Abstract
Diffusion cell devices have made an enormous contribution to basic physiology and the understanding of the transport of substances between two compartments separated by a biological membrane. Although much of the earlier development of such devices was to characterize absorption and secretion in the gut using isolated sheets of gastrointestinal mucosa; diffusion chamber systems have been much more extensively used to measure the dermal absorption of drugs and chemicals using resected skin from animals and humans. The challenge for physiologists when using tissue ex vivo is normally the viability of the specimen, and how this may impact on the function it normally performs in vivo. Fortunately, with skin, the primary barrier to the entry of a substance from the external surface to the living epidermis is a non-viable compacted desquamed structure, the stratum corneum. This makes it a highly suitable tissue for in vitro chamber work. Dermal absorption is essentially the measurement of the mass of a substance transferred across the skin over a specific time period. The chamber device provides key components of the experiment, namely a fixed surface area, a rigid platform for surface dosing, and a seal between the donor and receptor chamber compartments. Its vertical orientation also allows each side of the skin to face different environmental conditions. Various modifications to the basic design of these devices have been developed to improve the prediction of dermal absorption in humans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
This chapter will cover aspects of skin diffusion cell design and how various types of devices have evolved over the years for the purpose of measuring the absorption of drugs and chemicals across the skin. Much of this relates to the author’s own experience, but to do justice to this topic, the historical aspects of flux chamber devices and adaptations to conventional designs, including a new generation of automated diffusion cell equipment will be covered. Recognising that the rest of this book covers the dermal absorption of compounds and formulated products used in the pharmaceutical sector where dermal exposure is intentional, the author has focused mainly on this area. However, much of the published work that utilizes skin diffusion cells and how they have evolved over the last 50 years actually comes from the area of human safety assessment of industrial chemicals, where in vitro methodology is used extensively for predicting dermal absorption in humans. Absorption data generated in these models is a key element of human risk assessment for a broad range of chemical products that come in contact with human skin, either for cosmetic purposes [30] or in the case of pesticides [7, 9] and industrial chemicals [40], during occupational exposure that may occur during manufacture or intended use. The basic chamber design is similar for the approved methods used across the different industrial sectors including the pharmaceutical industry.
2 Historical Perspective
Diffusion cell devices that use resected skin for estimating dermal absorption in man have been around for many decades. Of all the portals of entry to the human body, the skin is a relatively accessible organ to study in the laboratory. The skin from many animal species has been investigated in vitro using chamber approaches with the pig being probably the most extensively studied animal species as well as a widely accepted surrogate model for human skin [5, 32].
As far as the use of flux chambers as devices for measuring the transport of chemicals from external (environmental) compartments to internal (systemic) compartments goes, they have been used to provide the rigid structure to support a functional epithelial layer or some other form of biological barrier, such as the skin, in a situation where the exposed surface is fixed and of known engineered area, and each side of the biological specimen faces a different environmental or biological condition. The most commonly used chambers are designed to replicate the polarized nature of the skin, where the external surface faces an air environment and the internal surface faces the systemic circulation. However, much of the history of chamber devices actually comes from in vitro experiments using isolated mucosal sheets from the gastrointestinal tract, where ion transport and gut absorption (not dermal absorption) have been investigated in great detail for many decades. One of the classical chamber approaches with isolated gut was developed primarily by Hans Ussing in Denmark for the study of gastric ion transport using amphibian mucosa [41]. Much of the understanding on how the gastric mucosa transports hydrogen ions into the lumen of the stomach in order to create a pH gradient between the serosal side of the gastric mucosa and the luminal side came from the classical ‘Ussing’ chamber studies. These chamber systems were constructed using Lucite or Perspex, and usually had discs of gut mounted on pins to stretch and flatten the tissue over the circular exposed aperture of the chamber. This allowed a fixed surface area of tissue to be bathed with separate circulating media in the two halves of the chamber. Such chamber devices were used mainly in the pharmaceutical industry to study the effect of drugs on both mucosal secretion and absorption. These gut mucosal chambers were also used to characterize mucosal protection. One such device that the author developed in the 1980s was a dual mucosal chamber. This device incorporated two discs of gut orientated such that their blood sides were in contact with a common bathing solution. The luminal sides of the membranes were bathed with separate oxygenated solutions. This chamber device demonstrated that the control of bicarbonate secretion, the protective mechanism against acid autodigestion, was via a humoral mechanism, as acidification of one mucosa caused a protective secretory response of the other mucosa, despite the physical separation of the two tissue discs [16].
