Abstract
This chapter presents a critical analysis of the behavioral alterations reported in the offspring of women exposed to stress and/or depression during pregnancy and the neurochemical and structural changles underlying them. Among the alterations attributed to prenatal stress in humans and experimental rats of both sexes is impaired regulation of the hypothalamic–pituitary–adrenal (HPA) axis, anxiety and exaggerated fear of novelty, and decreased social interaction. Learning and attention deficits are more prevalent in boys and male rats. Fear of novelty and anxiety are associated with enlargement of the amygdala and its corticotropin-releasing factor content, and decreased socialization, with lower oxytocin activity in the amygdala. Learning deficits are associated with a decrease in neurogenesis, dendritic complexity, and spine number in the dorsal hippocampus. Fostering prenatally stressed (PS) pups onto control mothers prevents the dysregulation of the HPA axis and heightened anxiety, indicating a role for postnatal factors in their etiology. By contrast, learning impairment and decreased socialization are not affected by this fostering procedure and are therefore prenatally mediated.
In spite of their widespread use in depressed pregnant women, selective serotonin reuptake inhibitor (SSRI) antidepressants do not normalize the behavior of their children. When administered during gestation to stressed rats, SSRIs do not reduce anxiety or learning deficits in their offspring. Moreover, when given to unstressed mothers, SSRIs induce anxiety in the offspring. The detrimental effect of SSRIs may result from inhibition of the serotonin transporter exposing the brain to excess amounts of 5-hydroxytryptamine (5-HT) at a critical time during fetal development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Amygdala
- Antidepressants
- Anxiety
- Cognitive deficits
- Corticotropin-releasing hormone
- Dorsal hippocampus
- Gestational stress
- Glucocorticoids
- Serotonin
1 Introduction
Observations from retrospective studies starting in the 1960s suggested that prolonged uncontrollable stress during pregnancy may cause alterations in the development and behavior of the offspring which can be detected in infancy, childhood, and adulthood. These alterations include a reduction in birth weight, delay in early developmental milestones, withdrawn or disruptive behavior, attention and learning deficits, anxiety , depression, and schizophrenia (see reviews by Koenig et al. 2002; Kofman 2002; Weinstock 1997, 2001, 2008) . In the past decade, prospective studies were initiated in women who had been exposed to natural (Laplante et al. 2008) or man-made disasters (Imamura et al. 1999) , marital discord (Lereya and Wolke 2012) , and adverse social or work-related conditions (Khashan et al. 2008) . Exposure to such adverse risk factors can also increase the incidence of depression in pregnant women (Giardinelli et al. 2012; Husain et al. 2012; Miszkurka et al. 2012; Qu et al. 2012) . Both depression and gestational stress can each adversely affect child development and behavior. Therefore, other studies focused on offspring from birth through to adolescence of women with anxiety and depression during and after pregnancy (Bergman et al. 2007; Davis and Sandman 2012; Van den Bergh and Marcoen 2004; Van den Bergh et al.2008) . However, these prospective studies underscored the difficulty in defining maternal stress and allowing for differences in the reaction of women to the same objective stress. While some reported an association between maternal distress and behavioral changes in children at different ages, none could differentiate unequivocally between prenatal, genetic, and postnatal factors in mediating the behavioral outcome.
A clearer assessment of the contribution of pre- and postnatal factors to the behavioral outcome that is less influenced by genetic factors can be achieved by studies in experimental animals. The majority has been performed in rats in which more comprehensive behavioral, morphological, and histological information is available than in other species. Several were able to replicate the increased anxiety , depressive-like behavior (Alonso et al. 1991; Morley-Fletcher et al. 2004; Poltyrev et al. 2005) , learning (Yaka et al. 2007; Yang et al. 2006) and attention deficits (Wilson et al. 2012) , reduced social interaction (Lee et al. 2007), and some of the characteristic neuronal changes of schizophrenia (Koenig et al. 2005) . Like in humans (Van den Bergh et al. 2008), gestational stress in rats impaired the regulation of the response to stress of the hypothalamic–pituitary–adrenal (HPA) axis in the offspring (Barbazanges et al. 1996; Weinstock et al. 1992) .
By fostering prenatally stressed (PS) pups onto control mothers, it was also possible to differentiate behavioral alterations arising from gestational stress per se from those ascribed to inadequate mother–infant interactions (Barros et al. 2006; Yang et al. 2006) . Other procedures like housing the stressed mothers (Li et al. 2012) or their offspring in an enriched environment were able to reduce the effects of gestational stress on several aspects of the offspring behavior (Lui et al. 2011; Yang et al. 2007) . This chapter discusses more recent research that has examined the effect of gestational stress on neurochemical, structural, gene, and proteomic changes in different brain regions of the offspring of both sexes. It also describes procedures that have been used to prevent or reverse the behavioral and structural changes induced by prenatal stress.
2 Gestational Stress and Activity of the HPA Axis in the Mother and Her Offspring
Subjects with anxiety and depression have hypercortisolemia and impairment of negative feedback by cortisol on the HPA. This has been attributed to the increased action of corticotropin-releasing factor (CRF; Keck 2006; Reul and Holsboer 2002) . Hypercortisolemia also occurs after chronic stress. It has been postulated that prenatal stress produces alterations in brain structure and behavior through the action of “stress” hormones, CRF, glucocorticoids, and catecholamines arising in the maternal adrenal gland and placenta (reviewed in Jansson and Powell 2007; Sandman et al. 2011; Weinstock 2005) . During a normal pregnancy, very little cortisol (in humans) and corticosterone (COR; in rodents) reaches the fetal brain because they are converted to inactive metabolites by the placental enzyme 11β-hydroxy steroid dehydrogenase-2 (11β-HSD-2). In addition, about 90 % of circulating corticosteroids are sequestered by a corticosteroid-binding globulin (CBG), thereby limiting their access to the fetus. However, chronic gestational stress reduces the level of CBG in rats (Takahashi et al. 1998) and downregulates the activity of 11β-HSD-2 in humans (O’Donnell et al. 2012) and rats (Jensen Pena et al. 2012) . This is accomplished by DNA methylation at specific sites within the 11β-HSD-2 gene promoter, thereby increasing the concentration of free steroids that can reach the developing fetal brain. Gestational stress also releases adrenaline and noradrenaline into the circulation which can reduce placental blood flow causing hypoxia and ischemia that could adversely influence fetal brain development (Delcour et al. 2012; Fan et al. 2009) . Higher levels of these catecholamines have been found in the fetal circulation in response to maternal stress (Ohkawa et al. 1991) and can reach the brain because of the absence of a blood brain barrier.
Several clinical studies have attempted to relate elevations in cortisol to the presence of chronic stress, anxiety, and/or depression (assessed by questionnaires) during pregnancy. No relation was found between the magnitude of the increase in maternal cortisol in plasma (Baibazarova et al. 2012) or saliva between gestational weeks 15–37 and the level of stress, anxiety, depression, or pregnancy-specific anxiety at any of the times that cortisol was measured (Davis and Sandman 2010) . However, a significant relation between salivary cortisol and maternal mood was found in subjects with comorbidity of anxiety and depression but not in those with only one of these conditions (Evans et al. 2008) . It is not clear why most studies failed to relate maternal anxiety and/or depression at a specific time during pregnancy to elevation of plasma cortisol. This may depend on the method of sample collection or its timing during the day, which may differ in subjects with alterations in their circadian rhythms due to depression. It is probable that the ongoing chronic emotional state of anxious, depressed women does not lend itself to the detection of a clearly defined increase in plasma cortisol, unlike that in response to stress.
