Abstract
For many years, surgical resection of the esophagus and subsequent reconstruction of enteral continuity was a formidable challenge for surgeons and patients alike. Since the first esophageal resection, much has been learned about the anatomical and physiological aspects of esophagectomy. One type of esophageal resection and reconstruction has been referred to as Ivor Lewis esophagectomy, after a British surgeon who utilized an abdominal incision and right thoracotomy to resect the cancer of the esophagus. Herein, we describe our modified approach with this technique.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Historical Perspective
The first successful resection of the thoracic esophagus was performed by Franz Torek in Germany on March 14, 1913, under chloroform and ether anesthesia [1]. The patient, who suffered from squamous cell carcinoma of the esophagus, was effectively cured by this resection and survived for an additional 12 years. He died of pneumonia without cancer recurrence. Unfortunately, many esophageal cancer patients who underwent esophagectomy at the beginning of the twentieth century succumbed in the early postoperative period. However, Torek’s success gave first hope that resection of the esophagus for carcinoma may become a feasible treatment modality as anesthesia and perioperative care evolved.
The main complexity of esophageal surgery at the beginning of the twentieth century was related to the intrathoracic location of the esophagus and patient tolerance of intraoperative pneumothorax, since positive pressure ventilation or selective lung ventilation did not exist. Another complex issue was associated with a reliable reconstruction of the alimentary tract following esophagectomy. Since then many operative techniques have been developed for the resection and reconstruction of the esophagus. The one technique that is commonly used and has withstood the test of time was presented by British surgeon Ivor Lewis in 1946 at the Royal College of Surgeons Hunterean Lecture [2]. Lewis proposed the combination of laparotomy and right thoracotomy for the resection of cancer of the esophagus. The operation was performed in two stages. First, laparotomy was performed with gastric mobilization followed by right thoracotomy 10–15 days later. During right thoracotomy the esophagus and tumor were resected, and alimentary continuity was reestablished with esophagogastric anastomosis. Lewis’ series described successful postoperative outcome in five of seven patients, a rare surgical triumph at the time. Nearly 70 years later, although performed as a single-stage procedure and with additional modifications, Ivor Lewis esophagectomy continues to be an applicable and widely used technique for the resection of middle or distal esophageal carcinoma.
Anatomical Highlights
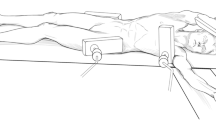
Ivor Lewis esophagectomy consists of abdominal dissection followed by right thoracotomy (Fig. 1.1). Understanding the abdominal and thoracic anatomy is therefore paramount to the performance of the operation.
Abdominal Anatomy
Knowledge of the upper abdominal anatomy is crucial to safe and effective esophageal and gastric mobilization, performance of adequate lymphadenectomy, and creation of the gastric conduit. Relationship of the esophagus and stomach to solid organs, vascular structures, and ligamentous attachments must be considered. The esophagus enters the abdomen via the esophageal hiatus located at the level of the T8 vertebra. It is attached to the diaphragmatic crus via the phreno-esophageal ligament, which extends onto the proximal portion of the stomach. The structures considered during the dissection on the right side of the crus include the inferior vena cava, left lobe of the liver, caudate lobe of the liver, pars flaccida, duodenum, porta hepatis, and right gastric artery.
Posteriorly, the abdominal esophagus and stomach are closely related to the abdominal aorta, celiac axis, left gastric artery and vein, common hepatic and splenic arteries, pancreas, and branches of the cisterna chyle (Fig. 1.2). Inferiorly, the important structures include the right gastro-epiploic artery, greater omentum, and transverse colon and mesocolon. On the left side, the greater curvature of the stomach has attachments to the colon and spleen. After mobilization of the short gastric arteries, the tail of the pancreas and left crus come into view.
