Abstract
The adult central nervous system (CNS) responds to myelin loss by generating new myelin sheaths in a robust regenerative process mediated by local stem/precursor cells. This process, termed remyelination, represents an exciting ability of the CNS to repair itself and is the default response to demyelination. Remyelination reinstates rapid axonal conduction and resolves functional deficits, although it is compromised in a number of important diseases. Delayed or incomplete remyelination results in exposed axons that lack trophic support from the myelin sheath and so become vulnerable to progressive degeneration with resultant neurological dysfunction. Therapeutically enhanced remyelination in the treatment of demyelinating disease is an important and attractive goal, and current research focuses on two categories of potential therapeutics: those that stimulate endogenous stem/precursor cells and those in which new stem cells are introduced to the patient to promote remyelination. A prerequisite for successful development of such therapeutics is a detailed understanding of the major cellular players and molecular mechanisms that underpin this biological process. In this chapter, we will review the progress made in unraveling the details of remyelination, followed by a discussion on how such information is being utilized to develop stem cell therapeutics for demyelinating disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neural stem cells
- Oligodendrocyte progenitor cells
- Myelin sheath
- Remyelination
- Functional recovery
- Multiple sclerosis
Functions of the Myelin Sheath
To understand the pathological significance of myelin loss, it is important to appreciate the functions of the myelin sheath. The oligodendrocyte is responsible for generating CNS myelin sheaths, which consist of lipid-rich membrane layers arranged concentrically around the axon. Their presence alters the electrical properties of the ensheathed region of the axon by creating an area of high resistance and low capacitance. The action potential effectively jumps across these regions of high resistance to the intervening regions of exposed axolemma in a process known as saltatory conduction—a form of rapid and efficient neuro transmission. Therefore, a primary function of myelin is to optimize electrical transmission (Kotter et al. 2011).
In addition to enabling saltatory conduction, there is growing evidence that the myelin sheath is required for long-term axonal survival. Transgenic mice lacking the integral myelin proteolipid protein (PLP) show long-term axonal degeneration, despite the presence of functional myelin that harbors only subtle ultrastructural alterations in compaction (Boison and Stoffel 1994; Klugmann et al. 1997). Axons were shown to develop focal accumulation of organelles at distal paranodal regions (Griffiths et al. 1998), indicative of impaired fast axonal transport (Edgar and Nave 2009). Similar degenerative changes were found in transgenic mice lacking other single myelin proteins, such as myelin-associated glycoprotein (MAG) (Pan et al. 2005) or 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNP) (Lappe-Siefke et al. 2003), and in patients with Pelizaeus–Merzbacher disease, caused by mutations in the Plp gene (Edgar and Nave 2009). These studies revealed that minor perturbations of the oligodendrocyte can have significant long-term consequences for the axon (Franklin et al. 2012).
Currently, studies are examining the mechanisms by which the oligodendrocyte supports the neuron and its axon, and these mechanisms are likely to be varied. Ultrastructural observations suggest that myelinating glia can sequester aged axonal organelles (Spencer and Thomas 1974) and transfer trophic factors to the axon via direct transport (Novotny 1984) or secreted exosomes (Kramer-Albers et al. 2007; Wilkins et al. 2003). Furthermore, the oligodendrocyte appears to provide direct support to the axon by conveying glycolysis products to fuel oxidative phosphorylation (Nave and Trapp 2008; Rinholm and Bergersen 2012). A recent study demonstrated that the myelin sheath provides the axon with lactate via the monocarboxylate transporter 1 (MCT1) located in the myelin sheath; this provision is essential for neuronal survival (Lee et al. 2012).
A growing body of evidence for the role of the oligodendrocyte in preserving axonal integrity, independent from the ability to generate the myelin sheath, provides an explanation for the progressive atrophy observed in chronically demyelinated axons. It would also suggest that the therapeutic promotion of remyelination could prevent the neurodegenerative component of demyelinating disease (Franklin and Ffrench-Constant 2008).
Demyelination
Demyelination is the pathological loss of myelin sheaths from around axons. Sometimes referred to as “primary demyelination” it is the loss of myelin as a direct consequence of injury to the oligodendrocyte, and should be distinguished from Wallerian degeneration, in which secondary demyelination occurs as a consequence of primary axonal loss. From a clinical perspective, there are two major causes of primary demyelination in the central nervous system (CNS): genetic abnormalities that affect glia (leukodystrophies) and inflammatory damage to myelin and oligodendrocytes (Franklin and Ffrench-Constant 2008; Popescu and Lucchinetti 2012). Regardless of its cause, demyelination impairs neuronal function.
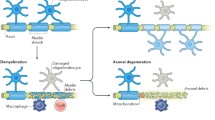
Chronic demyelination is the hallmark feature of multiple sclerosis (MS), an inflammatory demyelinating disease of the CNS, which remains among the most prominent and devastating disorders in contemporary neurology. Autoimmune-mediated attacks lead to oligodendrocyte destruction and the development of multiple diffuse foci of primary demyelination and acute axonal damage within the CNS. During the early stages of disease, the demyelinated regions are effectively remyelinated, but over time this process becomes progressively less effective (Fig. 1). Ultimately, areas of chronic demyelination develop in which exposed axons are susceptible to degeneration, leading to the secondary progressive neurological dysfunction characteristic of the later stages of MS (Peru et al. 2008; Ruckh et al. 2012).
Schematic representation of the CNS response to demyelination. (a) Oligodendrocyte extending processes to provide myelin internodes to ensheath multiple axons. (b) Demyelinating insult causes oligodendrocyte death, myelin sheath destruction, and axonal exposure. (c) Effective remyelination can replace lost myelin sheaths and enable functional recovery. However, in various demyelinating diseases, including multiple sclerosis, the remyelination response is incomplete, and axons are left exposed and susceptible to degeneration, with resultant clinical neurological decline
The etiology of MS remains poorly understood and the pathogenesis has been incompletely depicted. Genetic susceptibility is likely to be a major contributing factor, with additional influences from environmental factors and, potentially, as yet unidentified pathogens (Sotgiu et al. 2004). MS is a clinically heterogeneous disorder, and symptoms may manifest themselves in a relapsing–remitting or a progressive fashion, with the ultimate trend toward deteriorating neurological function and disability (Lublin et al. 2003). In cases of failed myelination, stimulation of remyelination could alleviate the major underlying causes of disability: impaired conduction by demyelinated neurons and axonal degeneration (Ruckh et al. 2012).
Understanding Developmental Myelination and Regenerative Remyelination
The developmental process of myelination and the adult regenerative process of remyelination share the common objective of ensheathing nerve axons with myelin. They may, therefore, be expected, as a consequence of evolutionary efficiency, to use the same mechanisms (Fancy et al. 2011). The “recapitulation hypothesis” of myelin regeneration states that mechanisms that underlie remyelination following injury are essentially the reinstating of a developmental myelination program (Franklin and Hinks 1999). Therefore, a better understanding of this developmental program is an important objective, which could provide a crucial baseline from which to study remyelination and the reasons for its failure.
In the developing spinal cord, oligodendrocyte precursor cells (OPCs) arise in the motor neuron progenitor (pMN) domain of the ventral neuroepithelium from embryonic day 12.5 (E12.5) under the control of the ventral midline signal Sonic Hedgehog (Shh) (Richardson et al. 1997; Rowitch 2004). A later population of OPCs develops in the dorsal spinal cord, in dorsal neural progenitor domains 1 to 5 from E15.5 (Cai et al. 2005; Vallstedt et al. 2005). Soon after their generation, the OPCs migrate into the surrounding gray and white matter, reaching all regions of the developing CNS. Some OPCs identify axonal targets and differentiate into myelinating oligodendrocytes, while others remain reserved as immature cells throughout the parenchyma.
Given the widespread distribution of oligodendrocytes throughout the CNS, it is somewhat surprising that the founder cells of the oligodendrocyte lineage are highly localized in the developing neural tube while also raising the question of the functional rationale of producing oligodendrocytes at several dorsoventral positions (Miller 2005). Potentially, multiple origins are needed to produce a sufficient number of oligodendrocytes for effective myelination of all axons. Alternatively, separate embryonic origins could signify different functional subgroups of oligodendrocytes that have developed to meet specific needs. Different classes of neurons develop from different regions of the neural tube, so it is possible that glial products also differ (Richardson et al. 2006). Recent data suggests that both dorsal and ventral OPC populations contribute to remyelination (Zhu et al. 2011), but further work is needed to quantify and compare their roles in remyelination and to determine whether both populations are equally affected by disease and aging. Whether one population proves to be more (or less) effective at remyelinating particular tracts, or at remyelinating in general, could influence strategic approaches to myelin regeneration (Tripathi et al. 2011). Furthermore, if oligodendrocytes could be generated using multiple methods, then the potential for harnessing that capacity to promote recovery in the adult CNS would be significantly enhanced (Miller 2005).
OPC Development in the Forebrain
In a similar fashion to the spinal cord, OPCs are generated from multiple locations within the developing forebrain. Their initial production begins in the Nkx2.1-expressing ventral territories, beginning around E12.5. These cells disperse into all regions of the developing brain and are followed by a second wave of developing OPCs emerging from the Gsh2-expressing lateral ganglionic eminence. A final wave of Emx1-positive oligodendrocyte precursors originates dorsally in the cortex itself. Selective destruction of separate waves of OPCs via expression of a diphtheria toxin transgene resulted in neighboring populations spreading into the vacant territory to restore normal oligodendrocyte distribution. Ultimately, mice in this study developed normally, suggesting that any functional difference between the three OPC populations was not crucial. Perhaps this compensatory redundancy serves to ensure rapid, fail-safe myelination and remyelination of the complex mammalian brain (Kessaris et al. 2006; Ventura and Goldman 2006).
Adult OPCs
Cultures of perinatal rat optic nerve cells lead to the first identification of glial progenitors (Raff et al. 1983). By exploiting the expression of a range of molecular markers, including nerve/glial antigen 2 (NG2) and platelet-derived growth factor receptor alpha subunit (PDGFRa), the natural history of glial progenitors was revealed (Ffrench-Constant and Raff 1986; Miller 1996; Richardson et al. 2006). Analysis of the adult CNS subsequently confirmed the presence of multiprocessed glial progenitor cells dispersed throughout the white and gray matter at a density similar to microglial cells, creating a continuous network of cells and cell processes (Chang et al. 2000; Dawson et al. 2003). The existence of these adult progenitors was immediately recognized to have important implications for the repair of demyelinating damage.