Others have tried to relate the time of occurrence of stress, anxiety , and/or depression during gestation to the behavioral outcome in the offspring. Here too, there is little consensus among the earlier studies. For example, low birth weight, increased infant anxiety, and fear of novelty were associated with stress at 28–30 (Wadhwa et al. 1993), 15–17, 27–28, and 37–38 weeks (Huizink et al. 2003) and at 18 and 32 weeks of gestation (O’Connor et al. 2002) . More recently, high maternal anxiety and elevated cortisol early in pregnancy were shown to be associated with a deleterious effect on infant cognitive development , while those occurring towards the end of pregnancy were associated with improved cognitive development (Davis and Sandman 2010) . Likewise, maternal anxiety at 12–22 weeks of pregnancy was a significant predictor of symptoms of attention deficit hyperactivity disorder (ADHD), aggressive and delinquent behavior, and anxiety in 8–9-year-old children (Van den Bergh and Marcoen 2004). Alterations in the reactivity of the HPA axis were found in adolescent boys and girls, but depressive symptoms, only in girls (Van den Bergh et al. 2008). More recently, this group has shown that prenatal maternal-state anxiety measured around the 16th week of gestation resulted in hyperactivity/inattention, emotional symptoms, problems with peer relationship, and social interaction, which were more prevalent in boys than in girls aged 5 years (Loomans et al. 2011) . Pregnancy-specific anxiety and a higher level of maternal cortisol measured at 20, 25, and 30 weeks were associated with increased anxiety in preadolescent children of both sexes (Davis and Sandman 2012). The fetal cortical and limbic systems develop during the first 10 weeks of pregnancy (Bayer et al. 1993) . It is therefore most probable that any changes in their programming by elevated cortisol, and the resulting effects on behavior, occur during that period. Cortisol levels may remain elevated as long as maternal anxiety and depression continue. If they increase only at a later stage of fetal brain development, the outcome may be different, and improvement in cognition can occur, as indicated in the study by Davis and Sandman (2010) .
2.1 Experimental Animals
In the rat, the HPA axis, cortex, and limbic systems develop from day 13 of gestation (Bayer et al. 1993) ; therefore, in most studies, stress was administered during the 3rd (last) week of pregnancy. When the rats were stressed randomly, thrice weekly by noise and flashing lights (Weinstock et al. 1988), or on alternate days throughout gestation (Takahashi et al. 1998), COR levels increased in the maternal and fetal blood after each stress. However, when the rats were subjected to noise and flashing lights once daily at the same time during the last week of gestation, COR no longer increased in the mother or fetuses by the 3rd day (Weinstock et al. 1988). Very few studies have assessed whether or not the rats adapted to the form of stress that was used. Varied short-acting stressors during the last week of gestation (Salomon et al. 2011), or psychosocial stress on days 16–20 (Brunton and Russell 2010), continued to increase plasma COR until the last day of stress. Adaptation to the stress after 2 or 3 days could partially explain the inconsistency in the behavioral data in the offspring when different stress paradigms were used.
In order to obtain direct evidence that maternal adrenal hormones mediate the alterations induced by gestational stress in the offspring, pregnant rats were adrenalectomized prior to the initiation of stress and given saline and maintenance levels of COR. This prevented the dysregulation of the response of the HPA axis to stress (Barbazanges et al. 1996) and the heightened anxiety and learning deficits in the offspring (Zagron and Weinstock 2006) . Administration of COR to the pregnant rats to mimic the increase induced by stress reinstated the altered response of the HPA axis to stress (Barbazanges et al. 1996) and the increased anxiety but did not restore the learning deficits in the offspring (Salomon et al. 2011). Thus, while glucocorticoids mediate the anxiety and impaired regulation of the HPA axis induced by prenatal stress, other adrenal hormones appear to be responsible for the genesis of learning deficits.
The slower return of COR to baseline levels in response to stress in PS rats (McCormick et al. 1995; Weinstock et al. 1992; Weinstock et al. 1998) results from reduced efficiency of the feedback mechanism because of downregulation of hippocampal glucocorticoid (Weinstock et al. 1992) and mineralocorticoid receptors (Barbazanges et al. 1996; Tamura et al. 2011) . Increased activation of the HPA axis in PS rats is also associated with a higher expression of CRF mRNA in the paraventricular nucleus (PVN) of the hypothalamus in females and a reduction in its corticotropin-releasing factor binding protein (CRF-BP) in males which is believed to limit the action of CRF (Zohar and Weinstock 2011) .
3 Anxiety and Depressive-Like Behavior in Rats
The heightened anxiety seen in infants of stressed, anxious, and depressed mothers is paralleled in 10-day-old PS infant rats detected by the increase in ultrasonic vocalizations in response to isolation from the mother (Laloux et al. 2012) . PS males are also more anxious than control rats, as indicated by the longer latency to emerge from a dark cage into a brightly lit box (Ward et al. 2000), or by avoidance of the center area in the field (Abe et al. 2007) . In 1988, following the description by Pellow and File (1986) of the use of the elevated plus maze (EPM) for detecting anxiolytic drugs, we used the test to demonstrate heightened anxiety in the offspring of both sexes of mothers subjected to unpredictable noise throughout gestation (Fride and Weinstock 1988) . This finding was replicated in both sexes after variable forms of stress during the last week of gestation by Richardson et al. (2006) and Zohar and Weinstock (2011) , but only in female offspring in a study by Schulz et al. (2011). When maternal stress consisted of thrice daily restraint, anxiety was detected in the EPM in which it was tested only in males (Baker et al. 2008; Estanislau and Morato 2005; Li et al. 2012; Vallee et al. 1997) , or was found selectively in males, but not in females (Zuena et al. 2008) or in neither sex (Richardson et al. 2006; Rimondini et al. 2003). Maternal psychosocial stress also produced conflicting results in male offspring. These were found to be either less anxious (Gotz and Stefanski 2007) or more anxious than controls (Brunton and Russell 2010) .
The disparate effect of prenatal stress demonstrated in these studies may arise from the amount by which plasma COR increased in response to the stress, and if this remained elevated during the period of the development of the limbic system. They could also result from the environmental conditions in which anxiety was assessed in the offspring, as demonstrated in the following experiment. Offspring of control mothers and those subjected to varied stress from day 14 of gestation were tested in the EPM under bright light, under dim light, or were housed from weaning under a reversed light cycle and tested under red light during the active phase of their cycle (Fig. 1.1). No difference was detected in the behavior of PS and controls of either sex in the EPM under bright light since the controls spent relatively little time in the open arms of the maze. However, under dim light, rats of both sexes ventured more into the open arms and a significant anxiogenic effect was detected only in females. In the third group, male and female controls spent even more time in the open arms, enabling clearer detection of anxiety in PS rats. A similar difference between PS and controls was also reported by others who assessed behavior in rats housed under reversed light (Brunton and Russell 2010; Zohar and Weinstock 2011) .