Thoracic Anatomy
Important structures within the right thorax related to the esophagus include the thoracic aorta, azygos vein and azygos arch, thoracic duct, inferior pulmonary vein, posterior pericardium, left and right mainstem bronchi, vagus nerves along with their bronchial branches, trachea, and left recurrent laryngeal nerve. Important lymphatic basins include mediastinal level 8 and 9 lymph nodes, subcarinal level 7 lymph nodes, and paratracheal level 4 lymph nodes.
Indications for the Operation
Currently, there are several surgical techniques utilized for the resection and reconstruction of the esophagus. Ivor Lewis esophagectomy is one of those techniques; however, it does not fit all clinical scenarios in which esophageal resection may be indicated. The main decision points about the utility of the Ivor Lewis approach are the location of tumor within the esophagus and subsequently the level of anticipated esophagogastric anastomosis. Generally, the most common indication for Ivor Lewis esophagectomy is carcinoma of the esophagus located in the middle or distal esophagus and as low as the gastroesophageal junction (GEJ) and upper cardia. Other potential indications include severe esophageal stricture from gastroesophageal reflux disease or end-stage achalasia. Tumors located proximal to the level of the carina or azygos arch, or less than 25 cm from the incisors on esophagoscopy require an anastomosis in the neck and are not suitable for the Ivor Lewis approach.
Preoperative Evaluation and Imaging
Considering that esophageal carcinoma is the most frequent indication for Ivor Lewis esophagectomy, the preoperative evaluation includes adequate clinical staging along with the assessment of physiologic fitness and nutritional status of the patient. Staging of esophageal carcinoma includes esophago-gastro-duodenoscopy (EGD) with endoscopic ultrasound (EUS) to define the location of the tumor within the esophagus and the depth of esophageal wall penetration. EUS is also useful for the assessment of regional and some non-regional lymph nodes. Positron emission tomography combined with computed tomography (PET/CT) supplements staging by searching for distant metastatic disease. Ivor Lewis esophagectomy may be utilized in patients with Tis-4a, N0-3 stages.
Evaluation of the physiologic fitness and suitability for the operation is based on the surgeon’s judgment which is guided by a thorough medical history and physical examination. Adjunctive studies such as pulmonary function tests and stress echocardiogram may further help with the decision-making process. The preoperative nutritional status is very important to the overall success of the operation. Patients with esophageal adenocarcinoma usually demonstrate more robust physiognomy than patients with squamous cell carcinoma who often present in an emaciated condition. However, prolonged dysphagia due to tumor obstruction or esophagitis related to neoadjuvant radiation therapy are frequent reasons for weight loss, and must be addressed prior to definitive surgical procedure.
Perioperative Preparation
Bowel preparation or chlorhexidine shower the day before the operation are left to the discretion of the operating surgeon; they are likely more dogmatic than necessary. Clinical judgment is the best guide. While formal bowel prep is likely not necessary, preoperative constipation may manifest as significant postoperative ileus that may hinder nutrition and progress toward discharge. Our practice is to place patients on a liquid diet for 2 days prior to surgery and to use a cathartic if there is history of constipation. From an anesthesia standpoint, patients should be prepared to tolerate single lung ventilation. Epidural analgesia has become an extremely useful tool for postoperative pain control but its use must be tempered to the downside of perioperative hypotension from a sympatholytic effect. Long acting local blocks as part of a pain control cocktail are also acceptable.
Description of the Operation
Ivor Lewis esophagectomy consists of a series of operative steps and maneuvers, some which have been the source of fervent discussion and even randomized trials. Herein, we describe our modifications and approach to open Ivor Lewis esophagectomy, which can be divided into following steps: abdominal incision, mobilization of the abdominal esophagus and stomach, mobilization of the greater omental pedicle flap, dissection of the left gastric artery and D2 lymphadenectomy, pylorus draining procedure, gastric conduit creation, and feeding jejunostomy. The thoracic part of the operation can be divided into: muscle-sparing right thoracotomy incision, esophageal mobilization, thoracic duct ligation, lymphadenectomy, esophagogastric anastomosis, and omental pedicle transposition and envelope.
