Abstract
Addiction inflicts large personal, social, and economic burdens, yet its etiology is poorly defined and effective treatments are lacking. As with other neuropsychiatric disorders, addiction is characterized by a core set of symptoms and behaviors that are believed to be influenced by complex gene–environment interactions. Our group focuses on the interaction between early stress and genetic background in determining addiction vulnerability. Prior work by our group and others has indicated that a history of prenatal stress (PNS) in rodents elevates adult drug seeking in a number of behavioral paradigms. The focus of the present chapter is to summarize work in the area of PNS and addiction models as well as our recent studies of PNS on drug seeking in different strains of mice as a strategy to dissect gene–environment interactions underlying cocaine addiction vulnerability. These studies indicate that ability of PNS to elevate adult cocaine seeking is strain dependent. Further, PNS also alters other nondrug behaviors in a fashion that is dependent on different strains and independent from the strain dependence of drug seeking. Thus, it appears that the ability of PNS to alter behavior related to different psychiatric conditions is orthogonal, with similar nonspecific susceptibility to prenatal stress across genetic backgrounds but with the genetic background determining the specific nature of the PNS effects. Finally, the advent of recombinant inbred mouse strains is allowing us to determine the genetic bases of these gene–environment interactions. Understanding these effects will have broad implications to determining the nature of vulnerability to addiction and perhaps other disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Addiction to drugs of abuse, including cocaine , is a leading health problem in Western society. The prevalence of substance-use disorders among the US general population is estimated to be approximately 9.2 % (Aldworth et al. 2007) . In the USA, approximately 2 % of the general population has used cocaine at least once in their life time, of which 2 million are current users and 1.5 million are cocaine dependent (NIDA 2004). Addiction produces significant costs to both the individual addicts, their family, and friends, as well as economic and social burdens to society in general. The addiction-associated costs resulting from productivity loss, traffic accidents, related diseases (e.g., spread through infected needles), and drug-related crime, including domestic violence, are estimated to be up to 3.5 % of the gross domestic product (GDP) in Western countries (Pouletty 2002) . Accordingly, given the US$ 13.86 trillion estimated GDP of the USA in 2007 (CIA 2008) , the cost of drug addiction to the country is around US$ 485 billion.
The reasons why some individuals become addicted to cocaine or other drugs, while others do not, are poorly understood, but it appears that cocaine addiction results from a complex interaction between genetic and environmental factors (for reviews see, e.g., Crabbe 2002; Enoch 2006; Kendler et al. 2007; Kreek 2001) . For instance, Ellenbroek and colleagues (Ellenbroek et al. 2002; van der Kam et al. 2005) argue that addiction , as with other neuropsychiatric disorders, is mediated by the interaction of genetic polymorphisms, early environment, and current environment. In both humans and animals, there are large individual differences on a wide range of measures following acute and repeated exposure to and/or level of intake of psychostimulants such as cocaine and d-amphetamine (DeWit et al. 1986, Cohen 1990) . Thus, it is presumed that there are polygenetic backgrounds which interact with specific experiences to determine cocaine responsiveness, which will make an individual more or less vulnerable to developing cocaine addiction. Our ultimate goal is to elucidate genes that modulate the ability of early environmental experiences to alter adult responsiveness to cocaine. Recent research has focused on delineating specific early environmental conditions that increase cocaine responsiveness. In particular, maternal stress during gestation or neonatal isolation produces an adult phenotype that has increased sensitivity to cocaine, greater cocaine intake during cocaine self-administration, and higher cocaine seeking during extinction and reinstatement procedures (Kippin et al. 2008; Thomas et al. 2009; Kosten et al. 2000, 2004, 2006; Lynch et al. 2005; Zhang et al. 2005; see also Deminiere et al. 1992) , suggesting that a history of early life stress increases vulnerability to cocaine addiction . Conversely, genetic background, namely gene polymorphism, also plays a role in determination of cocaine responsiveness. For example, substantial differences exist between inbred mouse strains regarding the behavioral and physiological effects of cocaine (Crawley et al. 1997; Crabbe et al. 1999) . Further, the advent of recombinant inbred (RI) mouse strains, in conjunction with the mapping of the mouse genome, now allows quantitative analyses of the contribution of specific genetic loci to traits (Belknap and Crabbe 1992; Chesler et al. 2003) and these analyses have been performed for several behavioral responses to acute or repeated cocaine exposure, including cocaine-induced convulsions and locomotion (see for reviews, e.g., Phillips and Belknap 2002; Crabbe 2002) . However, despite emerging evidence that gene–environment interactions (G × E) are critical determinants of cocaine responsiveness, there has been almost no systematic investigation that would allow determination of the specific genetic loci, and ultimately genes located within these loci, that facilitate or impede the capacity of early environmental experience to influence subsequent cocaine responsiveness. Accordingly, a major impediment to our ability to predict individual cocaine responsiveness, and potentially individual vulnerability to addiction , is a poor understanding of the interactions between genes and environment. The overall goal of the present review is to provide a broad analysis of the evidence implicating prenatal stress (PNS) in drug responsiveness and drug-seeking behavior, then to more narrowly review studies of the genetic bases of drug responsiveness/seeking behavior, followed by a description of our approach to provide a genetic analysis of the effects of early environmental experience, namely PNS, upon cocaine addiction vulnerability employing a mouse model of cocaine reward. We will also summarize what is known about the neurobiology of PNS-induced alterations in drug responsiveness and, finally, the relation of addiction vulnerability to vulnerability to other neuropsychiatric disorders will be addressed.
2 Early Environmental Stress and Neuropsychiatric Disease
Environmental stimuli during the embryonic and early postnatal periods have profound effects upon developmental processes, resulting in permanent alterations in nervous system structure and function. In humans, children of mothers experiencing stress during gestation show alterations in early motor development , anomalies in brain morphology, and behavioral abnormalities such as attention deficit hyperactivity disorder (ADHD), sleep disturbances, cognitive dysfunction, increased anxiety, and are at an increased risk of a variety of neuropsychiatric disorders, including higher incidence of substance abuse (reviewed in Huizink et al. 2004; Kofman 2002; Weinstock 2001) . Stress during gestation or PNS can be modeled in laboratory rodents using a number of procedures, of which a widely employed one involves repeated bouts of restraint stress to a pregnant dam during the last week of gestation. The data from animal studies using this PNS model are consistent with the epidemiological and clinical findings, but are far more extensive. In animals, PNS sequelae include (but are not limited to) increased anxiety, learning and memory impairments, altered circadian rhythm function, impaired sexual function, and increased psychomotor responsiveness to, as well as increased propensity to self-administer, drugs of abuse (for recent reviews see, e.g., Darnaudery and Maccari 2008; Weinstock 2008; Matthews 2002) . Thus, there is a remarkable parallel between the human literature and that derived from animal models regarding the long-term behavioral consequences resulting from stressful experiences during development.
Although the detailed mechanisms producing the PNS syndrome are not known, the intermediate cause appears to be maternal secretion of glucocorticoids during stressful events. The hypothalamic-pituitary-adrenal (HPA) axis is a primary element in the nervous system response to environmental stress and is used extensively as an index of nervous system stress output in animal models. Evidence from animal models has shown that maternal stress during gestation elevates glucocorticoids in the fetal brain in a variety of species (Lephart et al. 1997; Montano et al. 1991) . Removal of the adrenal glands, the maternal source of circulating glucocorticoids, prevents the development of the PNS syndrome (Barbazanges et al. 1996) . Systemic administration of exogenous glucocorticoids to the mother during gestation mimics the PNS syndrome (reviewed in Maccari et al. 2003; Takahashi 1998) . Similarly, in humans, exposure to glucocorticoids during development is associated with increased risk for psychiatric disorders (reviewed in, e.g., Bertram and Hanson 2002; Matthews et al. 2002; Sekyl and Meaney 2004) . Accordingly, it has been widely proposed that embryonic nervous system programming is highly sensitive to glucocorticoids with lifelong consequences that may compose a significant component of individual vulnerability to neuropsychiatric conditions, including drug addiction.
3 Early Environmental Stress Contributes to Adult Drug and Alcohol Responsiveness
In order to address the potential role of early environmental stress in addiction vulnerability, a number of groups have examined the role of PNS or prenatal glucocorticoid exposure (PNG) in modulating the responsiveness to cocaine and other drugs of abuse in adulthood. The common findings across studies are a general elevation in drug responsiveness as well as the motivation to take drugs, with these effects spanning psychostimulants, depressants/opiates, and alcohol.
