Abstract
Acute promyelocytic leukemia (APL) is a model disease for targeted therapy. APL is caused by a variety of fusion proteins, all implicating the retinoic acid receptor alpha (RARA). The promyelocytic gene (PML)/RARA fusion is by far the most frequent, present in 99 % of patients. Two unconventional drugs, retinoic acid (RA) and arsenic trioxide were first shown to exhibit extraordinary clinical activity and later found to directly target PML/RARA. RA binds PML/RARA via its RARA moiety, activates transcription and degrades PML/RARA. Arsenic only degrades the fusion protein by targeting its PML part. Mouse modeling in APL has allowed an unprecedented level of understanding of the disease pathogenesis and basis for therapy response, highlighting the key role of PML/RARA degradation in the latter. The combination of RA and arsenic definitively eradicate the disease in mice and in most patients. APL thus represents a paradigm for oncoprotein-targeted cures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
As detailed in the accompanying clinical chapter, few diseases have had such a dramatic change in treatment and prognosis as acute promyelocytic leukemia (APL). Indeed, it rose from being an hematologic emergency with less than 30 % 5-year survival, to a disease with a 95 % definitive cure rate, with some patient even no longer receiving chemotherapy.
Similarly, few diseases have been the focus of so many physio-pathological studies, as well as those addressing the basis for therapy response. A reasonably clear image of the disease is now emerging. Amusingly, in contrast to most kinase inhibitors, that were designed to block the activity of an oncogenic kinase activated by point mutation or translocation, the two miracle APL drugs were found empirically and only much later demonstrated to target the driving oncogene. They actually played a key role in unraveling the disease pathogenesis, starting with the identification of its underlying molecular defect. Finally, these drugs exert a curative activity allowing patients to go definitively off-treatment.
For all these reasons, despite its low frequency, APL is a key model for targeted therapies.
2 PML/RARA, the Driver of APL Pathogenesis
More than 98 % APL are associated with the balanced reciprocal translocation t(15;17)(q22;q11-12), fusing the promyelocytic gene (PML) with the retinoic acid receptor alpha gene (RARA; Fig. 23.1a) . Other APL patients harbor alternative translocations always involving RARA, the most common being t(11;17) that involves the promyelocytic leukemia zinc finger (PLZF) gene (Chen et al. 1993; Piazza et al. 2001).
a Schematic representation of promyelocytic gene/retinoic acid receptor alpha (PML/RARA). b The classic model of APL response to retinoic acid (RA). HDAC histone deacetylase. (Reprinted from de The and Chen 2010)
Other rare lesions, often shared with other leukemias or malignancies, have been implicated in APL progression, such as MYC amplification, Fms-like tyrosine kinase 3 activation, or RAS mutations (Akagi et al. 2009). However, APL has an almost constant incidence with age, suggesting that it arises from a single rate-limiting genetic event (Vickers et al. 2000). The recurrent presence of X-RARA fusions in APL patients and the fact that their sole expression initiates typical APL in transgenic mice strongly argue for a hierarchy in these genomic abnormalities, X-RARA being the primary actor of leukemogenesis. Still, because PML/RARA transgenic mice develop leukemias with long latencies and incomplete penetrance, the potential requirement for cooperating mutations and/or additional epigenetic changes to yield the full APL phenotype has been the object of many discussions (Welch et al. 2011). But recent studies have clearly demonstrated the absence of mutations actually contributing to transformation (Welch et al. 2012), while studies in APL that develop following chemotherapy have all demonstrated a short (typically 1 year) time interval between DNA-damaging chemotherapy and disease onset (Mistry et al. 2005). Thus, the authors consider APL as a monogenic, X-RARA-driven, disease (de The and Chen 2010) .
3 RARA, the Constant Partner of the Fusion
Retinoic acid (RA) has been involved in a variety of physiological regulatory mechanisms, in particular, morphogenesis, stem cell self-renewal, and myeloid differentiation (Kastner et al. 2001; Strickland and Mahdavi 1978).
RARA is a receptor for RA discovered in the early 1990. Like all other nuclear receptors, it has a highly conserved modular organization with a zinc-finger containing sequence-specific DNA-binding domain and a complex ligand-binding domain that also enables dimerization and transactivation (Kastner et al. 1995). RARA is normally bound to a member of the retinoid X receptor (RXR) family of nuclear receptors as an obligatory heterodimer. Within the retinoic acid receptor (RAR)/RXR complexes, both receptors contribute to DNA binding and transcriptional repression, but only RARs contribute to activation. The RAR and RXR DNA-binding domains each recognize a AGGTCA core motif, usually in a direct repeat orientation, separated by a spacing of 2 or 5 nucleotides. These retinoic acid response elements (RARE) confer RA sensitivity to the promoters where they are present (de The et al. 1990). Recent genomic experiments have outlined their distribution in the genome (Hua et al. 2009).
