Since malnutrition adversely affects clinical outcomes, guidelines of the European, American and Japanese Societies for Parenteral and Enteral Nutrition advocated nutritional support for cirrhotic patients [29, 30], recommending the following consensus nutrition standard: 35–40 kcal/kg/day in energy and 1.2–1.5 kcal/kg/day in proteins [29]. However, such standards are not always pertinent and should be altered depending on conditions such as race, intensity of daily activity, PEM, glucose intolerance, protein intolerance and obesity. Flexible handling of the ESPEN guideline is therefore necessary, and calorimetry might be the best way to assess the nutritional status of patients with liver cirrhosis. Possible treatments for PEM include BCAA supplementation and a late-evening snack (LES). BCAA effectively corrects protein malnutrition by increasing plasma albumin, and prolongs event-free survival in patients with advanced cirrhosis [4]. Since energy deficiency is related to worse survival rates, it is also important to address the early-morning energy starvation that is typical in cirrhosis and equivalent to a 3-day starvation period in healthy individuals. To this end, the ESPEN and ASPEN guidelines recommend a LES or divided meals to reduce the starvation period between dinner and breakfast. BCAA as a LES is an ideal supplement in patients with cirrhosis because it provides more energy and protein than the ordinary enteral formula or BCAA granules, significantly reduces fatigue, and improves energy metabolism, protein levels and nitrogen balance [31]. Furthermore, a Hong Kong study also showed that long-term dietary supplementation with BCAA twice daily significantly reduced complications such as ascites and peripheral edema, and improved survival in patients undergoing chemoembolization for HCC [32].
QOL After Curative Hepatectomy
Postoperative QOL
Since most liver neoplasms occur in patients with chronic liver disease, hepatic resection could potentially reduce QOL in these patients by further compromising liver function. QOL assessment has proven to be a valuable parameter for such patients and surgeons and may be helpful in determining the optimal treatment. As an outcome parameter, QOL is considered as important as disease-free and overall survival. In various benign and malignant liver diseases, surgical management is a common procedure with intent-to-cure treatment, as a result of recent advances in surgical technology and perioperative management. Although major and minor liver resections are both safe procedures, little is known about postoperative QOL in these patients [33]. Recent studies indicated that QOL returns to baseline within 3–6 months after liver resection for malignancies in most cases (Fig. 16.2) [34].
BCAA-Enriched Nutritional Support in Surgical Patients (Table 16.2)
In a prospective randomized clinical trial, the San-in Group of Liver Surgery [13] studied the effects of long-term oral administration of BCAA after curative resection of HCC. Between 2 and 3 weeks after surgery, 75 patients were randomized to receive oral BCAAs (Aminoleban EN) at 100 g/day for 1 year, and another 75 patients were assigned to a control group. Flapping tremor was less common, body weight was increased and performance status was better in the BCAA-treated group than in controls throughout the 1-year period (Fig. 16.3a). BCAA treatment also significantly increased red blood cell and serum albumin levels in patients with Child grade B and C disease. Substantially similar effects were observed in patients treated with major hepatic resection. The San-in Group summarized that long-term oral nutritional support with BCAAs after resection of HCC is beneficial in improving clinical features and laboratory data without increasing the rate of tumor recurrence, particularly in patients with advanced cirrhosis or after major hepatic resection (Fig. 16.3b).
The effect of BCAA [13]. Figure 16.3a, Percentage changes in body-weight in patients treated with BCAA (black circle) and controls (white circle). Values are mean (standard deviation). *P < 0.01, †P < 0.001 versus control group. Figure 16.3b, Changes in Fischer’s molar ratio in patients with BCAA (black circle) and controls (white circle). Values are mean (standard deviation). *P < 0.05, †P < 0.001 versus control group [13]
In a prospective study, Meng et al. [8] evaluated the effect of BCAA treatment in patients undergoing liver resection for HCC. A prospective randomized controlled clinical trial was conducted involving 44 patients. The BCAA group (21 patients) received Aminoleban EN in addition to a normal diet for 12 weeks and the control group (23 patients) received an isonitrogenous and isocaloric diet only. The BCAA group had a shorter hospital stay, and showed a significantly higher haemoglobin level, higher sodium level, higher albumin level and lower bilirubin level during the postoperative course (Fig. 16.4). The authors concluded that Aminoleban EN is safe to administer and does not have significant adverse effects, while contributing to a shorter hospital stay and quicker improvement of liver function in the early postoperative period (Fig. 16.4).
