Abstract
Immune stimulating complexes (ISCOMs) are lipid-based particles that have shown potential as adjuvants and carriers for antigens aiming at prophylactic or therapeutic vaccination upon injection as well as via mucosal and cutaneous administration. Both cellular and humoral immune responses have been reported after vaccination with antigens using ISCOM adjuvants, and some are in clinical trials. The adjuvant particles are formed by self-assembly of phospholipid, saponin, and cholesterol at well-defined ratios from mixtures of the components. In aqueous dispersion, they appear as cage-like structures with a hollow center and approximately 40–60 nm in size. The present chapter discusses state-of-the-art with regards to formulation design, characterization, and assessment of the mechanisms of action for ISCOMs with examples from our own research, along with addressing the different routes of administration referring to the clinical status of ISCOMs as adjuvants. The future perspectives of using ISCOMs as vaccine adjuvants are presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Vaccination utilizing adjuvants based on particles ranging from relatively small particles such as virus-like (VLP) particles or larger particles as liposomes and emulsion droplets is a feasible way to stimulate specific immune responses. Immune stimulating complexes (ISCOMs) can be categorized as small 40–60 nm lipid-based particles that have shown potential as adjuvants and carriers for antigens aiming at prophylactic or therapeutic vaccination. Both cellular and humoral immune responses have been reported after vaccination with antigens and ISCOM adjuvants (Sun et al. 2009; Morelli et al. 2012); some of which are in clinical trials (Hook and Rades 2013). Immune stimulation has been observed after administration by injection, via administration to mucosal sites and after cutaneous application (Morein et al. 1984; Sjölander et al. 1998; Pearse and Drane 2005; Sun et al. 2009; Alving et al. 2012; Morelli et al. 2012). The adjuvant particles are formed in solution by self-assembly at well-defined ratios of phospholipid, saponin, and cholesterol. In aqueous dispersion, they appear as cage-like structures with a hollow centre. The state-of-the-art with regards to formulation design, characterization, and assessment of the mechanisms of action for ISCOMs are summarized and discussed along with addressing the different routes of administration and the future perspectives of using ISCOMs as vaccine adjuvants.
2 Characteristics
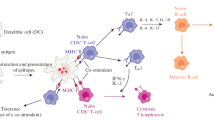
ISCOMs, similar to VLPs (Chap. 9), are self-assembling particles of a size comparable to most typical viruses; and are a type of vaccine adjuvant and delivery system that is attracting continuous attention for development of vaccines (Scheerlinck and Greenwood 2008). The resemblance to viruses in terms of geometry has been proposed to be a beneficial feature, as ISCOMs like VLPs may easily be recognized and taken up by antigen-presenting cells (APCs) due to their composition, size, and surface structure (Scheerlinck and Greenwood 2008), followed by processing and presentation of the antigen incorporated into the vaccine leading to induction of an immune response (Morein et al. 1984). ISCOMs may be comparable to other phospholipid-based vesicle adjuvants, as they have a hollow (albeit open) core, such as liposomes, niosomes, flexosomes, vesosomes, exosomes, and ethosomes although these are usually larger in size being in the size range of 100 nm and larger. Likewise, emulsions and other lipid-based nanoparticles with potential as future adjuvants (like solid lipid nanoparticles and cubosomes) usually appear larger (Nordly et al. 2009).
Microscopic characterization of ISCOMs dispersed in aqueous medium by cryotransmission electron microscopy (TEM) displays spherical hollow particles in the size range of 40–60 nm (Fig. 8.1a) with the presumed 3D hollow and open structure depicted in Fig. 8.1b. Recently, small angle X-ray scattering (SAXS) was employed to describe ISCOMs in suspension confirming the organization of the constituents in the expected structure (Fig. 8.1c) (Pedersen et al. 2012). Still, only qualified theories exist as to how the single constituents self-assemble and are organized to form spherical hollow particles. One hypothesis is that the constituents are placed in stacks with the hydrophobic parts of the molecules facing the interior of the particle bilayers and the more hydrophilic parts oriented towards the aqueous dispersion medium inside and outside the particle structure (Kersten et al. 1991; Kersten and Crommelin 1995). This hypothesis has been used to explain the organization of different types of ISCOM particles, irrespective of whether their net surface charge is negative or positive, as illustrated in Fig. 8.1d (Lendemans et al. 2005). The particles are thus organized into hollow structures with both locally charged areas and lipophilic bilayers, with which the antigens of choice may interact, and are shaped and stabilized by hydrophobic interactions, electrostatic repulsion, steric factors, and possibly hydrogen bonds (Kersten et al. 1991; Lendemans et al. 2005).