RARs are versatile transcriptional switches that can either repress or activate transcription. This is achieved by the binding of a family of proteins named coactivators or corepressors. At large, RAR/RXR complexes bind corepressors in their unliganded state and recruit coactivators in the presence of ligands. Interestingly, RARA appears to be a stronger binder for corepressors than other RARs. This may explain its constant implication in APL, as this will confer X-RARA fusions with stronger transcriptional repression (Farboud et al. 2003).
4 From the Classic to Refined Models for APL Pathogenesis
Different studies showed that PML/RARA or the rare PLZF/RARA variant bind co-repressors and histone deacetylase (HDAC) with higher affinity than RARA, due to their ability to homodimerize (Licht 2006). PML/RARA thus behaves as a super-repressor so that repression of basal retinoic acid signaling could contribute to the differentiation block (Fig. 23.1b). Pharmacological doses of RA could then release both the transcriptional and differentiation blocks (Melnick and Licht 1999) .
This model, primarily based on cell-line studies, was progressively refined, notably with the findings that RXRA plays an important role in the transformation process, most likely by enhancing DNA binding of X-RARA fusions (Zhu et al. 2007; Zeisig et al. 2007). PML/RARA homodimers, together with the RXRA co-receptor, have 4 domains able to recognize the AGGTCA cores and accordingly display highly degenerated binding-site specificity (Kamashev et al. 2004). Consequently, the PML/RARA binding site repertoire is considerably enlarged when compared to the one of RARA, as demonstrated with natural PML/RARA target genes in human APL cells (Martens et al. 2010). Importantly, some of the recognized sequences are targets of other nuclear receptors (vitamin D receptor (VDR), thyroid receptor (TR), peroxisome proliferator-activated receptor (PPAR)...) controlling myeloid differentiation or stem cell self-renewal. Similar properties were described for other myeloid leukemia-associated oncogenic fusions, suggesting that dimerization-enforced relaxation in DNA-binding site specificity may be a general mechanism of leukemic transformation (So and Cleary 2004) .
5 Is PML only a Dimerization Interface?
The first models for APL for APL pathogenesis viewed PML mainly as a provider of a strong dimerization interface. Indeed, all proteins fused to RARA in APL contain potent dimerization domains. While in cell lines RARA dimerization suffice to confer strong repressive ability on RARA signaling and some inhibition of differentiation, attempts to induce APL in vivo were largely unsuccessful (Sternsdorf et al. 2006). Importantly, these eventually succeeded only when using the PML dimerization domain (Occhionorelli et al. 2011), suggestive for an important contribution of PML beyond providing a dimerization interface.
PML protein initiates the formation of nuclear bodies (NBs), sub-nuclear spherical structures involved in the fine-tuning of several biological processes (Lallemand-Breitenbach and de The 2010) (Fig. 23.2, arrow in the left panel). A specific post-translational modification, sumoylation, controls recruitment onto NBs of a wide variety of partner proteins. In turn, partner recruitment into PML NBs finely modulates their post-translational modification and may result in protein sequestration or activation. Importantly, PML loss and/or NB disruption seem to be associated with enhanced self-renewal (Ito et al. 2008; Regad et al. 2009). In APL, PML/RARA dimerizes with PML, leading to the replacement of the normal speckled nuclear distribution of PML by a micro-speckled one (Fig. 23.2) (Koken et al. 1994). Thus, in addition to transcriptional deregulation, this alteration in nuclear architecture could participate in APL pathogenesis, notably in fostering aberrant self-renewal.
Left: disruption of the normal PML pattern (cell on the right, arrow) by PML/RARA expression (cells on the left). Right: the micro-speckeled pattern typical of APL cells, a feature that may be used for diagnosis (Dyck et al. 1995). (Adapted from Koken et al. 1994)
Moreover, some studies have found that PML actually contributes to transcriptional repression by PML/RARA, through its modification by SUMO, a post-transcriptional modification that confers transcriptional repression ability to transcription factors (Zhu et al. 2005; Verger et al. 2003).
6 Two Drugs for one Disease
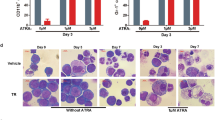
The introduction of RA for APL treatment in 1985 (Huang et al. 1988) constituted the first example of differentiation therapy (Degos et al. 1995). Ex vivo and in vivo, RA triggers rapid APL cell differentiation into granulocytes, which correlates with patient remissions (Fig. 23.3, top panel). With single-agent RA therapy, remissions are unfortunately usually transient (Warrell et al. 1993; Tallman and Altman 2009), suggesting that differentiation alone cannot abolish cancer cell self-renewal (Kogan 2009; de The and Chen 2010) (see the next chapter by M. Tallman).
