Abstract
Use of dietary supplements, especially among athletes is increasing. Among the amino acids, the branched-chain amino acids (BCAA-leucine, valine and isoleucine) are popular as dietary supplements especially among strength training athletes. In particular, leucine as a supplement is widely consumed because it has been implicated to be the key amino acid involved in stimulating muscle protein synthesis. This chapter will present current dietary leucine intakes, define the concept of safe upper limits (UL) and summarize a recent study in humans conducted to address leucine tolerability. In the 2005 Dietary References Intake (DRI) report, men 51 through 70 years of age had the highest intakes, at the 99th percentile for leucine, at 14,100 mg·d-1 (~ 201 mg·kg·d-1). In order to directly explore leucine tolerability in humans we proposed a novel model. We hypothesized that with increasing intakes of leucine above the estimated average requirement (EAR of 50 mg·kg-1·d-1) in adult men, the oxidation of leucine will increase and will reach a maximum, after which the leucine oxidation will achieve a plateau. This ‘metabolic limit’ to oxidize leucine may be used as a marker of an intake after which increasing intakes may result in increasing risk of adverse effects. Five healthy young men participated in the study. Each subject participated in a dose-escalation study design, where graded stepwise increases in leucine intake (150, 250, 500, 750, 1000 and 1250 mg·kg-1·d-1 corresponding to the EAR, EARx3, x5, x10, x15, x20 and x25) were provided on each study day. Oxidation of L-[1-13C]leucine to 13CO2 in breath (F13CO2) was measured on each study day. With increasing intakes of leucine, a dose-response in leucine oxidative capacity was observed, with a breakpoint estimated at 550 mg·kg-1·d-1 or 39 g·d-1. Simultaneous and significant increases in blood ammonia concentrations, plasma leucine concentrations and urinary leucine excretion were observed with leucine intakes higher than 500 mg·kg-1·d-1. These results taken together with the recent animal data suggest that under acute dietary conditions, as a cautious estimate, intakes greater than 500 mg leucine·kg-1·d-1 may potentially increase the risk of adverse events, and could be proposed as the UL for leucine in healthy adults.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Abbreviations
- α-KIC:
-
Alpha- ketoisocaproic acid
- ALT:
-
Alanine amino transferase
- BCAA:
-
Branched chain amino acids
- BCAT:
-
Branched chain aminotransferase
- BCDH:
-
Branched chain ketodehydrogenase
- CPS1:
-
Carbamoyl phosphate synthetase 1
- DRI:
-
Dietary Reference Intakes
- EAR:
-
Estimated average requirement
- F13CO2 :
-
Label tracer oxidation
- GDH:
-
Glutamate dehydrogenase
- NAGS:
-
N-acetylglutamate synthase
- NOAEL:
-
No-observed-adverse-effect level
- REE:
-
Resting energy expenditure
- UL:
-
Tolerable upper intake level
- VCO2 :
-
Carbon dioxide production
Key Points
-
Leucine supplements are popular especially among athletes, due to the perception that leucine promotes endurance and enhances athletic performance.
-
Current reported leucine intakes in developed countries among healthy adults is approximately 200 mg kg day−1.
-
Currently no safe upper limits (UL) for leucine intake has been set.
-
Recently using stable isotope labeled L-[1-13C]-Leucine and graded test intakes of leucine (50–1,250 mg kg−1 day−1), the upper limit to oxidize/metabolize leucine was tested in adult men.
-
The oxidation of leucine measured as a rate of tracer oxidation plateaued with increasing intakes of leucine above 550 mg kg day−1.
-
Simultaneously increases in plasma ammonia concentrations, plasma leucine concentrations, and urinary leucine excretion were observed with leucine intakes above 500 mg kg day−1.
-
Taken together the data suggest 500 mg kg day−1 as a cautious estimate of the UL for leucine intake under acute dietary conditions.