The relationship between OPCs in the perinatal CNS and in the adult was not immediately obvious, and it was not until the advent of genetic fate mapping approaches in transgenic mice that it was formally demonstrated that adult cells descended by lineage from their perinatal counterparts (Richardson et al. 2011; Zawadzka et al. 2010). The adult cells do, however, differ from perinatal OPCs in various ways, including specific antigenic markers and growth factor responsiveness. Additionally, the adult cells exhibit a slower basal motility rate and a longer basal cell cycle time (Fancy et al. 2011).
Speculation continues as to whether adult OPCs are, in fact, latent neural stem cells. One study demonstrated that OPCs purified from early postnatal rat optic nerves could be reprogrammed into multipotent stem cells. Primary treatment with fetal calf serum or bone morphogenetic proteins (BMPs) was followed by basic fibroblast growth factor (bFGF) to generate free-floating balls of cells (neurospheres) containing neural stem cells. Individual cells from these neurospheres could give rise to colonies containing a mixture of oligodendrocytes, astrocytes, and neurons, sparking hope that OPCs could be a regenerative resource for neurodegenerative diseases that involve neuronal and glial loss (Kondo and Raff 2000). However, further studies have shown that by far the most common differentiation products of parenchymal OPCs are oligodendrocytes, both in the normal and injured adult CNS. Some Schwann cell and astrocyte generation has also been documented (Richardson et al. 2011; Zawadzka et al. 2010).
It remains to be determined whether OPCs can generate neurons in vivo. Some PDGFRα-positive OPCs have been shown to accumulate in the piriform cortex and acquire NeuN reactivity and morphology resembling piriform projection neurons (Rivers et al. 2008). However, further fate mapping of OPCs in the corpus callosum, motor cortex, and anterior piriform cortex failed to identify neuronal generation (Clarke et al. 2012). Therefore, OPCs may have an underlying potential to generate neurons but may not readily reveal that potential in the environment of the developing or damaged CNS. Future studies are needed to determine whether pharmacological interventions could redirect cells toward a neuronal fate (Richardson et al. 2011).
Defining Adult OPCs
Is there a case for referring to OPCs as adult stem cells? They are self-renewing and multipotent—criteria that are used by some to assign the term “stem cell.” However, the absence of asymmetric cellular division in these cells and their rapid proliferative response to injury indicate that they have much in common with progenitor cells that amplify in transit in other stem cell-containing tissues, such as skin or bone marrow (Franklin and Ffrench-Constant 2008). As a result, the slightly ambiguous term of adult progenitor/stem cell is frequently used.
OPCs are abundant within the adult CNS, suggesting that they may have housekeeping roles beyond their function as a cell source for lesion repair, potentially by contributing to the maintenance of CNS homeostasis. An intriguing finding has been the identification of two distinct electrophysiological populations of OPC. One population expresses voltage-gated sodium and potassium channels, generates action potentials, and senses its environment by receiving excitatory and inhibitory synaptic input from axons while the other lacks action potentials and does not receive synaptic input (Karadottir et al. 2008). These two groups, however, do not correspond to dorsal and ventral developmental origins (Tripathi et al. 2011). Axonal-OPC communication has been shown to affect OPC proliferation and differentiation (Barres and Raff 1993; Li et al. 2010), and blocking action potentials retards myelination (Demerens et al. 1996). Thus, there is a great deal of cross-talk between axons and OPCs to potentially control myelination and remyelination, which could provide novel therapeutic targets (Bergles et al. 2000; Karadottir et al. 2008).
Germinal Niches
In the postnatal forebrain, “germinal niches” of neural stem cells have been identified that represent the remaining sites of neurogenesis that persist into adulthood. These niches are harbored in the sub-ventricular zone (SVZ) lining the lateral ventricles and in the subgranular zone (SGZ) of the dentate gyrus in the hippocampal formation, providing new neurons to the olfactory bulbs and hippocampus, respectively. In addition to neurogenesis, germinal niches act as a cortical source of astrocytes and oligodendrocytes during postnatal development (Kazanis 2009). At present, the extent to which mobilization of adult neural stem cells contributes to myelin repair after injury remains poorly understood (Peru et al. 2008). Demyelination in nearby white matter regions enhances SVZ proliferation, and these cells migrate toward the area of demyelination and differentiate into oligodendrocytes (Nait-Oumesmar et al. 1999). Recent data has demonstrated that the human SVZ becomes reactivated in MS and contains early glial progenitor cells expressing Olig2 and Sox10 transcription factors (Nait-Oumesmar et al. 2007). However, whether these cells can travel to distant and diffuse lesions and contribute to significant repair remains to be seen.
The Biology of Remyelination
Injuries involving oligodendrocyte loss with axonal preservation have the potential to undergo complete cytoarchitectural reconstruction and restoration of the glial compartment, albeit with a thinner and shorter myelin sheath than would be expected for a given axonal diameter. Remyelination involves the generation of new, mature oligodendrocytes. OPCs are the source of remyelinating oligodendrocytes, as shown by genetic fate mapping (Zawadzka et al. 2010), and transplantation studies in which OPCs have been shown to remyelinate areas of demyelination with great efficiency (Groves et al. 1993).
Experimental demyelination studies, involving toxin injection directly into the CNS to create a focal area of demyelination, have revealed characteristic age-dependent patterns of remyelination, which begins with the initiation of an inflammatory reaction in response to toxic damage. Local astrocytes and microglia recognize the disturbance in tissue homeostasis and release a plethora of inflammatory mediators that activate local cells, including OPCs. Upon activation, OPCs switch from a quiescent state to an activated phenotype, becoming sensitive to chemoattractants and mitogens, such as PDGF and fibroblast growth factor (FGF) released by microglia and astrocytes (Murtie et al. 2005; Woodruff et al. 2004). The activation process involves changes in OPC morphology (Levine and Reynolds 1999; Reynolds et al. 2002), as well as the upregulation of several genes. Many of the upregulated genes are also active during developmental oligodendrocyte generation, such as the transcription factors Olig2, Sox2, and Nkx2.2 (Franklin and Ffrench-Constant 2008).
Following activation, the recruitment phase of remyelination begins. OPCs proliferate and migrate into the damaged area to ensure rapid repopulation of the lesion. Once in situ, the OPCs differentiate into mature oligodendrocytes and begin the process of generating a myelin membrane to ensheath exposed axons. The oligodendrocytes must first establish contact with the axon to be remyelinated, before then generating the myelin protein membrane. The membrane is concentrically wrapped and tightly compacted to form the myelin sheath, which is typically thinner and shorter than the pre-lesion sheath, as shown in Fig. 2. However, despite its smaller dimensions, the newly formed myelin sheath appears sufficient to ensure full functional recovery of the axon (Franklin and Ffrench-Constant 2008).
Remyelination can be identified in resin-embedded tissue sections, as examined by light microscopy. The images in this series are transverse sections from the adult rat cerebellar white matter. Cross sections of normally myelinated axons of various diameters can be seen in the left-hand panel. The middle panel shows demyelinated axons, with debris-filled macrophages, 5 days after injection of ethidium bromide. In the right-hand panel, remyelinated axons with typically thin myelin sheaths 4 weeks after demyelination can be seen
Ultimately, remyelination serves to reinstate rapid axonal conduction, resolve functional deficits, and promote axon survival. Studies using OPC transplantation into demyelinated lesions have shown that remyelination protects axons (Irvine and Blakemore 2008), and analysis of MS autopsy samples have demonstrated that axonal survival is greatest in areas that have been remyelinated (Kornek et al. 2000). Future studies will determine whether therapeutic manipulation of remyelination can minimize or even reverse neurodegenerative changes.
The Impact of Aging on Remyelination
Almost every tissue studied has shown age-related decreases in the rate and/or efficiency of normal cellular turnover and regeneration in response to injury, strongly suggesting an age-related decline in stem cell function. The question arises as to whether age-associated declines in tissue regeneration are a consequence of intrinsic aging of stems cells or of impaired stem cell function in the aged tissue environment (Lombard et al. 2005; Park and Gerson 2005; Rando 2006).
The decline in remyelination that occurs with advancing age, compounded by an age-associated increase in the vulnerability of demyelinated axons to atrophy (Irvine and Blakemore 2008), poses a significant barrier to CNS therapy, particularly for long-term demyelinating diseases, such as MS. Although remyelination occurs more slowly in the aged CNS, it can nevertheless proceed to completion. This suggests that it is the rate of remyelination that is affected, rather than the extent (Shields et al. 2000). This declining rate is due to both a decreased efficiency of OPC recruitment into the demyelinated area, and of OPC differentiation into mature, remyelinating oligodendrocytes (Doucette et al. 2010; Shen et al. 2008).
What causes decreased OPC recruitment and differentiation with aging? Studies suggest that intrinsic determinants of OPC behavior and extrinsic environmental signals to which OPCs are exposed are altered in the aged CNS (Franklin and Ffrench-Constant 2008). For example, in vitro studies have revealed age-associated changes in growth factor responsiveness of adult OPCs (Tang et al. 2000; Zhao et al. 2006), while in vivo studies have demonstrated slower recruitment into precursor-depleted white matter of transplanted old adult precursor cells than of young adult cells (Chari et al. 2003). Furthermore, Shen et al. (2008) identified a critical age-associated change in the epigenetic regulation of OPC differentiation during remyelination. OPC differentiation is associated with recruitment of histone deacetylases (HDACs) to the promoter regions of differentiation inhibitors (Marin-Husstege et al. 2002). In aged animals, HDAC recruitment is impaired, resulting in prolonged expression of these inhibitors, delayed OPC differentiation and slower remyelination.