Cellular effects of retinoic acid (RA) or arsenic (AS) in a mouse model of APL. Note that both drugs induce differentiation, but with different kinetics and that AS also induces apoptosis at 2 days (arrow). Note the reappearance of normal bone marrow cells after 2 days of RA (arrow). (Reprinted from de The and Chen 2010)
The other potent anti-APL agent As2O3 (arsenic) , is considerably more efficient than RA as single agent (Chen et al. 2011; Zhu et al. 2002). It induces both apoptosis and differentiation in vivo (Fig. 23.3, bottom panel) and, in combination with RA may yield 90 % definitive cures, even without DNA-damaging chemotherapies (Hu et al. 2009; Estey et al. 2006; de The and Chen 2010; Wang and Chen 2008). Clinical trials in non-APL cancer patients have been largely disappointing, unexpectedly demonstrating that this notoriously toxic compound has a great specificity for APL cells (Zhu et al. 2002).
7 Molecular Basis for PML/RARA-Targeted APL Therapies?
Molecular studies performed after demonstration of their clinical efficacy have revealed that both RA and arsenic directly target the PML/RARA oncoprotein for degradation (Quignon et al. 1997; de The and Chen 2010; Zhu et al. 2001) (Fig. 23.4a). In a remarkable and completely unexpected symmetry, RA targets the RARA part of PML/RARA, while arsenic directly targets its PML part (Quignon et al. 1997) (Fig. 23.4b). Thus, these two agents discovered by chance actually directly target PML/RARA through its two constitutive moieties, making them a posteriori targeted therapies. This strongly suggested an important, if not essential, contribution of PML/RARA degradation to therapy response.
a Retinoic acid and arsenic both degrade promyelocytic gene/retinoic acid receptor alpha (PML/RARA) in APL cells after an overnight treatment ex vivo at therapeutic concentrations. Note that retinoic acid (RA) also degrades RARA, while arsenic (As) does not, pointing to distinct mechanisms. b Pathways of PML/RARA degradation. Domains in PML/RARA are indicated. Note that RA targets the RARA part of the fusion, while arsenic targets in PML moiety through oxidation, direct binding, and sumoylation and RNF4-mediated poly-ubiquitination. See text and (Lallemand-Breitenbach et al. 2012) for details
Concerning RA targeting of PML/RARA, RA: 1) releases co-repressor binding from PML/RARA, 2) induces transactivation through the PML/RARA-mediated recruitment of co-activators, and 3) induces PML/RARA degradation. Thus, RA reverses all PML/RARA properties but the contribution of each to APL clearance remains debated (Ablain and de The 2011; Ablain et al. 2011). It must be noted that the therapeutic concentrations of RA used against APL are several orders of magnitudes higher than its physiologic concentrations or its binding affinity, and the decrease in plasma concentration is constantly associated to clinical RA resistance (Muindi et al. 1992). Other cases of RA-resistance are associated with mutations in the RARA moiety of PM/RARA that preclude RA-binding, transactivation and/or degradation (Gallagher et al. 2006; Gallagher 2002). The fact that only pharmacologic levels of RA are associated with therapy response and full PML/RARA degradation, strongly supports an important role of the latter in disease remission (Zhu et al. 2001).
PML/RARA targeting by arsenic is enforced both by direct binding and arsenic-induced reactive oxygen species that elicit PML oxidation through the formation of disulfide bridges (Jeanne et al. 2010; Zhang et al. 2010; de The and Chen 2010). As extensively reviewed elsewhere, binding and oxidation initiate formation of a PML mesh, its hypersumoylation, then allowing recruitment of the SUMO-dependent ubiquitin ligase RNF4, which subsequently triggers PML or PML/RARA degradation (Geoffrey and Hay 2009; Lallemand-Breitenbach et al. 2008; Lallemand-Breitenbach et al. 2001; Tatham et al. 2008).
