Abstract
Pericardial effusion with tamponade physiology is an underrecognized and life-threatening but treatable cause of hemodynamic impairment in the critical care setting. As fluid accumulates in the pericardial space, the pericardial pressure exceeds the intracardiac pressure and diminishes cardiac output. Goal-directed critical care echocardiography performed at the bedside can promptly detect a pericardial effusion, which is visualized as an echo-free space surrounding the heart. In the proper clinical context, echocardiographic signs of tamponade, such as diastolic collapse of the right-sided chambers and plethora of the inferior vena cava, can help to establish the diagnosis of cardiac tamponade. This chapter will review technical considerations, imaging acquisition and interpretation, and pitfalls in the critical care ultrasound assessment of pericardial effusion and tamponade.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Inferior Vena Cava
- Right Ventricle
- Pericardial Effusion
- Cardiac Tamponade
- Left Ventricular Outflow Tract
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Echocardiography is a valuable tool in the comprehensive evaluation of critically ill patients with suspected pericardial effusion and cardiac tamponade. Cardiac tamponade is a life-threatening condition which requires timely identification and treatment. In recent years, conclusive data has emerged on adverse outcomes related to invasive hemodynamic monitoring in critically ill patients [1]. As a result, critical care physicians have increasingly been relying on noninvasive techniques, particularly ultrasonography, as preferred modalities for the hemodynamic assessment and management of critically ill patients [2]. Advances in ultrasound imaging and device portability have made bedside applications readily available in recent years [3]. Critical care echocardiography (CCE) provides real-time visualization of anatomic structures and also provides an assessment of functional parameters [4, 5]. CCE is increasingly being adopted by both intensivists and emergency medicine physicians and is particularly helpful in the acute diagnosis of pericardial effusion and tamponade [6, 7], allowing time-sensitive, lifesaving interventions to be performed at a rapid pace compared to traditional cardiologist-driven echocardiography [8, 9].
In this chapter, we will review the technical considerations, image acquisition techniques, and basic and advanced competencies required by intensivists when approaching a patient with suspected pericardial effusion or tamponade.
Technical and Patient Considerations
Ultrasound Principles
The improvements in transducer technology and miniaturization of the ultrasound probe size have helped bedside echocardiography become feasible and accessible to non-cardiologists. For optimal cardiac visualization, “microconvex” or phased array probes with smaller footprints and lower frequencies are commonly used (1–5 MHz). Phased array probes are more compact, allowing for easier imaging and maneuverability within the intercostal space. The commonly used ultrasound modes are “B-mode” or brightness mode, where structures are displayed in two-dimensional (2D) images. This is the default mode on most ultrasound machines and is optimal for evaluation of cardiac anatomy and function. A pericardial effusion can be seen in B-mode as a black, echo-free space surrounding the cardiac chambers (Fig. 7.1). The “M-mode” or motion mode displays images over time on a single plane when a scan line is placed over the point of interest in the 2D-mode. M-mode is better for assessing the rapid movements and excursion of cardiac structures in a single plane (Fig. 7.2). Although this is especially useful in diagnosing a pericardial effusion, advanced training is required to determine findings consistent with tamponade physiology in M-mode. The Doppler mode utilizes the “Doppler shift” resulting from interactions of the ultrasound waves with constantly moving particles to determine the direction and speed of tissue motion and blood flow. Doppler echocardiography is useful to measure respirophasic variations in blood flow velocities through right- and left-sided heart valves to diagnose cardiac tamponade.
Basic Anatomy of the Pericardium
The pericardium is normally 1–2 mm thick and consists of an outer parietal layer and an inner visceral layer. The parietal pericardium is a thicker fibrous layer that surrounds the heart and the visceral pericardium and is contiguous with the epicardium. The enclosed space between the two layers forms the pericardial sac, which normally contains around 5–10 mL of fluid. The parietal pericardium meets with the visceral pericardium around the great vessels to form the pericardial reflections. The pericardium encases all four chambers of the heart and extends 1–2 cm up the great vessels. The pericardial layers provide an enclosed chamber effectively isolating the heart from the rest of the structures in the mediastinum, allowing dynamic movements during the cardiac cycle, aiding cardiac filling during diastole, and providing lubrication [10]. Usually, the pericardial pressure reflects the pleural pressure and varies between – 5 cm H2O during inspiration and + 5 cm H2O during expiration [11, 12].
Pericardial Effusion and Tamponade Physiology
Pericardial effusions can result from various causes including, but not limited to, inflammatory or infectious etiologies, trauma, or malignancy (Table 7.1). Normally, the pericardium can stretch up to a point, termed the reserve volume, to accommodate changes in pericardial fluid volume; however, once the reserve volume is exceeded, pericardial compliance decreases. Further increases in the pericardial volume will then result in dramatic increases in pericardial pressure and hence affect atrioventricular filling, as described in detail below, with resultant hemodynamic effects.