A major difference between gut chambers and skin chambers is the requirement for tissue viability. The isolated gut requires a constant oxygen flow to the media bathing the tissue as even transient anoxia will arrest the active transport processes fundamental to the function of the tissue. In contrast, the skin surface, the stratum corneum is a dead cornified layer, which can still serve its main function of a barrier to the passage of substance through it, in the absence of an oxygenated media. As such, the design of a typical gut and skin chambers is similar in several respects such as a fixed surface area for application of a measured dose of test substance and expression of flux or other parameter relating to the proportion of the substance that has moved to the alternate compartment over a measured time period. However, the most obvious difference is the orientation of the device. Generally, a gut chamber is horizontal with a tissue held vertically. In contrast, a skin chamber is vertical with the tissue held horizontally. The gut mucosa is held between two aqueous physiological environments to measure the transport between the compartments, whereas the skin has an upper air-exposed surface and a lower aqueous-exposed dermal side bathed by a receptor fluid. There are, of course, some exceptions where some skin chambers were designed horizontally, but controlling the receptor temperature and mixing makes this system inherently more suitable to a vertical or upright design. In fact, these horizontal skin chambers can only be used for infinite dose type studies, where the chambers on both sides of the skin are filled with donor and receptor fluids [33]. A conventional skin diffusion cell uses a dose application that is typical of human exposure to a chemical or drug treatment. This normally involves a low (finite) volume of the product containing the active ingredient. This covers the skin surface in a vertical designed system [13, 33]. There are now a considerable number of publications on studies with skin diffusion cells across all industrial sectors [21]. These mainly stemmed from the original work in the area of drug absorption and skin disposition of pharmaceutical products. With the publication of OECD test guidelines in 2004, there are now increasing numbers of papers with literature on industrial chemicals , particularly pesticides, where the method is used extensively as part of the prediction of human exposure during the manufacture , handling and use of such chemicals [9]. The in vitro method, as used for chemicals and drug intermediates, has a guideline status and is used throughout the world as part of the human risk assessment process [25, 26]. This has now almost completely replaced the corresponding in vivo test method that used rats for such studies for pesticides only [24, 39]. In addition to the formal OECD test guidelines, the in vitro method using glass diffusion chambers has the endorsement of many different European industry bodies, many of whom have published their own specific guidance over the last two decades [7–9, 27, 30]. There are also several key publications detailing the methodology in the area of dermal absorption and how the in vitro approach fits into the overall risk assessment process for chemicals [17, 20, 21].
Probably the most comprehensive earlier publications comparing different permeants using diffusion chambers and resected human skin come from investigators such as Tom Franz, certainly on the static cells [13] and others such Bob Bronaugh on the flow-through type systems [1]. In order to gain credibility as a reliable model, the in vitro chamber system must be able to predict dermal absorption in humans with an acceptable degree of accuracy. A systematic evaluation of 12 organic compounds that all had in vivo data [10] was investigated in some of the earlier systematic evaluations of the glass chamber system. The static diffusion cell system was shown to predict the dermal absorption observed in humans very well [13]. Indeed, the author of this particular investigation, Tom Franz, did a lot of work developing the chamber system over the years, so much so that these devices are often referred to as ‘Franz’ chambers. Although skin chamber type studies were performed many years prior by investigators such as Burch and Winsor [2] and Malkinson [23], it was the work in the next two decades by others such as Scheuplein [31], Flynn and Yalkowsky [12], and Feldmann and Maibach [11] that investigated specific areas of both medicinal and industrial chemicals using chamber devices and identified the key methodological aspects that are important to the emerging field of in vitro dermal absorption at that time. A general understanding on how the physicochemical properties of the penetrant and the vehicle in which it was applied influenced the kinetics of skin penetration began to emerge. No formal validation of the chamber method was in place for the approach to be used as stand-alone in risk assessment, but many investigators from their own areas of industry, particularly for pesticides, were demonstrating the usefulness of the in vitro approach as a predictive model for humans. [28, 29, 34, 35].