(a) Cryo-TEM image of ISCOMs in suspension (Pedersen et al. 2012), (b) schematic 3D-model of the ISCOM cage-like structure, (c) structure of ISCOMs as derived from SAXS analysis (Pedersen et al. 2012), (d) proposed molecular alignment of components in lipid bilayers (Lendemans et al. 2005). Reprinted with permissions from Elsevier (Figure 8.1A and 8.1C) and from Wiley (Figure 8.1D)
3 Lipid Components
By formulation design and exchange of the traditionally used excipients, new generations of ISCOMs have appeared with slightly improved safety as well as immunostimulatory profiles. Such optimizations of the ISCOM properties have been done by partly or fully exchanging the neutral cholesterol component with the positively charged 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl](DC)-cholesterol (Kirkby and Samuelsen 2006; Lendemans et al. 2005) or by exchanging the zwitterionic phospholipids with the cationic dioleoyl-trimethyl-ammonium-propane (DOTAP) (Lendemans et al. 2007) (Fig. 8.2), to decrease the anionic surface charge (Posintros) or to provide them with a cationic surface charge (PLUSCOMs). The net cationic surface charge of PLUSCOMs (approximately +25 mV) (McBurney et al. 2008) will increase the likeliness of (electrostatic) interaction with antigens usually possessing a pI value below 7.4, and thus carrying an overall negative charge at physiological pH. This will also be the case for the Posintro nanoparticles (Kirkby and Samuelsen 2006), which are net anionic (approximately −30 mV), but contain a higher degree of cationic charges as compared to the first generation of ISCOMs with a net charge of −40 mV. Incorporation of other components with lipidic backbone structures into the ISCOM particle could also prove beneficial for the physical stability and/or the effect of the vaccine.
The presence of phospholipids in the ISCOMs has been reported to be important for the formation of the cage-like geometry (Myschik et al. 2006), yet also argued not to be crucial for particle formation, although important for the incorporation or association of antigens into the structure (Lövgren and Morein 1988). These discrepancies reflect the delicate balance between the molar ratios of the constituents that must be finely tuned when preparing ISCOMs by one of the various preparation methods, as will be described below. Also, the presence of cholesterol seems to be crucial in order to assemble the ISCOM structure together with the saponin adjuvant (Lövgren and Morein 1988).
4 Immunostimulating Component
In order to improve the efficacy of the formulation, also the immunogenic component may be changed. Saponins are natural products, which are surface active, negatively charged, possess strong adjuvant properties (Dalsgard 1974; Alving et al. 2012) and are used as the main adjuvant in the preparation of ISCOMs. The crude saponin mixture used for first generation ISCOMs is obtained from extracts from the Quillaja saponaria tree and is now partly purified to give the currently most often used, although still complex mixture, Quil A, or completely purified to obtain one of the main and most safe components, QS-21 from the extracts (Fig. 8.3) (Hook and Rades 2013). The QS-21 fraction has also been used as an adjuvant together with other adjuvants leading to non-ISCOM type structures. Examples of these are the adjuvants AS01, AS02, and AS15 from GSK, all containing QS21 and monophosphoryl lipid A (MPL) and one also CpG (AS15), in liposomal (AS01, AS15) or oil-in-water emulsion formulations (AS02). Structurally, these saponins consist of a rigid lipophilic backbone and two large polar head groups and belong to the group of bola surfactants. They are characterized as triterpene glycosides, and the presence of the glucoronic acid present in the sugar units of the molecule is the main contributor to the negative charge. Their surfactant properties seem to be important for their role in the structure formation of the ISCOMs. Saponins have the ability to bind cholesterol, which is the reason for their known effect on cell plasma membranes mediating lysis at high concentrations due to cholesterol depletion, but may also contribute to the stabilization of the ISCOM structure, which at the same time reduces the side effects of the saponin upon injection (Pham et al. 2006). Although incorporation of the saponins into the ISCOMs has been shown to reduce the cytotoxic effect of the compound (Cox et al. 1998; Kamstrup et al. 2000), the high content of the immune stimulating and negatively charged saponin is responsible for the often overall negative charge of the resulting ISCOM nanoparticles. The unique capacity to stimulate both the production of T-lymphocytes as well as to stimulate a Th1-based immune response makes saponins ideal adjuvants in therapeutic as well as prophylactic subunit vaccines (Sun et al 2009).
Molecular structure of QS-21. Reprinted with permission from Chea et al. 2012. Copyright (2012) American Chemical Society
The structure–activity relationship of the saponins in terms of adjuvanticity is influenced by the hydrophilic sugar side chains and the hydrophobic aglycone backbone, but it is also thought to be related to the aldehyde groups present in the lipophilic backbone of the molecules or to the acyl residue bearing the aglycone (Sun et al. 2009; Soltysik et al. 1995). Yet, the overall adjuvant mechanism is not completely understood. Modifications in the acyl backbone (Wang et al. 2013) have, however, been shown to induce specific alterations in the antibody and cytotoxic T-cell responses as well as in the hemolytic activity of QS-21 variants (Chea et al. 2012). Also, synthetic versions of QS-21 including carbohydrate modifications of the apiose and xylose moieties along with acyl chain modifications have been shown to have an impact on the immunological response and on how well the QS-21 derivative is tolerated (Chea et al. 2012). Besides altering the properties described above and improving the chemical stability of, e.g., the ester bond in the QS-21 acyl chain, the modified versions may incorporate better in the ISCOM particles and thus provide improved properties of the ISCOMs in terms of tailoring the formulation towards a specific immune response. However, this remains still to be proven. Also, despite the fact that the use of novel QS-21 derivatives may improve the safety profile, it may at the same time alter the immunostimulatory effect and thus, options to incorporate other potent immunostimulatory molecules could be explored, but has not yet been reported (Brito et al. 2013).