Pericardial Compliance
When pericardial fluid accumulates, the compliance of the parietal pericardium initially increases, resulting in a stable intrapericardial pressure. When the pericardial reserve volume is exceeded, pericardial compliance begins to fall and the intrapericardial pressure rises, and the central venous pressure must increase to maintain a gradient that allows cardiac filling. When the stretch threshold of the pericardium is exceeded, pericardial compliance markedly decreases, resulting in rapid increases in pericardial pressure with increasing volume (Fig. 7.3). At this point, the intrapericardial pressure may first equalize with the right ventricular diastolic pressure, and then with the left ventricular diastolic pressure. Critical tamponade occurs when all cardiac chambers have equal diastolic pressure. At this inflection point, there is collapse of the right-sided cardiac chambers and the cardiac output can suddenly drop (Fig. 7.3). Circulation at this point is maintained only through an increase in heart rate, contractility, and peripheral arteriolar vasoconstriction. Left untreated, this can result in circulatory failure and death [13].
Pericardial pressure-volume curves in relation to cardiac output. (a) Rapid accumulation of pericardial fluid leads to a rise in the pericardial pressure. Once the fluid overcomes the pericardial reserve volume and reaches the pericardial stretch threshold, the pressure in the pericardial space increases dramatically causing chamber collapse and impairing cardiac output. (b) With gradual accumulation of pericardial fluid, the pericardium is able to acclimate and stretch to a further extent prior to reaching the pericardial stretch threshold where cardiac output falls. Image courtesy of Guy Aristide, MD
Rate of Accumulation
The rate of accumulation of pericardial fluid and the total fluid volume determine the relative hemodynamic impairment caused by excess fluid in the pericardial space (Fig. 7.3). Conditions like uremic pericarditis or malignancy-related effusion can cause gradual accumulation of a large volume (>2 L) of fluid in the compliant pericardium, potentially causing minimal symptoms and no hemodynamic effects. However, in acute conditions, such as cardiac free wall rupture from pacemaker lead placement, rapid accumulation of even small amounts of pericardial fluid (50–80 mL) can exceed the pericardium’s ability to stretch and can cause hemodynamic compromise [14].
Interventricular Dependence
As the pericardial pressure exceeds the central venous and pulmonary venous pressure, diastolic filling is impaired and becomes increasingly influenced by the respiratory variation in intrathoracic pressures. During inspiration, as the intrathoracic pressure becomes more negative, central venous blood flow to the right atrium and right ventricle (RV) is augmented; at the same time, there is pooling of blood in the pulmonary veins, thereby reducing flow to the left atrium. As the RV is preferentially filled during inspiration, the interventricular septum bows into the left ventricle (LV), causing a drop in cardiac output and hence a drop in blood pressure with inspiration (Fig. 7.4). The opposite holds true with expiration, when the intrathoracic pressure increases and causes diminished return of blood from the vena cava to the right side of the heart and increased flow from the pulmonary veins into the left side of the heart. As more blood is accommodated in the LV, the ventricular septum bows toward the RV. This over-filling and under-filling of the heart with respect to respiration forms the basis for “pulsus paradoxus,” a classic clinical finding associated with cardiac tamponade. Although not specific for cardiac tamponade, an inspiratory drop in blood pressure of more than 10 mmHg in patients with pericardial effusion has a pooled sensitivity of 82 % for cardiac tamponade [13]. Moreover, the severity of the pulsus paradoxus correlates with the severity of hemodynamic compromise in cardiac tamponade [15]. However, caution should be used when interpreting this finding in patients with severe obstructive lung disease, severe volume depletion, right or left ventricular hypertrophy, or in those on invasive positive pressure ventilation. The clinician must be cognizant that cardiac tamponade is a clinical diagnosis and that neither presence of an elevated pulsus paradoxus nor an echocardiography finding of a pericardial effusion establishes the diagnosis of tamponade.
Illustration of respirophasic cardiac physiology in a spontaneously breathing patient with pericardial effusion and tamponade. With inspiration, the intrathoracic pressure becomes more negative leading to increased right heart filling and bulging of the interventricular septum into the left ventricle and decreased left ventricular filling and stroke volume. The converse is true with expiration where the left heart filling is increased, leading to septal bowing into the right ventricle. Image courtesy of Guy Aristide, MD
Patient Considerations
The patient should be examined in the supine or left lateral decubitus position. Suboptimal positioning of the patient will result in unsatisfactory images, especially in critically ill patients who are often on ventilatory support. It is worthwhile for the intensivist to take the time to reposition the patient in a left lateral decubitus position if adequate images are not obtained in a supine position. This position will move the cardiac structures away from the sternum, allowing for improved imaging. The ultrasound machine itself should be positioned such that the machine controls can be easily adjusted by the intensivist. The transducer should be held like a pencil and stabilized by resting the hand holding the transducer on the patient’s body (Fig. 7.5). If the patient is able to follow directions, the left arm should be positioned under the patient’s head when in the lateral decubitus position. Although electrocardiographic (EKG) monitoring is helpful in determining timing of systole and diastole, it is not an absolute necessity while performing emergency point-of-care ultrasonography, when time is of the essence.