There are many extrinsic environmental signals that change with aging, but the altered inflammatory response is one of the most intensively studied at present. An impaired macrophage response in the aged animal, which is associated with delayed expression of inflammatory cytokines and chemokines, leads to poor clearance of myelin debris and, therefore, to persistent myelin debris-associated differentiation inhibitory proteins (Kotter et al. 2006; Zhao et al. 2006). Changes also occur in the expression of remyelination-associated growth factors, such as IGF1 and TGF-β1, resulting in delayed OPC recruitment and differentiation (Hinks and Franklin 2000).
A key question relating to the development of remyelination therapies is the extent to which age-associated changes can be reversed. Intriguing experiments on skeletal muscle regeneration, using the technique of heterochronic parabiosis, provided clear proof of principle that poor regeneration in aged animals could be rejuvenated (Conboy et al. 2005). A more recent study utilized heterochronic parabiosis to show that remyelination in an aged animal was improved by exposure to a young systemic circulation. The study demonstrated that monocyte recruitment from the young parabiotic partner into the aged mouse lesion contributed to improved remyelination efficiency by enhancing myelin debris clearance (Ruckh et al. 2012). Thus, despite their intrinsic alterations, aged OPCs remain responsive to exogenous pro-differentiation signals and retain their competence for efficient repair. These results provide hope that the endogenous adult OPC is a valid therapeutic target for remyelination-enhancing therapies.
Remyelination Failure
Remyelination is the default repair process following loss of myelin sheaths. However, failure to remyelinate is a major feature of several neurological diseases. While the most profound factor affecting remyelination is aging, as discussed above, other factors play important roles. Theoretically, remyelination could fail due to a primary deficiency in precursor cells, a failure of precursor cell recruitment, or a failure of precursor cell differentiation and maturation. Data from experimental studies has disproved the first theory by demonstrating that OPCs are remarkably efficient at repopulating regions from which they have been depleted (Chari and Blakemore 2002). In addition, repeated episodes of focal demyelination in the same area neither deplete OPCs nor prevent subsequent remyelination (Penderis et al. 2003). OPC density in chronic lesions is, on average, lower than in normal white matter. However, OPC density can be as high as in normal white matter or remyelinated lesions, suggesting that OPC availability is not a limiting factor for remyelination (Kuhlmann et al. 2008). Nevertheless, the situation might be different when an area of tissue is exposed to a sustained demyelinating insult; in this case remyelination impairment seems to be due, at least in part, to decreased OPC availability (Mason et al. 2004).
In the second proposed mechanism, remyelination may fail due to inadequate OPC recruitment into the lesioned area, which involves proliferation, migration, and repopulation. Large lesions require a more protracted recruitment phase, and it has been calculated that it could take up to five months for a 2-cm lesion to become repopulated with OPCs (Chari and Blakemore 2002). OPC recruitment into areas of demyelination may fail owing to disturbances in local expression of OPC migration guidance cues, such as semaphorin 3A and 3F (Williams et al. 2007). This is likely to be more significant in aging, because older OPCs seem to be intrinsically less responsive to recruitment signals (Chari et al. 2003).
Most evidence, however, points to the importance of the third mechanism, whereby OPC differentiation and maturation is the rate-limiting step. OPCs unable to differentiate into mature oligodendrocytes were initially identified in MS lesions using the oligodendrocyte lineage marker O4 (Wolswijk 1998). This was subsequently verified with the OPC marker NG2 and most recently with Olig2 and Nkx2.2 (Chang et al. 2002; Kuhlmann et al. 2008). One possible explanation for failed differentiation is that chronically demyelinated lesions contain factors that inhibit precursor differentiation. The first factor to be implicated was the Notch–jagged pathway, a negative regulator of OPC differentiation (Wang et al. 1998). LINGO-1 and Wnt signaling have since been identified as negative regulators of differentiation (Fancy et al. 2009, 2010; Huang and Franklin 2011), while accumulation of glycosaminoglycan hyaluronan in MS lesions might further contribute to an environment that is not conducive to remyelination (Back et al. 2005).
In addition to the presence of inhibitory factors, the absence of stimulatory factors may play a role in remyelination failure. MS lesions are rarely devoid of any inflammatory activity. However, chronic lesions are relatively noninflammatory compared with acute lesions and constitute a less active environment in which OPC differentiation might become quiescent. The complexity of the environment needed for remyelination, along with the multitude of cell types, growth factors, and signaling molecules, would suggest that the presence or absence of a single factor is unlikely to make a significant difference. Additionally, efficient remyelination might depend as much on the precise timing of action of these cells and molecules, as on their presence or absence within the lesion. For example, a pro-recruitment environment must be maintained for a sufficient amount of time to allow the lesion to become sufficiently repopulated with OPCs to enable complete remyelination; only when repopulation is complete should the environment shift to one that supports differentiation (Franklin and Ffrench-Constant 2008). A process as intricate as remyelination relies on the carefully coordinated interplay of a wide spectrum of cells and signaling molecules, making it susceptible to failure at multiple stages and through numerous causes. Future studies of therapeutic strategies for promoting remyelination have an enormous playing field on which to work.
Enhancing Remyelination
At present, there are no clinical therapies that promote remyelination. Two major approaches are currently being tested in animal models of demyelination: cell replacement by transplantation (exogenous therapies) and promotion of repair by resident stem and precursor cell populations in the adult CNS (endogenous repair) (Franklin 2002).
Exogenous Therapies
The use of transplanted stem cells for treating demyelinating diseases is an exciting prospect for patients facing chronic, debilitating illness. The ideal stem cell should be capable of effective remyelination in the presence of an ongoing inflammatory insult. Additionally, it should provide positive effects on the local environment, such as neuroprotection, axonal trophic support, and immune modulation. Given the multifocal nature of many demyelinating diseases, an ability to travel within and between lesions following systemic administration would be beneficial.
Transplantation therapies entail administration of cells into the demyelinated lesion. The CNS presents a particularly inaccessible target for cell delivery, especially in diseases with diffuse lesions. Repeated local injections are technically demanding and carry the risk of intracerebral hemorrhage, advocating the development of alternative experimental approaches, including intracerebroventricular and intravenous injection (Ben-Hur 2011). Stem and precursor cells injected into the lateral ventricles have been shown to enter the CNS and generate myelin-forming oligodendrocytes in widely dispersed areas (Ben-Hur et al. 2003; Pluchino et al. 2003). This route presents a potential therapeutic approach, although the risk of cell aggregates blocking intraventricular flow of cerebrospinal fluid remains a theoretical concern. Intravenous administration is straightforward to perform, but significant subsequent stem cell recruitment into CNS lesions has yet to be demonstrated (Pluchino et al. 2005).
The effectiveness of any stem cell transplantation and remyelination therapy depends on the ability to suppress the effect of any ongoing disease process on new oligodendrocytes. In diseases such as periventricular leukomalacia (PvL), this is not an issue, because oligodendrocyte loss is due to an ischemic insult occurring in utero or at the time of birth and is, therefore, transient. Equally, for the leukodystrophies, such as Pelizaeus–Merzbacher disease, cells delivered by transplantation will not have the pathogenic genetic mutation and will, therefore, not be subjected to the cell autonomous effects of the genetic defect. For MS, however, the issue of ongoing damage is a major concern. Furthermore, injecting precursor cells into an environment that already contains an abundance of normal precursor cells does not seem wholly rational. Exogenous therapies are likely to show the most promise in specific demyelinating diseases that lack normal OPCs and do not have an ongoing disease insult (Franklin and Ffrench-Constant 2008).
Neural Stem Cells
The CNS contains a heterogeneous population of mitotically active stem and precursor cells that are capable of generating all postmitotic cell lineages of the CNS. Grouped together, these neural stem cells (NSCs) have been explored for their potential therapeutic use in demyelination (Gupta et al. 2012; Kingwell 2012; Uchida et al. 2012). NSCs can be obtained from embryonic (Fig. 3), fetal, neonatal, or adult CNS tissues. Induced pluripotent stem cells and autologous biopsies may become viable sources in the future (Ben-Hur 2011), with recent work validating the generation of functional postmitotic neurons and oligodendrocytes (Czepiel et al. 2011; Karumbayaram et al. 2009; Swistowski et al. 2010; Tsuji et al. 2010).
Intravenous administration of NSCs led to the amelioration of symptoms in experimental autoimmune encephalomyelitis (EAE), an animal model of MS (Pluchino et al. 2003), suggesting that these cells might enter the CNS from the cerebral vasculature. Magnetic resonance tracking has since shown incorporation of NSCs into CNS lesions following transplantation (Politi et al. 2007) and human NSCs, when implanted into demyelinated rat spinal cord, were shown to initiate a remyelination program (Akiyama et al. 2001). A recent study has shown that human NSCs can differentiate into myelin-producing cells when transplanted into the brains of myelin-deficient mice, with extensive migration of the transplanted cells along white matter tracts and increasing myelination, as detected by magnetic resonance imaging (MRI) (Uchida et al. 2012). Similar findings have been reported in humans, in which a phase I, open-label trial was undertaken in four young males with global hypomyelination due to the congenital leukodystrophy, Pelizaeus–Merzbacher disease. The patients underwent NSC transplantation into the superficial subcortical white matter. Over a 12-month follow-up period, MRI revealed enhanced myelination around implantation sites, which was associated with stable or modest gains in motor and cognitive function. The findings suggested donor-derived myelination in the cell transplantation region (Gupta et al. 2012), and a phase II efficacy study of human NSCs is currently being planned (Kingwell 2012).
Conflicting studies have, however, revealed poor NSC differentiation into neuronal and glial cells, with minimal replacement of damaged cells (Martino and Pluchino 2006). Growing evidence suggests that much of the beneficial effects of NSC transplantation result from modulation of the CNS microenvironment rather than by direct cell replacement. Systemically delivered NSCs have been shown to migrate to areas of CNS damage and subsequently release immunomodulatory soluble molecules, such as cytokines and chemokines, to ultimately promote recovery in EAE mice (Einstein et al. 2003; Pluchino et al. 2005) and nonhuman primates (Pluchino et al. 2009). Furthermore, an immunosuppressive effect of NSCs on T-cell populations has been documented in the perivascular space of the CNS and in peripheral lymph nodes, with a pro-apoptotic influence, as well as reduced proliferation of antigen-specific encephalitogenic T cells (Einstein et al. 2003, 2007; Pluchino et al. 2005).