5 Antigen Component
Due to the hollow geometry, the lipid bilayers and the presence of anionic (and sometimes cationic) patches in the ISCOM, the antigen may be enclosed in the pores, in the interior of the particle or closely associated to the surface of the particle, but cannot as such be encapsulated in the ISCOM structure like it is the case for, e.g., liposomes. The complexation with the ISCOM may thus be mediated by both electrostatic as well as hydrophobic interactions depending on the properties of the antigen. As mentioned above, a way to enhance the interaction between the adjuvant and the usually negatively charged antigen may be to modify the ISCOM to carry more positive charges, and it was indeed demonstrated that when using (partially) positively charged ISCOMs (e.g., PLUSCOMs), a high association of (negatively charged) antigen was achieved, likely due to electrostatic interactions between the ISCOM particles and the antigen (McBurney et al. 2008). Further, modification of the ISCOM by incorporating molecules that may bind directly with a given antigen is a strategy to improve the loading of hydrophilic peptides or proteins in ISCOM-adjuvanted vaccines (Andersson et al. 2000; Cruz-Bustos et al. 2012). Some antigens derived from membrane spanning proteins may by nature contain hydrophobic domains that are likely to interact with the hydrophobic parts of the ISCOM, and some antigens, such as tetanus toxoid, can be partly unfolded to expose hydrophobic patches (Morein et al. 1990) that can interact with the ISCOM lipid bilayers. Another viable approach is to conjugate lipophilic moieties to antigens to promote hydrophobic interactions with the ISCOM bilayers. An example of the latter approach includes conjugation of palmitic acid to ovalbumin (Könnings et al. 2002). However, care should be taken that this does not compromise the antigenicity of the molecule.
In efficacy studies, the antigen is usually co-administered with the adjuvant and detailed systematic studies regarding the localization of the antigen on or in the ISCOM structure prior to and after administration remains still to be reported. Electron microscopy does not provide sufficient resolution unless a thick antigen corona covers the ISCOMs, thus possible quantitative adsorption experiments along with biophysical analysis by, e.g., isothermal calorimetry may be used to describe the interaction between ISCOMs and antigens. Also, as recently demonstrated, modelling of SAXS data might provide valuable information about the localization and the amount of antigen interacting with the ISCOM particles (Pedersen et al. 2012).
6 ISCOM Terminology
The terminology used in literature for describing the various ISCOM nanoparticles is depending on the specific composition, and to some extent reflects their properties. In Table 8.1, the most often used terminologies are described. The differences mainly lie in whether a crude saponin mixture or the different fractions of the Quil A components are used resulting in different preparations that may be recommended for different species depending on the species sensitivity to the saponin component (Fossum et al. 2014).
7 Preparation and Characterization of ISCOM-Based Vaccines
The properties of ISCOM adjuvants or ISCOM-based particles depend on a variety of factors; some of which are described below.
7.1 Preparation Methods
The methods used for preparation of ISCOMs are similar to the methods used for preparation of liposomes, and include dialysis, ultracentrifugation, lipid film hydration, freeze drying, and ethanol or ether injection. The methodologies and their pros and cons are thoroughly described elsewhere (Hook and Rades 2013; Sun et al. 2009) and will not be further addressed in the present chapter. However, briefly it should be mentioned that although each of the mentioned methods results in colloidal dispersions, the main differences lie in (a) the time needed for reaching equilibrium mainly due to the need for removing the lipid solubilizing surfactant, (b) the sample yield, and (c) the homogeneity of the resulting dispersion. Overall, despite the relatively long dialysis time needed to remove the solubilizing surfactant, the dialysis method is often preferred due to the high homogeneity of the resulting ISCOMs.
7.2 Component Ratio
The need for including both phospholipid and cholesterol for the formation of ISCOMs is well recognized (Kersten et al. 1991; Myschik et al. 2006) although it has been extensively debated. The type of the three components, the exact ratio as well as the preparation method and conditions used determine the properties of the resulting self-assembled dispersed structures (Myschik et al. 2006; Hook and Rades 2013). Moreover, the total concentration of lipids is important for the outcome of the preparation. As mentioned, the molecular ratio of the different lipids and the saponin are important for the preparation of the ISCOMs. Thus, the narrow window of molar ratios that lead to ISCOM formation under the specified conditions must be identified, optimally by constructing ternary phase diagrams (Hook and Rades 2013). Briefly, as described in literature, in the absence of the saponin, liposomes are formed, whereas even relatively low amounts of saponin induce the formation of ISCOM structures. If the concentration of the saponin is too low, the dispersion will appear as a mixture of liposomes with ISCOM and/or ring-like micelles. Worm-like micelles and helical structures are typically formed if only cholesterol and saponin are present. Also, as the critical micellar concentration (CMC) of saponin is low compared to the large concentrations required for ISCOM preparation (e.g., the CMC for Quil A was determined to 0.03 % (Özel et al. 1989)), the appearance of saponin micelles may occur in the sample (Madsen et al. 2009). Further, lamellar structures are dominant if no or only a very little fraction of cholesterol is present.