Image Acquisition and Interpretation
Image acquisition and interpretation requires an awareness of the plane in which sound waves are transmitted by the footprint, or surface, of the ultrasound probe and the resulting orientation of the 2-D images of the cardiac structures that are generated by the reflected waves. On the monitor, structures closest to the transducer appear at the top of the screen (or near field) and deep structures appear on the bottom (or far field). The leading edge of the beam is marked on the probe and corresponds to the mark on the monitor. By convention, for cardiac imaging the marker is typically on the upper right-hand corner of the screen. The gain can be adjusted to amplify the sound beam and obtain a clearer view. Pericardial fluid can be visualized in a long axis, or sagittal, plane as well as in short axis, or transverse, plane.
Image Acquisition
The cardiac and pericardial structures must be visualized in multiple echocardiographic views to confidently rule out the presence of a pericardial effusion. The pericardial layers appear as a thin, bright, echogenic line around the myocardium (Fig. 7.6). The normal thickness of the pericardium is 1–2 mm. In the normal pericardium, there is less than 25 mL of serous fluid in the pericardial sac formed by the visceral and parietal pericardium. A simple pericardial effusion is visualized as an anechoic (black) area on B-mode, contiguous to the adjacent cardiac structures and separating the echogenic layers of the pericardium (Fig. 7.7). With advanced practice, M-mode and pulsed wave Doppler can be utilized to assess for findings of cardiac tamponade.
Parasternal Long Axis (PLAX)
In the parasternal long axis view, the transducer is placed on the third or fourth intercostal space along the left parasternal region with the probe marker pointing toward the patient’s right shoulder (Fig. 7.8). If the views are suboptimal in the supine position, the patient should be examined in left lateral decubitus position. The depth and gain should be adjusted to optimize the image. Subtle rotation and angling of the probe will ensure that the heart is visualized as a horizontal, elliptical-shaped structure in this view (Fig. 7.7). The normal pericardium is a highly reflective, bright echogenic layer surrounding the cardiac chambers. A pericardial effusion is visualized as a black, anechoic, or echo-free space within the pericardium, separating the two echogenic layers (Fig. 7.7). When the volume of fluid is small, it can be seen as an anechoic space present posterior to the left ventricle and may be seen only in systole. Using the caliper feature, the pericardial fluid can be measured both anteriorly and posteriorly. The right ventricular outflow tract (RVOT), which is seen in the near field, can display diastolic chamber collapse with tamponade physiology. This view is also useful to differentiate pericardial from pleural effusion, which appears posterior to the descending aorta (Fig. 7.9). Localized pericardial effusions, which sometimes can cause hemodynamic instability, can be seen with the PLAX view. Echogenic material with stranding between the pericardial layers may also be seen in this view, indicating the presence of an exudative pericardial effusion from malignant, infectious, or inflammatory etiologies. Additionally, M-mode can be employed from the PLAX 2-D view to closely assess for chamber collapse.
Parasternal Short Axis (PSAX)
From the PLAX view, the transducer is turned 90°, so that the probe marker is now pointing toward the patient’s left shoulder to achieve the PSAX (Fig. 7.10). A cross-sectional image of the left ventricle, with all four walls and part of the right ventricle, can be seen in this view (Fig. 7.11). The PSAX view is particularly useful to study respirophasic movements of the interventricular septum. The dynamic movements of the septum with respiratory variation should alert the clinician to the possibility of increased pressure in the pericardial space. By gently angling anteriorly to the aortic valve level such that the probe is facing the right shoulder, the RVOT, aortic, tricuspid, and pulmonic valves can be visualized. The RVOT can be seen at the top of the screen and can be assessed for diastolic chamber collapse.
Apical Four Chamber (A4C)
In the apical four-chamber view, the footprint of the probe is placed near the apex of the heart or point of maximum impulse with the probe marker pointing to the left shoulder (Fig. 7.12). A left lateral decubitus position and subtle adjustment of the probe will further optimize this view such that the heart appears elliptical and not foreshortened (Fig. 7.13). Both the PLAX and the A4C views are optimal for visualizing pericardial effusions. The A4C view can demonstrate RA and RV diastolic collapse and the paradoxical movement of the interventricular septum that occurs with tamponade physiology. The Doppler sample box can be placed over the mitral and tricuspid valves to assess Doppler velocities.