In a retrospective study involving 43 elective hepatectomized patients, Togo et al. [36] evaluated the usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. In the BCAA group (21 patients), postoperative ascites and edema tended to improve earlier than in the control group (22 patients), and nutritional status based on serum albumin and total protein levels recovered immediately after liver surgery in the BCAA group. Furthermore, the BCAA group showed a more rapid improvement in hyaluronic acid and type IV collagen 7S levels compared to controls.
In a large retrospective study involving 112 elective hepatectomized patients, Okabayashi et al. [37] evaluated the effects of BCAA-enriched nutrient support for patients undergoing liver resection for HCC. These patients were divided into two groups: 40 patients received perioperative supplementation of a BCAA-enriched nutrient mixture (BCAA group) and 72 patients had no supplement (control group). Laboratory data, postoperative complications, duration of hospitalisation and survival were compared between groups. The overall incidence of postoperative complications was lower in the BCAA group (17.5 %) than in the control group (44.4 %) (P = 0.01). Among the postoperative complications, surgical site infection and bile leakage were observed in 5 % of patients in the BCAA group and in 15.3 % and 12.5 % of patients in the control group, respectively. Ascites appeared after the surgery in 7.5 % of patients in the BCAA group and in 16.7 % of control patients, while the duration of hospitalisation was significantly shorter in the BCAA group than in the control group (P < 0.05). The authors suggested that their perioperative BCAA supplementation protocol is clinically beneficial in reducing the morbidity associated with postoperative complications and in shortening the duration of hospitalisation of patients with chronic liver disease who undergo liver resection for HCC.
In a prospective study involving 24 elective hepatectomized patients, including some with a non-hepatitis liver, Ishikawa et al. [38] studied the benefits of perioperative oral nutrition (ON) with BCAA. The patients (20 with malignant liver tumors and 4 with benign liver tumors) were randomly assigned to receive perioperative ON with BCAA (11 patients, BCAA group) or a usual diet (13 patients, control group). The BCAA group received a BCAA supplement twice daily plus a usual diet for 14 days before operation and on days 1–7 after operation. Two of the eleven patients in the BCAA group developed postoperative complications, as compared with 3 of the 13 patients in the control group (18.2 % vs. 23.1 %, P = 0.7686). Among patients with non-hepatitis, serum erythropoietin (EPO) levels on POD 3, 5 and 7 were significantly higher in the BCAA group than in the control group (P = 0.0174, P = 0.0141 and P = 0.0328, respectively). The short-term ON support with BCAA was thus associated with higher serum EPO levels in patients with non-hepatitis who underwent curative hepatic resection, and higher EPO levels might be beneficial in protecting liver cells from ischemic injury and preventing intraoperative haemorrhage associated with lower perioperative levels of alanine aminotransferase and aspartate aminotransferase in serum.
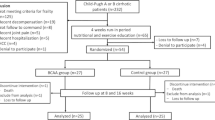
In a prospective randomized clinical study, Okabayashi et al. [35] assessed the impact of oral supplementation with BCAA-enriched nutrients on postoperative QOL in patients undergoing liver resection. To our knowledge, this was the first prospective clinical study evaluating an association between perioperative supplementation of BCAA and postoperative QOL. Patients were randomly assigned to receive BCAA supplementation (BCAA group, n = 48) or a conventional diet (control group, n = 48). Postoperative QOL and short-term outcomes were regularly and continuously evaluated in all patients using a short-form 36 (SF-36) health questionnaire and by measuring various clinical parameters. This study demonstrated a significant improvement in QOL after hepatectomy for liver neoplasm in the BCAA group based on the same patients’ preoperative SF-36 scores (Fig. 16.5). Perioperative BCAA supplementation preserved liver function and general patient health in the short term compared to a normal diet. The authors on this study concluded that BCAA supplementation improves postoperative QOL after hepatic resection over the long term by restoring and maintaining nutritional status and whole-body kinetics.