The optimal window for ISCOM preparation may thus more realistically be described as the ratios where mainly ISCOM structures are present after the complete preparation process rather than the more unlikely case of obtaining a sample with only ISCOM structures present. It should be emphasized that the theoretical ratios used may not represent the final ratios in the ISCOMs. As an example, the preparation of ISCOMs and Posintro by the dialysis method (Höglund et al. 1989) was done with an initial weight ratio of 5:1:1 for Quil A, POPC, and cholesterol and when exchanging some of the cholesterol with DC-cholesterol, quantitative lipid analysis showed that the relative amount of DC-cholesterol compared to the other lipids in the resulting particles was much higher than theoretically expected, due to loss during the dialysis process (Madsen, et al. 2010). In a study by Behboudi et al. (1995), it was also shown that for five different types of ISCOMs, the measured amount of lipids, especially the phospholipid, was much lower than the theoretical value, whereas the amount of saponin in most cases was close to the expected value.
Although the colloidal stability of some ISCOM dispersions has been reported to be longer than a year, the colloidal stability is critical to consider when handling ISCOM-based vaccines. Especially as it is a self-assembled particle in an equilibrium state, the colloidal stability of the dispersion will depend on the surrounding conditions, e.g., the storage medium and temperature, and changes to this as for example mixing and diluting with an antigen solution prior to use.
7.3 Structural Characterization
As the sample stability is dependent on, e.g., the ISCOM concentration and the ionic strength of the dispersion medium, care must be taken when preparing samples for analysis and when interpreting results obtained on for example diluted samples. Dilution of samples may be needed for proper determination of the size by using dynamic laser scattering (DLS) or of the zeta potential by using laser Doppler electrophoresis, which is often measured on diluted samples in low-ionic strength buffer. However, the size and shape characteristics may change upon storage or dilution, as results of in vitro studies have demonstrated that with higher dilution, the structure changes from ISCOM structures to more liposomal-like structures indicating a diffusion of the saponin out of the ISCOM (Lendemans et al. 2006). As an expression of negative surface charge density, the mobility of ISCOM particles has been monitored by titration with a cationic polymer, which clearly showed the change in surface charge density when incorporating the positively charged DC-cholesterol (Madsen et al. 2010).
7.4 Size and Structure
For confirmation of size and structural properties, transmission electron microscopy (TEM) or cryo-TEM has traditionally been applied elucidating ISCOMs as spherical particles in the size range of 40–60 nm and composed of ring-like subunits and a hollow centre (Özel et al. 1989; Kersten et al. 1991). The pores of around 7–10 nm in the structure (Kersten et al. 1988; Özel et al. 1989) has also been indicated by freeze fracture electron microscopy.
Recently, SAXS was applied on an undiluted ISCOM sample prepared by dialysis with a resulting size of 43.9 ± 0.2 nm and a polydispersity index of 0.14, measured by DLS, indicating a rather narrow and homogeneous size distribution of the ISCOM particles. Based on Monte Carlo simulation integrations, a novel modelling method was developed and implemented in order to describe the obtained SAXS data. The sample clearly showed a more polydisperse distribution with three types of perforated bilayer vesicles; namely icosahedral (29 nm), football (49 nm), and tennis ball (38 nm) structures. The predominant species that was named the tennis ball structure, accounted for 76–79 % of the ISCOMs in the dispersion by number and mass fractions, respectively. Modeling of these ISCOMs showed 20 pores per tennis ball of a diameter of 5–6 nm and a lipid bilayer membrane thickness of 4.6 nm (Pedersen et al. 2012), corresponding to the general perception of the ISCOM pore size. The structures determined by SAXS were very similar to structures observed in cryo-TEM images on the same batch (Fig. 8.4) strongly indicating that SAXS may be used to model ISCOM structures.
Cryo-TEM image of dispersed ISCOMs (scale bar 50 nm) and SAXS-derived suggested structures indicating three different populations of ISCOMs in the sample (not to scale). Reprinted from Pedersen et al. (2012) with permission from Elsevier
7.5 Association of Antigen
Co-administration of antigens to preformed ISCOM-based adjuvants likely broadens the use of the adjuvants as compared to ISCOM-based vaccines with antigens incorporated or chemically bound to the surface. In both cases, however, the stability and the localization of the antigen are considered key for the efficacy of the vaccine (Brito et al. 2013). How and to which extent the antigens may incorporate into the ISCOMs is speculated to depend strongly on the properties of both components. Only rarely, the ISCOMs with associated antigens are distinguishable from the ISCOMs without the antigen present, as visualized by electron microscopy (Barr and Mitchell 1996), which indicates that only a low number of antigen molecules may (partly) be incorporated in the adjuvant system (Hook and Rades 2013). This hypothesis was confirmed based on modeling of data obtained by using SAXS, as it was evident that only one molecule on average of the tetanus toxoid antigen monomer associated to one ISCOM structure with a size of 38 nm, which did not lead to a detectable change in the size as measured by using DLS. Further, and surprisingly, it was indicated that the tetanus toxoid was located just below the membrane inside the particles (Fig. 8.5). Thus, scattering may provide a useful tool to predict the further information on the interaction of specific antigens with ISCOMs.
Localization of tetanus toxoid on the predominant ISCOM species (38 nm, pore size 5–6 nm) derived from SAXS analysis. Surface representation (left) and cross-section (right) Reprinted from Pedersen et al. (2012) with permission from Elsevier
8 Administration and Mechanisms of Action
The exact mode of action for ISCOMs to induce an immune response is not completely understood, yet at the cellular level, the endocytotic uptake in APCs is stimulated by the particulate nature of the adjuvant/drug delivery system (Kersten and Crommelin 2003), which is likely to be dependent on the interaction with the plasma membrane of the cell.