The role of PML/RARA sumoylation and degradation in arsenic-based therapy is supported by significant genetic evidence. Mutation of the arsenic-sensitive sumoylation site in PML/RARA impairs response to arsenic ex vivo but not RA-induced differentiation (Zhu et al. 2005). Mutation of the cysteine residues required for arsenic binding impairs the response to As2O3 ex vivo (Jeanne et al. 2010) and neighboring mutations were observed in arsenic-resistant patients (Goto et al. 2011). Finally, in murine models of APL, vitamin E derivatives with mitochondrial toxicity generating oxidative stress induce prolonged remissions (Dos Santos et al. 2011), validating intracellular oxidation as a key anti-APL mechanism. Importantly, arsenic does not induce PLZF/RARA degradation and is accordingly inefficient in PLZF/RARA APL models (Rego et al. 2000; Jeanne et al. 2010).
8 Differentiation and/or Self-Renewal: Mice Come to the Stage
On the cellular side, differentiation-based therapy in APL primarily relies on the correlation between clinical remissions and morphological maturation of leukemia blasts (Warrell et al. 1993). However, this cannot explain why only few patients are cured by RA alone, neither why arsenic cures 70 % of APL patients, although it does not induce differentiation ex vivo. Accordingly, there have been controversies as to the exact contribution of differentiation to APL cure (Kogan 2009; Ablain and de The 2011).
The most recently proposed models have uncoupled APL differentiation and loss of self-renewal (Kogan 2009). Indeed, blast differentiation does not necessarily trigger loss of leukemia-initiating activity and self-renewal (Nasr et al. 2008). Yet, these are the only features predicting disease eradication in vivo (Ablain and de The 2011).
Mouse models have played a key role in understanding the mechanisms of RA and arsenic therapies, pointing to the importance of PML/RARA degradation and challenging the sole role of differentiation (Ablain and de The 2011). In PML/RARA-driven APL, complete differentiation of the leukemia is achieved even at low RA doses, but complete APL clearance only appears with treatments at the highest (toxic) concentrations (Nasr et al. 2008). This might explain the efficiency of liposomal RA, which has led to cures as single agent in patients (Tsimberidou et al. 2006). Complete loss of clonogenic activity was observed in PML/RARA-driven APL mice treated with the RA/arsenic combination, although the combination actually delays morphologic differentiation (Shao et al. 1998; Lallemand-Breitenbach et al. 1999; Nasr et al. 2008). Indeed, this combination rapidly abolishes the ability of APL cells to be transplanted and induce new APL, which explains the dramatic synergy of RA/arsenic for tumor regression and survival in mice (Lallemand-Breitenbach et al. 1999; Nasr et al. 2008; Rego et al. 2000) (Fig. 23.5). Moreover, RA and arsenic induce PML/RARA degradation by different mechanisms, predicting absence of cross-resistance in vivo. As detailed in the companion chapter, this was later successfully transposed to patients, with over 90 % of them definitively cured by the RA/arsenic combination (Shen et al. 2004; Hu et al. 2009; Estey et al. 2006; de The and Chen 2010; Tallman and Altman 2009; Wang and Chen 2008).
Synergistic effects of RA and arsenic when combined. Luciferase imaging of APL in vivo after 3 days of treatment. (Reproduced from Nasr et al. 2008)
9 What Actually Causes Loss of Clonogenic Activity?
Differentiation was proposed to reflect transcriptional activation while PML/RARA catabolism would entail loss of self-renewal (Kogan 2009; Ablain and de The 2011; Ablain et al. 2011). Yet, some recent studies have argued that even transcriptional derepression, for example through PML/RARA loss or reversal of histone deacetylation, may suffice for differentiation (Leiva et al. 2012). This may explain why arsenic (through PML/RARA degradation) may induce differentiation in vivo (Fig. 23.3) or ex vivo in combination with growth factors (Muto et al. 2001).
It remained to be determined how loss of clonogenic activity was entailed. Mechanistically, it is possible that degradation-induced derepression of some PML/RARA-specific target genes suffices for leukemia initiating cell (LIC) exhaustion. Alternatively, it is possible that efficacy of arsenic alone could rely on some other effects than PML/RARA degradation, either through PML itself or via other arsenic targets. One may thus envision that PML NB reassembly following PML-RARA degradation reassembly could activate specific pathways like apoptosis or modulate self-renewal .
In normal progenitors or in the context of other leukemic fusion proteins , PML controls self-renewal (Ito et al. 2008; Regad et al. 2009), consistent with the proposal that NBs tune several critical pathways involved in “stemness” and self-renewal, such as P53, AKT/PTEN, HIF1A (Ito et al. 2009; Song et al. 2008). Thus, PML/RARA loss and active enforcement of NB reformation (for example by arsenic) could directly contribute to loss of stemness. However, one should stress that studies on these biologically complex mechanisms will necessarily be performed in mice and that the regulation of self-renewal and the mode of interference by PML/RARA might be different between mice and humans (Fig. 23.6).