Introduction
Use of dietary supplements, especially among athletes is increasing. It has been reported that 80–85 % of professional athletes consume nutritional supplements, including amino acids due to the perception that they enhance performance or recovery [1–3]. In the United States approximately 3.4 % of the general population uses amino acid supplements, 62 % on a daily basis [4]. Among the amino acids, the branched chain amino acids (BCAA-leucine, valine, and isoleucine) are popular as dietary supplements especially among strength training athletes. In particular, leucine as a supplement is widely consumed because it has been implicated to be the key amino acid involved in stimulating muscle protein synthesis [5–8]. Therefore it is perceived that leucine might improve athletic endurance, performance [9–16], and increase lean body mass, although some results [17] have been inconclusive. From a public health perspective dietary supplementation of leucine in excess of requirement may have adverse health effects, and therefore additional knowledge is necessary regarding the highest possible intake of leucine at which no adverse effect occurs. This chapter will present current dietary leucine intakes, define the concept of safe upper limits (UL), and summarize recent studies in animals and humans conducted to address leucine tolerability.
Current Leucine Intakes
In the recent Dietary Reference Intakes (DRI) report [18], based on distribution data from the 1988–1994 NHANES III, mean daily intake of leucine for all life stage and gender groups from food and supplements were reported to be 6,100 mg day−1. Men 51 through 70 years of age had the highest intakes, at the 99th percentile for leucine, at 14,100 mg day−1 (~201 mg kg day−1). Similar results have been reported from the UK adult National Diet and Nutrition Survey, ranging from a median to 90th percentile intake of 108–138 mg kg day−1, respectively; the more recent UK survey [19] suggests a leucine intake of 200 mg kg day−1 in healthy adult men.
Defining Safe Upper Limits (UL) of Intakes for Leucine
The DRI’s refer to the safe upper limits of a nutrient as the Tolerable Upper Intake Level (UL), and is defined as, “the highest average daily nutrient intake level that is likely to pose no risk of adverse health effects to almost all individuals in the general population. As intake increases above the UL, the potential risk of adverse effects may increase.” [18]
The term tolerable was chosen to avoid implying a possible beneficial effect. Instead, the term is supposed to suggest a level of intake that can, with high probability, be tolerated biologically.
With respect to the BCAA supplements, earlier reviews [20, 21] have summarized studies in athletes, normal adults, and patients with clinical disorders, and reported that intakes of 15–60 g day−1 (~200–850 mg kg−1 day−1 for a 70 kg man) of total BCAA did not result in adverse event outcomes for the parameters monitored. A significant problem with interpreting the reported human studies is the great variability in approaches; these experiments include supplements of all BCAA, at many different doses, different ratios among the BCAA in intake, different routes of infusion/feeding (intravenous versus oral), and different athletic training regimes. Therefore in the 2005 DRI report, no UL for BCAA, including for leucine, was set, and it was noted that there was a paucity of data from well-designed dose–response studies in both animals and humans.
Development of a Novel Approach to Define UL
Based on the recommendations in the DRI (2005) report [18], we developed a conceptual model [22], which will help design studies to identify the UL for amino acids. We reasoned that markers for identifying excess intake of an amino acid should have specific dose–response characteristics. In particular, variation with intake should display an inflection point that would identify the onset of the amino acid excess situation [22]. Previously, in neonatal piglets [23], an upper inflection point was observed in the dose–response curve for phenylalanine retention and 14CO2 production from phenylalanine oxidation with graded phenylalanine intake. Once the maximum level of phenylalanine oxidation was reached then plasma phenylalanine levels, and retention, rose rapidly. Hence, it is reasonable that a suitable marker to define the upper limit of tolerance for a dietary amino acid would be the level of intake at which the maximum oxidation level was exceeded.