8.1 Interaction with Cells and Lipid Bilayer Membranes
The cellular interaction may also be unspecifically increased due to the interaction between the saponin carbohydrate and specific receptors on dendritic cells (DCs) (Jiang et al. 1995) or by interaction with the cholesterol component of the plasma membrane (Bangham et al. 1962). Also, the overall charge and thus composition of the particle may be important. An example is the clear increase in the interaction between net negatively charged stratum corneum-like liposomes and the Posintro, which has (theoretically) 25 % of the cholesterol exchanged with DC-cholesterol as compared to ISCOMs without DC-cholesterol and thus a higher degree of cationic charges (Madsen et al. 2010). This entropy-driven interaction was clearly dependent on the content of DC-cholesterol in the ISCOMs and resulted in interference with the lipid bilayer. In addition, the uptake specificity and kinetics may be modulated by formulation design to target, e.g., B-cells, by incorporating specific receptor ligands (Lycke 2004; Helgeby et al. 2006). The uptake and resulting specific cytokine responses may be dependent not only on the incorporated or co-administered antigen, but also on the adjuvant/carrier composition as well as the route and mode of administration.
8.2 Injection of ISCOM-Based Vaccines
The structure of the nanoparticles will inevitably be affected by the administration due to dilution or interaction with the surrounding biological matrix. Upon either subcutaneous (s.c.) or intramuscular (i.m.) injection, the ISCOMs may form a depot at the injection site and by this attract APCs although the results of some studies claim that the particles quickly disappear from the site of injection (Pearse and Drane 2005; Morein and Bengtsson 1998). Given the charged properties of ISCOMs, it is likely that some aggregation occurs as reported (Henriksen-Lacey et al. 2010) for the somewhat larger liposome adjuvant CAF01 currently in clinical trials, followed by disintegration and diffusion of individual components from the depot over time. The kinetics of the depot formation and disassembly will depend on the specific formulation and site of injection. However, it has been demonstrated that the size of nanoparticles in a range from 25 to 100 nm is a prerequisite for their ability to be transported via the lymphatic capillaries to the draining lymph nodes after injection, thus targeting lymph node-residing DCs (Reddy et al. 2007). This corresponds with reports that the DCs in the lymphoid organs and the spleen have been shown to be a target after s.c. or intraperitoneal injection of ISCOMs in mice (Sjölander et al. 1996, 1997).
Recent studies have shown promising results using ISCOMs as adjuvants administered by injection; one being a phase 1 clinical study in healthy adults demonstrating efficacy of influenza vaccination (Fries et al. 2013). Further, chickens were efficiently vaccinated by i.m. administration resulting in increased levels of antigen-specific intestinal IgA and CD4 and CD8 positive intestinal intraepithelial T-lymphocytes after a subsequent oral challenge with the antigen (Zhang et al. 2014).
8.3 Non-injectable Administration of ISCOM-Based Vaccines
Non-injectable administration of vaccines constitutes a more patient friendly, more convenient, and potentially also safer alternative to i.m. and s.c. vaccination strategies. At the same time it provides the possibility for induction of a local immune response at the site of dosing, e.g., to obtain a higher mucosal IgA response after dosing to mucosal sites. Mucosal administration of nanoparticle vaccines includes dosing primarily via the airway and oral routes, which are also the primary sites of infection.
Oral administration and single-dose vaccines have long been desired, yet major challenges remain to formulate a vaccine that is effectively delivered to the target, the gut-associated lymphoid tissue (GALT) within a time frame ensuring sufficient colloidal stability of the drug delivery system and also the appropriate chemical stability of the antigen or subunit antigen in the harsh environment of the gastrointestinal tract. Although the oral mucosa in general is considered to be relatively immune tolerant rather than mediating immune responses (Scheerlinck and Greenwood 2008) it is intriguing to aim for an oral vaccine with ISCOMs and indeed some are tested after oral administration (Gregory et al. 2013; Mowat et al. 1999).
Immunization via the airways may be achieved via the nose- or bronchial-associated lymphoid tissue (NALT and BALT, respectively) and ISCOMs are also a realistic option to be applied for this route of vaccination. Administration via the nose was recently demonstrated to be effective in boosting an existing immunity in draining nasal lymph nodes, whereas pulmonary administration induced strong immune responses in both the lung lavage as well as in the blood (Vujanic et al. 2012). Pulmonary administration of the ISCOMATRIX™ was also shown effective for influenza vaccination (Vujanic et al. 2010). Also, for vaccination against respiratory syncytial virus, the particle size of the nanoparticles was found to significantly influence the immune response (Mottram et al. 2007), which should attract attention for the development of future vaccines.