3.1 PNS Alters Response to and the Propensity to Take Stimulant Drugs
Psychostimulants are the class of drugs where the effects of PNS on the responsiveness to and the seeking or consumption of drugs of abuse has been most examined. These studies have formed the strongest basis for the linkage between PNS and changes in drug-use patterns. For amphetamine, Deminiere et al. (1992) found that while administration of a 0.3 mg/kg (intraperitoneal, IP) dose of amphetamine increased subsequent motor activity in all rats, PNS male rats given amphetamine produced double the locomotor counts of control males during the first 10 min after administration. Further, when rats self-administered amphetamine (30 µl/infusion; intravenous, IV), the PNS rats had higher intakes compared to controls on all trials after the first day (Deminiere et al. 1992). While others have not always found initial differences in the locomotor response to amphetamine (Henry et al. 1995; 1 mg/kg dose, IP) , they have seen greater sensitization of locomotor behavior in male PNS rats (Henry et al. 1995). Additionally, PNS female rats have increased rotational behavior (left turns) after amphetamine administration, indicating PNS asymmetries in motor system sensitivity to stimulants (Weinstock and Fride 1989) . There are also changes in the response to amphetamine after corticosterone is used to mimic a hormonal aspect of PNS. Diaz et al. (1995) implanted pregnant females with corticosterone pellets during late gestation, and the resulting corticosterone-exposed (prenatal corticosterone administration, PNC) weanlings were assessed for locomotor activity after amphetamine. Amphetamine increased locomotor activity in both normal and PNC offspring, but again there was an enhancement of movement in PNC animals. There was also a sex difference, in that male PNC pups increased all types of movement, while female PNC pups displayed increased rearing behavior only (Diaz et al. 1995), indicating a more robust effect in males. PNS status also interacts with behavior on cocaine. Kippin et al. (2008) found that a 15 mg/kg (IP) dose of cocaine enhanced locomotion in adult male PNS rats compared to adult male controls with similar levels of cocaine experience. On the other hand, Thomas et al. (2009) did not see pronounced differences between PNS and control males in locomotion after either acute or repeated cocaine exposure, but did find enhanced locomotor sensitization in PNS females. This change in female locomotor sensitization did not carry over to self-administration, where female PNS and control rats self-administered similar amounts of cocaine (Thomas et al. 2009) . In males, there were no differences on a constant (0.2 mg/kg/infusion, IV) cocaine dose, but on a weekly escalating (0.3–0.5 mg/kg/infusion, IV) schedule PNS males had significantly higher total intake (Thomas et al. 2009). In comparison, a study using a higher but constant dose (1.0 mg/kg) reported a trend towards PNS males self-administering more cocaine than controls (Kippin et al. 2008) . Yet during extinction, these same PNS males had significantly greater initial responding, took longer to reach extinction criterion, and exhibited greater cocaine-primed reinstatement (Kippin et al. 2008). Interestingly, noncontingent cocaine injections produced an extinction deficit following removal of brain self-stimulation in PNS but not control rats (Gao et al. 2011) suggesting that adult psychostimulant exposure may produce generalized cognitive deficits dependent upon early environmental stress.
PNS also increases responsiveness to other stimulant drugs. PNS increases the sensitivity to nicotine and the magnitude of nicotine-induced locomotor activity (Koehl et al. 2000) . For caffeine, adult PNS male rats displayed greater rearing and corner activity in an open field compared to controls, including at a dose where there was no difference in plasma caffeine concentrations between control and PNS animals (10 mg/kg, IP; Pohorecky et al. 1989) . For 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”; 5 mg/kg, IP), adolescent PNS female rats had fewer periods of inactivity while also demonstrating more slips and body twists on a runway, and had higher blood levels of MDMA after the same dose was administered compared to controls (Morley-Fletcher et al. 2004) . In summary, PNS increases responsiveness to and self-administration of a variety of psychostimulant drugs, and this response interacts with sex and task type. We will later discuss the PNS interaction with genetic background on measures thought to probe cocaine reward.
3.2 PNS Alters Response to and Preference for Morphine, and Response to Diazepam
PNS can also impact the response to therapeutics with abuse potential such as morphine and diazepam. PNS male rats are less sensitive than control males to the analgesic effects of morphine (5 mg/kg, IP) in the tail-flick test; conversely, PNS females are more sensitive than female controls to the analgesic effects of morphine, with longer latencies to remove the tail (Kinsley et al. 1988) . For both PNS and brief postnatal separations from the dam in mice , adult mice displayed greater morphine-induced analgesia 30 min after administration, with similar patterns with subtle sex-specific alterations after 60 min (Sternberg and Ridgeway 2003) . PNS male rats also show a difference in conditioned place preference (CPP) to morphine. While all male rats given morphine (10 mg/kg) showed a significant place preference for the morphine-paired side, the group males only exposed to PNS showed a significantly greater preference than the control group (Yang et al. 2006) .
PNS can also alter the response to diazepam. Male PNS rats responded to a low dose of diazepam (1 mg/kg, IP) that did not impact control behavior in the open field (Pohorecky and Roberts 1991) . Additionally, it has also been shown that PNS rats are hyperresponsive to bright-light-potentiated acoustic startle, and startle levels are normalized with diazepam treatment (3.2 mg/kg; Tazumi et al. 2005) .
3.3 PNS Alters Response to and the Propensity to Take Alcohol
There are several changes in the physiological response to alcohol after PNS. Adult PNS rats given alcohol in the 1–2 g/kg dose range display initial blunting of alcohol-induced hypothermia, a reduction in alcohol-induced corticosterone release, reduced free fatty acid release, and decreased alcohol-induced startle deficits, and they retain better motor control on the rotorod but less capability in a swim test (DeTurck and Pohorecky 1987) . Adolescent male PNS rats also exhibit this HPA axis blunting, such that a 1.5 g/kg dose of alcohol does not produce the sustained increase in serum adrenocorticotropic hormone (ACTH) and corticosterone levels in PNS rats versus controls (Van Waes et al. 2006) . Displaying a higher initial tolerance to alcohol increases the chance of later alcohol dependence in humans (for review, Schuckit 2009) , suggesting that PNS could predispose an individual to initial high alcohol consumption and increasing the risk of later addiction.
PNS also appears to impact voluntary alcohol consumption in animal models. In the two-bottle choice task, where animals are allowed access to a bottle of water and another bottle of alcohol solution, Darnaudery et al. (2007) found that a subpopulation of adult female rats showed a change due to PNS after a stressor in adulthood. After 2 weeks of two-bottle choice, these female rats were designated as high or low drinkers and they were subjected to an inescapable shock. Post-stress, PNS and control low-drinking females did not differ from each other, and high-drinking control females decreased their alcohol consumption from that point onwards, but high-drinking PNS females returned to their previous high level of consumption in the weeks following the stress (Darnaudery et al. 2007). PNS also increases the preference for a sweetened alcohol solution versus water in adolescent female rats (Van Waes et al. 2011a) , although PNS does not change two-bottle choice behavior in adolescent or adult male rats (Van Waes et al. 2011b).
Interestingly, there are effects of PNS on alcohol consumption which only emerge after alcohol exposure in the adult environment. Our studies indicate that there is not an effect of PNS on adult alcohol consumption under free-access conditions, but there is an impact of that free-access alcohol consumption on subsequent motivation for alcohol (Campbell et al., under review) . Briefly, PNS and control males and females without adult exposure to alcohol exhibited equivalent operant behavior for reinforcement with sucrose (15 %, 20 µl/infusion), sucrose–alcohol fading (decreasing sucrose from 15–0 % with concurrent increasing of alcohol from 0 to 10 %), or alcohol alone (10 %, 20 µl. infusion) even on a response schedule with increasing demand. In contrast, control males allowed prior continuous access to alcohol for 8 weeks exhibited a substantial decline in operant behavior for alcohol—a finding consistent with those indicating that alcohol experience by either injection (Carrara-Nascimento et al. 2012) or voluntary consumption (our own unpublished observations) reduces alcohol reward as measured in CPP. However, PNS males allowed alcohol access exhibited the same level of intake as controls but this alcohol exposure failed to diminish subsequent operant responsiveness for alcohol. PNS females only exhibited this difference from control females for the 10 % alcohol solution on the FR1 schedule. Again, these male and female C57BL/6J mice with either a PNS or control history exhibited the same level of alcohol consumption during continuous free access to alcohol in a two-bottle choice task over a period of 8 weeks, but then PNS males in particular did not show the expected behavior on the operant task. Accordingly, the ability of PNS to alter motivation for alcohol appears to be more subtle than the effects observed for psychostimulants as it is dependent upon fairly extensive exposure to alcohol in adulthood. Nonetheless, the altered alcohol-induced motivational plasticity of PNS-exposed individuals is consistent with the general findings of enhanced drug-seeking behavior following early environmental stress exposure.
In summary, PNS is known to change the response to a diverse number of drugs of abuse and increase seeking and consumptive behaviors towards drugs such as psychostimulants, morphine, and alcohol, with the behavioral alterations in many cases modulated by sex. However, these studies have largely occurred in parallel with separate lines of research indicating that genetic background modulates drug responsiveness and seeking behavior. To address the issue of genetic background influence, as well as interactions with early environment, on drug responsiveness and potential addiction vulnerability, the remainder of the chapter will focus more narrowly on cocaine.