4 Neurochemical and Structural Changes Associated with Increased Anxiety Induced by Prenatal Stress
The amygdala plays a primary role in the formation and storage of memories associated with emotional events that are imprinted in the lateral nuclei. Anxious behavior is elicited through connections between the lateral nuclei, the central nucleus of amygdala (CeA), and the bed nuclei of the stria terminalis. The volume of the amygdala is increased in children with generalized anxiety disorders (De Bellis et al. 2000) or in those born to women with anxiety and depression during pregnancy (Buss et al. 2012) , as shown by structural magnetic resonance imaging. In a study performed only in male offspring, prenatal stress caused an increase in the volume of the lateral amygdaloid nucleus which contained more neurons than that of controls (Salm et al. 2004). The CeA is a major extra hypothalamic site of CRF expression (Merchenthaler et al. 1982) . Injection of CRF into the amygdala was shown to induce anxiogenic behavior (Gray and Bingaman 1996), while administration of a CRF receptor antagonist into the brain selectively reduced the heightened anxiety of PS males in the cage emergence test (Ward et al. 2000). In keeping with their anxiogenic behavior, we showed that PS males and females had higher levels of CRF protein in amygdala extracts (Fig. 1.2). In comparison with controls, PS males also had a reduction in the levels of CRF-BP mRNA and of CRF 2 receptors (CRFR2) mRNA (Zohar and Weinstock 2011) , the activation of which reduces anxiety . The increase in CRF protein in the amygdala in PS rats could have resulted from excess COR activity via glucocorticoid receptors which are expressed in the CeA during development (Honkaniemi et al. 1992) . Maternal psychological stress that only increased anxiety in the male offspring also reduced the expression of CRFR2 and increased that of CRF 1 receptors (CRFR1), which mediates anxiety, in the medial nucleus of the amygdala of males (Brunton et al. 2011) .
5 Alterations in Cognitive Function and Spatial Learning Induced by Prenatal Stress in Humans and Rats
5.1 Humans
Cortical neurogenesis in humans occurs between gestational weeks 6 and 16 (Sidman and Rakic 1973) and neuronal migration and synaptogenesis leading to distinguishable cortical layers between weeks 24 and 26 (Meyer et al. 2000) .
It has been shown that prenatal stress affects cognitive abilities in humans from infancy to adulthood. Exposure of mothers to the severe stress of a freezing ice storm in Canada resulted in some degree of cognitive retardation in their 2-year-old children, while exposure to moderate levels of stress enhanced development of their cognitive ability (DiPietro et al. 2006) . At 5.5 years of age, severe stress exposure resulted in lower full-scale IQs and language abilities (Laplante et al. 2008) . Likewise, others found that high maternal antenatal anxiety , but not low or moderate levels, was associated with poorer performance of 17-year-old adolescent offspring when the cognitive load of the task was increased (Mennes et al. 2006) . Young adults whose mothers had experienced major adverse life events during pregnancy used a more rigid strategy to solve a navigational task that depended on the caudate nucleus, in contrast to the flexible, hippocampus-based one used by unstressed individuals (Schwabe et al. 2012). These studies did not provide any information about any possible sex differences in the effect of prenatal stress on cognitive performance. Nevertheless, data accumulated so far show that adverse life events may have permanent effects on cognitive function and on the way in which spatial problems are solved. The outcome of maternal stress appears to depend on its intensity and the time during pregnancy of its occurrence as confirmed by studies in experimental animals described below.
5.2 Rats
In the rat, neurogenesis begins in various cortical regions on day 14 and continues until birth on day 21; in hippocampal fields CA 1–3, it starts on day 15, and in the granule cells of the dentate gyrus (DG) on day 19, continuing until postnatal day 19 (Rice and Barone 2000). There are clear sex differences in normal brain morphology in rats, particularly in the hippocampus, both during development (Munoz-Cueto et al. 1990) and in adulthood (Andrade et al. 2000; Madeira and Lieberman 1995; see below). As in humans (Newhouse et al. 2007; Sneider et al. 2011) , sex differences underlie the performance of spatial learning in rodents and are related to different strategies used by each sex (Sandstrom et al. 1998). Several brain regions may participate in the execution of these strategies, including the medial prefrontal cortex (de Bruin et al. 2001) and the hippocampal formation. The latter is particularly involved in spatial navigation guided by distal cues to which females tend to respond better than males (Blokland et al. 2006) .
Most reports of the effect of prenatal stress on spatial memory are based on experiments in which pregnant rats were restrained once or thrice daily for periods ranging from 30 to 120 min during the last week of gestation when the cortical and hippocampal neurons develop. Spatial memory of their adult offspring was assessed in the Morris water maze (MWM) test. Unlike the effect of prenatal stress on anxiety , there was much more agreement between studies, with most of them reporting learning deficits in males (Hosseini-Sharifabad and Hadinedoushan 2007; Lemaire et al. 2000; Li et al. 2012; Lui et al. 2011; Salomon et al. 2011; Szuran et al. 2000; Zagron and Weinstock 2006) , and only one, selectively in females (Wu et al. 2007) . In another study in which control females performed the task less well than males, prenatal stress actually improved the performance of females (Zuena et al. 2008) . The relative immunity of adult PS females from impairment of spatial learning may result from the presence of estradiol which can increase neurogenesis, spine density, and their spatial performance (Gould et al. 1990; Phan et al. 2012) .
When the effect of varied forms of prenatal stress was examined in prepubertal or juvenile rats, spatial learning was selectively impaired in females (Li et al. 2008; Weinstock 2011) . However, the search strategy employed by both young PS male and female rats was shown to be less efficient than that of their respective controls (Wu et al. 2007). In adulthood, the same varied stress only caused spatial memory deficits in males (Weinstock 2011), possibly due to the positive influence of estradiol in females (Markham et al. 2010; Yaka et al. 2007) . In association with learning impairments in PS males, there was a decrease in hippocampal long-term potentiation (LTP) (Yaka et al. 2007; Yang et al. 2006) and in the expression of the NR2B subunit of the glutamate-type N-methyl-d-aspartate (NMDA) receptor (Lui et al. 2011; Yaka et al. 2007) and in the GluR1 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor (Yaka et al. 2007).
Attempts have been made to relate alterations induced by prenatal stress in spatial learning to those in hippocampal morphology. In accordance with the selective effect on learning, we found a reduction in dendritic length, complexity, and number of spines in the DG in juvenile PS females. In the hippocampal CA1 and CA3 regions, the length and complexity of apical dendrites were decreased in both sexes (Bock et al. 2011) . There do not appear to be any studies on the effect of prenatal stress on dendritic morphology in adult females. However, in adult males, a reduction was found in the complexity of dendrites and their length in the hippocampal CA3 region after once daily maternal restraint of 1 h (Hosseini-Sharifabad and Hadinedoushan 2007) and in the CA1, CA3, and DG after maternal restraint of 2 h (Fujioka et al. 2006) . By contrast, as in humans (DiPietro et al. 2006) , mild maternal restraint stress, which consisted in rats of only 30 min, improved cognitive function (Fujioka et al. 2001) , and increased dendritic length in the CA1 and DG and dendritic complexity in the CA3 region in PS males (Fujioka et al. 2006).
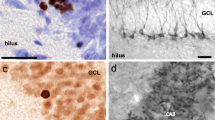
Neurogenesis in the subgranular zone (SGZ) of the DG of the hippocampus continues throughout life and plays an important role in cognition (Abrous et al. 2005) . When measured by the incorporation of cells labeled with 5-bromo-2ʹ-deoxyuridine (BrdU), neurogenesis was decreased in the DG of male PS rats that showed learning deficits (Lemaire et al. 2000) . When the same restraint stress used by this group failed to induce changes in spatial learning, BrdU was not decreased significantly in males in the dorsal hippocampus (that is involved in spatial learning) but only in the ventral part in females associated with increased anxiety (Zuena et al. 2008) . We used doublecortin (DCX), a reliable marker of newly generated neurons (Rao and Shetty 2004), and found that it was selectively reduced in the SGZ of the DG in adult males (Fig. 1.3). On the other hand, GAP 43, a protein that positively influences axonal guidance and synaptic plasticity, was increased in PS females compared to that in controls (Fig. 1.3). Prenatal stress also diminished neurogenesis in the DG of monkeys but no information was given about their spatial learning ability (Coe et al. 2003) .