Apical four-chamber view in a patient with small pericardial effusion. Note that the anechoic space adjacent to the LV apex. Note that the right ventricle is collapsed in diastole (mitral valves are open in diastole). RA right atrium, RV right ventricle, LA left atrium, LV left ventricle, PE pericardial effusion
Apical Five Chamber (A5C)
This view is obtained by slightly angling the probe anteriorly from the A4C view to bring out the left ventricular outflow tract (LVOT). Pulsed wave Doppler (PWD) recordings can reveal exaggerated respiration-dependent phasic changes in the LVOT outflow velocities reflecting changes in stroke volume.
Subcostal (SC)
The subcostal (or subxiphoid) view is especially useful in intubated patients. In the supine position, the probe is placed in the subxiphoid area with the marker pointing to the 3 o’ clock position and the probe angled at 15° cephalad (Fig. 7.14). The four chambers and part of the liver are visualized in the SC view (Fig. 7.15). Occasionally a pericardial cyst or epicardial fat pad can be seen and should be distinguished from an effusion. The entrance of the inferior vena cava (IVC) into the right atrium is seen by angling the probe further posteriorly and rotating counterclockwise. This view is critical when ruling out tamponade, as it is a quick and easy way to assess for inspiratory collapse of the IVC [16]. The SC view is of high utility in evaluating the right atrium for chamber collapse. Additionally, the SC view can be utilized for real-time echocardiographic guidance for pericardiocentesis via the subxiphoid approach.
Image Interpretation
Identification of Pericardial Effusion
Pericardial fluid is seen as an anechoic, or echo-free, space adjacent to the heart (Fig. 7.15). When evaluating for the presence of an effusion, using multiple echocardiographic views is prudent. In the absence of a prior history of cardiac surgery or pericardial disease, the effusion is usually diffuse and circumferential, separating the parietal and visceral pericardium. Small effusions are located posteriorly and, as the size increases, an echo-free space forms around the entire heart. Catastrophic causes of pericardial effusion, such as ventricular wall rupture or post-cardiac surgery effusions, can cause an echogenic effusion due to thrombus or fibrinous debris in the pericardial space. Effusions can also appear as a grayish, hypoechoic layer around the cardiac chambers. An isolated anterior, hypoechoic area should raise the possibilities of either epicardial fat or a loculated effusion. Other conditions such as a pericardial cyst or a pleural effusion can mimic pericardial fluid and should be ruled out in the hypotensive patient (Table 7.2). Pleural effusions are very common and are located posterior to the descending aorta in the PLAX view, whereas pericardial effusions are visualized anterior to the descending aorta and are contained within the echogenic pericardium. Pleural effusions can be further confirmed by dedicated ultrasonography of the thorax. Congenital pericardial cysts, on the other hand, are uncommon and can be unilocular or multilocular. Inflammatory pericardial cysts often present as a loculated pericardial effusion and are a result of connective tissue diseases, infections, or trauma. Chest radiography will often show loculated fluid in the right cardiophrenic angle. Computed tomography (CT) scan or magnetic resonance imaging can be used to further confirm this finding. Lastly, pericardial fat may also be seen on echocardiography, especially in patients with increased abdominal fat. Pericardial fat pads are usually localized anterior to the right ventricular outflow tract. Some clues to the diagnosis of a fat pad are absence of fluid posteriorly, a normal motion of the pericardium, and low intensity echoes or speckles in the pericardium.
Measuring the Size of a Pericardial Effusion
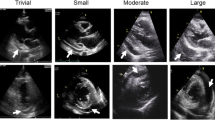
The separation between the heart and the parietal pericardium can be used to gauge the size of effusion. Pericardial effusions should be measured both in anterior and posterior dimensions. Different schemes have been used to quantify the volume of pericardial effusion. In general, a pericardial effusion is described as small if it is less than 1 cm; moderate, if 1 cm to 2 cm; and large, if greater than 2 cm in size [17, 18]. A trace pericardial effusion can be seen even with as little as 25 mL of pericardial fluid. It will appear as a small echo-free space in the posterior atrioventricular groove and may be visible only in systole, when the heart has pulled away from the pericardium. In a small effusion, as much as 1 cm of posterior fluid can be seen with or without fluid accumulation elsewhere. Moderate effusions have 1–2 cm of echo-free space (Fig. 7.16). Large effusions have more than 2 cm of maximal separation, and the heart may be seen swinging within the pericardial fluid (Fig. 7.1). Cardiac tamponade can be seen with moderate to large pericardial effusions but can also be present with small and/or loculated effusions.
Distribution of Pericardial Fluid
Pericardial effusions are usually diffuse, but in instances of prior inflammation or surgery, the effusion can be loculated. Identifying these effusions is important, as loculated effusions can cause hemodynamic compromise. Evaluation from multiple acoustic windows is fundamental in ruling out a loculated effusion (Fig. 7.17). The parasternal views help with excluding fluid collection at the base of the heart. In apical views, lateral, medial, and apical fluid collections can be observed.