4 Hereditability of Cocaine Responsiveness: Mouse Strain Differences
Studies using humans and animal models have suggested that genetic factors are important in substance abuse (DeWit et al. 1986; Cohen 1990; Crabbe et al. 1994) . Inbreeding has been performed in both rats and mice to reduce the allelic diversity between close genetic relatives and to produce a strain of animal in which same-sex members are almost monozygotic (identical) twins of all other members. There are currently over 100 inbred strains of mice available from the Jackson Laboratory and the stability of strain differences over decades of research has provided an extensive database concerning groups of genes that influence a particular phenotypic trait. Comparisons between different inbred mouse strains have provided substantial evidence for genetic contributions to an individual’s responsiveness to drugs of abuse, including cocaine . In this respect, strain differences between the C57BL/6J (B6) and DBA/2J (D2) strains have been the most thoroughly studied (see for a review, Crabbe et al. 1999; Crawley et al. 1997; Wahlsten et al. 2003) . In most studies, the D2 strain exhibits greater acute cocaine-induced locomotion and/or greater cocaine-induced locomotor sensitization upon repeated treatment (Cunningham et al. 1999; de Jong et al. 2007, 2008; Orsini et al. 2005; Tolliver and Carney 1994a, 1994b, 1995; but see also Kafkafi and Elmer 2005) . Conversely, a strain comparison of the reinforcing effects of cocaine indicates that B6 mice exhibit higher cocaine intake than D2 mice during both IV cocaine self-administration under operant conditions (van der Veen et al. 2007, 2008) and access to cocaine in drinking water (George and Goldberg 1989; Seale and Carney 1991) . Similarly, CPP has also been used to compare cocaine reward among these strains and B6 mice show a cocaine CPP with lower doses of cocaine , shorter conditioning trials, and/or a greater magnitude preference than do D2 (Cunningham et al. 1999; Seale and Carney 1991; Orsini et al. 2005, 2008) . Accordingly, comparison of the genetic differences between these strains is an approach to understanding individual differences in both the psychomotor stimulant responsiveness to drug exposure and the motivational components of drug-seeking behavior.
5 Search for Genetic Substrates Mediating Hereditability of Cocaine Responsiveness
Determining the genetic influences that contribute to hereditability of cocaine responsiveness has proven difficult because individual differences in behavioral responses to drugs are highly complex. In addition to substantial nongenetic factors, including potential intergenerational epigenetic hereditability, the range of drug responsiveness across different genotypes tends to exhibit a continuous distribution (see, e.g., Miner and Marley 1995a; Phillips et al. 1998) . This indicates a polygenetic pattern of inheritance involving many genes rather than one or two major genes. Quantitative trait loci (QTL) analyses have high utility for determining the relation between genetic influences that account for relatively small amounts of variation in a given behavior (Crabbe et al. 1999) . One strategy that has been employed in the search for QTLs influencing cocaine responsiveness is the employment of RI strains of mice derived from the systematic brother–sister matings of the F2 generation of two progenitor inbred mouse strains, which results in a panel of new inbred strains having unique recombinations of parental chromosomal material distinct from the two progenitor strains and all other RI strains (Belknap and Crabbe 1992; Complex Trait Consortium 2003) . RI strains have allowed significant advances in behavioral genetics because these mice have fixed genomes and genotypes of known genetic markers are already stored in a database; thus, they can be used for behavioral tests and then QTL can be performed without additional molecular analysis facilitating genetic mapping of behavioral traits (Belknap 1998) . The BXD and DXB panels of RI strains are derived from the B6 and D2 inbred parental strains and are ideal for genetically dissecting responsiveness to cocaine and other abused drugs because of the distinct phenotypes exhibited by the original strains (see, e.g., Chesler et al. 2003) . Accordingly, this panel has been employed to genetically dissect several distinct cocaine-related behaviors which include the acute locomotor stimulant effect of cocaine (Miner and Marley 1995b; Phillips et al. 1998; Tolliver et al. 1994) , sensitization to the locomotor stimulant effect of cocaine with repeated treatment (Phillips et al. 1998; Tolliver et al. 1994), cocaine-induced seizures (Miner and Marley 1995a) , cocaine-induced CPP (Philip et al. 2010) , and even cocaine self-administration in a small subset of available strains (Cervantes et al. 2013) . As the available RI strains are unable to resolve individual gene polymorphisms, additional studies need to employ fine mapping strategies utilizing loci-directed production of additional RI strains, accompanied by nucleotide sequences, to determine the specific genes within a loci that exhibit polymorphisms within RI panels that could contribute to phenotypic variability (e.g., Fehr et al. 2002; Hood et al. 2006) . Thus, it is possible to utilize rodent models to obtain fine-detail genetic analysis of specific aspects of cocaine responsiveness. Critically, the approaches outlined above are amenable to investigation of the impact of early environmental factors and can be used as a basis for forward and reverse genetic approaches to determine the nature of G × Es in cocaine-addiction-related behavior.
Of relevance to such approaches to studying interactions between early environmental stress and strain differences are strain differences in the stress response during adulthood. Although B6 mice are often characterized as stress resistant, both B6 and D2 mice show substantial changes in plasma corticosterone levels in response to psychological stressors (e.g., Prakash et al. 2006; Belzung et al. 2001; Crawley et al. 1997) and show stress-related modulation of programming in early development (reviewed in, e.g., Weinstock 2001; Zhang et al. 2006) —notably , we have recently compared changes in stress-induced glucocorticoid levels during pregnancy (i.e., during application of the PNS procedure) and both strains appear to have similar HPA activation during the PNS procedure indicating the differences in PNS-induced changes in adult behavior between these strains are unlikely to be due to stress responses of the mother during gestation. Be that as it may, RI strains may have different response profiles than either of the parental lines (e.g., Phillips et al. 2002); thus, the magnitude and duration of stress-induced changes in plasma corticosterone will be monitored during the PNS procedure in all strains allowing determination of its relation to adult behavior changes induced by PNS.
6 Interactions Between PNS and Genetic Background on Drug Seeking
With respect to studies of genetic background–environment interactions, D2 mice show greater changes in cocaine responsiveness following adult stress exposure whereas B6 mice appear to be relatively insensitive to adult stress-induced changes in cocaine responsiveness (Badiani et al. 1992; Cabib and Bonaverntura 1997; Cabib et al. 2000; van der Veen et al. 2007) . As discussed above, PNS procedures that enhance cocaine responsiveness in rats (Kippin et al. 2008; Thomas et al. 2009) also increase the level of responding for, and consumption of, alcohol during operant self-administration procedures in B6 mice with extensive alcohol experience (Campbell et al., under review) . Accordingly, our studies investigating the impact of PNS on cocaine responsiveness in B6 and D2 mice are summarized below. These studies employ standard PNS procedures of repeated restraint stress during late gestation and CPP that we have standardized between strain to be optimally sensitive to PNS–gene interactions.
6.1 Impact of Conditioning Parameters on Cocaine-Induced CPP in Male and Female B6 and D2 Mice
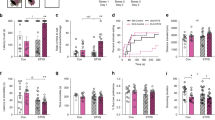
Prior work indicates that strain differences (between B6 and D2 mice) in cocaine-induced CPP exist and are sensitive to the duration of conditioning trials (Cunningham et al. 1999); , however, this evidence employed discreet conditioning stimuli (distinct tactile flooring) and did not examine females. Accordingly, we evaluated the impact of conditioning session duration on cocaine-induced CPP magnitude employing contextual conditioning stimuli in both B6 and D2 males and females as these stimuli were employed in our preliminary studies demonstrating PNS-induced potentiation of CPP. This experiment was a necessary first step to our investigation into the impact of PNS on cocaine seeking because it determined the procedures that allow assessment of cocaine-induced CPP in both male and female mice in the B6 and D2 parental strains. Specifically, both male and female B6 and D2 mice (obtained from Jackson Laboratories, Bar Harbor, ME) were tested on an unbiased CPP procedure that it is sensitive to psychomotor stimulant responsiveness and cocaine reward, extinction and reinstatement of CPP (e.g., Szumlinski et al. 2008) . Briefly, these experiments used a two-compartment chamber with distinct compartments (based on wall/flooring pattern and texture) that can be divided using an insert and supports acquisition and expression CPP as measured by video tracking. Mice were first allowed to explore the entire apparatus without the divider insert allowing assessment of novelty-induced locomotion. Next, each mouse received injections of cocaine (10 mg/kg, IP) and saline on alternating days and then were placed into one of the two compartments (with the divider insert in place) such that each compartment was repeatedly paired with cocaine or saline. Next, mice were allowed access to the entire apparatus on a “posttest” in order to determine direction and magnitude of place conditioning produced by the cocaine injections relative to the saline ones. In order to examine the rate of extinction, mice were subsequently placed into the undivided apparatus for 15-min sequential sessions and finally subjected to a “primed test” during which they first received a saline injection and CPP was reassessed for 15 min, and finally a cocaine injection (same dose as given during conditioning) and CPP was reassessed for 15 min. We have completed behavioral testing of both male and female B6 and D2 mice at session durations of 15, 20, and 30 min. The results of this experiment revealed a significant sex × strain interaction on the magnitude of CPP (exhibited during the initial posttest) with females exhibiting a larger CPP than males in the D2 strain but a smaller CPP than males in the B6 strain. Further, there was a significant strain effect (independent of sex) on extinction with B6 mice exhibiting rapid extinction (within two to three 15-min sessions) but D2 mice failing to exhibit significant extinction (even after 12 × 15-min sessions), thus, the absence of extinction in one of the strains is unfortunate as it complicates examination of PNS–gene interactions on this measure. However, neither a strain × session duration interaction nor strain effect was detected on the magnitude of CPP under these conditions. These findings indicate that strain effects on acquisition of CPP are minor when contextual stimuli (in contract to discreet stimuli) are employed and, accordingly, the subsequent PNS–gene interaction experiments employ a 15-min conditioning duration in the above-described apparatus.