Abdominal Incision and Exposure
An upper midline laparotomy incision extending from just above the xiphoid process to the level of the umbilicus provides adequate exposure of the upper abdominal viscera and the omentum. In patients with central obesity, a bilateral subcostal incision with the division of the bilateral rectus abdominis muscles is an excellent alternative of exposing the upper abdomen to avoid subsequent risk of ventral incisional hernia. The blades of the Thompson retractor are placed in bilateral subcostal regions to aid the exposure of the diaphragm and the GEJ. To avoid tearing the liver capsule from excessive retractor traction, the falciform ligament may be divided to the level of the diaphragmatic attachments.
Gastric and Esophageal Mobilization
The left lobe of the liver overlying the GEJ is retracted anteriorly. Mobilization of the triangular ligament is also an option but is rarely necessary. The pars flaccida is opened, exposing the caudate lobe of the liver and the right diaphragmatic crus within the lesser sac. Then, the phreno-esophageal ligament overlying the diaphragmatic crus is incised and the crus is dissected free from the GEJ. If involved with carcinoma, a part of the crus is resected en bloc with the operative specimen to ensure negative radial margins. A penrose drain is passed around the GEJ to aid with manipulation of the esophagus and further dissection proceeds in the superior and lateral periaortic planes and along the pleural surfaces laterally reaching up into the mediastinum. This maneuver facilitates intrathoracic esophageal mobilization; however, care must be taken not to inadvertently injure or partially divide the inferior pulmonary veins on either side.
Mobilization of the Omental Pedicle
The transverse colon is lifted out of the body cavity and retracted inferiorly as the omentum is retracted superiorly. The avascular plane between the omentum and colon is opened while carefully avoiding injury to the colon, mesocolon, omentum, and greater curvature blood supply (i.e., the right gastro-epiploic vessels). The omentum is fully mobilized off the transverse colon and mesocolon after entering the lesser sac to the level of the gastroduodenal artery. Care is taken to preserve the entire course of the right gastro-epiploic artery during omental mobilization by careful palpation and/or visualization of this artery. The omental pedicle flap, based on 2–3 perforating omental arterial branches off the right gastro-epiploic artery, is created along the left side of the greater curvature (Fig. 1.2). Gastric mobilization along the greater curvature is completed with the division of the short gastric arteries staying relatively close to the stomach to avoid inadvertent splenic injury.
Abdominal D2 Lymphadenectomy and Left Gastric Artery Division
With the aid of a nasogastric tube in the stomach stretching along the greater curvature, the stomach is elevated to approach the celiac axis and left gastric artery from the lesser sac in the dissection plane created above the mesocolon. The peritoneum overlying the superior edge of the pancreas is incised to reveal the common hepatic and splenic arteries. The tissues anterior and cranial to the common hepatic and splenic arteries is included in the lymph node dissection and lymph node tissues along the left gastric artery is dissected and swept anteriorly with the specimen. The left gastric vein and artery are then divided at their origin (doubly ligated or stapled) after ensuring a good palpable pulse in both splenic and common hepatic arteries. The boundaries of dissection from right to left are the porta hepatis/vena cava to the splenic hilum, respectively; and periaortic tissues from the celiac artery inferiorly up to the extent of the mediastinal resection (Fig. 1.3). Clips should be placed on the larger lymphatics in the area of the porta hepatis to avoid chylous ascites.
Pylorus Draining Procedure
Controversy surrounds this portion of the procedure [3]. Some experts leave the pylorus completely intact, whereas others favor pyloromyotomy, botox injection, selective balloon dilation postoperatively, or formal pyloroplasty. Emptying of the gastric conduit through the pylorus may be related to the width of the conduit, however conclusive evidence is lacking. Our preference has been to perform either pyloromytomy or pyloroplasty.