In addition to protecting the CNS from autoimmunity, NSCs have been shown to reduce astroglial scarring, enhance endogenous progenitor cell function, and increase survival of mature neural cells (Martino et al. 2010; Martino and Pluchino 2006; Pluchino et al. 2005). The induction of an environment permissive to axonal regeneration has been documented (Pluchino et al. 2005), as well as neurotrophic growth factor production, including nerve growth factor and brain-derived neurotrophic factor (Lu et al. 2003). Additionally, transplanted NSC induction of matrix metalloproteinases has been detected, potentially with the purpose of degrading the extracellular matrix, thereby facilitating axonal outgrowth and passage through the inhibitory glial scar (Zhang et al. 2007).
Enhanced remyelination efficiency has been reported following NSC administration in a spinal cord injury model, with both endogenous OPCs and the transplanted cells contributing to this enhanced repair (Keirstead et al. 2005). In aged rodent models, endogenous OPCs underwent increased proliferation and differentiation following NSC transplantation, hence improving the rate and extent of remyelination (Einstein et al. 2009). Therefore, growing evidence would suggest that NSCs promote CNS repair and that they act not through direct cell replacement but by modulation of the immune response and support of endogenous repair activities exerted by resident precursor cells. Translating these initial findings into controlled clinical trials to obtain rigorous safety and efficacy data will be the next challenge.
Oligodendrocyte Precursor Cells
When transplanted into regions of demyelination, OPCs elicit remyelination and restore neurological function (Windrem et al. 2004, 2008) and thus may represent an alternative source for stem cell therapies. One significant disadvantage, however, is the apparent limited ability for OPCs to migrate through normal intact regions of the CNS (Blakemore et al. 2000; Gensert and Goldman 1997), thereby necessitating direct targeting of individual lesions for transplantation. Sourcing sufficient numbers of autologous OPCs presents another difficulty, although the potential future use of autologous-induced pluripotent stems cells may provide a solution.
Adult OPCs exhibit a protracted cell cycle time and can be considered to exist in a somewhat dormant state, requiring prolonged growth factor stimulation before becoming proliferative, responsive cells (Psachoulia et al. 2009; Wolswijk and Noble 1992). As a result, precursors derived from embryonic (Fig. 4) or early postnatal CNS tissues may offer superior migratory, proliferative, and differentiation capabilities and may, therefore, represent a more effective remyelinating cell source than endogenous cells (Ben-Hur 2011). Results from one study demonstrated that fetal OPCs, following transplantation into the congenitally dysmyelinated brain, migrated more widely and engrafted more efficiently compared with adult OPCs. However, the adult cells generated oligodendrocytes more efficiently and were able to myelinate the recipient brains more rapidly (Windrem et al. 2004). Further work is needed to determine the potential efficacy of OPCs within the clinically diseased CNS, as well as to clarify the range of therapeutic effects. At present, there is no evidence to indicate that transplanted OPCs, whether administered systemically or locally into the CNS, have immunomodulatory properties (Archer et al. 1997; Ben-Hur 2011; Brustle et al. 1999; Jadasz et al. 2012).
Olfactory Ensheathing Cells
Olfactory ensheathing cells (OECs) are differentiated glial cells that ensheath the axons of the first cranial nerve and can be isolated from nasal mucosal biopsies, thereby providing a readily accessible autologous source. OECs appear capable of remyelinating experimentally demyelinated axons (Barnett et al. 2000; Franklin et al. 1996; Imaizumi et al. 1998; Kato et al. 2000), with additional neurotrophic factor secretion (Lipson et al. 2003; Woodhall et al. 2001) and promotion of axonal growth (Chung et al. 2004; Jiao et al. 2011; Ramon-Cueto et al. 2000; Runyan and Phelps 2009). Embryonic-derived OECs were found to achieve the most efficient remyelination within the rat spinal cord, compared with fetal and adult sources (Coutts et al. 2012). Preliminary trials of autologous OEC transplantation into the injured spinal cord in both humans and dogs have demonstrated the feasibility and safety of this technique (Feron et al. 2005; Jeffery et al. 2005; Lima et al. 2006; Mackay-Sim et al. 2008), while a recent study demonstrated improved locomotor outcome in chronic, clinical spinal cord injury in the dog (Granger et al. 2012). Further work is needed to ascertain the long-term benefits of such therapies and their potential role as a component of a multifaceted, therapeutic approach to demyelinating disease.
Schwann Cells
Schwann cells are neural crest-derived myelinating cells of the peripheral nervous system (PNS). However, they have been found to mediate CNS remyelination in both experimental models and human demyelinating disease (Dusart et al. 1992; Itoyama et al. 1983). It was presumed that these central Schwann cells were derived from the peripheral nervous system, from sites such as the spinal and cranial nerve roots, and that they migrated into the CNS after disruption to the astrocytic glia limitans (Duncan and Hoffman 1997; Franklin and Blakemore 1993). However, fate mapping has confirmed that the majority of Schwann cells in areas of CNS remyelination are derived from OPCs (Zawadzka et al. 2010). What could cause CNS-resident precursors to become Schwann cells, which are normally found only in the PNS? In a study using the EAE model, it was found that OPCs robustly generate oligodendrocytes, but very few Schwann cells, suggesting that the inflammatory microenvironment may exert a strong influence on the direction of OPC differentiation (Tripathi et al. 2010). In particular, BMPs, members of the transforming growth factor-β family of secreted growth factors, may stimulate Schwann cell differentiation. Pretreatment of adult OPCs with BMPs in vitro—prior to transplantation into demyelinated spinal cord—results in enhanced Schwann cell remyelination (Crang et al. 2004). Conversely, overexpression of Noggin, an inhibitor of BMP signaling, reduces Schwann cell remyelination (Talbott et al. 2005).
The implications of Schwann cell remyelination of CNS axons remain unclear. The major constituent of Schwann cell myelin is myelin protein zero (Mpz), compared with proteolipid protein (PLP) in oligodendrocyte myelin. Peripheral nerve myelin is generally not targeted in the autoimmune pathology of MS, potentially due to various myelin constituents providing a reduced antigenic target for immune cells (Duncan and Hoffman 1997). Thus, theoretically, remyelination with peripheral myelin may hinder progression of further autoimmune-mediated demyelination and so its promotion may prove beneficial in the treatment of MS. Both Schwann cell and oligodendrocyte remyelination are associated with a return of saltatory conduction (Smith et al. 1979); however, the relative ability of Schwann cells or oligodendrocytes to promote axon survival, a major function of myelin (Nave and Trapp 2008), has yet to be established. Further work is needed to ascertain whether OPC differentiation into Schwann cells has a beneficial or deleterious effect compared to oligodendrocyte remyelination.
Schwann cells isolated from a sural or sciatic nerve biopsy, expanded in culture, and transplanted into the CNS resulted in the production of compact myelin and restored normal conduction velocity (Bai et al. 2012; Baron-Van Evercooren et al. 1992; Blakemore and Crang 1985; Honmou et al. 1996; Kohama et al. 2001). Further work has assessed Schwann cell precursors isolated from the neural crest—specifically the boundary cap cells, which form the border between the developing PNS and CNS—and found these cells demonstrated enhanced migratory and myelinating properties (Aquino et al. 2006; Zujovic et al. 2010). Taken together, these studies suggest that Schwann cells may represent a promising candidate for cell replacement therapy in myelin disorders.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) reside in the bone marrow and most connective tissues and are characterized by their ability to differentiate into cells of the mesenchymal lineage, including osteoblasts, adipocytes, and chondrocytes (Minguell et al. 2001). MSCs have powerful immunomodulatory effects when administered systemically, and demonstrate “therapeutic plasticity” by adapting their fate and function to the specific environmental needs arising from different pathological conditions. Intravenously infused MSCs have been shown to reduce demyelination, increase neuroprotection, modulate inflammation, and enhance endogenous OPC differentiation and remyelination in the EAE model (Bai et al. 2009; Barhum et al. 2010; Gerdoni et al. 2007; Gordon et al. 2008; Kassis et al. 2008; Lanza et al. 2009; Lu et al. 2003; Zhang et al. 2005, 2006). The immunomodulatory effects of MSCs include promotion of T-cell anergy, stimulation of regulatory T-cell production, reduction of B-cell proliferation and differentiation, and interference with the antigen-presenting function of dendritic cells, with overall reductions in encephalitogenic T-cell proliferation, inflammatory CNS infiltrates, and, ultimately, demyelination (Beyth et al. 2005; Gordon et al. 2008; Kassis et al. 2008; Selmani et al. 2008; Uccelli et al. 2006; Zappia et al. 2005). In a phase I/II, open-safety clinical trial, MSCs were administered intravenously and intrathecally into patients with chronic MS or amyotrophic lateral sclerosis, a motor neuron disease. No major adverse effects were encountered following treatment, but minor adverse effects that included headache and transient fever were reported. An immunomodulatory effect was detected in the patients, including an increased proportion of regulatory T cells and a decreased lymphocyte proliferative response, while clinical follow-up showed an improvement in the mean expanded disability status scale (Karussis et al. 2010).
Several lines of evidence suggest that MSCs directly protect neural tissues through paracrine mechanisms (Li et al. 2002; Minguell et al. 2001; Uccelli et al. 2008) and in EAE mice that received MSCs axonal loss was significantly reduced (Zhang et al. 2006). MSCs are presumed to promote survival of damaged tissues by secreting large amounts of bioactive factors that inhibit scarring and apoptosis, as well as stimulating angiogenesis and mitosis of tissue-intrinsic stem or precursor cells. Studies have confirmed the presence of soluble factors that promote oligodendrocyte and neuronal survival, including hepatocyte growth factor, in MSC-conditioned medium (Bai et al. 2012; Steffenhagen et al. 2012), as well as having a strong antioxidant effect (Lanza et al. 2009). In a recent phase II clinical trial, autologous MSCs were safely given to patients with secondary progressive MS involving the visual pathways. Treated patients showed evidence of structural, functional, and physiological improvement in some visual endpoints, suggesting that the transplanted cells were inducing neuroprotection (Connick et al. 2012).