3 Static Diffusion Cell
Practically all the earlier studies on diffusion chambers that utilized human or animal skin were of the ‘static’ design [6, 14, 33] . These devices were simple and based on a two-part glass cell, the donor and the receptor compartments engineered to accommodate a fixed circular area of skin tissue between the compartments. Some of the designs had water-jacketed outer glass compartment in order to maintain the receptor fluid at skin temperature (32 °C). Other systems used open water baths in which the static cell was semi immersed so that the receptor compartment was maintained at this skin temperature but the donor compartment was placed above the waterline. A key design feature that is often overlooked is the thorough mixing of the receptor fluid during the experiment. A typical dermal absorption study normally involves a 24 h time period, whether for a pharmaceutical investigation, such as drug delivery, or for a safety investigation such as the systemic exposure to a potentially toxic agent. Indeed, a number of years ago these experiments often involved leaving a chemical on the skin for up to 3 days [39]. This is no longer a guideline requirement where the maximum duration of a correctly designed in vitro study is now 24 h [9, 25, 26]. The experiment relies on sampling of the receptor fluid at regular (or prescribed test guideline) time periods from the time of application of the test material to the skin till the end of the study. The small sample taken at each time point, which may be as low as 50 µl from a 4.5 ml receptor chamber volume, must be representative of the bulk concentration in the whole receptor volume of the test substance being evaluated . Therefore, rapid stirring of the receptor fluid to ensure the concentration of the test compound immediately below the dermal side of the tissue is the same as the point of entry into the collection device is essential. There are several interlinking aspects here such as the solubility of the test substance in the receptor fluid, the binding of the test compound to any component of the system (such as the glass wall of the chamber or sampling tubing) and the type of skin preparation (epidermal sheet versus split thickness skin). These can all affect the measured concentration of test substance that has permeated into the receptor fluid at any given time. These aspects are discussed in the respective test guidelines and guidance documents for in vitro percutaneous absorption [9, 25–27, 30], and form the basis for inclusion or otherwise of the so-called skin dose or mass of test substance that has crossed the stratum corneum, but not reached the receptor fluid , as being potentially systemically available .
The conventional type static diffusion cell must also accommodate the resected skin sample so that the seal between the glass donor and receptor chambers is effective. Most cells have an engineered ground glass joint so that even the thinnest heat-separated epidermal can be supported by a nickel (or other inert) grid and effectively sealed, and no lateral migration of the test application from its site of application can occur. A strong clamp with a steel edge in contact with the full circumference on either side of the joint ensures that this donor–receptor chamber seal is maintained until the chamber is dismantled. This same clamp is engineered with a wheel-locking device and base supports under the glass chamber, so that it sits perfectly horizontal in a water bath [33] .
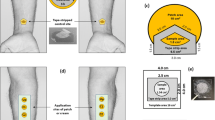
The surface area of exposed skin is a key element of diffusion cell design , and in the author’s experience the most fundamental aspect of a successful OECD 428 mass balance recovery in vitro percutaneous absorption study. The signal-to-noise ratio or that of skin surface-to-glass chamber should be as high as possible, to allow good surface spreading of a finite low volume dose of a formulated product, but without bowing or distortion of the skin sample and, of course, without utilizing too much precious biological material. With static cells, the exposed cell area is usually between 1 and 5 cm2. The smaller ‘mini’ cells around 1 cm2 are often used for nonregulatory investigations, or for other functional end points, including skin irritation assessment [18, 19]. The larger ‘standard’ static cells, around 2–3 cm2, are most common in regulatory dermal absorption studies. Figure 5.1 shows the diffusion cell designed and used by the Dermal Technology Laboratory (DTL). It has a relatively large surface area of skin to the size of the glassware. A key design feature is the broad rim of ground glass joint that evokes a good seal preventing lateral compound migration, and importantly any transfer round the rim of the specimen. The device is clamped with a spring holder and the skin sits on a thin nickel porous support to prevent any bowing or stretching of the skin. The design also allows easy access to the skin, post exposure, to facilitate sponge washing and tape stripping of the stratum corneum .