A model for pathogenesis and treatment efficiency in APL. Promyelocytic gene/retinoic acid receptor alpha (PML/RARA) has a dual function to repress transcription and disrupt PML nuclear bodies (NBs). Therapies that degrade PML/RARA induce differentiation through derepression, while PML/RARA loss allows NB reformation. (Reproduced from Ablain et al. 2011)
10 Could APL become a Model for other Targeted Therapies?
In APL, the extraordinary clinical potency of RA and arsenic reflects the fact that RARA and PML are both dispensable (in mice), while APL cells are addicted to the continuous expression of PML/RARA. These agents fully degrade RARA, PML, and PML/RARA, which exerts a maximal efficacy on APL cells without any toxicity on normal cells, hence the extremely high therapeutic index of these agents or their association (Lallemand-Breitenbach et al. 2005; de The and Chen 2010; Nardella et al. 2011).
APL is a paradigm for targeted therapies and probably the only leukemia where combination of non-cross reactive agents has led to definitive cures (Wang and Chen 2008; de The and Chen 2010; de The et al. 2012). It underscores the power of proteolysis rather than enzymatic inhibition. Indeed, complete proteolysis abolishes all of the functions of oncoproteins, including those linked to protein/protein interactions, which may be very important in LICs. Collectively, this suggests that agents targeting the stability of other dominant oncoproteins could be of high therapeutic value, in particular, in translocation-driven leukemias or sarcomas driven by a single dominant oncoprotein fusing two unessential genes (Ablain et al. 2011).
References
Ablain J, de The H (2011) Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood 117(22):5795–5802. doi:10.1182/blood-2011-02-329367
Ablain J, Nasr R, Bazarbachi A, de The H (2011) Oncoprotein proteolysis, an unexpected Achille’s Heel of cancer cells? Cancer Discov 1:117–127
Akagi T, Shih LY, Kato M, Kawamata N, Yamamoto G, Sanada M, Okamoto R, Miller CW, Liang DC, Ogawa S, Koeffler HP (2009) Hidden abnormalities and novel classification of t(15;17) acute promyelocytic leukemia (APL) based on genomic alterations. Blood 113 (8):1741–1748. doi:blood-2007-12-130260 [pii] 10.1182/blood-2007-12-130260
Chen Z, Brand N, Chen A, Chen S, Tong J, Wang Z, Waxman S, Zelent A (1993) Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor a locus due to a variant t(11,17) translocation in acute promyelocytic leukemia. EMBO J 12:1161–1167
Chen SJ, Zhou GB, Zhang XW, Mao JH, de The H, Chen Z (2011) From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood 117(24):6425–6437. doi:10.1182/blood-2010-11-283598
de The H, Chen Z (2010) Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer 10(11):775–783. doi:nrc2943 [pii] 10.1038/nrc2943
de Thé H, Vivanco-Ruiz MdM, Tiollais P, Stunnenberg H, Dejean A (1990) Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature 343:177–180
de The H, Le Bras M, Lallemand-Breitenbach V (2012) The cell biology of disease: acute promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol 198(1):11–21. doi:10.1083/jcb.201112044
Degos L, Dombret H, Chomienne C, Daniel MT, Miclea JM, Chastang C, Castaigne S, Fenaux P (1995) All-trans retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood 85(10):2643–2653.