Intravenously administered MSCs have been visualized within demyelinated CNS lesions (Azizi et al. 1998; Gordon et al. 2010; Zhang et al. 2006), and some studies suggest that MSCs can transdifferentiate into cells of the neuroectodermal lineage (Deng et al. 2001; Li et al. 2000; Mahmood et al. 2001; Padovan et al. 2003; Sanchez-Ramos et al. 2000; Woodbury et al. 2000). These results suggest that MSCs could contribute to cell replacement in the injured CNS. However, there is a lack of clear evidence that transdifferentiation occurs with any frequency within the damaged CNS, and there is no data to suggest that MSCs differentiate into oligodendrocytes (Gerdoni et al. 2007; Gordon et al. 2010; Jadasz et al. 2012; Uccelli et al. 2011). Nevertheless, various studies have demonstrated the ability of MSCs to recruit local OPCs to the site of demyelination, thereby inducing oligodendrogenesis and promoting remyelination (Bai et al. 2009; Constantin et al 2009; Cristofanilli et al. 2011; Zhang et al. 2005). Further work has revealed that MSCs activate oligodendrogenic differentiation and the maturation program in NSCs (Rivera et al. 2006), and when NSCs are transplanted with MSCs, they acquire an oligodendrocytic phenotype (Rivera et al. 2009).
Irrespective of little or no integration into the CNS, MSCs not only act in the periphery to inhibit autoimmune attack of the CNS, but they can also support tissue repair within the CNS (Uccelli et al. 2011). Given the abundance and accessibility of MSCs, the potential for autologous transplant purposes, feasibility of systemic administration, as well as relative simplicity of in vitro cultures, MSCs represent an attractive cellular tool for remyelination therapies. However, in cases of chronic inflammation and neural damage, MSCs appear unable to ameliorate the clinical course of disease and improve neurological parameters, with their beneficial effects seemingly limited to acute disorders involving active inflammation (Uccelli et al. 2011; Zappia et al. 2005). Thus, the timing of transplantation may prove to be crucial. Cells introduced during the non-active phase of the disease could fail to survive until a time when their actions are required, while those introduced later in the disease progression may miss the window of active inflammation for which their immunomodulatory properties are adapted (Ben-Hur 2011; Uccelli et al. 2011).
Further work is now needed to ascertain the most appropriate diseases for MSC use, as the therapeutic dose range, optimal frequency, timing, and route of administration, with future rigorous controlled clinical trials focusing on these parameters in combination with the generation of improved safety and efficacy data.
In summary, the stem cell approach to the treatment of demyelinating diseases has shifted from focal injection of cells toward a systemic delivery and from the regenerative potential of precursor cells toward their immunomodulatory properties. However, it remains to be determined whether cellular injection is a better immunomodulatory strategy than the many pharmacological approaches to immunomodulation that currently exist. Furthermore, long-term survival of transplanted cells remains to be seen, particularly in an environment as “hostile” as MS. Encouraging results have emerged from preliminary clinical trials, but markers for myelin repair and neuroprotection are still lacking (Franklin et al. 2012). Their development would facilitate the determination of clinical efficacy of stem cell therapies in demyelinating disease.
Endogenous Therapies
Because remyelination can be complete, and because the precursor cells responsible for remyelination are abundant throughout the adult CNS, a conceptually attractive approach to enhancing remyelination is to target the endogenous regenerative process. Such an approach necessitates a detailed understanding of remyelination mechanisms and why it fails to then pinpoint areas suitable for therapeutic manipulation. Identifying the key regulatory pathways involved should enable the design of regenerative medicines that enhance remyelination, thereby also protecting axons. At present, research efforts are focused on stimulating OPCs directly and creating the appropriate local environment conducive to effective remyelination.
The inhibition of OPC differentiation in chronically demyelinated lesions presents a vital therapeutic target whereby direct OPC stimulation or antagonism of inhibitory factors could encourage effective remyelination. Several negative regulators of OPC differentiation have recently been identified, including LINGO-1 and Wnt signaling. LINGO-1 is a protein made by oligodendrocytes and neurons that inhibits oligodendrocyte differentiation and myelination. Intrathecal delivery of a LINGO-1 antibody in the EAE model was shown to enhance remyelination and increase axonal integrity, suggesting that it could serve as a candidate for therapeutic remyelination enhancement (Mi et al. 2004). Wnt signaling controls temporal and spatial regulation of cell proliferation, migration, and survival and has been shown to negatively regulate OPC differentiation during remyelination (Fancy et al. 2009). Given the growing interest to develop Wnt inhibitors as cancer therapeutics, it may prove possible in the future to utilize these therapies for OPC differentiation stimulation and remyelination enhancement (Huang and Franklin 2011).
While most studies have focused on the negative regulators of OPC differentiation, positive regulators are now emerging, including leukemia inhibitory factor (LIF) and retinoid X receptors (RXRs). LIF is produced by myelin-reactive T cells infiltrating demyelinating lesions and has been shown to enhance oligodendrocyte survival and myelin repair while additionally decreasing demyelination (Deverman and Patterson 2012; Marriott et al. 2008). RXRs are nuclear receptors that regulate cell proliferation and differentiation and are highly expressed in active and remyelinating MS lesions. RXR knockout mice show an accumulation of undifferentiated OPCs in demyelinated lesions, while RXR agonists, such as 9-cis-retinoic acid, stimulate OPCs to differentiate in culture and enhance remyelination following focal demyelination in the rat CNS. RXR agonists can also attenuate inflammation by regulating macrophage activity. These agonists could provide dual benefits of immunomodulation and acceleration of OPC differentiation (Huang et al. 2011).
Pharmacological manipulation to generate a favorable environment for endogenous stem/precursor cells of the CNS is likely to generate new and valuable treatment options for demyelinating diseases. Furthermore, “environment-enhancing” therapeutics could be combined with stem cell transplantation therapies to improve survival and function of transplanted cells. In particular, manipulation of the inflammatory response to recreate the active environment needed for remyelination has been under considerable investigation. Much success has already been achieved with treatments such as interferon-β, which acts to shift a detrimental Th1 or Th17 immune response into a protective Th2 response, as well as alemtuzumab, an anti-CD52 humanized monoclonal antibody that causes long-term T-cell depletion (Cohen et al. 2012; Coles et al. 2012). The true complexity of the signaling environment needed for successful remyelination is being uncovered, as well as the enormous lesion diversity between diseases and between individuals with the same disease (Franklin et al. 2012). As this understanding expands, new and exciting treatment options are likely to come to light.
Conclusions
Since the discovery that OPCs are widely dispersed throughout the adult CNS, there has been a growing interest in regenerative therapies for demyelinating diseases. Much of the research has, and continues to, focus on unraveling the complex biology of remyelination to identify new means of therapeutic manipulation. Pharmacological approaches to enhance endogenous OPC differentiation and to create a pro-remyelination environment could ultimately be combined with exogenous cell transplantation therapies to provide an array of treatments to tackle these heterogeneous diseases and provide the best possible outcome for affected patients.
References
Akiyama, Y., Honmou, O., Kato, T., Uede, T., Hashi, K., & Kocsis, J. D. (2001). Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Experimental Neurology, 167, 27–39.
Aquino, J. B., Hjerling-Leffler, J., Koltzenburg, M., Edlund, T., Villar, M. J., & Ernfors, P. (2006). In vitro and in vivo differentiation of boundary cap neural crest stem cells into mature Schwann cells. Experimental Neurology, 198, 438–449.
Archer, D. R., Cuddon, P. A., Lipsitz, D., & Duncan, I. D. (1997). Myelination of the canine central nervous system by glial cell transplantation: A model for repair of human myelin disease. Nature Medicine, 3, 54–59.
Azizi, S. A., Stokes, D., Augelli, B. J., DiGirolamo, C., & Prockop, D. J. (1998). Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats–similarities to astrocyte grafts. Proceedings of the National Academy of Sciences of the United States of America, 95, 3908–3913.
Back, S. A., Tuohy, T. M., Chen, H., Wallingford, N., Craig, A., Struve, J., et al. (2005). Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nature Medicine, 11, 966–972.
Bai, L., Lennon, D. P., Caplan, A. I., DeChant, A., Hecker, J., Kranso, J., et al. (2012). Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nature Neuroscience, 15, 862–870.
Bai, L., Lennon, D. P., Eaton, V., Maier, K., Caplan, A. I., Miller, S. D., et al. (2009). Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia, 57, 1192–1203.
Barhum, Y., Gai-Castro, S., Bahat-Stromza, M., Barzilay, R., Melamed, E., & Offen, D. (2010). Intracerebroventricular transplantation of human mesenchymal stem cells induced to secrete neurotrophic factors attenuates clinical symptoms in a mouse model of multiple sclerosis. Journal of molecular neuroscience, 41, 129–137.
Barnett, S. C., Alexander, C. L., Iwashita, Y., Gilson, J. M., Crowther, J., Clark, L., et al. (2000). Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain, 123(Pt 8), 1581–1588.
Baron-Van Evercooren, A., Gansmuller, A., Duhamel, E., Pascal, F., & Gumpel, M. (1992). Repair of a myelin lesion by Schwann cells transplanted in the adult mouse spinal cord. Journal of Neuroimmunology, 40, 235–242.
Barres, B. A., & Raff, M. C. (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature, 361, 258–260.
Ben-Hur, T. (2011). Cell therapy for multiple sclerosis. Neurotherapeutics, 8, 625–642.
Ben-Hur, T., Einstein, O., Mizrachi-Kol, R., Ben-Menachem, O., Reinhartz, E., Karussis, D., et al. (2003). Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia, 41, 73–80.
Bergles, D. E., Roberts, J. D., Somogyi, P., & Jahr, C. E. (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature, 405, 187–191.
Beyth, S., Borovsky, Z., Mevorach, D., Liebergall, M., Gazit, Z., Aslan, H., et al. (2005). Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood, 105, 2214–2219.
Blakemore, W. F., & Crang, A. J. (1985). The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. Journal of the Neurological Sciences, 70, 207–223.
Blakemore, W. F., Gilson, J. M., & Crang, A. J. (2000). Transplanted glial cells migrate over a greater distance and remyelinate demyelinated lesions more rapidly than endogenous remyelinating cells. Journal of Neuroscience Research, 61, 288–294.