6.2 Impact of PNS and Cocaine Dose on Cocaine-Induced CPP—PNS × Genetic Background Effect but Not Sex Effects
We used the procedures described above (with a 15-min conditioning session duration) to determine the impact of PNS on CPP induced by 3, 10, or 30 mg/kg (IP) in male and female B6 and D2 mice. Briefly, B6 and D2 mice (obtained from the same source as above) were mated and then pregnant dams were subject to either repeated restraint stress (3 × 1 h per day from E14 to delivery) or left alone for the entire gestation period. Offspring were weaned at 3 weeks of age and housed into same-sex groups until testing at 8–10 weeks of age. The results indicate that PNS potentiates cocaine-induced CPP at all employed doses in B6, but not D2, mice . Specifically, there was a significant effect of cocaine dose with all groups exhibiting increased CPP magnitude with increasing cocaine dose. Moreover, there was an interaction between PNS and strain with B6 mice subject to PNS exhibiting greater CPP than control B6 mice (independent of sex or dose) but no differences between PNS and control D2 mice (in either sex or at any dose). The finding that PNS increases CPP in both males and females was somewhat surprising as it contrasts with other research indicating greater sensitivity to PNS potentiation of drug seeking in males relative to females in experiments employing operant self-administration of cocaine in rats (Thomas et al. 2009) as well as our own research employing operant self-administration of alcohol in B6 mice (Campbell et al. 2009) . Accordingly, we replicated this experiment (at the 10 mg/kg dose) and produced the same results in a second cohort of PNS and control B6 males and females. Thus, we are highly confident in this effect and, consistent with other measures of drug seeking, PNS does increase the magnitude of cocaine-induced CPP in B6, but not D2, mice indicating that our approach is sufficient to detect gene–early environment interactions in adult drug responsiveness. Moreover, this strain × PNS interaction indicates that utilization of inbred strains, including RI strains, will be a feasible approach to determine the genetic factors which permit early environmental stress to increase drug-seeking behavior.
6.3 Impact of PNS in B6 and D2 Strains on Alcohol-Induced CPP—PNS × Genetic Background × Sex Effects
As described above, it was surprising that both male and female B6 mice exhibited greater CPP following PNS because sex-dependent effects of PNS are observed on the rate of operant drug self-administration. In order to determine whether the effects of PNS are consistent between CPP and operant self-administration models of drug-seeking, we examined the impact of PNS on alcohol-induced CPP in male and female B6 and D2 mice . Mice were generated as above and then conditioning was performed using the same apparatus and procedures except that each mouse received eight alcohol (2 g/kg, i.p.) and eight vehicle pairings prior to the posttest. The results of this experiment mirror those of our operant alcohol self-administration study. Specifically, we observed a sex × strain × PNS interaction with male B6 mice exhibiting greater alcohol-induced CPP than control B6 mice but PNS and control differences were not observed in other sex/genotype conditions. Accordingly, these results indicate that PNS male, but not female, B6 mice exhibit greater alcohol-seeking behavior, that the impact of PNS on drug seeking is specific to the drug abuse examined, and that the effects of PNS on drug seeking are consistent across operant self-administration and CPP models.
Mouse strains appear to be ideal for elucidating gene–early environment interactions for several reasons. First, the impact of early environmental stress , namely PNS, appears to be consistent across species, including inbred mouse strains, as well as a variety of measures of drug-seeking behavior (i.e., CPP and operant). Second, strain differences are generally stable across laboratories. Third, the availability of RI strains allows detailed dissections of gene–early environment interactions and is an approach currently being employed in our laboratory. Fourth, the availability of a host of well-established behavioral models for a variety of indices of neuropsychiatric disorders which can be utilized for investigation into the relation between heightened cocaine responsiveness and alterations in nondrug behaviors which will further our understanding of nature of comorbid neuropsychiatric vulnerability with addiction.
7 Interactions Between PNS and Genetic Background on Nondrug Behaviors
Direct evidence for interactions between genetic background and early environmental stress is observed in a small number of studies that have examined the impact of PNS in different strains of rodents. PNS increases aggression in male and female B6 mice , but decreases this measure in female D2 mice and does not affect aggression in male D2 mice (Kinsley and Svare 1987) . PNS increases exploratory behavior in a novel environment in B6 mice but decreases it in BALB/c mice (DeFries 1964; DeFries et al. 1967; Weir and DeFries 1964) . Similarly, strain specificity in the effects of PNS upon the behavior of adult rats also appears to be strain specific. For instance, PNS effects upon active avoidance and forced swim-induced immobility are observed in Lewis, but not Fisher 344, strains of rats (Stöhr et al. 1998) . Further, PNS produces different changes in emotionality and/or neuroendocrine responsiveness in “high” versus “low” novelty-seeking substrains of Sprague-Dawley rats (Clinton et al. 2008) or “high” versus “low” anxiety substrains of Wistar rats (Bosch et al. 2006; Neumann et al. 2005) . Further, analyses of the impact of PNS in B6 × BALB/c F1 progeny generated by bidirectional crossing of males and females revealed contributions of both maternal and paternal genetic background to the expression of PNS-induced changes in open-field behavior (DeFries 1964; DeFries et al. 1967) . Importantly, these data indicate that maternal genes are important for, but cannot account for all of, the gene–environment effects observed in PNS-induced changes across genetic background. Such data further the notion that G × Es are complex and likely involve polygenetic influences.
Given widely reported comorbidity of addiction with heritable traits (e.g., novelty seeking) as well as other neuropsychiatric disorders (e.g., schizophrenia), we conducted experiments to assess open-field behavior and prepulse inhibition as measures of reactivity to novel environments and of altered sensory-motor gating observed in schizophrenics. B6 and D2 mice were mated and then pregnant dams were either subject to repeated restraint stress (3 × 1 h per day from E14 to delivery) or left alone for the entire gestation period. Adult offspring were tested for acoustic startle and prepulse inhibition (PPI) and then 3–5 days later were tested for open-field behavior (as reported previously by our group; see Szumlinski et al. 2005) . All subjects exhibited robust acoustic startle which was not altered by PNS. However, the ability of a sub-startle acoustic prepulse to attenuate response to a startle tone was altered by PNS in a strain-specific fashion. Importantly, on this measure the D2, not the B6, strain exhibited attenuated PPI following a history of PNS. Thus, these findings with those of our CPP experiments indicate that both B6 and D2 strains are sensitive to the enduring effects of PNS but that the different genetic backgrounds produced sensitivity to PNS on different behavioral measures. Similarly, open-field behavior was altered in a strain-specific fashion by PNS, with PNS B6 mice exhibiting increased explorations and reduced episodes of immobility compared to their controls whereas PNS D2 mice exhibited increased immobility compared to their controls. Accordingly, our findings suggest that the ability of PNS to modify neuropsychiatric disease vulnerability interacts with genetic background such that specific outcomes are altered in a genetic background-specific fashion, rather than simply having some backgrounds sensitive and others resilient to the effects of PNS. We believe that this finding is critical for proper conceptualization of how the interaction between genetic vulnerability and environment interact across disease vulnerability as well as the potential comorbidity of a disease.
8 Towards a Defined Neurobiology of PNS-Induced Alterations in Drug Seeking
In addition to establishing the pattern of interaction between genes and environment, another major benefit of animal studies of drug responsiveness is the ability to elucidate the underlying neurobiology of that altered responsiveness. Although the impact of drugs of abuse on brain function has been extensively studied, the way in which PNS alters these functions have not been well elucidated. Moreover, there has been even more limited attention on how gene–PNS interactions may be mediated at a neurobiological level. The following section will review what is known about PNS-induced changes in the neurobiology, particularly at a neurochemical level, and how PNS modulates neurochemical responses to drugs, as well as introduce our approach to further this literature by examination of PNS across mouse strains towards an understanding of G × E neurobiology.