Behavior in the elevated plus maze of stressed and control mothers with and without citalopram treatment. White column: control mothers; black column: stressed mothers; hatched column: controls with citalopram (10 mg/kg/day); cross-hatched column: stressed with citalopram (10 mg/kg/day). Significantly different from controls; *p < 0.05; significantly different from stressed # p < 0.05
Prenatal stress has been shown to reduce the size of the anogenital distance in prepubertal males (Holson et al. 1995; Pereira et al. 2006; Salomon et al. 2011) , a sign of a relative lack of testosterone. If the amounts of testosterone synthesized by their hippocampal neurons are also reduced in PS males, it would adversely affect their synaptic plasticity (Ooishi et al. 2012) and could explain their greater susceptibility to learning and memory deficits. In summary, the majority of studies support a selective reduction in spatial learning by prenatal stress in adult males but not females. This is associated with a reduction of dendritic length, complexity, and spines in the DG, and of neurogenesis in the SGZ which may occur through a relative lack of testosterone at a critical time during development.
6 Contribution of Pre- and Postnatal Factors to Human Offspring Behavioral Pathology
A clear association was found between the presence of anxiety disorder during pregnancy and depressive symptoms after birth, which together affected the outcome, increasing the incidence of separation anxiety disorder and ADHD in the offspring (Martini et al. 2010) . It was also shown that maternal anxiety before birth was also associated with anxiety and depression during and after pregnancy and resulted in anxiety in the children, making it impossible to differentiate genetic personality traits from pregnancy-related stress in this outcome (Martini et al. 2010) . In an attempt to assess the relative contributions of prenatal and postnatal maternal anxiety, a full range of child psychopathology and functioning was assessed in over 3000 mother–child pairs. Maternal depression was found to have a more significant impact on different types of child maladjustment than maternal anxiety in either the prenatal and postnatal periods. Internalizing difficulties in the child were linked to postnatal depression, while externalizing difficulties and impaired verbal IQ were associated with adverse prenatal factors, like low socioeconomic status and substance abuse, but not with maternal depression (Barker et al. 2011) . Since smoking and drug abuse are themselves risk factors for infant pathology, it would be important in future studies to separate adverse factors associated with stress from depression and drug abuse in determining their impact on child development and behavior.
7 Experiments Differentiating Pre- and Postnatal Effects of Maternal Stress in Rats
7.1 Maternal Behavior
In experimental studies in rats, a clear separation can be made between the influence of prenatal and postnatal factors wih no confounds due to drug intake by the use of cross-fostering of pups from a stressed onto a control mother and vice versa. Thus, we found that chronic variable stress during the last week of pregnancy increased the anxiogenic behavior of stressed mothers in the EPM test measured 2 days after their pups are weaned (Fig. 1.4), and this may reduce their maternal behavior towards their pups (Moore and Power 1986; Power and Moore 1986; Smith et al. 2004) . This may have resulted from excess levels of maternal COR released in response to stress since diminished maternal care was also seen when COR was administered during pregnancy (Brummelte and Galea 2010) . The presence (Smith et al. 2004) or absence (Poltyrev and Weinstock 1999) of an alteration in maternal care appears to depend on the magnitude of the increase in COR and its duration (Brummelte and Galea 2010).
Effect of prenatal stress on neurogenesis and synaptic plasticity in the dentate gyrus of the hippocampus. a Represents the % area of immunoreactivity in cells labeled with an antibody to doublecortin. b Represents the intensity of staining with an antibody to GAP43. Significantly different from controls; *p <0.05; **p<0.01
7.2 Cross-fostering
Fostering PS pups onto control mothers reduced their anxiety in the EPM test but increased anxiety in controls pups reared by a stressed mother (Barros et al. 2006) . This testifies to the influence of postnatal factors in the etiology of anxiety in the offspring. The fostering procedure of PS rats onto control mothers also normalized the activity of the HPA axis of the males and prevented the downregulation of glucocorticoid receptors (Maccari et al. 1995) . In contrast to the amelioration of anxiety, rearing by a control foster mother had no effect on the learning deficits or the reduction in LTP in PS males (Yang et al. 2006) . This indicated that the effect on learning and synaptic plasticity is prenatally mediated.
It has been shown that the anxiety and abnormal regulation of the HPA axis, but not the learning deficits, in PS rats are brought about by raised levels of maternal COR (Barbazanges et al. 1996; Salomon et al. 2011) . Chronic maternal stress was shown to increase COR in the mothers’ milk for periods of up to 3 weeks (Pfister and Muir 1989) . Rearing of PS pups by control mothers would also prevent their exposure to such raised levels of COR. The finding that heightened anxiety in PS rats is associated with the quality of postnatal maternal mood, and rearing ability accords well with the findings in human subjects. The closer association between maternal stress during the prenatal period and the cognitive outcome is also in agreement with the human data (Laplante et al. 2008) .
7.3 Rearing in an Enriched Environment
Environmental enrichment (EE) consists in modifying the rat’s housing conditions to provide enhanced sensory motor and cognitive stimulation. This is probably only significant for laboratory rats that are normally housed in small cages with no source of stimulation or little room for movement. EE has been shown to increase total brain weight (Wainwright et al. 1993) and the number of dendritic branches in the hippocampus (Greenough and Volkmar 1973) . Housing PS male rats in an EE from weaning restored the response of the HPA axis to stress to that in control rats (Morley-Fletcher et al. 2003) and reduced their anxiety (Li et al. 2012) . In contrast to the lack of effect of fostering, an EE also normalized the spatial performance of PS rats in the MWM test, and hippocampal LTP (Lui et al. 2011; Yang et al. 2007) . However, the reduction in social interaction in adolescent and adult male PS rats, which was associated with a decrease in oxytocin in the PVN and was restored to that of controls by injection of oxytocin into the amygdala , was not improved by EE (Lee et al. 2007) . Others housed the pregnant rats themselves in an EE while they were stressed and found that their male offspring showed less anxiety , performed like controls in the MWM, and had an increase in spine density in the hippocampal CA1 and DG regions (Li et al. 2012) . It is not yet known if maternal housing in an EE would prevent the reduction in social interaction induced by prenatal stress. The mechanism by which EE reverses some of the effects of prenatal stress is not clear, but it may occur through stimulation of neurogenesis and formation of more dendritic spines to overcome their loss in PS rats.
8 Effect of Antidepressant Treatment
During ontogenesis, 5-HT serves as a developmental signal for both serotonergic neurons and target tissues (Lauder 1990) . In the mature brain, 5-HT acts as a neurotransmitter and also modulates neuronal function and plasticity (Lesch 2001) . In order to exert its proper function during development, serotonin must be present in various brain regions in optimal concentrations which are controlled through the levels of 5-HT synthesis and metabolism. The level of 5-HT in the brain is largely regulated via its reuptake through the serotonin transporter (SERT) which appears in humans and rats during midgestation (Daws and Gould 2011) . Alterations in serotoninergic activity are believed to occur in subjects with depression (Ressler and Nemeroff 2000).