Dos Santos GA, Abreu ELRS, Pestana CR, Lima AS, Scheucher PS, Thome CH, Gimenes-Teixeira HL, Santana-Lemos BA, Lucena-Araujo AR, Rodrigues FP, Nasr R, Uyemura SA, Falcao RP, de The H, Pandolfi PP, Curti C, Rego EM (2011) (+)alpha-Tocopheryl succinate inhibits the mitochondrial respiratory chain complex I and is as effective as arsenic trioxide or ATRA against acute promyelocytic leukemia in vivo. Leukemia: Off Leukemia Soc Am, Leukemia Res Fund, UK 26(3):451–460. doi:10.1038/leu.2011.216
Dyck JA, Warrell RP, Evans RM, Miller WH (1995) Rapid diagnosis of acute promyelocytic leukemia by immunohistochemical localization of PML/RAR alpha protein. Blood 86:862–867
Estey E, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D, Kantarjian H (2006) Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 107(9):3469–3473
Farboud B, Hauksdottir H, Wu Y, Privalsky ML (2003) Isotype-restricted corepressor recruitment: a constitutively closed helix 12 conformation in retinoic acid receptors beta and gamma interferes with corepressor recruitment and prevents transcriptional repression. Mol Cell Biol 23(8):2844–2858
Gallagher RE (2002) Retinoic acid resistance in acute promyelocytic leukemia. Leukemia: Off Leukemia Soc Am, Leukemia Res Fund, UK 16(10):1940–1958. doi:10.1038/sj.leu.2402719
Gallagher RE, Schachter-Tokarz EL, Zhou DC, Ding W, Kim SH, Sankoorikal BJ, Bi W, Livak KJ, Slack JL, Willman CL (2006) Relapse of acute promyelocytic leukemia with PML-RARalpha mutant subclones independent of proximate all-trans retinoic acid selection pressure. Leukemia: Off Leukemia Soc Am, Leukemia Res Fund, UK 20(4):556–562. doi:10.1038/sj.leu.2404118
Geoffroy MC, Hay RT (2009) An additional role for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol 10(8):564–568. doi:nrm2707 [pii] 10.1038/nrm2707
Goto E, Tomita A, Hayakawa F, Atsumi A, Kiyoi H, Naoe T (2011) Missense mutations in PML-RARA critical for the lack of responsiveness to arsenic trioxide treatment. Blood 118(6):1600–1609 doi:10.1182/blood-2011-01-329433
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, Li JM, Tang W, Zhao WL, Wu W, Sun HP, Chen QS, Chen B, Zhou GB, Zelent A, Waxman S, Wang ZY, Chen SJ, Chen Z (2009) Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A 106(9):3342–3347. doi:10.1073/pnas.0813280106
Hua S, Kittler R, White KP (2009) Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell 137(7):1259–1271. doi:10.1016/j.cell.2009.04.043
Huang M, Ye Y, Chen R, Chai J, Lu J, Zhoa L, Gu L, Wang Z (1988) Use of all trans retinoic acid in the treatment of acute promyelocytic leukaemia. Blood 72:567–572
Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP (2008) PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453(7198):1072–1078. doi:nature07016 [pii] 10.1038/nature07016
Ito K, Bernardi R, Pandolfi PP (2009) A novel signaling network as a critical rheostat for the biology and maintenance of the normal stem cell and the cancer-initiating cell. Curr Opin Genet Dev 19(1):51–59. doi:S0959-437X(09)00017-3 [pii]10.1016/j.gde.2009.01.004
Jeanne M, Lallemand-Breitenbach V, Ferhi O, Koken M, Le Bras M, Duffort S, Peres L, Berthier C, Soilihi H, Raught B, de The H (2010) PML/RARA oxidation and arsenic binding initiate the antileukemia response of As2O3. Cancer Cell 18(1):88–98. doi:S1535-6108(10)00241-2 [pii] 10.1016/j.ccr.2010.06.003
Kamashev DE, Vitoux D, De Thé H (2004) PML/RARA-RXR oligomers mediate retinoid- and rexinoid-/cAMP in APL cell differentiation. J Exp Med 199:1–13
Kastner P, Mark M, Chambon P (1995) Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83(6):859–869
Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S (2001) Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood 97(5):1314–1320
Kogan SC (2009) Curing APL: differentiation or destruction? Cancer Cell 15 (1):7–8. doi:S1535-6108(08)00413-3 [pii] 10.1016/j.ccr.2008.12.012
Koken MHM, Puvion-Dutilleul F, Guillemin MC, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, Degos L, Puvion E, de Thé H (1994) The t(15;17) translocation alters a nuclear body in a RA-reversible fashion. EMBO J 13:1073–1083
Lallemand-Breitenbach V, de The H (2010) PML nuclear bodies. Cold Spring Harb Perspect Biol 2:a000661
Lallemand-Breitenbach V, Guillemin M-C, Janin A, Daniel M-T, Degos L, Kogan SC, Bishop JM, de The H (1999) Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med 189:1043–1052