The pylorus is identified and a 00 silk suture is placed at the superior and inferior border of the pylorus. With the cutting cautery current at 15 V the muscle tissue of the pyloric sphincter is divided and pyloromyotomy is performed; or alternatively, a Heineke-Mikulicz pyloroplasty is performed. The pyloromyotomy or pyloroplasty may be covered with a piece of omentum or previously mobilized falciform ligament and secured in place with previously placed 00 silk sutures.
Gastric Pedicle Creation
The incisura is identified at the lesser curvature of the stomach and the right gastric artery is divided above the incisura. Multiple fires of green 4.5 mm linear stapler loads aiming towards the angle of His are used to create a gastric conduit approximately 4 cm in width. In cases where the tumor extends into the stomach, care must be taken to ensure an adequate distal margin. A second staple line can be fired parallel to the first to supply the pathologist with specimen to determine the distal margin status on frozen section examination. The conduit can be completely formed in the abdomen and transferred to the chest with traction sutures, or alternatively partially formed in the abdomen with completion of the tubularization in the chest after formation of the anastomosis. Using 000 silk sutures, the omental pedicle that is to be transferred into the chest is tacked to the staple line on the proximal stomach to facilitate transposition of the gastric conduit and omentum to the chest. A feeding jejunostomy catheter is placed 30 cm from the ligament of Treitz in Witzeled fashion. Finally, the abdomen is closed.
Right Thoracotomy
The patient is positioned in the left lateral decubitus position and right, muscle-sparing thoracotomy is performed (Fig. 1.1b). Neither the latissimus dorsi nor the serratus anterior muscles is divided. The chest cavity is entered in the fifth interspace and the sixth rib may be cut behind the paraspinous muscle to aid with exposure.
Esophageal Mobilization, Thoracic Duct Ligation, and Lymphadenectomy
Esophageal mobilization begins with incision of the inferior pulmonary ligament. The right lung is retracted anteriorly and the mediastinal pleura is incised along the anterior surface of the esophagus at the edge of the lung parenchyma. Continuing superiorly and staying close to the posterior pericardium, the subcarinal level 7 lymph node compartment is mobilized en bloc with the esophagus. Care must be used with any cautery device in the subcarinal region to avoid thermal airway injury. Scissor dissection is another option with vascular clip control of bronchial vessels supplying the level 7 LNs.
The azygos arch is then mobilized and divided with a vascular 2.5 mm linear stapler load. The mediastinal pleura above the azygos arch is then incised and the esophagus is further mobilized away from the trachea using both sharp and blunt dissection. Care must be taken in this region not to injure the left recurrent laryngeal nerve. If the tumor is in the distal esophagus or below, any dissection of the esophagus above the level of the arch should be right on the esophageal wall. The posterior pleura is incised just anterior to the azygos vein. This incision is extended inferiorly to the level of the diaphragmatic hiatus.
For thoracic duct ligation we focus on the area between the spine and aorta at the T10 vertebral level which will contain the duct. The duct traverses the diaphragm with the aorta in the aortic hiatus. Above the ninth interspace there is variability in the crossover level of the duct to the left chest, therefore attempts at duct ligation in the mid-thoracic area may not result in control of the duct. Just above the diaphragmatic hiatus, mass ligation of the paraspinous tissue to include the thoracic duct is performed by passing the right angle clamp along the periaortic plane to the vertebral body. Using 0 silk suture material, the tissue between the aorta and spine is ligated. Duct ligation may be optimized if the pleura is left intact in this area to “hold” the suture, as the thoracic duct is notoriously friable and the ligature itself can cut the duct and lead to a chylothorax.
The esophagus is further mobilized along the periaortic plane to the level of the left pleura with all periesophageal lymph node bearing tissues. Passing a penrose drain around the esophagus may facilitate the retraction and dissection. The boundaries of the modified en bloc thoracic esophageal dissection are from right to left, the pleura to pleura, diaphragm to azygos arch, and azygos vein/spine to pericardium, respectively (Figs. 1.4 and 1.5).