Drugs of abuse, like natural rewards, interact with the mesocorticolimbic dopamine system. This system is involved with providing incentive salience to stimuli and inducing the performance of goal-directed behavior. Dopamine cell bodies in the ventral tegmental area (VTA) project to area including the amygdala, hippocampus, nucleus accumbens (NAc), and areas of the prefrontal cortex (Feltenstein and See 2008) . Interestingly, in male and female PNS rats there is reduced dopamine turnover in the left dorsal striatum (Weinstock and Fride 1989) . There are also higher dopamine levels in dorsal striatum (Gerardin et al. 2005) and the ventral striatum (vSTR) of PNS rats (Alonso et al. 1994; McArthur et al. 2005) . This neurochemical change is similar to that of prenatal exposure to corticosterone levels, which increases basal dopamine metabolism in the dorsal and ventral striatum of both male and female offspring (Diaz et al. 1995) . Also, adolescent and adult male PNS rats show higher basal levels of extracellular dopamine in the NAc (Kippin et al. 2008; Silvagni et al. 2008) . There are changes in the expression and/or binding potential of dopamine D1 and D2 receptors, the dopamine transporter (DAT), and changes in the number of tyrosine hydroxylase-positive cells within the mesocorticolimbic dopamine system (i.e., NAc, medial PFC, hippocampal subregions) in PNS animals (Alonso et al. 1994; Berger et al. 2002; Henry et al. 1995; McArthur et al. 2005) . Furthermore, PNS reduces spine density of medium spiny cells of the NAc in adult male rats, but not in preadolescents (Martinez-Tellez et al. 2009) . These baseline changes in the dopamine system could contribute to reactions to drugs of abuse which may facilitate overuse, as discussed later. The dopamine systems of PNS animals react differently from controls to some drugs of abuse. Amphetamine stimulates greater dopamine output in the NAc of PNS adolescent and adult rats compared to controls (Silvagni et al. 2008). Conversely, amphetamine-stimulated dopamine output was blunted in the PFC in PNS animals (Carboni et al. 2010) . PNS males also show higher dopamine output in the NAc after first cocaine exposure, while cocaine-experienced PNS rats exhibit increased PFC dopamine at baseline with enhanced NAc and PFC dopamine output following cocaine administration (Kippin et al. 2008) .
Neurotransmitter systems beyond dopamine also contribute to drug seeking (Koob and Volkow 2010) , and these systems show differences in PNS animals at baseline and after drug administration (Baier et al. 2012) . In the NAc, basal levels of norepinephrine are lower in adolescent PNS rats, although this change is not present in adulthood (Silvagni et al. 2008) . Both adult and adolescent rats have decreased basal PFC norepinephrine output (Carboni et al. 2010) . When either amphetamine or nicotine is given, Nac norepinephrine output is increased in adult PNS rats (Silvagni et al. 2008). In the PFC, PNS increases amphetamine-stimulated norepinephrine output and PNS decreased nicotine-stimulated norepinephrine output in adults (Carboni et al. 2010). In contrast, there are no basal differences in hippocampal acetylcholine release between PNS and control male and female rats, although when exposed to injection stress or intracerebroventricular corticotropin-releasing factor (CRF) administration PNS rats have higher acetylcholine release compared to controls (Day et al. 1998) . As for CRF, PNS animals do have differences in that system, with PNS rats displaying higher amygdala levels of CRF and increased CRF release in response to depolarization (Cratty et al. 1995) , and increased CRF receptor binding (Ward et al. 2000) . The serotonin system also changes in PNS animals. PNS male rats show reduced baseline serotonin in the NAc (Kippin et al. 2008) . PNS also alters 5-HT1A receptor binding, with males showing significantly lower binding compared to controls in the ventral hippocampus, with a similar trend for PNS females (Van den Hove et al. 2006) . Considering the effects of prenatal corticosterone administration alone, autoradiographs of the dorsal hippocampus found significant decreases of the 5-HT1A receptor system in the hippocampal CA1 region (Meerlo et al. 2001) . Male PNS rats also show higher levels of serotonin and 5-HIAA in the dorsal striatum than controls (Gerardin et al. 2005).
The glutamate system and opiate system are also altered by PNS. PNS male rats show reductions in basal glutamate in the NAc compared to controls (Kippin et al. 2008). PNS also increases in N-methyl-D-aspartate (NMDA) receptors in several regions, including the medial PFC, dorsal frontal cortex (DFC), the CA1 region of the hippocampus, the medial striatum, and the NAc, with increases in group III metabotropic glutamate receptors in both the medial PFC and DFC (Berger et al. 2002) . PNS male rats also show reduced mGluR1/5 activity in the ventral hippocampus, while female PNS rats show increased mGluR1/5 activity in both the dorsal and ventral hippocampus (Zuena et al. 2008) . Further, the distribution of Homer proteins which are glutamatergic scaffolding proteins, e.g., are also altered throughout limbo-corticostriatal regions (Ary et al. 2007) . Additionally, on the first administration of cocaine adult male PNS rats show enhanced glutamate neurotransmission, while cocaine-experienced PNS rats show reduced NAc glutamate at baseline and enhanced NAc glutamate following a cocaine challenge (Kippin et al. 2008) . Finally, there are differences to note in the opiate system. Receptor autoradiography has shown that male and female PNS rats show decreased μ opiate receptor binding in the striatum, specifically in the anterior striatum, the NAc, the lateral amygdala, and the endopiriform cortex (Insel et al. 1990) .
PNS also produces morphological changes in brain structures associated with drug reward. In the amygdala, some subnuclei diverge between PNS and control males across several measures (Krazpulski et al. 2006) . By early adulthood, though, PNS individuals begin to match controls on obvious features such as nuclear volume, length, and neuron and glial cell number (Krazpulski et al. 2006). PNS animals may even surpass control volume in the lateral nucleus in later adulthood (Salm et al. 2004) , highlighting the importance of developmental time point on the effects of PNS. Hippocampal morphology changes over time as well, with preadolescent PNS males displaying increased spine density in the CA1 subregion and decreased density in the CA3 subregion (Martinez-Tellez et al. 2009) . By adulthood, PNS males display reductions in dendritic spines in both the CA1 and CA3 subregions (Martinez-Tellez et al. 2009) . PNS or prenatal corticosterone treatment causes a decrease in synaptophysin and an increase in GAP-43 and phosphorylated GAP-43 in the hippocampus, possibly contributing to the alteration in synapse formation in the hippocampus seen after PNS (Afadlal et al. 2010) . As we have shown, there are many ways in which PNS is known to change brain morphology, the basal state of neurotransmitter systems, and the reactions of these systems to drugs of abuse. Any of these changes, or even PNS changes not identified yet, might contribute to a greater individual predisposition towards addiction, and further research is needed to determine which of these changes are critical to the addiction process.
PNS also adversely impacts the ability of the adult brain to produce new neurons. New neurons are produced in high numbers within the adult olfactory bulbs and hippocampus with the ultimate precursors for these new cells being the proliferative and multipotential neural stem cells (Taupin and Gage 2002; Weiss and van der Kooy 1998) . PNS reduces the number of neural stem cells in the lateral ventricle subventricular zone (SVZ) across the lifespan (Kippin et al. 2004) . PNS also reduces overall proliferation in the dentate gyrus (DG; Fujioka et al. 2006; Kawamura et al. 2006; Lemaire et al. 2000, 2006; Odagiri et al. 2008; Rayen et al. 2011) and in the SVZ (Kippin et al. 2004) as well survival of new born precursors is reduced following PNS (Koo et al. 2003) in adolescent and adult rodents with similar findings reported in PNS adult primates (Coe et al. 2003) . Alternatively, a wide variety of drugs of abuse negatively impact adult neurogenesis (for reviews, see, e.g., Canales 2010; Eisch and Harburg 2006) and one study reported elevated cocaine self-administration following ablation of the hippocampal neurogenesis (Noonan et al. 2010) suggesting that PNS-induced changes in neurogenesis may be critically involved in PNS-induced changes in drug-seeking behavior. Other differences in the neurogenic system emerge between the sexes following PNS (Koehl et al. 2009; Schmitz et al. 2002; Biala et al. 2011; Mandyam et al. 2008; Zuena et al. 2008) and these effects may be relevant to sex-differentiated response to PNS modulation in responsiveness to drugs of abuse (Thomas et al. 2009) .