8.1 Humans
Selective serotonin reuptake inhibitors (SSRIs), fluoxetine, paroxetine, and citalopram, are most frequently prescribed antidepressants for maternal depression because they are generally considered to cause fewer adverse effects than the older tricyclic antidepressants (Cipriani et al. 2005; Westenberg and Sandner 2006) . The number of anxious and depressed pregnant women using these medications varies from 5 to 20 % (Marcus 2009; Moses-Kolko and Roth 2004; Nordeng et al. 2012) . A higher incidence of preterm births (Hayes et al. 2012; Klieger-Grossmann et al. 2012) , autism (Croen et al. 2011), irritability (Thormahlen 2006), and lower psychomotor development index (Casper et al. 2003) has been reported in infants of mothers treated with SSRIs, but these symptoms are also seen in those of untreated subjects with depression (Louik et al. 2007) . Others were able to differentiate an influence of SSRIs from that of depression on neuronal function. Infants from untreated depressed mothers had significantly lower attention scores than those of nondepressed mothers, while those of drug-treated mothers had a lower gestational age, more hypertonia, and a higher number of central nervous system stress signs than those of either untreated mothers or controls (Salisbury et al. 2011).
Only two studies have compared the effect on behavior of the children of depressed mothers with and without SSRI treatment. In a relatively small group of women (22) given either paroxetine, sertraline, or fluoxetine, no difference was found in maternal mood or the incidence of behavioral abnormalities in 4-year-old children of treated and untreated mothers (Oberlander et al. 2007) . In a larger group, depressed mothers were either untreated or given venlafaxine, sertraline, paroxetine, fluoxetine, or citalopram in the first semester or throughout pregnancy. There was a reduction in the number of women who experienced a depressive episode in the 1st year following childbirth in those receiving venlafaxine but not the other drugs. However, irrespective of drug treatment and maternal outcome, their children aged 3, 6, and 12 years had significantly higher rates of poor neonatal adaptation, problematic externalizing and internalizing behaviors, and lower verbal and performance IQs than those of nondepressed mothers (Nulman et al. 2012) . Thus, there is no evidence that treatment of pregnant women with these drugs produces any benefit in terms of the behavioral outcome in the children.
8.2 Rats
Administration of citalopram (10 mg/kg/day) to pregnant rats from day 7 of gestation 1 week before commencement of stress, until the pups were weaned, reduced the anxiogenic behavior of stressed mothers but had no effect on that of unstressed mothers (Fig. 1.4). However, like SSRI treatment in depressed pregnant women (Nulman et al. 2012), citalopram did not ameliorate anxiogenic behavior of PS male offspring or their spatial learning deficits. When given to control mothers, citalopram induced learning deficits in their offspring (Fig. 1.5). Maternal administration of fluoxetine from gestational day 11 to unstressed mothers resulted in a reduction in social play behavior in their juvenile offspring and anxiety at adulthood (Olivier et al. 2011) . However, when fluoxetine was administered to rat mothers after parturition, anxiogenic behavior of the PS male offspring was decreased and there was no increased anxiety in controls (Rayen et al. 2011). In addition, fluoxetine treatment restored the suppressed neurogenesis in the SGZ of the DG of PS males and females to that in controls. Imipramine or fluoxetine given chronically to PS males in adulthood reduced their anxiety in the open field and the levels of COR and glucocorticoid receptor in response to stress (Szymanska et al. 2009). How can one explain the difference in outcome when SSRIs are given pre- or postnatally? Prenatal administration of SSRIs inhibits the SERT during a critical period of neuron development and exposes the brain to excess amounts of 5-HT, as also shown in SERT knockout mice (Borue et al. 2007) . Once the serotoninergic systems are fully developed and neuronal guidance is complete, the actions of SSRIs on abnormal behavior result from readjustment of alterations induced by prenatal stress in pre- and postsynaptic 5-HT receptor activation. Taken together, the data from experiments in human subjects and rats suggest that treatment of pregnant mothers with SSRIs may improve their depressed mood in some subjects. However, SSRIs can adversely affect neuronal guidance and the development of serotoninergic systems in the offspring by inhibiting the SERT at a critical time during development.
Effect of maternal stress and citalopram treatment on spatial learning in the Morris water maze of adult male offspring. Open circles: controls, mothers untreated; closed circles: prenatally stressed, mothers untreated; open triangles: controls, mothers treated with citalopram (10 mg/kg/day); closed triangles: prenatally stressed, mothers treated with citalopram (10 mg/kg/day). Significantly different from controls; *p < 0.05; significantly different from prenatally stressed # p < 0.05
Conflict of Interest
The author declares no conflicts of interest.
Abbreviations
- ADHD:
-
Attention deficit hyperactivity disorder
- BrdU:
-
5-bromo-2ʹ-deoxyuridine
- CeA:
-
Central nucleus of the amygdala
- COR:
-
Corticosterone
- CRF:
-
Corticotropin-releasing factor
- CRF-BP:
-
Corticotropin-releasing factor binding protein
- CRFR1:
-
Corticotropin-releasing factor receptor 1
- CRFR2:
-
Corticotropin-releasing factor receptor 2
- DG:
-
Dentate gyrus
- DCX:
-
Doublecortin
- EE:
-
Environmental enrichment
- EPM:
-
Elevated plus maze
- HPA:
-
Hypothalamic pituitary adrenal
- 11β-HSD-2:
-
11β-hydroxy steroid dehydrogenase-2
- MWM:
-
Morris water maze
- PS:
-
Prenatally stressed
- SERT:
-
Serotonin transporter
- SSRI:
-
Selective serotonin reuptake inhibitor
References
Abe H, Hidaka N, Kawagoe C, Odagiri K, Watanabe Y, Ikeda T, Ishizuka Y, Hashiguchi H, Takeda R, Nishimori T, Ishida Y (2007) Prenatal psychological stress causes higher emotionality, depression-like behavior, and elevated activity in the hypothalamo-pituitary-adrenal axis. Neurosci Res 59(2):145–151. doi:10.1016/j.neures.2007.06.1465
Abrous DN, Koehl M, Le Moal M (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85(2):523–569. doi:10.1152/physrev.00055.2003
Alonso SJ, Arevalo R, Afonso D, Rodriguez M (1991) Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiol Behav 50(3):511–517
Andrade JP, Madeira MD, Paula-Barbosa MM (2000) Sexual dimorphism in the subiculum of the rat hippocampal formation. Brain Res 875(1-2):125–137
Baibazarova E, van de Beek C, Cohen-Kettenis PT, Buitelaar J, Shelton KH, van Goozen SH (2012) Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology 38(6):907–915. doi:10.1016/j.psyneuen.2012.09.015
Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C (2008) Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res 1213:98–110. doi:10.1016/j.brainres.2008.03.035
Barbazanges A, Piazza PV, Le Moal M, Maccari S (1996) Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 16(12):3943–3949
Barker ED, Jaffee SR, Uher R, Maughan B (2011) The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depress Anxiety 28(8):696–702. doi:10.1002/da.20856
Barros VG, Rodriguez P, Martijena ID, Perez A, Molina VA, Antonelli MC (2006) Prenatal stress and early adoption effects on benzodiazepine receptors and anxiogenic behavior in the adult rat brain. Synapse 60(8):609–618. doi:10.1002/syn.20336
Bayer SA, Altman J, Russo RJ, Zhang X (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14(1):83–144
Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V (2007) Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry 46(11):1454–1463. doi:10.1097/chi.0b013e31814a62f6