Differentiating Pleural from Pericardial Effusion
A left pleural effusion results in an echo-free space posterior to the heart and can be easily confused with a pericardial effusion. Pericardial reflections surround the pulmonary veins and tend to limit the potential space behind the left atrium. Hence, fluid appearing exclusively behind the left atrium is more likely to represent pleural rather than pericardial effusion. The pericardial reflection is typically anterior to the descending thoracic aorta, and therefore, fluid appearing anterior to the aorta is likely to be pericardial; whereas fluid appearing posterior to the descending thoracic aorta is more likely to be pleural (Fig. 7.9; see also Fig 14.16 in Chap. 14).
Basic Competencies
In the evaluation of patients with dyspnea and hypotension, detecting an anechoic area separating the pericardial layers on any of the basic echocardiographic views will establish the diagnosis of a pericardial effusion. However, the characteristic findings of pericardial tamponade may not always be present on echocardiography, and correlation with clinical findings is essential. Hence, the first step is always to obtain vital signs and to check for jugular venous distention and pulsus paradoxus in a patient suspected to have pericardial effusion and/or tamponade. Critical care echocardiography can then be correlated with the clinical findings. The critical care physician with basic echocardiographic training should be able to identify a pericardial effusion, to distinguish a pericardial effusion from mimics, to assess the distribution of the effusion, and to determine if the effusion is associated with echocardiographic signs of hemodynamic impairment (see Box 7.1). The basic assessment of hemodynamic compromise by CCE is discussed below.
Box 7.1 Suggested approach with suspected pericardial effusion
The critical care physician with the help of CCE should attempt to answer the following questions:
-
Is this pericardial effusion or a mimic?
-
Can the etiology of the effusion be determined with CCE?
-
Is the effusion localized or diffuse?
-
Is the effusion small, moderate, or large in size?
-
Is the effusion causing hemodynamic compromise?
-
Can it be safely drained percutaneously?
Determining Hemodynamic Impairment
Bedside CCE can be performed to determine if a pericardial effusion is causing hemodynamic impairment (Box 7.2). However, patients with pericardial effusion and hypotension but without typical tamponade physiology findings on CCE should still be considered for pericardial effusion drainage, if no other explanation for the hypotension is felt to be present.
Box 7.2 basic critical care echocardiographic findings suggestive of cardiac tamponade physiology
-
Moderate or large pericardial effusion
-
Right atrial collapse or inversion
-
Right ventricular diastolic collapse
-
Respiratory paradoxical septal motion
-
Respiratory variation of left and right ventricular size
-
IVC dilation and loss of respiratory variation
Right Atrial (RA) Systolic Collapse
The RA is a low pressure chamber, and the RA free wall is relatively thin. When the pericardial pressure surpasses the atrial pressure, the free wall may collapse during atrial systole (ventricular diastole) (Fig. 7.18). RA systolic collapse is best assessed in the A4C and SC views. The RA wall can remain collapsed through atrial diastole. This inversion of the RA, when present for more than a third of the cardiac cycle, has a very high sensitivity and specificity for diagnosis of tamponade [19]. RA collapse typically occurs at lower intrapericardial pressures than will cause right ventricular collapse. It may be seen as a lone finding in the absence of tamponade physiology, hence other echocardiographic features of tamponade must also be present to confirm cardiac tamponade [20].
Right Ventricular Diastolic Collapse
As the pericardial pressure exceeds the RV diastolic pressure, the free wall of the RV, which is less thick than the LV free wall, may collapse. This can be appreciated with PLAX, PSAX, A4C, or SC views. The RV outflow tract is the more compressible area of the RV and tends to collapse earlier with increasing intrapericardial pressure (Fig. 7.19). Diastolic collapse can be identified following the T wave by employing real-time EKG monitoring. Using echocardiography, the frames can be scanned back and forth to observe for the opening of the mitral or tricuspid valve to estimate the onset of diastole and to assess for RV collapse at that time point [4]. In the PLAX view, an M-mode recording through the RV free wall and anterior mitral valve leaflet helps with visualization and timing of the free wall collapse (Fig. 7.2). RV collapse may be absent in conditions causing RV hypertrophy, such as long-standing pulmonary hypertension. While the finding of RA systolic collapse is highly sensitive, diastolic collapse of the right ventricle is highly specific for diagnosing cardiac tamponade [10, 21].
Reciprocal Respiratory Changes
With cardiac tamponade, the total pericardial volume is fixed and there is an equalization of diastolic pressures within the pericardium and the cardiac chambers. As filling in the right-sided chambers increases with inspiration, there is a reciprocal decrease in the left-sided volume. This is best appreciated in the four-chamber view. During inspiration, there is a shift in the interventricular septum toward the LV and vice versa with expiration (Fig. 7.4). An M-mode tracing can be obtained from the PLAX view to delineate the movement of the septum.