Given our observed PNS × genetic background effects on behavior, we have initiated a project to identify associated molecular epigenetic changes. In our first experiment, we specifically targeted a region of the brain that plays an important role in emotional learning and memory, the ventromedial prefrontal cortex (mPFC; i.e., infralimbic and prelimbic cortices), and determined whether epigenetic mechanisms are impacted by PNS × genetic background interactions. To this end, genome-wide DNA methylation profiling was performed using a methyl-capture microarray approach on mPFC tissue derived from PNS and control mice of B6 and D2 strains (that received no further experimental manipulations, i.e., drug naïve). Indeed, widely varied differences in DNA methylation were observed in non-PNS DBA/2J and C57BL/6J adult offspring, suggesting that underlying genetic background influences the epigenotype, which was further impacted by PNS. Interestingly, many of the genes displaying differential DNA methylation are well known to play a role in the neurodevelopmental process and neuroplasticity, and altered epigenetic regulation of these genes might therefore be responsible for the distinct behavioral phenotypes observed in these offspring in later life. To verify the results of the genome-wide assay, select genes are being examined employing bisulfite conversion and mass spectrophotometry as well as quantitative polymerase chain reaction (PCR) to determine mRNA levels. Although this project is still in its infancy, we believe that it will be instrumental in explaining the ability of prenatal perturbations to alter adult brain function, both in terms of drug responsiveness as well as other types of behaviors, such as PPI deficits. Further, the potentially fortuitous finding that PNS effects are genetically dissociable on drug-induced CPP versus PPI allows for built-in controls for elucidating PNS-induced molecular changes that are relevant to specific neuropsychiatric outcomes, although these will need to be functionally verified.
9 Summary and Future Goals
Clinical and preclinical studies not only indicate that the genetic inheritance of cocaine addiction ranges from 0.30 to 0.70 (reviewed in, e.g., Agrawal and Lynskey 2008) but also have implicated early environmental factors as a major risk factor for cocaine addiction (see, e.g., Ellenbroek et al. 2005) . However, there is limited research on the impact of early environmental factors, such as stress , and genetic background, which comprises a large gap in our understanding of the determinants of individual vulnerability to cocaine addiction. For instance, contributions of individual genes for addiction vulnerability determined by genome-wide association studies (GWAS) typically account for a very small amount of variability (e.g., Visscher et al. 2012) . However, such analyses generally neglect and/or are insensitive to environmental contributing factors that may interact with specific genetic backgrounds. Evidence from our studies and others indicate that early environmental programming can modulate genetic background differences (i.e., across strains), and may be a reason for the weak contributions determined by GWAS studies. That is by looking at genetic contribution across environmental histories, the G × E contributions may confound such approaches and dramatically underestimate the actual importance of specific genes to neuropsychiatric vulnerability. Accordingly, one of the overall benefits of examining the effects of PNS across various genetic background in a parametric fashion (i.e., RI strains) is that it will enable a way to compare genetic alone versus G × E contributions to a phenotype for specific genetic factors (i.e., loci or ideally specific gene).
Another major benefit of our current studies is that it is providing fundamental insight into the nature and relation of G × E effects on indices of multiple neuropsychiatric diseases. The changes produced by PNS in behavior and neural function have been argued to be relevant to addiction (Campbell et al. 2009; Koehl et al. 2002) as well as many neuropsychiatric diseases, including schizophrenia, ADHD, depression, and anxiety disorders (see, e.g., Koenig et al. 2002; Kofman 2002; Van den Bergh et al. 2008; Glover 2011) . Further, there is a high comorbidity between addiction and other neuropsychiatric conditions (e.g., Siegfried 1998; Cuffel 1996) . Accordingly, it was somewhat surprising that we were able to find strain-independent G × E effects on drug seeking (in CPP) versus psychoses (PPI; response to novelty) following PNS in B6 and D2 mice , respectively, as this suggests independent genetic vulnerability for each disorder based on environmental history. Although this finding indicates that the role of PNS in different disorders is distinct, an extension of this suggests that disease comorbidity may be related to presence of multiple G × E effects which may exacerbate each other. Thus, when individuals have elevated drug responsiveness/reward/seeking with other disruptions, addiction may be more pronounced (e.g., exhibiting faster transition from recreational use to need for clinical treatment or being resistant to available clinical interventions). We feel understanding the relation of G × E effects on behaviors of relevance to different neuropsychiatric disorders will be a critical milestone in for identifying individuals at risk.
References
Afadlal S, Polaboon N, Surakul P, Govitrapong P, Jutapakdeegul N (2010) Prenatal stress alters presynaptic marker proteins in the hippocampus of rat pups. Neurosci Lett 470(1):24–27
Agrawal A, Lynskey MT (2008) Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction 103(7):1069–1081
Aldworth J, Ault K, Bishop E (2007) Results from the 2006 National Survey on Drug Use and Health: National Findings: Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293, Rockville, MD
Alonso SJ, Navarro E, Rodriguez M (1994) Permanent dopaminergic alterations in then accumbens after prenatal stress. Pharmacol Biochem Behav 49:353–358
Ary AW, Aguilar VR, Szumlinski KK, Kippin TE (2007) Prenatal stress alters limbo-corticostriatal Homer protein expression. Synapse 61(11):938–941
Badiani A, Cabib S, Puglisi-Allegra S (1992) Chronic stress induces strain-dependent sensitization to the behavioral effects of amphetamine in the mouse. Pharmacol Biochem Behav 43(1):53–60
Baier CJ, Katunar MR, Adrover E, Pallarés ME, Antonelli MC (2012) Gestational restraint stress and the developing dopaminergic system: an overview. Neurotox Res 22(1):16–32
Barbazanges A, Piazza PV, Le Moal M, Maccari S (1996) Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 16(12):3943–3949
Belknap JK (1998) Effect of within-strain sample size on QTL detection and mapping using recombinant inbred mouse strains. Behav Genet 28(1):29–38
Belknap JK, Crabbe JC (1992) Chromosome mapping of gene loci affecting morphine and amphetamine responses in BXD recombinant inbred mice. Ann N Y Acad Sci 654:311–323
Belzung C, El Hage W, Moindrot N, Griebel G (2001) Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology 41(3):400–408
Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC (2002) Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem Res 27(11):1525–1533
Bertram CE, Hanson MA (2002) Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction 124(4):459–467
Biala YN, Bogoch Y, Bejar C, Linial M, Weinstock M (2011) Prenatal stress diminishes gender differences in behavior and in expression of hippocampal synaptic genes and proteins in rats. Hippocampus 21(10):1114–1125
Bosch OJ, Krömer SA, Neumann ID (2006) Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur J Neurosci 23(2):541–551
Cabib S, Bonaventura N (1997) Parallel strain-dependent susceptibility to environmentally-induced stereotypies and stress-induced behavioral sensitization in mice. Physiol Behav 61(4):499–506
Cabib S, Orsini C, Le Moal M, Piazza PV (2000) Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science 289(5478):463–465
Campbell JC, Dunn CP, Nayak RR, Cornateuanu, RJ, Kippin TE (under review) Prenatal stress produces sex-specific effects on alcohol-induced motivational plasticity in C57Bl/6 J mice. Physiol Behav (submitted)
Campbell JC, Szumlinski KK, Kippin TE (2009) Contribution of early environmental stress to alcoholism vulnerability. Alcohol 43(7):547–554
Canales JJ (2010) Comparative neuroscience of stimulant-induced memory dysfunction: role for neurogenesis in the adult hippocampus. Behav Pharmacol 21(5–6):379–393
Carboni E, Barros VG, Ibba M, Silvagni A, Mura C, Antonelli MC (2010) Prenatal restraint stress: an in vivo microdialysis study on catecholamine release in the rat prefrontal cortex. Neuroscience 168(1):156–166
Carrara-Nascimento PF, Olive MF, Camarini R (2012) Ethanol pre-exposure during adolescence or adulthood increases ethanol intake but ethanol-induced conditioned place preference is enhanced only when pre-exposure occurs in adolescence. Dev Psychobiol (epub ahead of print)
Cervantes MC, Laughlin RE, Jentsch JD (2013) Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology (Berl) (Epub ahead of print)
Chesler EJ, Wang J, Lu L, Qu Y, Manly KF, Williams RW (2003) Genetic correlates of gene expression in recombinant inbred strains: a relational model system to explore neurobehavioral phenotypes. Neuroinformatics 1(4):343–357
CIA (2008) The world factbook: office of public affairs, CIA, Washington, DC
Clinton S, Miller S, Watson SJ, Akil H (2008) Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology 33(2):162–177
Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E (2003) Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile Rhesus monkeys. Biol Psychiatry 54(10):1025–1034