Blokland A, Rutten K, Prickaerts J (2006) Analysis of spatial orientation strategies of male and female Wistar rats in a Morris water escape task. Behav Brain Res 171(2):216–224. doi:10.1016/j.bbr.2006.03.033
Bock J, Murmu MS, Biala Y, Weinstock M, Braun K (2011) Prenatal stress and neonatal handling induce sex-specific changes in dendritic complexity and dendritic spine density in hippocampal subregions of prepubertal rats. Neuroscience 193:34–43. doi:10.1016/j.neuroscience.2011.07.048
Borue X, Chen J, Condron BG (2007) Developmental effects of SSRIs: lessons learned from animal studies. Int J Dev Neurosci 25(6):341–347. doi:10.1016/j.ijdevneu.2007.06.003
Brummelte S, Galea LA (2010) Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav 58(5):769–779. doi:10.1016/j.yhbeh.2010.07.012
Brunton PJ, Russell JA (2010) Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol 22(4):258–271. doi:10.1111/j.1365-2826.2010.01969.x
Brunton PJ, Donadio MV, Russell JA (2011) Sex differences in prenatally programmed anxiety behaviour in rats: differential corticotropin-releasing hormone receptor mRNA expression in the amygdaloid complex. Stress 14(6):634–643. doi:10.3109/10253890.2011.604750
Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA (2012) Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A 109(20):E1312–E1319. doi:10.1073/pnas.1201295109
Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE (2003) Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 142(4):402–408. doi:10.1067/mpd.2003.139
Cipriani A, Brambilla P, Furukawa T, Geddes J, Gregis M, Hotopf M, Malvini L, Barbui C (2005) Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst Rev (4):CD004185. doi:10.1002/14651858.CD004185.pub2
Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E (2003) Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 54(10):1025–1034
Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V (2011) Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 68(11):1104–1112. doi:10.1001/archgenpsychiatry.2011.73
Davis EP, Sandman CA (2010) The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev 81(1):131–148. doi:10.1111/j.1467-8624.2009.01385.x
Davis EP, Sandman CA (2012) Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology 37(8):1224–1233. doi:10.1016/j.psyneuen.2011.12.016
Daws LC, Gould GG (2011) Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther 131(1):61–79. doi:10.1016/j.pharmthera.2011.03.013
De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan ND (2000) A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry 48(1):51–57
de Bruin JP, Moita MP, de Brabander HM, Joosten RN (2001) Place and response learning of rats in a Morris water maze: differential effects of fimbria fornix and medial prefrontal cortex lesions. Neurobiol Learn Mem 75(2):164–178. doi:10.1006/nlme.2000.3962
Delcour M, Russier M, Amin M, Baud O, Paban V, Barbe MF, Coq JO (2012) Impact of prenatal ischemia on behavior, cognitive abilities and neuroanatomy in adult rats with white matter damage. Behav Brain Res 232(1):233–244. doi:10.1016/j.bbr.2012.03.029
DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP (2006) Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev 77(3):573–587. doi:10.1111/j.1467-8624.2006.00891.x
Estanislau C, Morato S (2005) Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behav Brain Res 163(1):70–77. doi:10.1016/j.bbr.2005.04.003
Evans LM, Myers MM, Monk C (2008) Pregnant women’s cortisol is elevated with anxiety and depression-but only when comorbid. Arch Womens Ment Health 11(3):239–248. doi:10.1007/s00737-008-0019-4
Fan JM, Chen XQ, Jin H, Du JZ (2009) Gestational hypoxia alone or combined with restraint sensitizes the hypothalamic-pituitary-adrenal axis and induces anxiety-like behavior in adult male rat offspring. Neuroscience 159(4):1363–1373. doi:10.1016/j.neuroscience.2009.02.009
Fride E, Weinstock M (1988) Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci 42(10):1059–1065
Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S (2006) Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience 141(2):907–915. doi:10.1016/j.neuroscience.2006.04.046
Fujioka T, Fujioka A, Tan N, Chowdhury GM, Mouri H, Sakata Y, Nakamura S (2001) Mild prenatal stress enhances learning performance in the non-adopted rat offspring. Neuroscience 103(2):301–307
Giardinelli L, Innocenti A, Benni L, Stefanini MC, Lino G, Lunardi C, Svelto V, Afshar S, Bovani R, Castellini G, Faravelli C (2012) Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health 15(1):21–30. doi:10.1007/s00737-011-0249-8
Gotz AA, Stefanski V (2007) Psychosocial maternal stress during pregnancy affects serum corticosterone, blood immune parameters and anxiety behaviour in adult male rat offspring. Physiol Behav 90(1):108–115. doi:10.1016/j.physbeh.2006.09.014
Gould E, Woolley CS, Frankfurt M, McEwen BS (1990) Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10(4):1286–1291
Gray TS, Bingaman EW (1996) The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol 10(2):155–168
Greenough WT, Volkmar FR (1973) Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol 40(2):491–504
Hayes RM, Wu P, Shelton RC, Cooper WO, Dupont WD, Mitchel E, Hartert TV (2012) Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol 207(1):49e41–e49. doi:10.1016/j.ajog.2012.04.028
Holson RR, Gough B, Sullivan P, Badger T, Sheehan DM (1995) Prenatal dexamethasone or stress but not ACTH or corticosterone alter sexual behavior in male rats. Neurotoxicol Teratol 17(4):393–401
Honkaniemi J, Pelto-Huikko M, Rechardt L, Isola J, Lammi A, Fuxe K, Gustafsson JA, Wikstrom AC, Hokfelt T (1992) Colocalization of peptide and glucocorticoid receptor immunoreactivities in rat central amygdaloid nucleus. Neuroendocrinology 55(4):451–459
Hosseini-Sharifabad M, Hadinedoushan H (2007) Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. Anat Sci Int 82(4):211–217. doi:10.1111/j.1447-073X.2007.00186.x
Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK (2003) Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry 44(6):810–818
Husain N, Cruickshank K, Husain M, Khan S, Tomenson B, Rahman A (2012) Social stress and depression during pregnancy and in the postnatal period in British Pakistani mothers: a cohort study. J Affect Disord 140(3):268–276. doi:10.1016/j.jad.2012.02.009
Imamura Y, Nakane Y, Ohta Y, Kondo H (1999) Lifetime prevalence of schizophrenia among individuals prenatally exposed to atomic bomb radiation in Nagasaki City. Acta Psychiatr Scand 100(5):344–349
Jansson T, Powell TL (2007) Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 113(1):1–13. doi:10.1042/CS20060339
Jensen Pena C, Monk C, Champagne FA (2012) Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One 7 (6):e39791. doi:10.1371/journal.pone.0039791
Keck ME (2006) Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino Acids 31(3):241–250. doi:10.1007/s00726-006-0333-y
Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB (2008) Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry 65(2):146–152. doi:10.1001/archgenpsychiatry.2007.20
Klieger-Grossmann C, Weitzner B, Panchaud A, Pistelli A, Einarson T, Koren G, Einarson A (2012) Pregnancy outcomes following use of escitalopram: a prospective comparative cohort study. J Clin Pharmacol 52(5):766–770. doi:10.1177/0091270011405524
Koenig JI, Kirkpatrick B, Lee P (2002) Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology 27(2):309-318. doi:10.1016/S0893-133x(01)00396–7
Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL (2005) Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res 156(2):251–261. doi:10.1016/j.bbr.2004.05.030
Kofman O (2002) The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev 26(4):457–470
Laloux C, Mairesse J, Van Camp G, Giovine A, Branchi I, Bouret S, Morley-Fletcher S, Bergonzelli G, Malagodi M, Gradini R, Nicoletti F, Darnaudery M, Maccari S (2012) Anxiety-like behaviour and associated neurochemical and endocrinological alterations in male pups exposed to prenatal stress. Psychoneuroendocrinology 37(10):1646–1658. doi:10.1016/j.psyneuen.2012.02.010