Anastomosis
The esophagus is divided for approximately 75 % of its circumference at or above the level of the azygos arch. We typically employ an intraluminal stapling device for an end-to-side esophagogastrostomy. Depending on the size of the esophagus, either a 25 or 29 mm anvil of a circular stapler is placed within the esophagus. 00 or 000 prolene suture is sewn in a continuous horizontal mattress fashion approximately 2 mm from the cut esophageal wall incorporating full thickness esophageal wall to purse-string the esophagus around the staple anvil. The esophagus is then fully divided and at this point a proximal esophageal margin can be sent for frozen section analysis as necessary. The same prolene suture is then continued as a second over-and-over layer (i.e., baseball stitch) to further align the esophageal wall around the anvil.
The resected portion of the esophagus, stomach, and the omentum are delivered to the chest cavity in the anatomic position. It is important to ensure that the conduit is not twisted. The staple line of the lesser curvature of the stomach should be pointing laterally, directly towards the surgeon’s view. The stitches between the surgical specimen and gastric conduit and omentum are cut and the specimen is removed. Alternatively, the gastric conduit may be partially created within the abdomen and delivered to the chest cavity, where the gastric conduit is completed in the chest after esophagogastric anastomosis.
The gastric conduit is inspected and stretched superiorly inside the thoracic cavity to avoid redundancy of the conduit. A suitable well perfused area for the anastomosis is selected on the greater curvature of the stomach. Gastrotomy is performed at the tip of the gastric conduit, a circular stapler is placed inside the conduit, and the stapler is opened with the spike penetrating the conduit in the preselected anastomotic area opposite the staple line on the lesser curvature. The anvil and spike of the stapler are aligned while ensuring that no other tissues such as lung parenchyma are trapped inside the anastomosis. The stapler is closed, fired, and removed through the gastrotomy. The anastomosis is visually inspected through the gastrotomy site and a nasogastric tube is advanced under direct vision across the anastomosis into the gastric conduit. An additional green linear stapler load is used to amputate the tip of the conduit thus removing the gastrotomy site. This staple line should be sufficiently away from the circular staple line of the esophagogastric anastomosis to avoid tissue ischemia between the staple lines (Fig. 1.6).
Omental Envelope
An omental pedicle flap is placed underneath, between the anastomosis and the airway to circumferentially envelop the anastomosis and gastric staple line. A few 000 silk sutures are used to secure the omentum around the anastomosis. The chest cavity is then irrigated and chest tubes are placed in the pleural spaces. Prior to approximating costal sutures, the lung is insufflated and it is ensured that all three lobes of the right lung are anatomically aligned and expanded without evidence of trapping or atelectasis. The thoracotomy incision is closed in routine fashion.
Postoperative Management
The patient is extubated in the operating room and transferred to the postanesthesia care unit (PACU) and then to a monitored step-downward. Portable chest x-ray is obtained in PACU and formal upright postero-anterior and lateral chest x-rays are obtained on postoperative day (POD) #1. Intravenous fluids are usually infused at a rate of 125 mL/h for the first 2 days. However, the fluid management is guided by the clinical status and urine output. It is important to maintain adequate blood pressure/perfusion to the newly created gastric conduit. The nasogastric tube is kept to continuous low wall suction for a few days, then placed to gravity and then removed on POD 4–5 as long as there are no signs of early anastomotic leak or ileus. Chest tubes are removed after nasogastric tube removal, as long as there is no evidence of chyle leak, bile leak, or air leak; and the volume of the output is less than 400 mL for 24 h.
Feeding via the jejunostomy catheter is initiated on POD #3, initially at a rate of 15 mL/h and advanced to goal by 15 mL daily. When bowel function returns, feeds can be advanced fairly rapidly to goal. Pain is transitioned to liquid pain medicines via the jejunostomy tube when the patient is tolerating feeds well. Generally, patients are discharged from the hospital on POD #7 after receiving instructions on tube feedings and jejunostomy tube care.