Lallemand-Breitenbach V, Zhu J, Puvion F, Koken M, Honore N, Doubeikovsky A, Duprez E, Pandolfi PP, Puvion E, Freemont P, de The H (2001) Role of Promyelocytic Leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As(2)O(3)-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med 193(12):1361–1372
Lallemand-Breitenbach V, Zhu J, Kogan S, Chen Z, de The H (2005) Opinion: how patients have benefited from mouse models of acute promyelocytic leukaemia. Nat Rev Cancer 5(10):821–827
Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, Zhou J, Zhu J, Raught B, de The H (2008) Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol 10(5):547–555. doi:ncb1717 [pii] 10.1038/ncb1717
Lallemand-Breitenbach V, Zhu J, Chen Z, de The H (2012) Mechanisms of APL cure through PML/RARA degradation by As2O3. Trends Mol Med 18:36–42
Leiva M, Moretti S, Soilihi H, Pallavicini I, Peres L, Mercurio C, Dal Zuffo R, Minucci S, de The H (2012) Valproic acid induces differentiation and transient tumor regression, but spares leukemia-initiating activity in mouse models of APL. Leukemia: Off Leukemia Soc Am, Leukemia Res Fund, UK 26(7):1630–1637. doi:10.1038/leu.2012.39
Licht JD (2006) Reconstructing a disease: what essential features of the retinoic acid receptor fusion oncoproteins generate acute promyelocytic leukemia? Cancer Cell 9(2):73–74
Martens JH, Brinkman AB, Simmer F, Francoijs KJ, Nebbioso A, Ferrara F, Altucci L, Stunnenberg HG (2010) PML-RARalpha/RXR alters the epigenetic landscape in acute promyelocytic leukemia. Cancer Cell 17(2):173–185. doi:S1535-6108(10)00005-X [pii] 10.1016/j.ccr.2009.12.042
Melnick A, Licht JD (1999) Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 93:3167–3215
Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, Parry A, Walz C, Wiemels JL, Segal MR, Ades L, Blair IA, Osheroff N, Peniket AJ, Lafage-Pochitaloff M, Cross NC, Chomienne C, Solomon E, Fenaux P, Grimwade D (2005) DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med 352(15):1529–1538
Muindi J, Frankel SR, Miller WH Jr, Jakubowski A, Scheinberg DA, Young CW, Dmitrovsky E, Warrell RP, Jr. (1992) Continuous treatment with all-trans retinoic acid causes a progressive reduction in plasma drug concentrations: implications for relapse and retinoid "resistance" in patients with acute promyelocytic leukemia. Blood 79(2):299–303
Muto A, Kizaki M, Kawamura C, Matsushita H, Fukuchi Y, Umezawa A, Yamada T, Hata J, Hozumi N, Yamato K, Ito M, Ueyama Y, Ikeda Y (2001) A novel differentiation-inducing therapy for acute promyelocytic leukemia with a combination of arsenic trioxide and GM-CSF. Leukemia 15(8):1176–1184
Nardella C, Lunardi A, Patnaik A, Cantley LC (2011) The APL paradigm and the co-clinical trial project. Cancer Discov 1:108–116
Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C, Rousselot P, Robledo-Sarmiento M, Lallemand-Breitenbach V, Gourmel B, Vitoux D, Pandolfi PP, Rochette-Egly C, Zhu J, de The H (2008) Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med 14(12):1333–1342. doi:nm.1891 [pii] 10.1038/nm.1891
Occhionorelli M, Santoro F, Pallavicini I, Gruszka A, Moretti S, Bossi D, Viale A, Shing D, Ronzoni S, Muradore I, Soncini M, Pruneri G, Rafaniello P, Viale G, Pelicci PG, Minucci S (2011) The self-association coiled-coil domain of PML is sufficient for the oncogenic conversion of the retinoic acid receptor (RAR) alpha. Leukemia: Off Leukemia Soc Am, Leukemia Res Fund, UK 25(5):814–820. doi:10.1038/leu.2011.18
Piazza F, Gurrieri C, Pandolfi PP (2001) The theory of APL. Oncogene 20(49):7216–7222
Quignon F, Chen Z, de Thé H (1997) Retinoic acid and arsenic: towards oncogene targeted treatments of acute promyelocytic leukaemia. Biochim Biophys Acta 1333:M53–M61
Regad T, Bellodi C, Nicotera P, Salomoni P (2009) The tumor suppressor Pml regulates cell fate in the developing neocortex. Nat Neurosci 12(2):132–140. doi:nn.2251 [pii] 10.1038/nn.2251
Rego EM, He LZ, Warrell RP Jr, Wang ZG, Pandolfi PP (2000) Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc Natl Acad Sci U S A 97:10173–10178
Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, Lamph WW, Waxman S, Pelicci PG, Lo Coco F, Avvisati G, Testa U, Peschle C, Gambacorti-Passerini C, Nervi C, Miller WHJ (1998) Arsenic trioxide as an inducer of apoptosis and loss of PML/RARalpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst 90:124–133
Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, Shi JY, Zheng PZ, Yan H, Liu YF et al (2004) All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A 101:5328–5335