Laplante DP, Brunet A, Schmitz N, Ciampi A, King S (2008) Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry 47(9):1063–1072. doi:10.1097/CHI.0b013e31817eec80
Lauder JM (1990) Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci 600:297–313; discussion 314
Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI (2007) Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res 1156:152–167. doi:10.1016/j.brainres.2007.04.042
Lemaire V, Koehl M, Le Moal M, Abrous DN (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A 97(20):11032–11037
Lereya ST, Wolke D (2012) Prenatal family adversity and maternal mental health and vulnerability to peer victimisation at school. J Child Psychol Psychiatry. doi:10.1111/jcpp.12012
Lesch KP (2001) Variation of serotonergic gene expression: neurodevelopment and the complexity of response to psychopharmacologic drugs. Eur Neuropsychopharmacol 11(6):457–474
Li H, Li X, Jia N, Cai Q, Bai Z, Chen R, Song T, Zhu Z, Liu J (2008) NF-kappaB regulates prenatal stress-induced cognitive impairment in offspring rats. Behav Neurosci 122(2):331–339. doi:10.1037/0735-7044.122.2.331
Li M, Wang M, Ding S, Li C, Luo X (2012) Environmental enrichment during gestation improves behavior consequences and synaptic plasticity in hippocampus of prenatal-stressed offspring rats. Acta Histochem Cytochem 45(3):157–166. doi:10.1267/ahc.11054
Loomans EM, van der Stelt O, van Eijsden M, Gemke RJ, Vrijkotte T, den Bergh BR (2011) Antenatal maternal anxiety is associated with problem behaviour at age five. Early Hum Dev 87(8):565–570. doi:10.1016/j.earlhumdev.2011.04.014
Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA (2007) First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. New Engl J Med 356(26):2675–2683. doi:10.1056/NEJMoa067407
Lui CC, Wang JY, Tain YL, Chen YC, Chang KA, Lai MC, Huang LT (2011) Prenatal stress in rat causes long-term spatial memory deficit and hippocampus MRI abnormality: differential effects of postweaning enriched environment. Neurochem Int 58(3):434–441. doi:10.1016/j.neuint.2011.01.002
Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M (1995) Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci 15(1 Pt 1):110–116
Madeira MD, Lieberman AR (1995) Sexual dimorphism in the mammalian limbic system. Prog Neurobiol 45(4):275–333
Marcus SM (2009) Depression during pregnancy: rates, risks and consequences-Motherisk Update 2008. Can J Clin Pharmacol 16(1):e15-e22
Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI (2010) Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci 4:173. doi:10.3389/fnbeh.2010.00173
Martini J, Knappe S, Beesdo-Baum K, Lieb R, Wittchen HU (2010) Anxiety disorders before birth and self-perceived distress during pregnancy: associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Hum Dev 86(5):305–310. doi:10.1016/j.earlhumdev.2010.04.004
McCormick CM, Smythe JW, Sharma S, Meaney MJ (1995) Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res 84(1):55–61
Mennes M, Stiers P, Lagae L, Van den Bergh B (2006) Long-term cognitive sequelae of antenatal maternal anxiety: involvement of the orbitofrontal cortex. Neurosci Biobehav Rev 30(8):1078–1086. doi:10.1016/j.neubiorev.2006.04.003
Merchenthaler I, Vigh S, Petrusz P, Schally AV (1982) Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat 165(4):385–396. doi:10.1002/aja.1001650404
Meyer G, Schaaps JP, Moreau L, Goffinet AM (2000) Embryonic and early fetal development of the human neocortex. J Neurosci 20(5):1858–1868
Miszkurka M, Goulet L, Zunzunegui MV (2012) Antenatal depressive symptoms among Canadian-born and immigrant women in Quebec: differential exposure and vulnerability to contextual risk factors. Soc Psychiatry Psychiatr Epidemiol 47(10):1639–1648. doi:10.1007/s00127-011-0469-2
Moore CL, Power KL (1986) Prenatal stress affects mother-infant interaction in Norway rats. Dev Psychobiol 19(3):235–245. doi:10.1002/dev.420190309
Morley-Fletcher S, Rea M, Maccari S, Laviola G (2003) Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci 18(12):3367–3374
Morley-Fletcher S, Darnaudery M, Mocaer E, Froger N, Lanfumey L, Laviola G, Casolini P, Zuena AR, Marzano L, Hamon M, Maccari S (2004) Chronic treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT1A receptor mRNA in prenatally stressed rats. Neuropharmacology 47(6):841–847. doi:10.1016/j.neuropharm.2004.06.011
Moses-Kolko EL, Roth EK (2004) Antepartum and postpartum depression: healthy mom, healthy baby. J Am Med Womens Assoc 59(3):181–191
Munoz-Cueto JA, Garcia-Segura LM, Ruiz-Marcos A (1990) Developmental sex differences and effect of ovariectomy on the number of cortical pyramidal cell dendritic spines. Brain Res 515(1-2):64–68
Newhouse P, Newhouse C, Astur RS (2007) Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res 183(1):1–7. doi:10.1016/j.bbr.2007.05.011
Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M (2012) Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol 32(2):186–194. doi:10.1097/JCP.0b013e3182490eaf
Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D, Feldman B (2012) Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry 169(11):1165–1174. doi:10.1176/appi.ajp.2012.11111721
O’Connor TG, Heron J, Golding J, Beveridge M, Glover V (2002) Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon longitudinal study of parents and children. Br J Psychiatry 180:502–508
O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V (2012) Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology 37(6):818–826. doi:10.1016/j.psyneuen.2011.09.014
Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE (2007) Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 161(1):22–29. doi:10.1001/archpedi.161.1.22
Ohkawa T, Takeshita S, Murase T, Okinaga S, Arai K (1991) The effect of an acute stress in late pregnancy on hypothalamic catecholamines of the rat fetus. Nihon Sanka Fujinka Gakkai Zasshi 43(7):783–787
Olivier JD, Valles A, van Heesch F, Afrasiab-Middelman A, Roelofs JJ, Jonkers M, Peeters EJ, Korte-Bouws GA, Dederen JP, Kiliaan AJ, Martens GJ, Schubert D, Homberg JR (2011) Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology 217(3):419–432. doi:10.1007/s00213-011-2299-z
Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H (2012) Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol 131(1-2):37–51. doi:10.1016/j.jsbmb.2011.10.004
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24(3):525–529
Pereira OC, Bernardi MM, Gerardin DC (2006) Could neonatal testosterone replacement prevent alterations induced by prenatal stress in male rats? Life Sci 78(24):2767–2771. doi:10.1016/j.lfs.2005.10.035
Pfister HP, Muir JL (1989) Psychological stress and administered oxytocin during pregnancy: effect corticosterone and prolactin response in lactating rats. Int J Neurosci 45(1-2):91–99
Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E (2012) Low doses of 17beta-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37(10):2299–2309. doi:10.1038/npp.2012.82
Poltyrev T, Weinstock M (1999) Effect of gestational stress on maternal behavior in response to cage transfer and handling of pups in two strains of rat. Stress 3(1):85–95. doi:10.3109/10253899909001114
Poltyrev T, Gorodetsky E, Bejar C, Schorer-Apelbaum D, Weinstock M (2005) Effect of chronic treatment with ladostigil (TV-3326) on anxiogenic and depressive-like behaviour and on activity of the hypothalamic-pituitary-adrenal axis in male and female prenatally stressed rats. Psychopharmacology 181(1):118–125. doi:10.1007/s00213-005-2229-z
Power KL, Moore CL (1986) Prenatal stress eliminates differential maternal attention to male offspring in Norway rats. Physiol Behav 38(5):667–671