IVC Plethora
Elevated right atrial pressures are reflected by a dilation of the IVC. In a spontaneously breathing patient, a dilated IVC without an inspiratory decrease in size, along with dilated hepatic veins, is termed IVC plethora. This can be visualized best on the SC view (Fig. 7.20). An M-mode tracing of the IVC shows the change in diameter with the respiratory cycle (Fig. 7.21). Less than 50 % inspiratory reduction in the IVC diameter within 2 cm of the entrance into the RA is very sensitive but not specific for diagnosing tamponade [22]. For other causes of IVC plethora, see Pitfalls and Precautions below.
Advanced Competencies
Advanced critical care echocardiography involves the use of Doppler recordings to assess the volume of inflow across the mitral and tricuspid valves and RV/LV diastolic filling to exclude tamponade physiology (see Box 7.3). During inspiration, early diastolic filling velocity in the RV is augmented, while LV diastolic filling is reduced. Normally, there is some variation in flow across the valves due to respiration. In cardiac tamponade, this respiratory variation in blood flow across the valves is exaggerated. If the respiratory variation in flow across the mitral or tricuspid valve is more than 25 %, tamponade is suggested. The tissue Doppler through the mitral annulus will show a reduced E′ when tamponade is present. Additionally, analysis of Doppler flow provides information regarding velocities and direction of flow. Velocities toward the transducer are displayed above the baseline, and velocities away from the transducer are displayed below the baseline. The returning Doppler signal is a spectral tracing of velocity against time. The area under the curve of the spectral trace is known as the Velocity Time Integral (VTI). In tamponade, the VTI in the pulmonary artery increases with inspiration, while the stroke volume and VTI of aortic velocity decrease. Once the diagnosis of tamponade is established, the management should focus on maintaining hemodynamics while preparing for percutaneous or surgical drainage.
Box 7.3 Advanced CCE features of cardiac tamponade
2-D Assessment
-
Moderate or large pericardial effusion
-
Right atrial collapse or inversion (>1/3 cardiac cycle)
-
Right ventricular diastolic collapse
-
Respiratory “paradoxical” septal motion
-
Respiratory variation of left and right ventricular size
-
Inferior vena cava plethora
M-Mode
-
Interventricular septal shift with respiration
-
Diastolic collapse of RVOT/RV
-
IVC dilation (>2 cm) with no respiratory variation
Pulsed-Wave Doppler
-
Increased respiratory variation of inflow across mitral valve >25 % or tricuspid >35 %
-
Phasic variation of LVOT and RVOT outflow velocities
-
IVC with low-velocity flow
Doppler Flow Velocities Across Mitral and Tricuspid Valve
Proceeding with the Doppler tracing, the ventricular inflow velocities are traced across the tricuspid and mitral valve. A 2D image on A4C view is recorded for several beats to correctly align the intercept angle of the Doppler beam parallel to the direction of inflow [10]. Note that as the Doppler intercept angle increases from the direction of inflow, Doppler flow velocities may potentially be underestimated. Inflow velocities can be calculated using the caliper feature of the machine. With cardiac tamponade, there is an increased respiratory variation in flow across the mitral valve of >25 % and across the tricuspid valve of >35 % (Figs. 7.22 and 7.23).
Velocity Time Integral Across the Left Ventricular Outflow Tract
VTI is measured with PWD of LVOT velocities and is recorded from the apical five-chamber view, with the sample volume positioned just proximal to the AV. It denotes the distance that blood travels with each stroke. In patients with cardiac tamponade, VTI across the LVOT is reduced with inspiration. However, measurement of VTI may be time consuming and difficult in the critically ill patient.
Echocardiography-Guided Pericardiocentesis
With the patient in supine position, 2-D echo is performed in multiple views. The angle and depth of penetration to reach the fluid is measured, and a site is marked. Most physicians use the subcostal approach with the needle placed between the xiphoid process and the left costal margin and advanced toward the left scapula in the same orientation as the probe. Real-time echocardiography, using a sterile probe cover, has been used for pericardiocentesis via other approaches including apical and left parasternal, with a 97 % success rate [23]. Care must be taken to avoid the internal mammary artery when using these alternative approaches. Visualization of the needle tip may be difficult, so agitated saline can be injected to confirm that the needle is in the pericardial space prior to insertion of the catheter. Post-procedure echocardiography should be performed to determine residual fluid volume or complications from the procedure.
Transesophageal Echocardiography (TEE)
TEE has a limited role in the spontaneously breathing patient with cardiac tamponade, who may be decompensating. However, patients on positive pressure ventilation, particularly those who are post-sternotomy and have suboptimal 2-D views, may be good candidates for TEE. Postoperative effusions can be loculated and localized to the posterior and lateral aspects of the heart. Postoperative effusions are by definition hemorrhagic in the immediate postoperative setting. An intrapericardial hematoma will have an echogenic appearance similar to the myocardium. They can cause isolated compression of one or more chambers. Transesophageal echocardiography may be the best imaging modality of pericardial effusion or hematoma in these circumstances.