The above described response pattern for increasing phenylalanine intake should be observable for most amino acids and we proposed [22] that it can be applied to define the UL. When the amino acid intake is low, protein synthesis, oxidation, and excretion of the amino acid and related metabolites will be low (Fig. 1.1a). With increasing intake of the limiting amino, retention of the amino acid will increase as a result of increasing utilization of the limiting amino acid for protein synthesis and other required metabolic functions. Therefore a positive slope in amino acid retention will be observed. Once the requirement for the amino acid is met for protein synthesis and related functions, additional increments of the test amino acid will be primarily catabolized in proportion to the extra intake. This increasing catabolism in proportion to intake will occur because each additional increment in intake will be in excess of the requirements for metabolism, and will be oxidized. In this zone, the catabolic pathways are sufficient to deal with the excess intake and the excess amino acid is broken down and used for energy. For the range of amino acid intakes in this zone, a minimum slope or no slope in the retention of the amino acid will probably be observed, depending on the amino acid being tested. Further increases in amino acid intake will lead to a positive slope in the amino acid retention curve, due to increased retention of the amino acid in body pools. This increase in retention is a result of dietary intake exceeding the metabolic capacity to catabolize the amino acid in direct proportion to intake. This point, at which the metabolic capacity to catabolize or oxidize the excess amino acid is exceeded, can be regarded as one estimate of the UL because it represents the intake where the normal regulatory mechanisms are no longer sufficient to dispose of the excess (Fig. 1.1b). The amino acid intake corresponding to this inflection point does not represent a toxic intake level, but rather suggests that with increasing dietary intakes above this level the potential or risk for adverse events will increase. Also, amino acid intakes above this point are usually characterized by an increasing rate of accumulation in blood and excretion of the amino acid, and its secondary catabolites in urine (Fig. 1.1a, b).
An approach to define the safe upper limits of amino acids. (a) Schematic to describe body responses to increasing amino acid intake. (b) Patterns of response expected due to increasing intakes of amino acids. With increasing intakes of the amino acid, oxidation of the amino acid will increase. Once the “metabolic limit” to oxidize the excess amino acid is reached, amino acid oxidation will plateau and this inflection point will identify the upper tolerable intake limit (UL) for the test amino acid. As amino acid intake increases above the UL, the potential risk of adverse effects and toxicity may increase. Source: Adapted from J Nutr, Pencharz et al. J Nutr. 2008;138:1996S–2002S [22]
Leucine UL in Animals
Sakai et al. [24] used the above-described approach to identify an excess intake of leucine in rats. They identified the “metabolic limit” to catabolize leucine by measuring 13CO2 production arising from graded leucine intakes ranging from 0 to 30 % of the diet. The maximum limit to oxidize excess leucine was reached at 10 % of dietary intake; the oxidation achieved a plateau above the intakes of 10 % dietary leucine or 8.9 g leucine kg−1. This inflection point was identified as the UL for leucine intake in rats [24]. They also reported that plasma leucine and other plasma amino acid concentrations were not significantly different among all the leucine intakes. Therefore, they were not able to identify accumulation of excess leucine and other metabolites as a potential biomarker, or surrogate marker. In an earlier study using a similar strain of rats and receiving a diet similar in composition, they observed significant growth inhibition in rats fed 15 % leucine or 12.4 g leucine kg−1. This suggests that the inflection point, at which the maximum limit to oxidize excess leucine is reached, is an early marker to identify the potential for an adverse event (in this case – growth inhibition) and may identify the UL more appropriately.
Tsubuku et al. [25] in a controlled week-long experiment, with moderate or high amounts of protein in the diet, studied oral supplements of leucine excess in 4-week-old rats. They estimated ~3,500 mg kg−1 day−1 to be the no-observed-adverse-effect level (NOAEL), based on body weight, food consumption, and hematological measurements. Mawatari et al. [26] in 10-week-old female rats estimated that oral leucine at 1,000 mg kg−1 day−1 did not affect the outcome of pregnancy and did not cause fetal toxicity. Recently, Imamura et al. [27] under conditions of low-protein intake (6 %), suggested that 2 % leucine in the diet may be the NOAEL, using a novel gene marker panel. Thus, as concluded earlier by Baker [28] young experimental animals are able to tolerate a considerable dietary excess of leucine, when consumed in diets containing sufficient levels of protein and the other two BCAA.
Leucine UL in Humans
We hypothesized [29] that with increasing intakes of leucine above the estimated average requirement (EAR of 50 mg kg−1 day−1) in adult men [30], the oxidation of leucine will increase and will reach a maximum, after which the leucine oxidation will achieve a plateau. This ‘metabolic limit’ to oxidize leucine may be used as a marker of an intake after which increasing intakes may result in increasing risk of adverse effects.