Cohen P (1990) Desires for cocaine. In: Warburton DM (ed) Addiction controversies. Chur, Harwood, 212–222
Complex Trait Consortium (2003) The nature and identification of quantitative trait loci: a community’s view. Nat Rev Genet 4(11):911–916
Crabbe JC (2002) Genetic contributions to addiction. Annu Rev Psychol 53:435–462
Crabbe JC, Belknap JK, Buck KJ, Metten P (1994) Use of recombinant inbred strains for studying genetic determinants of responses to alcohol. Alcohol Alcohol Suppl 2:67–71
Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK (1999) Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci 22(4):173–179
Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL (1995) Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res, 675(1–2):297–302
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132(2):107–124
Cuffel BJ (1996) Comorbid substance use disorder: prevalence, patterns of use, and course. New Dir Ment Health Serv 70:93–105
Cunningham CL, Dickinson SD, Grahame NJ, Okorn DM, McMullin CS (1999) Genetic differences in cocaine-induced conditioned place preference in mice depend on conditioning trial duration. Psychopharmacology (Berl) 146(1):73–80
Darnaudéry M, Maccari S (2008) Epigenetic programming of the stress response in male and female rats by prenatal restrain stress. Brain Res Rev 57(2):571–585
Darnaudery M, Louvart H, Defrance L, Leonhardt M, Morley-Fletcher S, Gruber SH et al (2007) Impact of an intense stress on ethanol consumption in female rats characterized by their prestress preference: modulation by prenatal stress. Brain Res 1131:181–186
Day JC, Koehl M, Deroche V, Le Moal M, Maccari S (1998) Prenatal stress enhances stress- and corticotropin-releasing factor-induced stimulation of hippocampal acetylcholine release in adult rats. J Neurosci 18(5):1886–1892
DeFries JC (1964) Prenatal maternal stress in mice: differential effects on behavior. J Hered 55:289–925
DeFries JC, Weir MW, Hegmann JP (1967) Differential effects of prenatal stress on offspring behavior in mice as a function of genotype and stress. J Comp Physiol Psychol 63(2):332–334
de Jong IE, Oitzl MS, de Kloet ER (2007) Adrenalectomy prevents behavioural sensitization of mice to cocaine in a genotype-dependent manner. Behav Brain Res 177(2):329–339
de Jong IE, Steenbergen PJ, de Kloet ER (2008) Strain differences in the effects of adrenalectomy on the midbrain dopamine system: implication for behavioral sensitization to cocaine. Neuroscience 153(3):594–604
Deminiere JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M et al (1992) Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res 586:135–139
DeTurck KH, Pohorecky LA (1987) Ethanol sensitivity in rats: effect of prenatal stress. Physiol Behav 40:407–410
DeWit J, Uhlenhuth EH, Johanson CE (1986) Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend 16:341–360
Diaz R, Ogren SO, Blum M, Fuxe K (1995) Prenatal corticosterone increases spontaneous and d-amphetamine induced locomotor activity and brain dopamine metabolism in prepubertal male and female rats. Neuroscience 66:467–473
Eisch AJ, Harburg GC (2006) Opiates, psychostimulants, and adult hippocampal neurogenesis: insights for addiction and stem cell biology. Hippocampus 16(3):271–286
Ellenbroek BA, van der Kam EL, van der Elst MC, Cools AR (2005) Individual differences in drug dependence in rats: the role of genetic factors and life events. Eur J Pharmacol 526(1–3):251–258
Enoch MA (2006) Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann N Y Acad Sci, 1094:193–201
Fehr C, Shirley RL, Belknap JK, Crabbe JC, Buck KJ (2002) Congenic mapping of alcohol and pentobarbital withdrawal liability loci to a <1 centimorgan interval of murine chromosome 4: identification of Mpdz as a candidate gene. J Neurosci 22:3730–3738
Feltenstein MW, See RE (2008) The neurocircuitry of addiction: an overview. Br J Pharmacol 154(2)261–274
Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S (2006) Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience 141(2), 907–915
Gao S, Suenaga T, Oki Y, Yukie M, Nakahara D (2011) Cocaine enhances resistance to extinction of responding for brain-stimulation reward in adult prenatally stressed rats. Behav Brain Res 223(2):411–416
George FR, Goldberg SR (1989) Genetic approaches to the analysis of addiction processes. Trends Pharmacol Sci 10(2):78–83
Gerardin DC, Pereira OC, Kempinas WG, Florio JC, Moreira EG, Bernardi MM (2005) Sexual behavior, neuroendocrine, and neurochemical aspects in male rats exposed prenatally to stress. Physiol Behav 84(1):97–104
Glover V (2011) Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry 52(4):356–367
Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M et al (1995) Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res 685 179–186
Hood HM, Metten P, Crabbe JC, Buck KJ (2006) Fine mapping of a sedative-hypnotic drug withdrawal locus on mouse chromosome 11. Genes Brain Behav 5(1):1–10
Huizink AC, Mulder EJ, Buitelaar JK (2004) Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull 130:115–142
Insel TR, Kinsley CH, Mann PE, Bridges RS (1990) Prenatal stress has long-term effects on brain opiate receptors. Brain Res 511:93–97
Kafkafi N, Elmer GI (2005) Activity density in the open field: a measure for differentiating the effect of psychostimulants. Pharmacol Biochem Behav 80(2):239–249
Kawamura T, Chen J, Takahashi T, Ichitani Y, Nakahara D (2006) Prenatal stress suppresses cell proliferation in the early developing brain. Neuroreport 17(14):1515–1518
Kendler KS, Myers J, Prescott CA (2007) Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry 64(11):1313–1320
Kinsley C, Svare B (1987) Genotype modulates prenatal stress effects on aggression in male and female mice. Behav Neural Biol 47(2):138–150
Kinsley CH, Mann PE, Bridges RS (1988) Prenatal stress alters morphine- and stress-induced analgesia in male and female rats. Pharmacol Biochem Behav 30:123–128
Kippin TE, Cain SW, Masum Z, Ralph MR (2004) Neural stem cells show bidirectional experience-dependent plasticity in the perinatal mammalian brain. J Neurosci 24:2832–2836
Kippin TE, Szumlinski KK, Kasparova Z, Rezner B, See RE (2008) Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology 33(4):769–782
Koehl M, Bjijou Y, Le Moal M, Cador M (2000) Nicotine-induced locomotor activity is increased by preexposure of rats to prenatal stress. Brain Res 882(1–2):196–200
Koehl M, Lemaire V, Mayo W, Abrous DN, Maccari S, Piazza PV, Le Moal M, Vallée M (2002) Individual vulnerability to substance abuse and affective disorders: role of early environmental influences. Neurotox Res 4(4):281–296
Koenig JI, Kirkpatrick B, Lee P (2002) Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology 27(2):309–318
Kofman O (2002) The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev 26(4):457–470
Koo JW, Park CH, Choi SH, Kim NJ, Kim HS, Choe JC, Suh YH (2003) The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. FASEB J 11:1556–1558
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238
Koehl M, Lemaire V, Le Moal M, Abrous DN (2009) Age-dependent effect of prenatal stress on hippocampal cell proliferation in female rats. Eur J Neurosci 29:635–640
Kosten TA, Miserendino MJ, Kehoe P (2000) Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res 875(1–2):44–50
Kosten TA, Sanchez H, Zhang XY, Kehoe P (2004) Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res 151(1–2):137–149
Kosten TA, Zhang XY, Kehoe P (2006) Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology 31(1):70–76
Krazpulski M, Dickerson PA, Salm AK (2006) Prenatal stress affects the developmental trajectory of the rat amygdala. Stress 9(2):85–95
Kreek MJ (2001) Drug addictions: molecular and cellular endpoints. Ann N Y Acad Sci 937:27–49
Lemaire V, Koehl M, Le Moal M, Abrous DN (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus Proc Natl Acad Sci U S A 97(20):11032–11037
Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN (2006) Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry 59(9):786–792
Lephart ED, Watson MA, Rhees RW, Ladle DR, Jacobson NA (1997) Developmental expression of calretinin in the medial basal hypothalamus and amygdala from male and female rats. Neurosci Res 28(3):269–273
Lynch WJ, Mangini LD, Taylor JR (2005) Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology 30:322–329
Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O (2003) Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev 27(1–2):119–127
Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN (2008) Stress experiences in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobio 68(5):575–589
Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G (2009) Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse 63(9):794–804
Matthews SG (2002) Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab 13(9):373–380
McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE (2005) Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol 17:475–482
Meerlo P, Horvath KM, Luiten PG, Angelucci L, Catalani A, Koolhaas JM (2001) Increased maternal corticosterone levels in rats: effects on brain 5-HT1A receptors and behavioral coping with stress in adult offspring. Behav Neurosci 115(5):1111–1117
Miner LL, Marley RJ (1995a) Chromosomal mapping of loci influencing sensitivity to cocaine-induced seizures in BXD recombinant inbred strains of mice. Psychopharmacology (Berl) 117(1):62–66
Miner LL, Marley RJ (1995b) Chromosomal mapping of the psychomotor stimulant effects of cocaine in BXD recombinant inbred mice. Psychopharmacology (Berl) 122(3):209–214