Transcutaneous immunization by cutaneous application of ISCOM-based vaccines has also been investigated (Combadiere and Mahe 2008). This is mediated by the fact that the strongly immune competent Langerhans cells (LC) are present in high numbers in the epidermis and thus covering a large area underneath the skin surface (Huang 2007). Upon stimulation, these LCs, and activated DCs residing in the dermis, migrate to the lymph nodes resulting in cellular immune responses and antibody production resulting in also mucosal immunity (Frech et al. 2008). Penetration of the adjuvant and an antigen through the outermost layer of the skin, the stratum corneum, constitutes a delivery challenge and considerable efforts are put into creation of novel devices and strategies for expanding the repertoire of skin-breaching modalities, such as the use of microneedles (Bal et al. 2010). Also, the development of novel adjuvants suitable for transcutaneous immunization is a focus area, and it was demonstrated that the application of Posintro particles to human skin in vitro significantly enhanced the penetration of an incorporated dye into stratum corneum, and that the application of ISCOMs using a hydrogel patch resulted in ultrastructural changes in the human stratum corneum (Madsen et al. 2009). Previously, indications that the hair follicles may be a route of entry for ISCOM-based vaccines were given when fluorophore-labeled Posintro particles were observed to localize in the hair follicles of mouse skin after cutaneous application in vivo (Madsen 2010). Especially, since the appearance of LC protrusions is pronounced close to the hair follicles, this finding is valuable.
9 Summary and Perspectives
Since the first description of the potential of ISCOMs as adjuvants by Morein et al. (1984), the technology has matured significantly, and several vaccines with this adjuvant have been tested in clinical trials (Hook and Rades 2013). Extensive modification of the basic technology was first made to mitigate the toxicity due to the presence of the saponin, which included use of purified fractions of the crude mixture of saponins, a different Quil A-to-lipid ratio (Brito et al. 2013) as well as synthetic, more specific immunopotentiators prepared from chemical modifications of the saponin skeleton. Recent mechanistic insight into the influence of the glycol moieties on the adjuvant is opening new perspectives for improved design of carbohydrate-based vaccines (Berti and Adamo 2013) and by use of the progressing biosynthetic technologies, novel glycoconjugates may be pursued for use in future ISCOM-based vaccines.
Research is ongoing with regards to optimizing the formulation design of the ISCOM-based adjuvants and vaccines, especially on the control of the type and specificity, as well as efficacy of the immune response generated, which seem to depend on both the properties of the nanoparticles, the administration route, the dose and dosing regimen as well as the antigen used. The stability and localization of the co-delivered antigens are key factors for the concept to be a success, and since the performance of ISCOM-based vaccines appears to be partially dependent on antigen association (Brito et al. 2013) various approaches to improve the association and binding of the antigens to the particles have been described. The successful use of for example Matrix-M as an adjuvant simply admixed with the antigen, poses the question as to what extent incorporation of the antigen into the ISCOM structure is really needed (Bengtsson et al. 2011). The concept of using preformed ISCOM adjuvants to which the antigen of choice is added prior to use seems therefore promising both from a manufacturing perspective as well as with regards to broader application ranges. However, also in this case it is of importance to investigate the antigen-to-particle association behavior and the importance of this to the efficacy of the vaccine. Many open or only partially answered questions still remain in order to fully understand and develop ISCOM adjuvants and vaccines further: The molecular level mechanism of ISCOM adjuvanticity is still not well understood, nor is the fate of ISCOMS after administration. Also the question of what importance the different colloidal structures, which are found when slightly modifying the component ratios in ISCOM formulations, are for adjuvanticity or immunogenicity needs to be further investigated. The question regarding in which cases co-localization of the antigen and the ISCOM is advantageous and in which cases co-administration is sufficient should be investigated further. Finally, alternative application routes need to be explored and exploited in more detail. These are only a few of the remaining challenges that inspire current application of ISCOM as adjuvants and require future research. Despite these open questions, as ISCOM-based vaccines are generally well tolerated and only inducing minor local side effects upon injection, they are likely to be a part of the future adjuvants and vaccines also for, e.g., cancer vaccines and for both human and veterinary use.
References
Alving CR, Peachman KK, Rao M, Reed SG (2012) Adjuvants for human vaccines. Curr Opin Immunol 24:310–315
Andersson C, Sandberg L, Wernérus H, Johansson M, Lövgren-Bengtsson K, Ståhl S (2000) Improved systems for hydrophobic tagging of recombinant immunogens for efficient iscom preparation. J Immunol Methods 238(1–2):181–193