Five healthy young men participated in the study [29]. Each subject participated in a dose-escalation study design, where graded stepwise increases in leucine intake were provided on each study day. This study design was chosen to ensure that with each increasing dose of leucine intake, subject safety could be monitored. Each subject was initially studied at a leucine intake of 50 mg kg−1 day−1. Following this baseline study subjects received increased dietary leucine in a graded stepwise intake of 150, 250, 500, 750, 1,000, and 1,250 mg kg−1 day−1 corresponding to the EAR, EAR ×3, ×5, ×10, ×15, ×20, and ×25 on separate study days. The study diets were adequate in protein (1 g kg−1 day−1), energy (1.5 × resting energy expenditure, REE), and all other nutrients including carbohydrates and fats [29]. The study days were separated by a minimum of 2 weeks to ensure a sufficient washout period between the leucine excess study day diets. On each study day a baseline, and end of study blood and urine sample was collected for analysis of blood and urine biochemistry.
Leucine Oxidation
Oxidation of L-[1-13C]leucine to 13CO2 in breath (F13CO2) was measured with increasing intakes of dietary leucine. F13CO2 increased with increasing intakes of leucine until 500 mg kg−1 day−1, after which oxidation remained at a plateau (Fig. 1.2a). Two-phase linear regression analysis [31, 32] identified a breakpoint at a leucine intake of 550 mg kg−1 day−1, and this represents the maximum leucine oxidative potential in vivo in adult men [29]. Leucine oxidation (measured from plasma enrichment of leucine) and KIC oxidation (a surrogate measure of intracellular leucine enrichment and oxidation) showed a dose response with increasing leucine intakes, which appeared to reach a plateau after 500 mg kg−1 day−1 although absolute leucine oxidation seemed to be increasing (Fig. 1.2b, c). Thus the metabolic capacity to dispose of excess leucine intake occurs between 500 and 700 mg kg−1 day−1 and the efficiency of leucine oxidation appears to have reached a plateau. Nevertheless, plasma and urinary biomarkers as discussed in the following sections displayed a simultaneous change that correlated with the 500 mg leucine kg−1 day−1.
Graded dietary excess leucine intake and metabolic oxidative capacity in healthy young men (n = 29 observations). (a) Production of 13CO2 from the oxidation of orally administered L-[1-13C]-Leucine in young men (F13CO2). The oxidation of L-[1-13C]leucine to 13CO2, calculated as F13CO2, showed a significant response (P < 0.0001) to increasing leucine intakes. Statistical analysis performed using mixed models ANOVA, followed by two-phase linear regression analysis. Using two-phase linear regression analysis, a ‘breakpoint’ in F13CO2 was identified at 550 mg kg−1 day−1 (r 2 = 0.85). The lower and upper 95 % CI was calculated to be 454 and 647 mg kg−1 day−1, respectively. (b) Leucine oxidation. (c) KIC1 oxidation. 1 KIC, alpha-keto isocaproic acid. Source: Elango et al. Am J Clin Nutr. 2012;96:759–767 [29]
Plasma Ammonia and Biochemical Measures
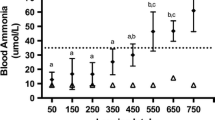
There was a concomitant increase in blood ammonia concentrations (Fig. 1.3a, b) above normal values (<35 μmol/L) with significant increases in plasma leucine concentration (Fig. 1.4a) and urinary leucine excretion (Fig. 1.4b) in all subjects with intakes of leucine above 500 mg kg−1 day−1, suggesting that higher intakes might be harmful. Urinary excretion of KIC increased, although the increases were not significant until 1,250 mg kg−1 day−1 (Fig. 1.4c). Taken together the data suggest 500 mg kg−1 day−1 as a cautious estimate of the UL for leucine intake under acute dietary conditions [29, 33].