Boison, D., & Stoffel, W. (1994). Disruption of the compacted myelin sheath of axons of the central nervous system in proteolipid protein-deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 91, 11709–11713.
Brustle, O., Jones, K. N., Learish, R. D., Karram, K., Choudhary, K., Wiestler, O. D., et al. (1999). Embryonic stem cell-derived glial precursors: A source of myelinating transplants. Science, 285, 754–756.
Cai, J., Qi, Y., Hu, X., Tan, M., Liu, Z., Zhang, J., et al. (2005). Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron, 45, 41–53.
Chang, A., Nishiyama, A., Peterson, J., Prineas, J., & Trapp, B. D. (2000). NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. The Journal of Neuroscience, 20, 6404–6412.
Chang, A., Tourtellotte, W. W., Rudick, R., & Trapp, B. D. (2002). Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. The New England Journal of Medicine, 346, 165–173.
Chari, D. M., & Blakemore, W. F. (2002). New insights into remyelination failure in multiple sclerosis: Implications for glial cell transplantation. Multiple Sclerosis, 8, 271–277.
Chari, D. M., Crang, A. J., & Blakemore, W. F. (2003). Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. Journal of Neuropathology and Experimental Neurology, 62, 908–916.
Chung, R. S., Woodhouse, A., Fung, S., Dickson, T. C., West, A. K., Vickers, J. C., et al. (2004). Olfactory ensheathing cells promote neurite sprouting of injured axons in vitro by direct cellular contact and secretion of soluble factors. Cellular and Molecular Life Sciences, 61, 1238–1245.
Clarke, L. E., Young, K. M., Hamilton, N. B., Li, H., Richardson, W. D., & Attwell, D. (2012). Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. The Journal of Neuroscience, 32, 8173–8185.
Cohen, J. A., Coles, A. J., Arnold, D. L., Confavreux, C., Fox, E. J., et al. (2012). Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet, 380, 1819–1828.
Coles, A. J., Twyman, C. L., Arnold, D. L., Cohen, J. A., Confavreux, C., Fox, E. J., et al. (2012). Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet, 380, 1829–1839.
Conboy, I. M., Conboy, M. J., Wagers, A. J., Girma, E. R., Weissman, I. L., & Rando, T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature, 433, 760–764.
Connick, P., Kolappan, M., Crawley, C., Webber, D. J., Patani, R., Patani, R., et al. (2012). Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurology, 11, 150–156.
Constantin, G., Marconi, S., Rossi, B., Angiari, S., Calderan, L., Anghileri, E., et al. (2009). Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells, 27, 2624–2635.
Coutts, D. J., Humphries, C. E., Zhao, C., Plant, G. W., & Franklin, R. J. (2012). Embryonic-derived olfactory ensheathing cells remyelinate focal areas of spinal cord demyelination more efficiently than neonatal or adult derived cells. Cell Transplantation, 22(7), 1249–1261.
Crang, A. J., Gilson, J. M., Li, W. W., & Blakemore, W. F. (2004). The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS. The European Journal of Neuroscience, 20, 1445–1460.
Cristofanilli, M., Harris, V. K., Zigelbaum, A., Goossens, A. M., Lu, A., Rosenthal, H., et al. (2011). Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells and Development, 20, 2065–2076.
Czepiel, M., Balasubramaniyan, V., Schaafsma, W., Stancic, M., Mikkers, H., Huisman, C., et al. (2011). Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia, 59, 882–892.
Dawson, M. R., Polito, A., Levine, J. M., & Reynolds, R. (2003). NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Molecular and Cellular Neurosciences, 24, 476–488.
Demerens, C., Stankoff, B., Logak, M., Anglade, P., Allinquant, B., Couraud, F., et al. (1996). Induction of myelination in the central nervous system by electrical activity. Proceedings of the National Academy of Sciences of the United States of America, 93, 9887–9892.
Deng, W., Obrocka, M., Fischer, I., & Prockop, D. J. (2001). In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochemical and Biophysical Research Communications, 282, 148–152.
Deverman, B. E., & Patterson, P. H. (2012). Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. The Journal of Neuroscience, 32, 2100–2109.
Doucette, J. R., Jiao, R., & Nazarali, A. J. (2010). Age-related and cuprizone-induced changes in myelin and transcription factor gene expression and in oligodendrocyte cell densities in the rostral corpus callosum of mice. Cellular and Molecular Neurobiology, 30, 607–629.
Duncan, I. D., & Hoffman, R. L. (1997). Schwann cell invasion of the central nervous system of the myelin mutants. Journal of Anatomy, 190(Pt 1), 35–49.
Dusart, I., Marty, S., & Peschanski, M. (1992). Demyelination, and remyelination by Schwann cells and oligodendrocytes after kainate-induced neuronal depletion in the central nervous system. Neuroscience, 51, 137–148.
Edgar, J. M., & Nave, K. A. (2009). The role of CNS glia in preserving axon function. Current Opinion in Neurobiology, 19, 498–504.
Einstein, O., Fainstein, N., Vaknin, I., Mizrachi-Kol, R., Reihartz, E., Grigoriadis, N., et al. (2007). Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Annals of Neurology, 61, 209–218.
Einstein, O., Friedman-Levi, Y., Grigoriadis, N., & Ben-Hur, T. (2009). Transplanted neural precursors enhance host brain-derived myelin regeneration. The Journal of Neuroscience, 29, 15694–15702.
Einstein, O., Karussis, D., Grigoriadis, N., Mizrachi-Kol, R., Reinhartz, E., Abramsky, O., et al. (2003). Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Molecular and Cellular Neurosciences, 24, 1074–1082.
Fancy, S. P., Baranzini, S. E., Zhao, C., Yuk, D. I., Irvine, K. A., Kaing, S., et al. (2009). Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes & Development, 23, 1571–1585.
Fancy, S. P., Chan, J. R., Baranzini, S. E., Franklin, R. J., & Rowitch, D. H. (2011). Myelin regeneration: A recapitulation of development? Annual Review of Neuroscience, 34, 21–43.
Fancy, S. P., Kotter, M. R., Harrington, E. P., Huang, J. K., Zhao, C., Rowitch, D. H., et al. (2010). Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Experimental Neurology, 225, 18–23.
Feron, F., Perry, C., Cochrane, J., Licina, P., Nowitzke, A., Urquhart, S., et al. (2005). Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain, 128, 2951–2960.
Ffrench-Constant, C., & Raff, M. C. (1986). Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature, 319, 499–502.
Franklin, R. J. (2002). Why does remyelination fail in multiple sclerosis? Nature reviews. Neuroscience, 3, 705–714.
Franklin, R. J., & Blakemore, W. F. (1993). Requirements for Schwann cell migration within CNS environments: A viewpoint. International Journal of Developmental Neuroscience, 11, 641–649.
Franklin, R. J., & Ffrench-Constant, C. (2008). Remyelination in the CNS: From biology to therapy. Nature Reviews. Neuroscience, 9, 839–855.
Franklin, R. J., Ffrench-Constant, C., Edgar, J. M., & Smith, K. J. (2012). Neuroprotection and repair in multiple sclerosis. Nature Reviews. Neurology, 8, 624–634.
Franklin, R. J., Gilson, J. M., Franceschini, I. A., & Barnett, S. C. (1996). Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia, 17, 217–224.
Franklin, R. J., & Hinks, G. L. (1999). Understanding CNS remyelination: Clues from developmental and regeneration biology. Journal of Neuroscience Research, 58, 207–213.
Gensert, J. M., & Goldman, J. E. (1997). Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron, 19, 197–203.
Gerdoni, E., Gallo, B., Casazza, S., Musio, S., Bonanni, I., Pedemonte, E., et al. (2007). Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Annals of Neurology, 61, 219–227.
Gordon, D., Pavlovska, G., Glover, C. P., Uney, J. B., Wraith, D., & Scolding, N. J. (2008). Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neuroscience Letters, 448, 71–73.
Gordon, D., Pavlovska, G., Uney, J. B., Wraith, D. C., & Scolding, N. J. (2010). Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. Journal of Neuropathology and Experimental Neurology, 69, 1087–1095.
Granger, N., Blamires, H., Franklin, R. J. M., & Jeffery, N. D. (2012). Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain: a journal of neurology, 135, 3227–3237.
Griffiths, I., Klugmann, M., Anderson, T., Yool, D., Thomson, C., Schwab, M. H., et al. (1998). Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science, 280, 1610–1613.
Groves, A. K., Barnett, S. C., Franklin, R. J., Crang, A. J., Mayer, M., Blakemore, W. F., et al. (1993). Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature, 362, 453–455.
Gupta, N., Henry, R. G., Strober, J., Kang, S. M., Lim, D. A., Bucci, M., et al. (2012). Neural stem cell engraftment and myelination in the human brain. Science Translational Medicine, 4, 155ra37.
Hinks, G. L., & Franklin, R. J. (2000). Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Molecular and Cellular Neurosciences, 16, 542–556.
Honmou, O., Felts, P. A., Waxman, S. G., & Kocsis, J. D. (1996). Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. The Journal of Neuroscience, 16, 3199–3208.
Huang, J. K., & Franklin, R. J. (2011). Regenerative medicine in multiple sclerosis: Identifying pharmacological targets of adult neural stem cell differentiation. Neurochemistry International, 59, 329–332.
Huang, J. K., Jarjour, A. A., Nait Oumesmar, B., Kerninon, C., Williams, A., Krezel, W., et al. (2011). Retinoid X receptor gamma signaling accelerates CNS remyelination. Nature Neuroscience, 14, 45–53.
Imaizumi, T., Lankford, K. L., Waxman, S. G., Greer, C. A., & Kocsis, J. D. (1998). Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. The Journal of Neuroscience, 18, 6176–6185.
Irvine, K. A., & Blakemore, W. F. (2008). Remyelination protects axons from demyelination-associated axon degeneration. Brain, 131, 1464–1477.
Itoyama, Y., Webster, H. D., Richardson, E. P., Jr., & Trapp, B. D. (1983). Schwann cell remyelination of demyelinated axons in spinal cord multiple sclerosis lesions. Annals of Neurology, 14, 339–346.