Trends of Norm-based score by SF-36 in the group administered BCAA and the control group [35]. Trends of Norm-based score by SF-36 in the group administered BCAA (solid line) and the control group (broken line) (*P < 0.05, compared to preoperative score) [35]. All scores decreased immediately after the operation. However, general health measures including perceptions of health and well-being, vitality, social functioning, and mental health improved by 6 months postoperative to at least preoperative levels in the AEN group [35]
In a prospective randomized clinical study, Ichikawa et al. [39] studied the effect of oral supplementation with BCAA on the development of liver tumorigenesis after hepatic resection in HCC patients. Fifty-six patients were randomly assigned to receive either BCAA supplementation orally for 2 weeks before and 6 months after hepatic resection (BCAA group, n = 26) or a conventional diet (control group, n = 30). Postoperative tumor recurrence was continuously evaluated in all patients by measuring various clinical parameters. Recurrence rate at 30 months after surgery was significantly better in the BCAA group than in controls. Interestingly, tumour markers, including AFP and PIVKA-II, significantly decreased at 36 months after liver resection in the BCAA group in comparison to the control group. These findings therefore indicated that oral supplementation of BCAA also reduces the incidence of early recurrence after hepatic resection in patients with HCC, and this treatment regimen offers potential benefits for clinical use in such patients, even in cases with a well-preserved preoperative liver function.
BCAA Improved Perioperative Insulin Resistance
HCV infection causes insulin resistance [40], which is a known risk factor for HCC and reduced long-term survival. Insulin resistance is therefore a potential therapeutic target in patients with HCV infection. BCAA may also play an important role in improving insulin resistance, and in experimental studies using rodents, BCAA induced glucose uptake in skeletal muscle, adipocytes and hepatocytes. Furthermore, in a rat model of liver cirrhosis induced by CCl4, leucine and isoleucine promoted glucose uptake in skeletal muscle [41]. This effect might occur due to up-regulation of the glucose transporters 4 and 1 (GLUT4 and GLUT1) and/or the rapamycin-dependent activation of glucose synthase in skeletal muscle. Interestingly, a recent human study [42] found that oral supplementation of BCAA for 4 and 6 weeks reduced HOMA-IR in two cases with HCV-related liver disease.
Glucose metabolism is generally adversely affected in patients following major surgery, with hyperglycemia a possible result of postoperative insulin resistance due to reduced glucose uptake by skeletal muscle, adipose tissue and liver [43]. Patients may also develop hyperglycemia due a combination of surgical stress and postoperative insulin resistance. Indeed, insulin resistance after major surgery is well documented and its development is related to the magnitude of surgery [44]. Insulin infusion support to maintain normal glucose levels thus reduces morbidity and mortality rates in critically ill patients, and preoperative management of whole-body insulin resistance is required. Interestingly, preoperative oral administration of carbohydrate reduces postoperative insulin resistance in patients with colorectal resection [43]. Furthermore, short-term infusion of amino acids following colorectal surgery can reduce insulin resistance since endogenous glucose production and glucose clearance is decreased [45]. However, it is uncertain whether preoperative dietary supplementation with carbohydrates and BCAA improves postoperative insulin resistance.