Graded dietary excess leucine intake and blood ammonia levels. Values are means ± SD; n = 2 to 5 per mean; symbols with different superscript letters are significantly different, P < 0.05. Statistical analysis performed using mixed models ANOVA and post-hoc analysis using Tukey-Kramer’s multiple comparison tests. Dotted lines indicate normal blood ammonia levels in young men (35 μmol/L). (a) Comparison of blood ammonia concentrations between fasted (pre) and end of study day (post) blood samples; (b) Blood ammonia concentrations at the end of each study day. Source: Elango et al. Am J Clin Nutr. 2012;96:759–767 [29]
Graded dietary excess leucine intake and plasma leucine concentrations, urinary leucine excretion, and plasma KIC concentrations. (a) Plasma leucine concentrations. (b) Urinary leucine excretion. (c) Plasma KIC1 concentrations. 1 KIC, alpha-keto isocaproic acid. Source: Elango et al. Am J Clin Nutr. 2012;96:759–767 [29]
Plasma and urine samples were analyzed for other biochemical measures as potential markers of leucine UL. Although plasma ammonia concentrations significantly increased with increasing leucine intakes (Fig. 1.3a, b), no significant changes were observed for urea, creatinine, ALT, glucose, insulin, electrolytes (sodium, potassium, chloride), complete blood count including hematocrit, WBC, RBC, and hemoglobin [29]. Leucine has been suggested to act as an insulin secretagogue [34, 35], but we did not observe significant changes in plasma insulin due to increasing leucine intakes and plasma glucose remaining within the normal range of 3.3–6.1 μmol/L. Plasma leucine concentration increased significantly with leucine intakes greater than 500 mg kg−1 day−1, and plasma valine and isoleucine concentrations decreased significantly with increasing leucine intakes (Fig. 1.5a, b), as reported previously [36–39]. The BCAA share a common catabolic pathway with the branched chain ketodehydrogenase (BCDH) controlling the irreversible catabolic step, which commits the carbon skeleton of the BCAA to the TCA cycle. Leucine concentrations have been shown to stimulate BCDH, as well as to compete with the other two BCAA for metabolism in vivo [40]; this phenomenon, referred to as BCAA antagonism, is well documented [36].
Graded dietary excess leucine intake and plasma isoleucine, valine concentrations. (a) Plasma isoleucine concentrations. (b) Plasma valine concentrations. Source: Elango et al. Am J Clin Nutr. 2012;96:759–767 [29]
Potential Mechanism to Explain Plasma Ammonia Increase
Leucine has been shown to be an activator of glutamate dehydrogenase (GDH) [41], which results in alpha-ketoglutarate and ammonia production (Fig. 1.6). Leucine concentrations above 800 μM activates GDH to dispose of the surplus amino acids [42]; in the current study plasma leucine concentrations increased well above 2,000 μM, potentially leading to increased blood ammonia concentrations. Furthermore accumulation of isovaleryl CoA, a metabolite formed from leucine catabolism, has been shown to inhibit N-acetylglutamate synthase (NAGS) [43]. NAGS is an activator of carbamoyl phosphate synthetase 1 (CPS1), which regulates the urea cycle [43]. With inhibition of NAGS, CPS activation would not have occurred (Fig. 1.6), and possibly explains the increase in blood ammonia with no change in blood urea concentrations.
Potential mechanism to explain hyperammonemia due to increasing leucine intakes. Leucine has been shown to activate glutamate dehydrogenase (GDH), which results in alpha-ketoglutarate and ammonia production [41]. In addition, catabolism of leucine yields isovaleryl CoA, a known inhibitor of N-acetylglutamate synthase (NAGS) [43]. NAGS activates carbamoyl phosphate synthetase 1 (CPS1), which regulates the urea cycle [43]. With inhibition of NAGS, CPS activation would not have occurred, and possibly explains the increase in blood ammonia with no change in blood urea concentrations
Leucine UL in Relation to Current Leucine Intakes
An analysis of the current habitual leucine intake was conducted in strength-training athletes who are chronic amino acid supplement users. The average protein intake [44] in these athletes was reported to be ~2 g kg−1 day−1. Mean leucine content in food is ~15 % [45]. Therefore, for an athlete weighing 80 kg, the dietary leucine intake is 300 mg kg−1 day−1. BCAA supplements available contain a maximum 1,800 mg/serving [29], with a suggested dose of 3 doses per day; this equals ~67.5 mg kg−1 day−1. Therefore, the habitual total exposure of adults consuming 2 g protein kg−1 day−1 and this amino acid supplement is at ~367 mg kg−1 day−1. These calculations reveal that most people, including athletes, consume less than the UL for leucine oxidation determined (500 mg kg−1 day−1). However, there is probably a range in protein and leucine intake amongst athletes, with some consuming more than the recommended dose and thus at potential risk of adverse effects. The impact of chronic consumption of excess leucine by humans remains unknown, as the study was conducted with an acute dietary supply of leucine. A high chronic intake may either reduce the risk of adverse effects, by increasing the basal leucine oxidation rate, or increase the risk of adverse effects by gradual accumulation of metabolic events associated with excess intake, and such effects need to be confirmed in the future with long-term leucine supplementation, as recently recommended [46].