Bal SM, Ding Z, van Riet E, Jiskoot W, Bouwstra JA (2010) Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J Control Release 148:266–282
Bangham AD, Horne RW, Glauert AM, Dinglejt LJA (1962) Action of saponin on biological cell membranes. Nature 196:952–955
Barr IG, Mitchell GF (1996) ISCOMs (immunostimulating complexes): the first decade. Immunol Cell Biol 74:8–25
Behboudi S, Morein B, Rönnberg B (1995) Isolation and quantification of Quillaja saponaria Molina saponins and lipids in iscom-matrix and iscoms. Vaccine 13:1690–1696
Bengtsson KL, Morein B, Osterhaus ADME (2011) ISCOM technology-based Matrix M™ adjuvant: success in future vaccines relies on formulation. Expert Rev Vaccines 10:401–403
Berti F, Adamo R (2013) Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem Biol 8:1653–1663
Brito LA, Malyala P, O’Hagan DT (2013) Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol 25:130–145
Chea EK, Fernández-Tejada A, Damani P, Adams MM, Gardner JR, Livingston PO, Ragupathi G, Gin DY (2012) Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J Am Chem Soc 134:13448–13457
Combadiere B, Mahe B (2008) Particle-based vaccines for transcutaneous vaccination. Comp Immunol Microbiol Infect Dis 31:293–315
Cox JC, Sjölander A, Barr IG (1998) ISCOMs and other saponin based adjuvants. Adv Drug Deliv Rev 32(3):247–271
Cruz-Bustos T, González-González G, Morales-Sanfrutos J, Megía-Frenández A, Santoyo-González F, Osuna A (2012) Functionalization of immunostimulating complexes (ISCOMs) with lipid vinyl sulfones and their application in immunological techniques and therapy. Int J Nanomedicine 7:5941–5956
Dalsgard K (1974) Saponin adjuvants. 3. Isolation of a substance form Quillaja saponaria Molina with adjuvant activity in food-and-mouth disease vaccines. Arch Gesamte Virsuforschung 44:243–254
DiStefano D, Antonello JM, Bett AJ, Medi MB, Casimiro D, ter Meulen J (2013) Immunogenicity of a reduced-dose whole killed rabies vaccine is significantly enhanced by ISCOMATRIX™ adjuvant, Merck amorphous aluminium hydroxylphosphate sulfate (MAA) or a synthetic TLR9 agonist in rhesus macaques. Vaccine 31:4888–4893
Fossum C, Hjertner B, Ahlberg V, Charerntantanakul W, McIntosh K, Fuxler L, Balagunaseelan N, Wallgren P, Bengtsson KL (2014) Early inflammatory response to the saponin adjuvant Matrix-M in the pig. Vet Immunol Immunopathol 158:53–61
Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, Okhuysen PC, Guerrero NH, Martinez-Sandoval FG, Meléndez-Romero JH, Jiang ZD, Halpern J, Torres OR, Hoffman AS, Villar CP, Kassem RN, Flyer DC, Andersen BH, Kazempour K, Breisch SA, Glenn GM (2008) Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhea: a phase II, randomized, double-blind, placebo-controlled field trial. Lancet 371:2019–2025
Fries LF, Smith GE, Glenn GM (2013) A recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J Med 369:2564–2566
Gregory AE, Titball R, Williamson D (2013) Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 3:1–13
Helgeby A, Robson NC, Donachie AM, Beacock-Sharp H, Lövgren K, Schön K, Mowat A, Lycke NY (2006) The combined CTA1-DD/ISCOM adjuvant vector promotes priming of mucosal and systemic immunity to incorporated antigens by specific targeting of B-cells. J Immunol 15:3697–3706
Henriksen-Lacey M, Bramwell VW, Agger EM, Andersen P, Perrie Y (2010) Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release 142:180–186
Höglund S, Dalsgaard K, Lovgren K, Sundquist B, Osterhaus A, Morein B (1989) ISCOMs and immunostimulation with viral antigens. Subcell Biochem 15:39–68
Hook S, Rades T (2013) Immune stimulating complexes (ISCOMs) and Quil-A containing particulate formulations as vaccine delivery systems. In: Flower DR, Perrie Y (eds) Immunomic discovery of adjuvants and candidate subunit vaccines. Immunomics reviews, vol 5. Springer, New York, pp 233–261. doi:10.1007/978-1-4614-5070-2_12
Huang CM (2007) Topical vaccination: the skin as a unique portal to adaptive immune responses. Semin Immunopathol 29:71–80
Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC (1995) The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375:151–155
Kamstrup S, San MR, Doberti A, Grande H, Dalsgaard K (2000) Preparation and characterisation of quillaja saponin with less heterogeneity than Quil-A. Vaccine 18:2244–2249
Kersten GF, Crommelin DJ (1995) Liposomes and ISCOMs as vaccine formulations. Biochim Biophys Acta 1241:117–138
Kersten GF, Crommelin DJ (2003) Liposomes and ISCOMs. Vaccine 21:915–920
Kersten GF, Teerlink T, Derks HJ, Verkleij AJ, van Wezel TL, Crommelin DJ, Beuvery EC (1988) Incorporation of the major outer membrane protein of Neisseria gonorrhoeae in saponin-lipid complexes (iscoms): chemical analysis, some structural features, and comparison of their immunogenicity with three other antigen delivery systems. Infect Immun 56:432–438
Kersten GF, Spiekstra A, Beuvery EC, Crommelin DJ (1991) On the structure of immune-stimulating saponin-lipid complexes (iscoms). Biochim Biophys Acta 1062:165–171
Kirkby N, Samuelsen P (2006) Composition for vaccination. US 2006/0147509 A1, 6 July 2006
Könnings C, Copland MJ, Davies NM, Rades T (2002) A method for the incorporation of ovalbumin into immune stimulating complexes prepared by the hydration method. Int J Pharm 241:385–389
Lendemans DG, Myschik J, Hook S, Rades T (2005) Cationic cage-like complexes formed by DC-cholesterol, Quil-A, phospholipid. J Pharm Sci 94:1794–1807