To address this question, Okabayashi et al. [40] conducted a randomized clinical trial in which 26 patients undergoing a hepatectomy for the treatment of a hepatic neoplasm either received a supplement of carbohydrate and BCAA prior to surgery or had no supplement. The postoperative blood glucose level and the total insulin requirement for normoglycemic control during the 16 h following hepatic resection were determined using a closed-loop glycemic control system. Postoperative insulin requirements for normoglycemic control in the group with preoperative nutritional support was significantly lower than that in the control group (P = 0.039), indicating that preoperative oral administration of carbohydrate and BCAA is clinically beneficial and reduces postoperative insulin resistance in patients undergoing hepatic resection.
Future: Improving Postoperative QOL According to Liver Regeneration Following the Administration of BCAA
Liver failure is a potentially life-threatening condition for which organ transplantation is the only definitive therapy. However, the current shortage of available livers for transplant results in the death of many patients while awaiting transplantation. Thus, it is imperative that new approaches for repairing the liver are developed, so that the need for transplanting a partial or complete human liver to cure the patient can be eliminated. Presently, cell-based therapies represent one of the most promising alternative solutions to entire or partial liver transplantation. Unfortunately, human livers would still be required as a source of cells and the isolation of human hepatocytes remains difficult and inefficient. Furthermore, differentiated hepatocytes cannot yet be effectively expanded in culture, greatly limiting the cell numbers obtained from each liver. Numerous studies have therefore concentrated on culturing and differentiating stem cells from different sources that can be readily isolated using non-invasive procedures, to give rise to hepatocytes both in vitro and in vivo. An added advantage of a stem-cell approach is that many of these cell populations can be expanded significantly in vitro, making it possible to generate large numbers of cells for transplantation from a fairly small initial number. Since some of these stem cell populations are present within the adult, and could thus be isolated from the patient to be treated, the production of personalized, immunologically matched hepatocytes is possible [46].
Liver regeneration is unique among organ systems [47]. In the adult liver, there are two major populations of cells that have been thought to explain liver regeneration and/or repair. The first consists of unipotential cells, specifically hepatocytes and bile duct epithelial cells that regenerate during normal tissue turnover. The second population consists of bipotential cells, intrahepatic liver stem cells and/or oval cells that can differentiate into hepatocytes and bile duct epithelial cells. Recent reports suggest that bone marrow (BM) stem cells may harbor unexpected developmental plasticity [48], although it remains unclear precisely how and to what extent BM cells contribute to liver regeneration, and whether it is by cell fusion, transdifferentiation or both. Previous studies reported that under certain conditions BM cells are recruited into the liver and become not only Kupffer cells, endothelial cells, oval cells, stromal cells and cholangiocytes, but also functioning hepatocytes [48]. Further examination is required to address the possible association between BCAA and liver regeneration, especially the mechanisms by which BM-derived extra-hepatic stem cell develop after liver resection with perioperative BCAA supplementation.
Liver cirrhosis negatively impacts the patient’s nutritional status, with derangements in energy expenditure and in protein, carbohydrate and fat metabolism. Common complications of cirrhosis such as ascites, hepatic encephalopathy and esophageal varices require appropriate nutritional intervention. However, for patients with hepatic encephalopathy, the evidence is controversial; while some studies indicate that protein restriction is beneficial, others found that this strategy does not have apparent benefits in acute encephalopathy. For patients unable to tolerate animal proteins, other protein sources should be considered, such as proteins from vegetables or BCAA-enriched formulations [46]. In general, it is recommended that nutrition is individualized according to a patient’s nutritional status and monitored to ensure well being and nutritional adequacy. Following the assessment, dieticians should educate patients and carers about sodium and fluid restriction and appropriate food choices. Nutrient-dense meals, snacks and oral supplements are recommended.
Conclusions
Supplementation of BCAA has been shown to improve the nutritional status and QOL in patients with cirrhosis, preventing complications and prolonging survival, including in those patients undergoing chemoembolization and liver resection for HCC. BCAA may also inhibit carcinogenesis in heavier patients with cirrhosis and play a key role in liver regeneration. Individualized intervention is recommended based on each patient’s nutritional status.