Conclusions
In summary, in the recent human study [29] with increasing intakes of leucine, a dose–response in leucine oxidative capacity was observed, with a breakpoint estimated at 550 mg kg−1 day−1 or 39 g day−1. Simultaneous and significant increases in blood ammonia concentrations, plasma leucine concentrations, and urinary leucine excretion were observed with leucine intakes higher than 500 mg kg−1 day−1. These results taken together with the recent animal data [27] suggest that under acute dietary conditions, as a cautious estimate, intakes greater than 500 mg leucine kg−1 day−1 may potentially increase the risk of adverse events, and could be proposed as the UL for Leucine in healthy adults [47].
References
Maughan RJ, Depiesse F, Geyer H, International Association of Athletics Federations. The use of dietary supplements by athletes. J Sports Sci. 2007;25:S103–13.
Lun V, Erdman KA, Fung TS, et al. Dietary supplementation practices in Canadian high-performance athletes. Int J Sport Nutr Exerc Metab. 2012;22:31–7.
Braun H, Koehler K, Geyer H, et al. Dietary supplement use among elite young German athletes. Int J Sport Nutr Exerc Metab. 2009;19:97–109.
Young VR. Introduction to the 2nd amino acid assessment workshop. J Nutr. 2003;133:2015S–20.
Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–61.
Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9.
Crozier SJ, Kimball SR, Emmert SW, et al. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–82.
Nair KS, Short KR. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135:1547S–52.
Pitkänen HT, Oja SS, Rusko H, et al. Leucine supplementation does not enhance acute strength or running performance but affects serum amino acid concentration. Amino Acids. 2003;25:85–94.
Mero A. Leucine supplementation and intensive training. Sports Med. 1999;27:347–58.
Crowe MJ, Weatherson JN, Bowden BF. Effects of dietary leucine supplementation on exercise performance. Eur J Appl Physiol. 2006;97:664–72.
Coburn JW, Housh DJ, Housh TJ, et al. Effects of leucine and whey protein supplementation during eight weeks of unilateral resistance training. J Strength Cond Res. 2006;20:284–91.
Shimomura Y, Inaguma A, Watanabe S, et al. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr Exerc Metab. 2010;20:236–44.
Walker TB, Smith J, Herrera M, et al. The influence of 8 weeks of whey-protein and leucine supplementation on physical and cognitive performance. Int J Sport Nutr Exerc Metab. 2010;20:409–17.
Thomson JS, Ali A, Rowlands DS. Leucine-protein supplemented recovery feeding enhances subsequent cycling performance in well-trained men. Appl Physiol Nutr Metab. 2011;36:242–53.
Ispoglou T, King RF, Polman RC, et al. Daily L-leucine supplementation in novice trainees during a 12-week weight training program. Int J Sports Physiol Perform. 2011;6:38–50.
Verhoeven S, Vanschoonbeek K, Verdijk LB, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–75.
Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, Carbohydrate, Fiber, fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC: The National Academy Press; 2005.
Millward DJ. Knowledge gained from studies of leucine consumption in animals and humans. J Nutr. 2012;142:2212S–9.
Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135:1539S–46.
Matthews DE. Observations of branched-chain amino acid administration in humans. J Nutr. 2005;135:1580S–4.
Pencharz PB, Elango R, Ball RO. An approach to defining the upper safe limits of amino acid intake. J Nutr. 2008;138:1996S–2002.