So CW, Cleary ML (2004) Dimerization: a versatile switch for oncogenesis. Blood 104(4):919–922
Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP (2008) The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455(7214):813–817. doi:nature07290 [pii] 10.1038/nature07290
Sternsdorf T, Phan VT, Maunakea ML, Ocampo C, Sohal J, Siletto A, Galimi F, Le Beau MM, Evans R, Kogan S (2006) Forced retinoic acid receptor a homodimer prime mice for APL-like leukemia. Cancer Cell 9:81–94
Strickland S, Mahdavi V (1978) The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell 15(2):393–403. doi:0092-8674(78)90008-9 [pii]
Tallman MS, Altman JK (2009) How I treat acute promyelocytic leukemia. Blood 114 (25):5126–5135. doi:blood-2009-07-216457 [pii] 10.1182/blood-2009-07-216457
Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT (2008) RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol 10(5):538–546. doi:ncb1716 [pii] 10.1038/ncb1716
Tsimberidou AM, Tirado-Gomez M, Andreeff M, O’Brien S, Kantarjian H, Keating M, Lopez-Berestein G, Estey E (2006) Single-agent liposomal all-trans retinoic acid can cure some patients with untreated acute promyelocytic leukemia: an update of The University of Texas M. D. Anderson Cancer Center Series. Leuk Lymphoma 47(6):1062–1068. doi:K54M427781W6874X [pii] 10.1080/10428190500463932
Verger A, Perdomo J, Crossley M (2003) Modification with SUMO. A role in transcriptional regulation. EMBO Rep 4(2):137–142
Vickers M, Jackson G, Taylor P (2000) The incidence of acute promyelocytic leukemia appears constant over most of a human lifespan, implying only one rate limiting mutation. Leukemia 14 (4):722–726
Wang ZY, Chen Z (2008) Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111(5):2505–2515. doi:111/5/2505 [pii] 10.1182/blood-2007-07-102798
Warrell R, de Thé H, Wang Z, Degos L (1993) Acute promyelocytic leukemia. New Engl J Med 329:177–189
Welch JS, Yuan W, Ley TJ (2011) PML-RARA can increase hematopoietic self-renewal without causing a myeloproliferative disease in mice. J Clin Invest 121(4):1636–1645. doi:10.1172/JCI42953
Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, Kandoth C, Fulton RS, McLellan MD, Dooling DJ, Wallis JW, Chen K, Harris CC, Schmidt HK, Kalicki-Veizer JM, Lu C, Zhang Q, Lin L, O’Laughlin MD, McMichael JF, Delehaunty KD, Fulton LA, Magrini VJ, McGrath SD, Demeter RT, Vickery TL, Hundal J, Cook LL, Swift GW, Reed JP, Alldredge PA, Wylie TN, Walker JR, Watson MA, Heath SE, Shannon WD, Varghese N, Nagarajan R, Payton JE, Baty JD, Kulkarni S, Klco JM, Tomasson MH, Westervelt P, Walter MJ, Graubert TA, Dipersio JF, Ding L, Mardis ER, Wilson RK (2012) The origin and evolution of mutations in acute myeloid leukemia. Cell 150(2):264–278. doi:10.1016/j.cell.2012.06.023
Zeisig BB, Kwok C, Zelent A, Shankaranarayanan P, Gronemeyer H, Dong S, So CW (2007) Recruitment of RXR by homotetrameric RARalpha fusion proteins is essential for transformation. Cancer Cell 12(1):36–51. doi:S1535-6108(07)00175-4 [pii] 10.1016/j.ccr.2007.06.006
Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, de The H, Chen SJ, Chen Z (2010) Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 328(5975):240–243. doi:328/5975/240 [pii] 10.1126/science.1183424
Zhu J, Lallemand-Breitenbach V, de The H (2001) Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission. Oncogene 20(49):7257–7265
Zhu J, Chen Z, Lallemand-Breitenbach V, de Thé H (2002) How acute promyelocytic leukemia revived arsenic. Nat Rev Cancer 2:705–713
Zhu J, Zhou J, Peres L, Riaucoux F, Honore N, Kogan S, de The H (2005) A sumoylation site in PML/RARA is essential for leukemic transformation. Cancer Cell 7(2):143–153
Zhu J, Nasr R, Peres L, Riaucoux-Lormiere F, Honore N, Berthier C, Kamashev D, Zhou J, Vitoux D, Lavau C, de The Hs (2007) RXR is an essential component of the oncogenic PML/RARA complex in vivo. Cancer Cell 12(1):23–35
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag New York
About this chapter
Cite this chapter
de Thé, H., Zhu, J., Nasr, R., Ablain, J., Lallemand-Breittenbach, V. (2015). PML/RARA as the Master Driver of APL Pathogenesis and Therapy Response. In: Andreeff, M. (eds) Targeted Therapy of Acute Myeloid Leukemia. Current Cancer Research. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1393-0_23
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1393-0_23
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1392-3
Online ISBN: 978-1-4939-1393-0
eBook Packages: MedicineMedicine (R0)