A barium swallow is performed between POD #10 to 14 prior to the first outpatient visit. If there is no sign of anastomotic leak, a diet is initiated and the patient has a formal consultation with a nutritionist to discuss oral transitioning, weaning tube feeds, and postesophagectomy dietary habits.
Complications
Esophagectomy is associated with a relatively high potential risk (approximately 50 %) for postoperative morbidity and a relatively small but significant (approximately 4 % 90-day) risk of mortality [4]. Postoperative morbidity may be related to virtually any organ system. Pneumonia, atelectasis, acute respiratory failure, atrial fibrillation, ileus, wound complications, recurrent laryngeal nerve injury, myocardial infarction, stroke, pulmonary embolism, bowel ischemia, and conduit necrosis are all possible postoperatively. However, the most common complication, which most often determines the postoperative course, is anastomotic leak. Successful healing of the esophagogastric anastomosis depends on many factors, of which relative ischemia is likely the most important. To maximize the chance for healing and minimize potential anastomosis related complications, surgeons have experimented with constructing the anastomosis in the neck or chest, stapling the anastomosis linearly or circularly, or sewing the anastomosis in one or two layers. The Ivor Lewis esophagectomy anastomosis is constructed within the right chest, and our preference has been a circular anastomosis at or above the level of the azygos vein to minimize postoperative reflux. If neoadjuvant therapy was employed, placing the anastomosis in an area of nonirradiated esophagus may help to avoid a leak. We also favor enveloping the anastomosis with an omental pedicle [5]. Using this technique, our leak rate has decreased from 8 to 4 %. Importantly, severe leaks requiring reoperative intervention are now extremely rare with the use of the omental buttress.
The management of an anastomotic leak varies depending on the severity of the leak and the clinical and hemodynamic characteristics. Experience and clinical judgment is required in this setting. The management strategy may range from nonoperative to endoscopic to operative treatments. Contained anastomotic leaks without signs of systemic inflammation or sepsis are usually treated with nil per os and occasional antibiotics. Anastomotic leaks draining into the pleural cavity or stimulating systemic inflammatory response or sepsis require more aggressive management. The main principle is sepsis control, which may be accomplished with endoscopic, image-guided, or operative techniques. Over the last decade, endoscopic stenting of an anastomotic leak has become popular, and few studies suggest success with this approach [6]. Operative approach is occasionally necessary and usually necessitates decortication of the lung, along with anastomotic reinforcement with a vascularized muscle flap. Intercostal, serratus anterior, or latissimus dorsi muscles provide excellent choices for coverage of the defect [7]. Necrosis of the gastric conduit is a rare but life threatening complication [8]. Early recognition is important, and treatment involves resection of the conduit and esophagostomy. Enteral reconstruction can be subsequently achieved either with colon or jejunal interposition depending on the institutional experience and expertise [9].
Conclusion
Open Ivor Lewis esophagectomy remains an excellent and reproducible procedure for the treatment of middle and distal esophageal carcinoma. With the addition of omental transposition, the perioperative anastomosis leak rate and leak-associated complications have further declined. However, principles of careful gastric mobilization based on the right gastro-epiploic artery, conduit creation, and meticulous anastomosis construction within the chest remain the core maneuvers of this time-honored surgical procedure.
References
Dubecz A, Schwartz SI. Franz John A. Torek. Ann Thorac Surg. 2008;85(4):1497–9. doi:10.1016/j.athoracsur.2007.10.106.
Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18–31.
Gaur P, Swanson SJ. Should we continue to drain the pylorus in patients undergoing an esophagectomy? Dis Esophagus. 2013. doi:10.1111/dote.12035.
Low DE, Bodnar A. Update on clinical impact, documentation, and management of complications associated with esophagectomy. Thorac Surg Clin. 2013;23(4):535–50. doi:10.1016/j.thorsurg.2013.07.003.