Montano MM, Wang MH, Even MD, vom Saal FS (1991) Serum corticosterone in fetal mice: sex differences, circadian changes, and effect of maternal stress. Physiol Behav 50(2):323–329
Morley-Fletcher S, Puopolo M, Gentili S, Gerra G, Macchia T, Laviola G (2004) Prenatal stress affects 3,4-methylenedioxymethamphetamine pharmacokinetics and drug-induced motor alterations in adolescent female rats. Eur J Pharmacol 489(1–2):89–92
Neumann ID, Krömer SA, Bosch OJ (2005) Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology 30(8):791–806
Noonan MA, Bulin SE, Fuller DC, Eisch AJ (2010) Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci 30(1):304–315
Odagiri K, Abe H, Kawagoe C, Takeda R, Ikeda T, Matsuo H, Nonaka H, Ebihara K, Nishimori T, Ishizuka Y, Hashiguchi H, Ishida Y (2008) Psychological prenatal stress reduced the number of BrdU immunopositive cells in the dorsal hippocampus without affecting the open field behavior of male and female rats at one month of age. Neurosci Lett 446(1):25–29
Orsini C, Bonito-Oliva A, Conversi D, Cabib S (2005) Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 181(2):327–336
Orsini C, Bonito-Oliva A, Conversi D, Cabib S (2008) Genetic liability increases propensity to prime-induced reinstatement of conditioned place preference in mice exposed to low cocaine. Psychopharmacology (Berl) 198(2):287–296
Philip VM, Duvvuru S, Gomero B, Ansah TA, Blaha CD, Cook MN, Hamre KM, Lariviere WR, Matthews DB, Mittleman G, Goldowitz D, Chesler EJ (2010) High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav 9(2):129–159
Phillips TJ, Belknap JK (2002) Complex-trait genetics: emergence of multivariate strategies. Nat Rev Neurosci 3(6):478–485
Phillips TJ, Huson MG, McKinnon CS (1998) Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci 18(8):3023–3034
Phillips TJ, Belknap JK, Hitzemann RJ, Buck KJ, Cunningham CL, Crabbe JC (2002) Harnessing the mouse to unravel the genetics of human disease. Genes Brain Behav 1(1):14–26
Pohorecky LA, Roberts P (1991) Activity in a modified open-field apparatus: effect of diazepam and prenatal stress. Neurotoxicol Teratol 13:129–133
Pohorecky LA, Roberts P, Cotler S, Carbone JJ (1989) Alteration of the effects of caffeine by prenatal stress. Pharmacol Biochem Behav 33(1):55–62
Pouletty P (2002) Drug addictions: towards socially accepted and medically treatable diseases. Nat Rev Drug Discov 1(9):731–736
Prakash P, Merali Z, Kolajova M, Tannenbaum BM, Anisman H (2006) Maternal factors and monoamine changes in stress-resilient and susceptible mice: cross-fostering effects. Brain Res 1111(1):122–133
Rayen I, Van den Hove DL, Prickaerts J, Steinbusch HW, Pawluski JL (2011) Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS ONE 6(9):e24003
Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle D (2004) Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Dev Brain Res 148:159–167
Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA (2002) Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry 7(7):810–813
Schuckit MA (2009) An overview of genetic influences in alcoholism. J Subst Abuse Treat 36(1):S5–S14
Seale TW, Carney JM (1991) Genetic determinants of susceptibility to the rewarding and other behavioral actions of cocaine. J Addict Dis 10(1–2):141–162
Seckl JR, Meaney MJ (2004) Glucocorticoid programming. Ann N Y Acad Sci 1032:63–84
Siegfried N (1998) A review of comorbidity: major mental illness and problematic substance use. Aust N Z J Psychiatry 32:707–717
Silvagni A, Barros VG, Mura C, Antonelli MC, Carboni E (2008) Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ‘in vivo’ microdialysis study in adolescent and young adult rats. Eur J Neurosci 28(4):744–758
Sternberg WF, Ridgeway CG (2003) Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav 78:375–383
Stöhr T, Schulte Wermeling D, Szuran T, Pliska V, Domeney A, Welzl H, Weiner I, Feldon J (1998) Differential effects of prenatal stress in two inbred strains of rats. Pharmacol Biochem Behav 59(4):799–805
Szumlinski KK, Lominac KD, Kleschen M, Oleson EB, Dehoff M, Schwartz M, Seeberg P, Worley PF, Kalivas PW (2005) Behavioural and neurochemical phenotyping of Homer1 mutant mice: possible implications for schizophrenia. Genes Brain Behav 4:273–288
Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE (2008) Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6 J mice. Neuropsychopharmacology 33(6):1365–1378
Takahashi LK, Turner JB, Kalin NH (1998) Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology 23(6):571–581
Taupin P, Gage FH (2002) Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res 69(6):745–749
Tazumi T, Hori E, Uwano T, Umeno K, Tanebe K, Tabuchi E, Ono T, Nishijo H (2005) Effects of prenatal maternal stress by repeated cold environment on behavioral and emotional development in the rat offspring. Behav Brain Res 162:153–160
Thomas MB, Hu M, Lee TM, Bhatnagar S, Becker JB (2009) Sex-specific susceptibility to cocaine in rats with a history of prenatal stress. Physiol Behav 97(2):270–277
Tolliver BK, Carney JM (1994a) Comparison of cocaine and GBR 12935: effects on locomotor activity and stereotypy in two inbred mouse strains. Pharmacol Biochem Behav 48(3):733–739
Tolliver BK, Carney JM (1994b) Sensitization to stereotypy in DBA/2 J but not C57BL/6 J mice with repeated cocaine. Pharmacol Biochem Behav 48(1):169–173
Tolliver BK, Carney JM (1995) Locomotor stimulant effects of cocaine and novel cocaine analogs in DBA/2 J and C57BL/6 J inbred mice. Pharmacol Biochem Behav 50(2):163–169
Tolliver BK, Belknap JK, Woods WE, Carney JM (1994) Genetic analysis of sensitization and tolerance to cocaine. J Pharmacol Exp Ther 270(3):1230–1238
Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L (2008) Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology 33(3):536–545
Van den Hove DL, Lauder JM, Scheepens A, Prickaerts J, Blanco CE, Steinbusch HW (2006) Prenatal stress in the rat alters 5-HT1A receptor binding in the ventral hippocampus. Brain Res 1090(1):29–34
van der Kam EL, Ellenbroek BA, Cools AR (2005) Gene-environment interactions determine the individual variability in cocaine self-administration. Neuropharmacology 48(5):685–695
van der Veen R, Piazza PV, Deroche-Gamonet V (2007) Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2 J but not in C57BL/6 J mice. Psychopharmacology (Berl) 193(2):179–186
van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V (2008) Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS ONE 3(5):e2245
Van Waes V, Enache M, Dutriez I, Lesage J, Morley-Fletcher S, Vinner E et al (2006) Hypo-response of the hypothalamic-pituitaryadrenocortical axis after an ethanol challenge in prenatally stressed adolescent male rats. Eur J Neurosci 24:1193–1200
Van Waes V, Darnaudery M, Marrocco J, Gruber SH, Talavera E, Mairesse J, Van Camp G, Casolla B, Nicoletti F, Mathe AA, Maccari S, Morley-Fletcher S (2011a) Impact of early life stress on alcohol consumption and on the short- and long-term responses to alcohol in adolescent female rats. Behav Brain Res 221(1):43–49
Van Waes V, Enache M, Berton O, Vinner E, Lhermitte M, Maccari S, Darnaudery M (2011b) Effects of prenatal stress on alcohol preference and sensitivity to chronic alcohol exposure in male rats. Psychopharmacology (Berl) 214(1):197–208
Visscher PM, Brown MA, McCarthy MI, Yang J (2012) Five years of GWAS discovery. Am J Hum Genet 90(1):7–24
Wahlsten D, Metten P, Phillips TJ, Boehm SL 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC (2003) Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol 54(1):283–311
Ward HE, Johnson EA, Salm AK, Birkle DL (2000) Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav 70(3–4):359–366
Weinstock M (2001) Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 65:427–451
Weinstock M (2008) The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32:1073–1086
Weinstock M, Fride E (1989) Alterations in behavioral and striatal dopamine asymmetries induced by prenatal stress. Pharmacol Biochem Behav 32(2):425–430
Weir MW, DeFries JC (1964) Prenatal maternal influence of behavior in mice: evidence of a genetic basis. J Comp Physiol Psychol 58:412–417
Weiss S, van der Kooy D (1998) CNS stem cells: where’s the biology (a.k.a. beef)? J Neurobiol 36(2):307–314
Yang J, Li W, Liu X, Li Z, Li H, Yang G, Xu L, Li L (2006) Enriched environment treatment counteracts enhanced addictive and depressive-like behavior induced by prenatal chronic stress. Brain Res 1125:192–197
Zhang XY, Sanchez H, Kehoe P, Kosten TA (2005) Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology (Berl) 177(4):391–399
Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ (2006) Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol 73(1):72–89
Zuena AR, Mairesse J, Casolini P, Cinque C, Alema GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S (2008) Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE 3(5):e2170
Acknowledgments
This work was supported by National Institute on Drug Abuse (NIDA) grant DA-027525 and a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award to TEK.
Conflict of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kippin, T., Campbell, J., Ploense, K., Knight, C., Bagley, J. (2015). Prenatal Stress and Adult Drug-Seeking Behavior: Interactions with Genes and Relation to Nondrug-Related Behavior. In: Antonelli, M. (eds) Perinatal Programming of Neurodevelopment. Advances in Neurobiology, vol 10. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1372-5_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1372-5_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1371-8
Online ISBN: 978-1-4939-1372-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)