House JD, Pencharz PB, Ball RO. Phenylalanine requirements determined by using L-[1-14C]phenylalanine in neonatal piglets receiving total parenteral nutrition supplemented with tyrosine. Am J Clin Nutr. 1997;65:984–93.
Sakai R, Miura M, Amao M, et al. Potential approaches to the assessment of amino acid adequacy in rats: a progress report. J Nutr. 2004;134:1651S–5.
Tsubuku S, Hatayama K, Katsumata T, et al. Thirteen-week oral toxicity study of branched-chain amino acids in rats. Int J Toxicol. 2004;23:119–26.
Mawatari K, Katsumata T, Uematsu M, et al. Prolonged oral treatment with an essential amino acid L-leucine does not affect female reproductive function and embryo-fetal development in rats. Food Chem Toxicol. 2004;42:1505–11.
Imamura W, Yoshimura R, Takai M, et al. Adverse effects of excessive leucine intake depend on dietary protein intake: a transcriptomic analysis to identify useful biomarkers. J Nutr Sci Vitaminol (Tokyo). 2013;59:45–55.
Baker DH. Tolerance for branched-chain amino acids in experimental animals and humans. J Nutr. 2005;135:1585S–90.
Elango R, Chapman K, Rafii M, et al. Determination of the tolerable upper intake level of leucine in acute dietary studies in young men. Am J Clin Nutr. 2012;96:759–67.
Riazi R, Wykes LJ, Ball RO, et al. The total branched-chain amino acid requirement in young healthy adult men determined by indicator amino acid oxidation by use of L-[1-13C]phenylalanine. J Nutr. 2003;133:1383–9.
Littell R, Milliken G, Stroup W, Wolfinger R. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996.
Seber GAF. Linear regression analysis. Wiley: New York, NY; 1977.
Pencharz PB, Elango R, Ball RO. Determination of the tolerable upper intake level of leucine in adult men. J Nutr. 2012;142:2220S–4.
Nair KS, Matthews DE, Welle SL, et al. Effect of leucine on amino acid and glucose metabolism in humans. Metabolism. 1992;41:643–8.
Kalogeropoulou D, Lafave L, Schweim K, et al. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism. 2008;57:1747–52.
Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54.
Block KP, Harper AE. Valine metabolism in vivo: effects of high dietary levels of leucine and isoleucine. Metabolism. 1984;33:559–66.
Eriksson S, Hagenfeldt L, Wahren J. A comparison of the effects of intravenous infusion of individual branched-chain amino acids on blood amino acid levels in man. Clin Sci (Lond). 1981;60:95–100.
Hambraeus L, Bilmazes C, Dippel C, et al. Regulatory role of dietary leucine on plasma branched-chain amino acid levels in young men. J Nutr. 1976;106:230–40.
Aftring RP, Block KP, Buse MG. Leucine and isoleucine activate skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1986;250:E599–604.
Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am J Clin Nutr. 2009;90:862S–6.
Erecińska M, Nelson D. Activation of glutamate dehydrogenase by leucine and its nonmetabolizable analogue in rat brain synaptosomes. J Neurochem. 1990;54:1335–43.
Coude FX, Sweetman L, Nyhan WL. Inhibition by propionyl-coenzyme A of N-acetylglutamate synthetase in rat liver mitochondria. A possible explanation for hyperammonemia in propionic and methylmalonic acidemia. J Clin Invest. 1979;64:1544–51.
Phillips SM. Protein requirements and supplementation in strength sports. Nutrition. 2004;20:689–95.
Gleeson M. Interrelationship between physical activity and branched-chain amino acids. J Nutr. 2005;135:1591S–5.
Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care. 2010;13:265–70.
Cynober L, Bier DM, Kadowaki M, et al. A proposal for an upper limit of leucine safe intake in healthy adults. J Nutr. 2012;142:2249S–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Elango, R., Ball, R.O., Pencharz, P.B. (2015). Tolerability of Leucine in Humans. In: Rajendram, R., Preedy, V., Patel, V. (eds) Branched Chain Amino Acids in Clinical Nutrition. Nutrition and Health. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1914-7_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1914-7_1
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1913-0
Online ISBN: 978-1-4939-1914-7
eBook Packages: MedicineMedicine (R0)