Sepesi B, Swisher SG, Walsh GL, Correa A, Mehran RJ, Rice D, et al. Omental reinforcement of the thoracic esophagogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg. 2012;144(5):1146–50. doi:10.1016/j.jtcvs.2012.07.085.
Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259(5):852–60. doi:10.1097/sla.0000000000000564.
Martin LW, Hofstetter W, Swisher SG, Roth JA. Management of intrathoracic leaks following esophagectomy. Adv Surg. 2006;40:173–90.
Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin. 2006;16(1):11–22. doi:10.1016/j.thorsurg.2006.01.003.
Marks JL, Hofstetter WL. Esophageal reconstruction with alternative conduits. Surg Clin North Am. 2012;92(5):1287–97. doi:10.1016/j.suc.2012.07.006.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Key Operative Steps
Key Operative Steps
-
1.
Create upper midline incision from the xiphoid to the umbilicus.
-
2.
Retract the left lobe of the liver anteriorly and superiorly over the gastroesophageal junction.
-
3.
Open the pars flaccida exposing the caudate lobe and right diaphragmatic crus.
-
4.
Incise the phreno-esophageal ligament over the diaphragmatic crus and dissect the crus free from the gastroesophageal junction.
-
5.
Pass a penrose drain around the gastroesophageal junction to aid with dissection.
-
6.
Divide the avascular plane between the omentum and colon. Preserve the entire course of the right gastro-epiploic artery.
-
7.
Create an omental pedicle flap, based on 2–3 perforating omental arterial branches off the right gastro-epiploic artery.
-
8.
Complete gastric mobilization along the greater curvature by dividing short gastric arteries.
-
9.
Perform D2 lymphadenectomy and divide the left gastric vessels.
-
10.
Perform either pyloromyotomy or pyloroplasty.
-
11.
Create the gastric conduit with multiple fires of linear stapler from incisura towards the angle of His.
-
12.
Create feeding jejunostomy 30 cm from the ligament of Treitz.
-
13.
Close the abdomen.
-
14.
Perform right thoracotomy.
-
15.
Mobilize the esophagus by incising the inferior pulmonary ligament, retracting the lung anteriorly and medially, and incising the mediastinal pleura along the anterior surface of the esophagus.
-
16.
Mobilize the subcarinal/level 7 lymph node compartment en bloc with the esophagus.
-
17.
Mobilize the azygos arch and divide it with vascular stapler.
-
18.
Mobilize the esophagus away from the trachea.
-
19.
Incise the posterior pleura anterior to the azygos vein and extend inferiorly to the diaphragmatic hiatus.
-
20.
Ligate the thoracic duct between the spine and aorta at T10.
-
21.
Mobilize the esophagus along the periaortic plane to the left pleura with all periesophageal lymphatic tissues.
-
22.
Divide the esophagus at or above the level of the azygos arch.
-
23.
Purse-string the esophagus around the anvil of the stapler.
-
24.
Create gastrotomy at the tip of the gastric conduit and place the circular stapler into the conduit.
-
25.
Open the stapler extending the spike along the greater curvature of the stomach. Align the anvil with the spike and staple the anastomosis.
-
26.
Amputate the tip of the conduit removing the gastrotomy site.
-
27.
Place the omental pedicle flap between the anastomosis and the airway and circumferentially envelop the anastomosis and gastric staple line.
-
28.
Irrigate the chest cavity and place chest tubes in the pleural spaces.
-
29.
Close thoracotomy incision in routine fashion.
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sepesi, B., Hofstetter, W.L. (2015). Open Technique for Ivor Lewis Esophagectomy. In: Kim, J., Garcia-Aguilar, J. (eds) Surgery for Cancers of the Gastrointestinal Tract. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1893-5_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1893-5_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1892-8
Online ISBN: 978-1-4939-1893-5
eBook Packages: MedicineMedicine (R0)