Lendemans DG, Egert AM, Myschik J, Hook S, Rades, T (2006) On the dilution behaviour of immuno-stimulating complexes (ISCOMs). Pharmazie 61:689–695
Lendemans DG, Egert AM, Hook S, Rades T (2007) Cage-like complexes formed by DOTAP, Quil-A and cholesterol. Int J Pharm 332:192–195
Lövgren K, Morein B (1988) The requirement of lipids for the formation of immunostimulating complexes (iscoms). Biotechnol Appl Biochem 10:161–172
Lycke N (2004) From toxin to adjuvant: the rational design of a vaccine adjuvant vector, CTA1-DD/ISCOM. Cell Microbiol 6:23–32
Madsen HB (2010) Penetration and interaction of ISCOM nanoparticles with the skin. PhD thesis, University of Copenhagen. ISBN 978-87-92199-87-4
Madsen HB, Ifversen P, Madsen F, Brodin B, Hausser I, Nielsen HM (2009) In vitro cutaneous application of ISCOMs on human skin enhances delivery of hydrophobic model compounds through the stratum corneum. AAPS J 4:728–739
Madsen HB, Arboe-Andersen HM, Rozlosnik N, Madsen F, Ifversen P, Kasimova MR, Nielsen HM (2010) Investigation of the interaction between modified ISCOMs and stratum corneum lipid model systems. Biochim Biophys Acta 1798:1779–1789
McBurney WT, Lendemans DG, Myschik J, Hennessy T, Rades T, Hook S (2008) In vivo activity of cationic immune stimulating complexes (PLUSCOMs). Vaccine 26:4549–4556
Morein B, Bengtsson KL (1998) Functional aspects of iscoms. Immunol Cell Biol 76:295–299
Morein B, Sundquist B, Höglund S, Dalsgaard K, Osterhaus A (1984) ISCOM, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 308:457–460
Morein B, Ekström J, Lövgren K (1990) Increased immunogenicity of a non-amphipathic protein (BSA) after infusion into iscoms. J Immunol Methods 128:177–181
Morelli AB, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E (2012) ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J Med Microbiol 61:935–943
Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M (2007) Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm 4:73–84
Mowat AM, Smith RE, Donachie AM, Furrie E, Grdic D, Lycke N (1999) Oral vaccination with immune stimulating complexes. Immunol Lett 65:133–140
Myschik J, Lendemans DG, McBurney WT, Demana PH, Hook S, Rades T (2006) On the preparation, microscopic investigation and application of ISCOMs. Micron 37:724–734
Nordly P, Madsen HB, Nielsen HM, Foged C (2009) Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Exp Opin Drug Deliv 6:1–16
Özel M, Hoglund S, Gelderblom HR, Morein B (1989) Quaternary structure of the immunostimulating complex (iscom). J Ultrastruct Mol Struct Res 102:240–248
Pearse MJ, Drane D (2005) ISCOMATRIX adjuvant for antigen delivery. Adv Drug Deliv Rev 57:465–474
Pedersen JS, Oliveira CLP, Hübschmann HB, Arleth L, Manniche S, Kirkby N, Nielsen HM (2012) Structure of ISCOM matrices and ISCOMs in suspension determined by small-angle X-ray scattering. Biophys J 102:2372–2380
Pham HL, Ross BP, McGeary RP, Shaw PN, Hewavitharana AK, Davies NM (2006) Saponins from Qillaja saponina Molina: isolation, characterization and ability to form immune stimulatory complexes (ISCOMs). Curr Drug Deliv 3:389–397
Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA (2007) Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 25:1159–1164
Scheerlinck J-PY, Greenwood DLV (2008) Virus-sized vaccine delivery systems. Drug Discov Today 13:882–887
Sjölander A, Bengtsson KL, Johansson M, Morein B (1996) Kinetics, localization and isotype profiling of antibody responses to immune stimulating complexes (iscoms) containing human influenza virus envelope glycoproteins. Scand J Immunol 43:164–172
Sjölander A, Bengtsson KL, Morein B (1997) Kinetics, localization and cytokine profile of T-cell responses to immune stimulating complexes (iscoms) containing human influenza virus envelope glycoproteins. Vaccine 15:1030–1038
Sjölander A, Cox JC, Barr IG (1998) ISCOMs: an adjuvant with multiple functions. J Leukoc Biol 64:713–723
Soltysik S, Wu J-Y, Recchia J, Wheeler DA, Newman MJ, Coughlin RT, Kensil CR (1995) Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 13:1403–1410
Sun H-X, Xie Y, Ye Y-P (2009) ISCOMs and ISCOMATRIX™. Vaccine 27:4388–4401
Vujanic A, Wee JLK, Snibson KJ, Edwards S, Pearse M, Quinn C, Moloney M, Taylor S, Scheerlinck J-PY, Sutton P (2010) Combined mucosal and systemic immunity following pulmonary delivery of ISCOMATRIX™ adjuvanted recombinant antigens. Vaccine 28:2593–2597
Vujanic A, Sutton P, Snibson KL, Yen H-H, Scheerlinck J-PY (2012) Mucosal vaccination: lung versus nose. Vet Immunol Immunopathol 148:172–177
Wang P, Dai Q, Thogaripally P, Zhang P, Michalek SM (2013) Synthesis of QS-21 based immunoadjuvants. J Org Chem 78:11525–11534
Zhang Y, Wang L, Ruan W, Zhang J, Yao P, Zhou S, An J (2014) Immunization with recombinant 3-1E protein in AbISCO®-300 adjuvant induced protective immunity against Eimeria acervulina infection in chickens. Exp Parasitol 141:75–81
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nielsen, H.M., Hübschmann, H.B., Rades, T. (2015). ISCOMs as a Vaccine Delivery System. In: Foged, C., Rades, T., Perrie, Y., Hook, S. (eds) Subunit Vaccine Delivery. Advances in Delivery Science and Technology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1417-3_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1417-3_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1416-6
Online ISBN: 978-1-4939-1417-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)