References
Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59.
Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, Ellis E, Lakey AF, Burt AD, Douglass A, Wright MC, White SA, Jaffré F, Maroteaux L, Mann DA. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med. 2011;17:1668–73.
Caregaro L, Alberino F, Amodio P, Merkel C, Bolognesi M, Angeli P, et al. Malnutrition in alcoholic and virus-related cirrhosis. Am J Clin Nutr. 1996;63:602–9.
Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313:405–9.
Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R, Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–801.
Sorbye H, Mauer M, Gruenberger T, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Van Cutsem E, Scheithauer W, Lutz MP, Nordlinger B, EORTC Gastro-Intestinal Tract Cancer Group, Cancer Research UK (CRUK), Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO), Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann Surg. 2012;255:534–9.
The San-in Group of Liver Surgery. Long-term oral administration of branched chain amino acids after curative resection of hepatocellular carcinoma: a prospective randomized trial. Br J Surg. 1997;84:1525–31.
Meng WC, Leung KL, Ho RL, Leung TW, Lau WY. Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Aust NZ J Surg. 1999;69:811–5.
Testa M, Simonson D. Assessment of quality of life outcomes. N Engl J Med. 1996;334:835–40.
Wilson J, Cleary P. Linking clinical variables with health-related quality of life. JAMA. 1995;273:59–65.
McNeil BJ, Weichselbaum R, Pauker SG. Speech and survival. Tradeoffs between quality and quantity of life in laryngeal cancer. N Engl J Med. 1981;305:982–7.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9.
Kondo Y, Yoshida H, Tateishi R, Shiina S, Mine N, Yamashiki N, Sato S, Kato N, Kanai F, Yanase M, Yoshida H, Akamatsu M, Teratani T, Kawabe T, Omata M. Health-related quality of life of chronic liver disease patients with and without hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:197–203.
Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51:1025–36.
Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, Loguercio C, Apolone G, Niero M, Abbiati R, Italian Study Group for quality of life in cirrhosis. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–8.
Cash WJ, McConville P, McDermott E, McCormick PA, Callender ME, McDougall NI. Current concepts in the assessment and treatment of hepatic encephalopathy. QJM. 2010;103:9–16.
Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106:307–16.
Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–23.
Das A, Dhiman RK, Saraswat VA, Verma M, Naik SR. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531–5.
Amodio P, Del Piccolo F, Marchetti P, Angeli P, Iemmolo R, Caregaro L, Merkel C, Gerunda G, Gatta A. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662–7.
Hamer HM, Knake S, Schomburg U, Rosenow F. Valproate-induced hyperammonemic encephalopathy in the presence of topiramate. Neurology. 2000;54:230–2.
Bodega G, Suárez I, López-Fernández LA, García MI, Köber M, Penedo M, Luna M, Juárez S, Ciordia S, Oria M, Córdoba J, Fernández B. Ammonia induces aquaporin-4 rearrangement in the plasma membrane of cultured astrocytes. Neurochem Int. 2012;61:1314–24.
Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45:806–16.
Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–9.
Solà E, Watson H, Graupera I, Turón F, Barreto R, Rodríguez E, Pavesi M, Arroyo V, Guevara M, Ginès P. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol. 2012;57:1199–206.
Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002–10.
Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43:1187–90.
Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, DGEM (German Society for Nutritional Medicine), Ferenci P, Holm E, Vom Dahl S, Müller MJ, Nolte W, ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr. 2006;25:285–94.
Bankhead R, Boullata J, Brantley S, Corkins M, Guenter P, Krenitsky J, Lyman B, Metheny NA, Mueller C, Robbins S, Wessel J, A.S.P.E.N. Board of Directors. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr. 2009;33:122–1267.
Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H, Tsubouchi H, Kato S, Kaito M, Watanabe A, Habu D, Ito S, Ishikawa T, Kawamura N, Arakawa Y, Hepatic Nutritional Therapy (HNT) Study Group. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113–20.