Jadasz, J. J., Aigner, L., Rivera, F. J., & Kury, P. (2012). The remyelination Philosopher’s Stone: Stem and progenitor cell therapies for multiple sclerosis. Cell and Tissue Research, 349, 331–347.
Jeffery, N. D., Lakatos, A., & Franklin, R. J. (2005). Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. Journal of Neurotrauma, 22, 1282–1293.
Jiao, Y., Novozhilova, E., Karlen, A., Muhr, J., & Olivius, P. (2011). Olfactory ensheathing cells promote neurite outgrowth from co-cultured brain stem slice. Experimental Neurology, 229, 65–71.
Karadottir, R., Hamilton, N. B., Bakiri, Y., & Attwell, D. (2008). Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nature Neuroscience, 11, 450–456.
Karumbayaram, S., Novitch, B. G., Patterson, M., Umbach, J. A., Richter, L., Lindgren, A., et al. (2009). Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells, 27, 806–811.
Karussis, D., Karageorgiou, C., Vaknin-Dembinsky, A., Gowda-Kurkalli, B., Gomori, J. M., Kassis, I., et al. (2010). Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Archives of Neurology, 67, 1187–1194.
Kassis, I., Grigoriadis, N., Gowda-Kurkalli, B., Mizrachi-Kol, R., Ben-Hur, T., Slavin, S., et al. (2008). Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Archives of Neurology, 65, 753–761.
Kato, T., Honmou, O., Uede, T., Hashi, K., & Kocsis, J. D. (2000). Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia, 30, 209–218.
Kazanis, I. (2009). The subependymal zone neurogenic niche: A beating heart in the centre of the brain: How plastic is adult neurogenesis? Opportunities for therapy and questions to be addressed. Brain, 132, 2909–2921.
Keirstead, H. S., Nistor, G., Bernal, G., Totoiu, M., Cloutier, F., Sharp, K., et al. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of Neuroscience, 25, 4694–4705.
Kessaris, N., Fogarty, M., Iannarelli, P., Grist, M., Wegner, M., & Richardson, W. D. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nature Neuroscience, 9, 173–179.
Kingwell, K. (2012). White matter disease: Myelination achieved by transplanted neural stem cells. Nature Reviews. Neurology, 8, 659.
Klugmann, M., Schwab, M. H., Puhlhofer, A., Schneider, A., Zimmermann, F., Griffiths, I. R., et al. (1997). Assembly of CNS myelin in the absence of proteolipid protein. Neuron, 18, 59–70.
Kohama, I., Lankford, K. L., Preiningerova, J., White, F. A., Vollmer, T. L., & Kocsis, J. D. (2001). Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. The Journal of Neuroscience, 21, 944–950.
Kondo, T., & Raff, M. (2000). Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science, 289, 1754–1757.
Kornek, B., Storch, M. K., Weissert, R., Wallstroem, E., Stefferl, A., Olsson, T., et al. (2000). Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. The American Journal of Pathology, 157, 267–276.
Kotter, M. R., Li, W. W., Zhao, C., & Franklin, R. J. (2006). Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. The Journal of Neuroscience, 26, 328–332.
Kotter, M. R., Stadelmann, C., & Hartung, H. P. (2011). Enhancing remyelination in disease–can we wrap it up? Brain, 134, 1882–1900.
Kramer-Albers, E. M., Bretz, N., Tenzer, S., Winterstein, C., Mobius, W., Berger, H., et al. (2007). Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clinical Applications, 1, 1446–1461.
Kuhlmann, T., Miron, V., Cui, Q., Wegner, C., Antel, J., & Bruck, W. (2008). Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain, 131, 1749–1758.
Lanza, C., Morando, S., Voci, A., Canesi, L., Principato, M. C., Serpero, L. D., et al. (2009). Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. Journal of Neurochemistry, 110, 1674–1684.
Lappe-Siefke, C., Goebbels, S., Gravel, M., Nicksch, E., Lee, J., Braun, P. E., et al. (2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genetics, 33, 366–374.
Lee, Y., Morrison, B. M., Li, Y., Lengacher, S., Farah, M. H., Liu, Y., et al. (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature, 487, 443–448.
Levine, J. M., & Reynolds, R. (1999). Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Experimental Neurology, 160, 333–347.
Li, Q., Brus-Ramer, M., Martin, J. H., & McDonald, J. W. (2010). Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neuroscience Letters, 479, 128–133.
Li, Y., Chen, J., Chen, X. G., Wang, L., Gautam, S. C., Xu, Y. X., et al. (2002). Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology, 59, 514–523.
Li, Y., Chopp, M., Chen, J., Wang, L., Gautam, S. C., Xu, Y. X., et al. (2000). Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. Journal of Cerebral Blood Flow and Metabolism, 20, 1311–1319.
Lima, C., Pratas-Vital, J., Escada, P., Hasse-Ferreira, A., Capucho, C., & Peduzzi, J. D. (2006). Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. The Journal of Spinal Cord Medicine, 29, 191–203. discussion 04-6.
Lipson, A. C., Widenfalk, J., Lindqvist, E., Ebendal, T., & Olson, L. (2003). Neurotrophic properties of olfactory ensheathing glia. Experimental Neurology, 180, 167–171.
Lombard, D. B., Chua, K. F., Mostoslavsky, R., Franco, S., Gostissa, M., & Alt, F. W. (2005). DNA repair, genome stability, and aging. Cell, 120, 497–512.
Lu, P., Jones, L. L., Snyder, E. Y., & Tuszynski, M. H. (2003). Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Experimental Neurology, 181, 115–129.
Lublin, F. D., Baier, M., & Cutter, G. (2003). Effect of relapses on development of residual deficit in multiple sclerosis. Neurology, 61, 1528–1532.
Mackay-Sim, A., Feron, F., Cochrane, J., Bassingthwaighte, L., Bayliss, C., Davies, W., et al. (2008). Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain, 131, 2376–2386.
Mahmood, A., Lu, D., Wang, L., Li, Y., Lu, M., & Chopp, M. (2001). Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery, 49, 1196–1203. discussion 203-4.
Marin-Husstege, M., Muggironi, M., Liu, A., & Casaccia-Bonnefil, P. (2002). Histone deacetylase activity is necessary for oligodendrocyte lineage progression. The Journal of Neuroscience, 22, 10333–10345.
Marriott, M. P., Emery, B., Cate, H. S., Binder, M. D., Kemper, D., Wu, Q., et al. (2008). Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia, 56, 686–698.
Martino, G., Franklin, R. J., Baron Van Evercooren, A., & Kerr, D. A. (2010). Stem cell transplantation in multiple sclerosis: Current status and future prospects. Nature Reviews. Neurology, 6, 247–255.
Martino, G., & Pluchino, S. (2006). The therapeutic potential of neural stem cells. Nature Reviews. Neuroscience, 7, 395–406.
Mason, J. L., Toews, A., Hostettler, J. D., Morell, P., Suzuki, K., Goldman, J. E., et al. (2004). Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. The American Journal of Pathology, 164, 1673–1682.
Mi, S., Lee, X., Shao, Z., Thill, G., Ji, B., Relton, J., et al. (2004). LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature Neuroscience, 7, 221–228.
Miller, R. H. (1996). Oligodendrocyte origins. Trends in Neurosciences, 19, 92–96.
Miller, R. H. (2005). Dorsally derived oligodendrocytes come of age. Neuron, 45, 1–3.
Minguell, J. J., Erices, A., & Conget, P. (2001). Mesenchymal stem cells. Experimental Biology and Medicine, 226, 507–520.
Murtie, J. C., Zhou, Y. X., Le, T. Q., Vana, A. C., & Armstrong, R. C. (2005). PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiology of Disease, 19, 171–182.
Nait-Oumesmar, B., Decker, L., Lachapelle, F., Avellana-Adalid, V., Bachelin, C., & Baron-Van, E. A. (1999). Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. The European Journal of Neuroscience, 11, 4357–4366.
Nait-Oumesmar, B., Picard-Riera, N., Kerninon, C., Decker, L., Seilhean, D., Hoglinger, G. U., et al. (2007). Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proceedings of the National Academy of Sciences of the United States of America, 104, 4694–4699.
Nave, K. A., & Trapp, B. D. (2008). Axon-glial signaling and the glial support of axon function. Annual Review of Neuroscience, 31, 535–561.
Novotny, G. E. (1984). Formation of cytoplasm-containing vesicles from double-walled coated invaginations containing oligodendrocytic cytoplasm at the axon-myelin sheath interface in adult mammalian central nervous system. Acta Anatomica, 119, 106–112.
Padovan, C. S., Jahn, K., Birnbaum, T., Reich, P., Sostak, P., Strupp, M., et al. (2003). Expression of neuronal markers in differentiated marrow stromal cells and CD133+ stem-like cells. Cell Transplantation, 12, 839–848.
Pan, B., Fromholt, S. E., Hess, E. J., Crawford, T. O., Griffin, J. W., Sheikh, K. A., et al. (2005). Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: Neuropathology and behavioral deficits in single- and double-null mice. Experimental Neurology, 195, 208–217.
Park, Y., & Gerson, S. L. (2005). DNA repair defects in stem cell function and aging. Annual Review of Medicine, 56, 495–508.
Penderis, J., Shields, S. A., & Franklin, R. J. (2003). Impaired remyelination and depletion of oligodendrocyte progenitors does not occur following repeated episodes of focal demyelination in the rat central nervous system. Brain, 126, 1382–1391.
Peru, R. L., Mandrycky, N., Nait-Oumesmar, B., & Lu, Q. R. (2008). Paving the axonal highway: From stem cells to myelin repair. Stem Cell Reviews, 4, 304–318.
Pluchino, S., Gritti, A., Blezer, E., Amadio, S., Brambilla, E., Borsellino, G., et al. (2009). Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Annals of Neurology, 66, 343–354.
Pluchino, S., Quattrini, A., Brambilla, E., Gritti, A., Salani, G., Dina, G., et al. (2003). Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature, 422, 688–694.
Pluchino, S., Zanotti, L., Rossi, B., Brambilla, E., Ottoboni, L., Salani, G., et al. (2005). Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature, 436, 266–271.
Politi, L. S., Bacigaluppi, M., Brambilla, E., Cadioli, M., Falini, A., Comi, G., et al. (2007). Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem Cells, 25, 2583–2592.