Evidence Review and Evidence-Based Use
Goal-directed critical care echocardiography in the intensive care unit (ICU) setting is increasingly performed at the bedside to determine the cause of hemodynamic impairment and to provide critical information about the cardiovascular status of the patient [24]. In many instances, pocket/handheld devices can only provide 2-D images and may not be able to perform Doppler studies [25]. Newer devices available on the market have overcome this limitation and are increasingly being used by intensivists to perform goal-directed CCE.
Many common presentations of ICU patients, including shock, dyspnea, respiratory failure, and suspected pericardial effusion, are listed by the American Society of Echocardiography as indications for transthoracic echocardiography [26]. Major professional societies such as the American College of Chest Physicians, European Society of Intensive Care Medicine, and networks such as WINFOCUS (World Interactive Network Focused on Critical UltraSound) ECHO-ICU Group all recommend that intensivists obtain necessary echocardiographic training such that they are able to practice this anatomical and physiological investigation at a high level [27–29]. Training in ultrasound techniques for intensivists should aim to achieve competencies in three main areas: general critical care ultrasound, basic critical care echocardiography, and advanced CCE [28]. The training in focused echocardiographic techniques should be adopted as part of fellowship training or, if not available at the time of fellowship training, through training from relevant endorsing professional societies.
A significant number of patients admitted to the medical ICU with noncardiac illness can have cardiac abnormalities. In one series, up to 77 % of patients who had surveillance echocardiography had cardiac abnormalities, including 5 % who had moderate to large pericardial effusions [30]. In another series by Joseph et al., up to 63 % of patients who presented with shock were found to have a cardiac etiology. After severe systolic dysfunction, cardiac tamponade was the second most common cause of shock (17 %) in this series and prompted therapeutic intervention. Of note, transthoracic echocardiography provided adequate imaging in nearly all the cases [31]
Vignon and colleagues studied the use of handheld echocardiography performed by critical care physicians and compared the exam to conventional transthoracic echocardiography (TTE) interpreted by a cardiologist in 103 intubated patients [32]. The diagnostic capacity of the 2-D imaging and the therapeutic impact based on imaging were similar in both groups (44 % vs. 46 %, p = 0.9). However, the mean time required for completing the examination was shorter with the portable device (5 ± 4 min vs. 9 ± 5 min, P = 0.001). Both the portable device and conventional TTE had similar capability in diagnosing pericardial effusion and tamponade (75 % each). In another study involving 515 patients at high risk for pericardial effusion, emergency medicine physicians trained in bedside echocardiography, as compared to trained cardiologists, were able to identify patients with pericardial effusion with a sensitivity of 96 %, specificity of 98 %, and overall accuracy of 97.5 % (95 % CI 95.7–98.7 %) [5].
Biais and associates performed a single-center, prospective study to compare the diagnostic ability of ultra-miniaturized handheld ultrasound devices used by intensivists to conventional TTE, read by a cardiologist blinded to the initial study [33]. Focused examinations showed a high concordance with conventional TTE and adequately identified RV dilation, RV dysfunction, pericardial effusion, and tamponade with good to excellent agreement (κ values 0.63 – 1).
Pitfalls and Precautions
There are several potential pitfalls while performing CCE in diagnosing pericardial effusion and tamponade [34].
-
Diastolic chamber collapse can be seen with severe hypovolemia and large pleural effusion. On the other hand, patients with right ventricular hypertrophy, such as with longstanding pulmonary hypertension, may not demonstrate diastolic chamber collapse even in the presence of cardiac tamponade.
-
An echo-free space limited to the anterior surface is a pericardial fat pad, which appears as an isolated dark area with brighter speckles.
-
A pleural effusion is usually located posterior to the descending thoracic aorta and unlike a pericardial effusion, would not surround the heart on all sides.
-
In post-surgical patients, a loculated or eccentric effusion may cause regional cardiac compression and hemodynamic compromise.
-
Rapid accumulation of even relatively small effusions can cause tamponade physiology and should not be discounted based on size alone.
-
Intrapericardial hematoma arising as a complication of cardiac surgery or procedures may not be apparent on the initial examination, and serial echocardiographic evaluations may be necessary to determine the cause of hypotension.
-
IVC plethora may be seen with other loading conditions causing elevated right atrial pressure such as pulmonary embolism and volume overload states.
-
Cardiac tamponade is ultimately a clinical diagnosis established by a combination of physical exam and echocardiographic findings.
References
Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–24.
Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749–57.
Beaulieu Y. Bedside echocardiography in the assessment of the critically ill. Crit Care Med. 2007;35(5 Suppl):S235–49.
Nagdev A, Stone MB. Point-of-care ultrasound evaluation of pericardial effusions: does this patient have cardiac tamponade? Resuscitation. 2011;82(6):671–3.