Qu Z, Tian D, Zhang Q, Wang X, He H, Zhang X, Huang L, Xu F (2012) The impact of the catastrophic earthquake in China’s Sichuan province on the mental health of pregnant women. J Affect Disord 136(1-2):117–123. doi:10.1016/j.jad.2011.08.021
Rao MS, Shetty AK (2004) Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19(2):234–246
Rayen I, van den Hove DL, Prickaerts J, Steinbusch HW, Pawluski JL (2011) Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS One 6(9):e24003. doi:10.1371/journal.pone.0024003
Ressler KJ, Nemeroff CB (2000) Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety 12(Suppl 1):2–19. doi:10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4
Reul JM, Holsboer F (2002) On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues Clin Neurosci 4(1):31–46
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(Suppl 3):511–533
Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL (2006) Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology 147(5):2506–2517. doi:10.1210/en.2005-1054
Rimondini R, Agren G, Borjesson S, Sommer W, Heilig M (2003) Persistent behavioral and autonomic supersensitivity to stress following prenatal stress exposure in rats. Behav Brain Res 140(1-2):75–80
Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM (2011) Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety 28(11):1008–1019. doi:10.1002/da.20883
Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL (2004) Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res 148(2):159–167. doi:10.1016/j.devbrainres.2003.11.005
Salomon S, Bejar C, Schorer-Apelbaum D, Weinstock M (2011) Corticosterone mediates some but not other behavioural changes induced by prenatal stress in rats. J Neuroendocrinol 23(2):118–128. doi:10.1111/j.1365-2826.2010.02097.x
Sandman CA, Davis EP, Buss C, Glynn LM (2011) Prenatal programming of human neurological function. Int J Pept 2011:837596. doi:10.1155/2011/837596
Sandstrom NJ, Kaufman J, Huettel SA (1998) Males and females use different distal cues in a virtual environment navigation task. Brain Res Cogn Brain Res 6(4):351–360
Schulz KM, Pearson JN, Neeley EW, Berger R, Leonard S, Adams CE, Stevens KE (2011) Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol Behav 104(2):340–347. doi:10.1016/j.physbeh.2011.02.021
Schwabe L, Bohbot VD, Wolf OT (2012) Prenatal stress changes learning strategies in adulthood. Hippocampus 22(11):2136–2143. doi:10.1002/hipo.22034
Sidman RL, Rakic P (1973) Neuronal migration, with special reference to developing human brain: a review. Brain Res 62(1):1–35
Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW (2004) Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 29(2):227–244
Sneider JT, Sava S, Rogowska J, Yurgelun-Todd DA (2011) A preliminary study of sex differences in brain activation during a spatial navigation task in healthy adults. Percept Mot Skills 113(2):461–480
Szuran TF, Pliska V, Pokorny J, Welzl H (2000) Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav 71(3-4):353–362
Szymanska M, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Kubera M, Leskiewicz M, Regulska M, Lason W (2009) The effect of antidepressant drugs on the HPA axis activity, glucocorticoid receptor level and FKBP51 concentration in prenatally stressed rats. Psychoneuroendocrinology 34(6):822–832. doi:10.1016/j.psyneuen.2008.12.012
Takahashi LK, Turner JG, Kalin NH (1998) Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology 23(6):571–581
Tamura M, Sajo M, Kakita A, Matsuki N, Koyama R (2011) Prenatal stress inhibits neuronal maturation through downregulation of mineralocorticoid receptors. J Neurosci 31(32):11505–11514. doi:10.1523/JNEUROSCI.3447-10.2011
Thormahlen GM (2006) Paroxetine use during pregnancy: is it safe? Ann Pharmacother 40(10):1834–1837. doi:10.1345/aph.1H116
Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S (1997) Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci 17(7):2626–2636
Van den Bergh BR, Marcoen A (2004) High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev 75(4):1085–1097. doi:10.1111/j.1467-8624.2004.00727.x
Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L (2008) Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology 33(3):536–545. doi:10.1038/sj.npp.1301450
Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ (1993) The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol 169(4):858–865
Wainwright PE, Levesque S, Krempulec L, Bulman-Fleming B, McCutcheon D (1993) Effects of environmental enrichment on cortical depth and Morris-maze performance in B6D2F2 mice exposed prenatally to ethanol. Neurotoxicol Teratol 15(1):11–20
Ward HE, Johnson EA, Salm AK, Birkle DL (2000) Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav 70(3-4):359–366
Weinstock M (1997) Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neurosci Biobehav Rev 21(1):1–10
Weinstock M (2001) Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 65(5):427–451
Weinstock M (2005) The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 19(4):296–308. doi:10.1016/j.bbi.2004.09.006
Weinstock M (2008) The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32(6):1073–1086. doi:10.1016/j.neubiorev.2008.03.002
Weinstock M (2011) Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update. Stress 14(6):604–613. doi:10.3109/10253890.2011.588294
Weinstock M, Fride E, Hertzberg R (1988) Prenatal stress effects on functional development of the offspring. Prog Brain Res 73:319–331. doi:10.1016/S0079-6123(08)60513−0
Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS (1992) Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res 595(2):195–200
Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R (1998) Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav 64(4):439–444
Westenberg HG, Sandner C (2006) Tolerability and safety of fluvoxamine and other antidepressants. Int J Clin Pract 60(4):482–491. doi:10.1111/j.1368-5031.2006.00865.x
Wilson CA, Schade R, Terry AV Jr (2012) Variable prenatal stress results in impairments of sustained attention and inhibitory response control in a 5-choice serial reaction time task in rats. Neuroscience 218:126–137. doi:10.1016/j.neuroscience.2012.05.040
Wu J, Song TB, Li YJ, He KS, Ge L, Wang LR (2007) Prenatal restraint stress impairs learning and memory and hippocampal PKCbeta1 expression and translocation in offspring rats. Brain Res 1141:205–213. doi:10.1016/j.brainres.2007.01.024
Yaka R, Salomon S, Matzner H, Weinstock M (2007) Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav Brain Res 179(1):126–132. doi:10.1016/j.bbr.2007.01.018
Yang J, Han H, Cao J, Li L, Xu L (2006) Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus 16(5):431–436. doi:10.1002/hipo.20181
Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L (2007) Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem 87(2):257–263. doi:10.1016/j.nlm.2006.09.001
Zagron G, Weinstock M (2006) Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res 175(2):323–328. doi:10.1016/j.bbr.2006.09.003
Zohar I, Weinstock M (2011) Differential effect of prenatal stress on the expression of corticotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. J Neuroendocrinol 23(4):320–328. doi:10.1111/j.1365-2826.2011.02117.x
Zuena AR, Mairesse J, Casolini P, Cinque C, Alema GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S (2008) Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One 3(5):e2170. doi:10.1371/journal.pone.0002170
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Weinstock, M. (2015). Changes Induced by Prenatal Stress in Behavior and Brain Morphology: Can They Be Prevented or Reversed?. In: Antonelli, M. (eds) Perinatal Programming of Neurodevelopment. Advances in Neurobiology, vol 10. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1372-5_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1372-5_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1371-8
Online ISBN: 978-1-4939-1372-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)