Popescu, B. F., & Lucchinetti, C. F. (2012). Pathology of demyelinating diseases. Annual Review of Pathology, 7, 185–217.
Psachoulia, K., Jamen, F., Young, K. M., & Richardson, W. D. (2009). Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biology, 5, 57–67.
Raff, M. C., Miller, R. H., & Noble, M. (1983). A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature, 303, 390–396.
Ramon-Cueto, A., Cordero, M. I., Santos-Benito, F. F., & Avila, J. (2000). Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron, 25, 425–435.
Rando, T. A. (2006). Stem cells, ageing and the quest for immortality. Nature, 441, 1080–1086.
Reynolds, R., Dawson, M., Papadopoulos, D., Polito, A., Di Bello, I. C., Pham-Dinh, D., et al. (2002). The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. Journal of Neurocytology, 31, 523–536.
Richardson, W. D., Kessaris, N., & Pringle, N. (2006). Oligodendrocyte wars. Nature Reviews. Neuroscience, 7, 11–18.
Richardson, W. D., Pringle, N. P., Yu, W. P., & Hall, A. C. (1997). Origins of spinal cord oligodendrocytes: Possible developmental and evolutionary relationships with motor neurons. Developmental Neuroscience, 19, 58–68.
Richardson, W. D., Young, K. M., Tripathi, R. B., & McKenzie, I. (2011). NG2-glia as multipotent neural stem cells: Fact or fantasy? Neuron, 70, 661–673.
Rinholm, J. E., & Bergersen, L. H. (2012). Neuroscience: The wrap that feeds neurons. Nature, 487, 435–436.
Rivera, F. J., Couillard-Despres, S., Pedre, X., Ploetz, S., Caioni, M., Lois, C., et al. (2006). Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells, 24, 2209–2219.
Rivera, F. J., Siebzehnrubl, F. A., Kandasamy, M., Couillard-Despres, S., Caioni, M., Poehler, A. M., et al. (2009). Mesenchymal stem cells promote oligodendroglial differentiation in hippocampal slice cultures. Cellular Physiology and Biochemistry, 24, 317–324.
Rivers, L. E., Young, K. M., Rizzi, M., Jamen, F., Psachoulia, K., Wade, A., et al. (2008). PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature Neuroscience, 11, 1392–1401.
Rowitch, D. H. (2004). Glial specification in the vertebrate neural tube. Nature Reviews. Neuroscience, 5, 409–419.
Ruckh, J. M., Zhao, J. W., Shadrach, J. L., van Wijngaarden, P., Rao, T. N., Wagers, A. J., et al. (2012). Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell, 10, 96–103.
Runyan, S. A., & Phelps, P. E. (2009). Mouse olfactory ensheathing glia enhance axon outgrowth on a myelin substrate in vitro. Experimental Neurology, 216, 95–104.
Sanchez-Ramos, J., Song, S., Cardozo-Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., et al. (2000). Adult bone marrow stromal cells differentiate into neural cells in vitro. Experimental Neurology, 164, 247–256.
Selmani, Z., Naji, A., Zidi, I., Favier, B., Gaiffe, E., Obert, L., et al. (2008). Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4 + CD25highFOXP3+ regulatory T cells. Stem Cells, 26, 212–222.
Shen, S., Sandoval, J., Swiss, V. A., Li, J., Dupree, J., Franklin, R. J., et al. (2008). Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nature Neuroscience, 11, 1024–1034.
Shields, S., Gilson, J., Blakemore, W., & Franklin, R. (2000). Remyelination occurs as extensively but more slowly in old rats compared to young rats following fliotoxin-induced CNS demyelination. Glia, 29, 102.
Smith, K. J., Blakemore, W. F., & McDonald, W. I. (1979). Central remyelination restores secure conduction. Nature, 280, 395–396.
Sotgiu, S., Pugliatti, M., Fois, M. L., Arru, G., Sanna, A., Sotgiu, M. A., et al. (2004). Genes, environment, and susceptibility to multiple sclerosis. Neurobiology of Disease, 17, 131–143.
Spencer, P. S., & Thomas, P. K. (1974). Ultrastructural studies of the dying-back process. II. The sequestration and removal by Schwann cells and oligodendrocytes of organelles from normal and diseases axons. Journal of Neurocytology, 3, 763–783.
Steffenhagen, C., Dechant, F. X., Oberbauer, E., Furtner, T., Weidner, N., Kury, P., et al. (2012). Mesenchymal stem cells prime proliferating adult neural progenitors toward an oligodendrocyte fate. Stem Cells and Development, 21, 1838–1851.
Swistowski, A., Peng, J., Liu, Q., Mali, P., Rao, M. S., Cheng, L., et al. (2010). Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells, 28, 1893–1904.
Talbott, J. F., Loy, D. N., Liu, Y., Qiu, M. S., Bunge, M. B., Rao, M. S., et al. (2005). Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Experimental Neurology, 192, 11–24.
Tang, D. G., Tokumoto, Y. M., & Raff, M. C. (2000). Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. The Journal of Cell Biology, 148, 971–984.
Tripathi, R. B., Clarke, L. E., Burzomato, V., Kessaris, N., Anderson, P. N., Attwell, D., et al. (2011). Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts. The Journal of Neuroscience, 31, 6809–6819.
Tripathi, R. B., Rivers, L. E., Young, K. M., Jamen, F., & Richardson, W. D. (2010). NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. The Journal of Neuroscience, 30, 16383–16390.
Tsuji, O., Miura, K., Okada, Y., Fujiyoshi, K., Mukaino, M., Nagoshi, N., et al. (2010). Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America, 107, 12704–12709.
Uccelli, A., Laroni, A., & Freedman, M. S. (2011). Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurology, 10, 649–656.
Uccelli, A., Moretta, L., & Pistoia, V. (2006). Immunoregulatory function of mesenchymal stem cells. European Journal of Immunology, 36, 2566–2573.
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews. Immunology, 8, 726–736.
Uchida, N., Chen, K., Dohse, M., Hansen, K. D., Dean, J., Buser, J. R., et al. (2012). Human neural stem cells induce functional myelination in mice with severe dysmyelination. Science Translational Medicine, 4, 155ra36.
Vallstedt, A., Klos, J. M., & Ericson, J. (2005). Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron, 45, 55–67.
Ventura, R. E., & Goldman, J. E. (2006). Telencephalic oligodendrocytes battle it out. Nature Neuroscience, 9, 153–154.
Wang, S., Sdrulla, A. D., diSibio, G., Bush, G., Nofziger, D., Hicks, C., et al. (1998). Notch receptor activation inhibits oligodendrocyte differentiation. Neuron, 21, 63–75.
Wilkins, A., Majed, H., Layfield, R., Compston, A., & Chandran, S. (2003). Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: A novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. The Journal of Neuroscience, 23, 4967–4974.
Williams, A., Piaton, G., Aigrot, M. S., Belhadi, A., Theaudin, M., Petermann, F., et al. (2007). Semaphorin 3A and 3F: Key players in myelin repair in multiple sclerosis? Brain, 130, 2554–2565.
Windrem, M. S., Nunes, M. C., Rashbaum, W. K., Schwartz, T. H., Goodman, R. A., McKhann, G., 2nd, et al. (2004). Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature Medicine, 10, 93–97.
Windrem, M. S., Schanz, S. J., Guo, M., Tian, G. F., Washco, V., Stanwood, N., et al. (2008). Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell, 2, 553–565.
Wolswijk, G. (1998). Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. The Journal of Neuroscience, 18, 601–609.
Wolswijk, G., & Noble, M. (1992). Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. The Journal of Cell Biology, 118, 889–900.
Woodbury, D., Schwarz, E. J., Prockop, D. J., & Black, I. B. (2000). Adult rat and human bone marrow stromal cells differentiate into neurons. Journal of Neuroscience Research, 61, 364–370.
Woodhall, E., West, A. K., & Chuah, M. I. (2001). Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Research. Molecular Brain Research, 88, 203–213.
Woodruff, R. H., Fruttiger, M., Richardson, W. D., & Franklin, R. J. (2004). Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Molecular and Cellular Neurosciences, 25, 252–262.
Wren, D., Wolswijk, G., & Noble, M. (1992). In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. The Journal of Cell Biology, 116, 167–176.
Zappia, E., Casazza, S., Pedemonte, E., Benvenuto, F., Bonanni, I., Gerdonni, E., et al. (2005). Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood, 106, 1755–1761.
Zawadzka, M., Rivers, L. E., Fancy, S. P., Zhao, C., Tripathi, R., Jamen, F., et al. (2010). CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell, 6, 578–590.
Zhang, J., Li, Y., Chen, J., Cui, Y., Lu, M., Elias, S. B., et al. (2005). Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Experimental Neurology, 195, 16–26.
Zhang, J., Li, Y., Lu, M., Cui, Y., Chen, J., Noffsinger, L., et al. (2006). Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. Journal of Neuroscience Research, 84, 587–595.
Zhang, Y., Klassen, H. J., Tucker, B. A., Perez, M. T., & Young, M. J. (2007). CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. The Journal of Neuroscience, 27, 4499–4506.
Zhao, C., Li, W. W., & Franklin, R. J. (2006). Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiology of Aging, 27, 1298–1307.
Zhu, Q., Whittemore, S. R., Devries, W. H., Zhao, X., Kuypers, N. J., & Qiu, M. (2011). Dorsally-derived oligodendrocytes in the spinal cord contribute to axonal myelination during development and remyelination following focal demyelination. Glia, 59, 1612–1621.
Zujovic, V., Thibaud, J., Bachelin, C., Vidal, M., Coulpier, F., Charnay, P., et al. (2010). Boundary cap cells are highly competitive for CNS remyelination: Fast migration and efficient differentiation in PNS and CNS myelin-forming cells. Stem Cells, 28, 470–479.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Crawford, A.H., Franklin, R.J.M. (2015). Neural Stem Cells and Demyelinating Disease. In: Kuhn, H., Eisch, A. (eds) Neural Stem Cells in Development, Adulthood and Disease. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1908-6_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1908-6_8
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1907-9
Online ISBN: 978-1-4939-1908-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)