Mandavia DP, Hoffner RJ, Mahaney K, Henderson SO. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001;38(4):377–82.
Melamed R, Sprenkle MD, Ulstad VK, Herzog CA, Leatherman JW. Assessment of left ventricular function by intensivists using hand-held echocardiography. Chest. 2009;135(6):1416–20.
Vignon P, Dugard A, Abraham J, Belcour D, Gondran G, Pepino F, et al. Focused training for goal-oriented hand-held echocardiography performed by noncardiologist residents in the intensive care unit. Intensive Care Med. 2007;33(10):1795–9.
Labovitz AJ, Noble VE, Bierig M, Goldstein SA, Jones R, Kort S, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225–30.
Kaplan A, Mayo PH. Echocardiography performed by the pulmonary/critical care medicine physician. Chest. 2009;135(2):529–35.
Catherine M, Otto M. Pericardial Disease. In: Catherine M, Otto M, editors. Text book of clinical echocardiography. 4th ed. Philadelphia: Saunders Elsevier; 2009. p. 242–58.
Schairer JR, Biswas S, Keteyian SJ, Ananthasubramaniam K. A systematic approach to evaluation of pericardial effusion and cardiac tamponade. Cardiol Rev. 2011;19(5):233–8.
Bodson L, Bouferrache K, Vieillard-Baron A. Cardiac tamponade. Curr Opin Crit Care. 2011;17(5):416–24.
Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA. 2007;297(16):1810–8.
Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349(7):684–90.
Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA. Pulsus paradoxus: definition and relation to the severity of cardiac tamponade. Am Heart J. 1988;115(2):391–8.
Himelman RB, Kircher B, Rockey DC, Schiller NB. Inferior vena cava plethora with blunted respiratory response: a sensitive echocardiographic sign of cardiac tamponade. J Am Coll Cardiol. 1988;12(6):1470–7.
Maisch B, Seferovic PM, Ristic AD, Erbel R, Rienmuller R, Adler Y, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004;25(7):587–610.
William F, Armstrong TR. Feigenbaum’s echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2009.
Gillam LD, Guyer DE, Gibson TC, King ME, Marshall JE, Weyman AE. Hydrodynamic compression of the right atrium: a new echocardiographic sign of cardiac tamponade. Circulation. 1983;68(2):294–301.
Merce J, Sagrista-Sauleda J, Permanyer-Miralda G, Evangelista A, Soler-Soler J. Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. Am Heart J. 1999;138(4 Pt 1):759–64.
Kerber RE, Gascho JA, Litchfield R, Wolfson P, Ott D, Pandian NG. Hemodynamic effects of volume expansion and nitroprusside compared with pericardiocentesis in patients with acute cardiac tamponade. N Engl J Med. 1982;307(15):929–31.
Kircher B, Himmelman R. Non invasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–6.
Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77(5):429–36.
Schmidt GA, Koenig S, Mayo PH. Shock: ultrasound to guide diagnosis and therapy. Chest. 2012;142(4):1042–8.
Spevack DM, Tunick PA, Kronzon I. Hand carried echocardiography in the critical care setting. Echocardiography. 2003;20(5):455–61.
Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. 2011;24(3):229–67.
Price S, Via G, Sloth E, Guarracino F, Breitkreutz R, Catena E, et al. Echocardiography practice, training and accreditation in the intensive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49.
Cholley B. International expert statement on training standards for critical care ultrasonography. Intensive Care Med. 2011;37(7):1077–83.
Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050–60.
Bossone E, DiGiovine B, Watts S, Marcovitz PA, Carey L, Watts C, et al. Range and prevalence of cardiac abnormalities in patients hospitalized in a medical ICU. Chest. 2002;122(4):1370–6.
Joseph MX, Disney PJ, Da Costa R, Hutchison SJ. Transthoracic echocardiography to identify or exclude cardiac cause of shock. Chest. 2004;126(5):1592–7.
Vignon P, Chastagner C, Francois B, Martaille JF, Normand S, Bonnivard M, et al. Diagnostic ability of hand-held echocardiography in ventilated critically ill patients. Crit Care. 2003;7(5):R84–91.
Biais M, Carrie C, Delaunay F, Morel N, Revel P, Janvier G. Evaluation of a new pocket echoscopic device for focused cardiac ultrasonography in an emergency setting. Crit Care. 2012;16(3):R82.
Saito Y, Donohue A, Attai S, Vahdat A, Brar R, Handapangoda I, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3):321–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nair, G.B., Mathew, J.P. (2015). Critical Care Echocardiography: Pericardial Disease, Tamponade, and Other Topics. In: Jankowich, M., Gartman, E. (eds) Ultrasound in the Intensive Care Unit. Respiratory Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1723-5_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1723-5_7
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1722-8
Online ISBN: 978-1-4939-1723-5
eBook Packages: MedicineMedicine (R0)