Poon RT, Yu WC, Fan ST, Wong J. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Aliment Pharmacol Ther. 2004;19:779–88.
Farges O, Goutte N, Bendersky N, Falissard B, ACHBT-French Hepatectomy Study Group. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012;256:697–704.
Okabayashi T, Nishimori I, Sugimoto T, Iwasaki S, Akisawa N, Maeda H, Ito S, Onishi S, Ogawa Y, Kobayashi M, Hanazaki K. The benefit of the supplementation of perioperative branched-chain amino acids in patients with surgical management for hepatocellular carcinoma: a preliminary study. Dig Dis Sci. 2008;53:204–9.
Okabayashi T, Iyoki M, Sugimoto T, Kobayashi M, Hanazaki K. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids. 2011;40:1213–20.
Togo S, Tanaka K, Morioka D, Sugita M, Ueda M, Miura Y, Kubota T, Nagano Y, Matsuo K, Endo I, Sekido H, Shimada H. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition. 2005;21:480–6.
Okabayashi T, Nishimori I, Sugimoto T, Maeda H, Dabanaka K, Onishi S, Kobayashi M, Hanazaki K. Effects of branched-chain amino acids-enriched nutrient support for patients undergoing liver resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1869–73.
Ishikawa Y, Yoshida H, Mamada Y, Taniai N, Matsumoto S, Bando K, Mizuguchi Y, Kakinuma D, Kanda T, Tajiri T. Prospective randomized controlled study of short-term perioperative oral nutrition with branched chain amino acids in patients undergoing liver surgery. Hepatogastroenterology. 2010;57:583–90.
Ichikawa K, Okabayashi T, Maeda H, Namikawa T, Iiyama T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today. 2013;43:720–6.
Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Namikawa T, Maeda H, Yatabe T, Hanazaki K. Preoperative oral supplementation with carbohydrate and branched-chain amino acid-enriched nutrient improves insulin resistance in patients undergoing a hepatectomy: a randomized clinical trial using an artificial pancreas. Amino Acids. 2010;38:901–7.
Nishitani S, Takehana K, Fujitani S, Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1292–300.
Kawaguchi T, Taniguchi E, Itou M, Sumie S, Oriishi T, Matsuoka H, Nagao Y, Sata M. Branched-chain amino acids improve insulin resistance in patients with hepatitis C virus-related liver disease: report of two cases. Liver Int. 2007;27:1287–92.
Svanfeldt M, Thorell A, Hausel J, Soop M, Rooyackers O, Nygren J, Ljungqvist O. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342–50.
Thorell A, Efendic S, Gutniak M, Häggmark T, Ljungqvist O. Development of postoperative insulin resistance is associated with the magnitude of operation. Eur J Surg. 1993;159:593–9.
Donatelli F, Schricker T, Mistraletti G, Asenjo F, Parrella P, Wykes L, Carli F. Postoperative infusion of amino acids induces a positive protein balance independently of the type of analgesia used. Anesthesiology. 2006;105:253–9.
Almeida-Porada G, Zanjani ED, Porada CD. Bone marrow stem cells and liver regeneration. Exp Hematol. 2010;38:574–80.
Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–81.
Okabayashi T, Cameron AM, Hisada M, Montgomery RA, Williams GM, Sun Z. Mobilization of host stem cells enables long-term liver transplant acceptance in a strongly rejecting rat strain combination. Am J Transplant. 2011;11:2046–56.
Financial Support
Kochi organization for medical reformation and renewal grants.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Okabayashi, T., Kozuki, A., Sumiyoshi, T., Shima, Y. (2015). Branched Chain Amino Acids and Postoperative Quality of Life. In: Rajendram, R., Preedy, V., Patel, V. (eds) Branched Chain Amino Acids in Clinical Nutrition. Nutrition and Health. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1914-7_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1914-7_16
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1913-0
Online ISBN: 978-1-4939-1914-7
eBook Packages: MedicineMedicine (R0)