The static cell design has not changed much over the years. Some laboratories use cells that are slightly smaller than the one described above. This conserves the precious commodity, human skin. However, it has been shown that the interlaboratory concordance for a variety of drugs is very good when the studies are performed correctly using different size static diffusion cells. One example of this was a published interlaboratory investigation of caffeine, benzoic acid , and testosterone . The dermal absorption of these three reference chemicals in terms of both, the maximum absorption rate and the percentage absorption after 24 h, was shown to be very similar for each drug, despite the fact that the human skin was sourced, stored and prepared in different laboratories and by different technicians [15]. The most important aspect here to achieve this concordance was the measurement of the skin integrity for each skin diffusion cell. The laboratories used the same rejection criteria for tritiated water flux and electrical resistance measurement to assess the skin integrity [4]. Only skin samples that were deemed to have normal barrier function were used for drug application. This should apply, of course, to all in vitro dermal absorption studies, as specified by the OECD test guidelines [25] .
4 Flow-Through Diffusion Cell
By the late 1970s, many laboratories throughout the world were using resected skin mounted in static diffusion cells . As more and more papers on the predictive ability of these models came to press, further refinements in this approach were investigated . The drivers for moving towards a continuous flow-through receptor design included a general move towards physiological receptors and moved away from solvent-type systems in an attempt to replicate the normal physiological conditions of the skin and its draining blood supply that carries any absorbed material away from the skin [1]. This, of course, makes solubility in the receptor more of a challenge as it is important that sink conditions are maintained and the concentration of test substance in the receptor never reaches a point where back diffusion can occur. Recent guidance indicates that the maximum concentration of the test substance observed in the receptor fluid should not exceed 10 % of its saturated solubility in the receptor fluid selected [9] . The flow-through design with physiological receptor fluid means that for many studies the flow rate and replacement of fresh receptor needs careful attention. Another issue with the flow-through design relates to the analytical sensitivity. For non-radiolabelled investigations, the extraction and analysis of the test compound in a mass balance type investigation may be more challenging due to the greater volumes of receptor fluid compared to static cell designs.
Static diffusion cells are more commonly used than their flow-through counterparts. One distinct advantage of the static cell is that it runs independently from the other diffusion cells in the group. It is also much easier to undertake the mass balance washing and tape stripping procedures that form a part of the dermal absorption protocol [9, 30] than in a flow-through diffusion cell system .
Most flow-through type systems utilize a dry heated-block type design or water-jacketed approach to maintain a constant skin temperature of 32 °C . There is an electric pump to move the receptor under the dermal side of the skin at a constant flow rate into some form of collection device or autosampler vials. A typical flow rate is around 1.5 ml/h for such devices [1, 3]. Although most of the systems are quite reliable, one disadvantage of the flow-through system is that if the receptor flow system fails or the pump speed varies then all the diffusion cells in the group may be lost. In a group of static cells if there is a single blockage, say in the receptor collection line and the other samples are running independently then, the autosampler will continue to take accurate receptor volumes. As these in vitro experiments run for 24 h to obtain daily exposure data, and taking samples of receptor every 1–2 h through the night, it is important that the system is reliable .
5 Special Devices
5.1 Volatile Compounds
Diffusion cells can be used to study chemicals that are volatile at room temperature, or likely to be lost from the skin via evaporation during their normal use. However, care must be taken in the design of such studies to assess the likely proportion of an applied dose that is lost to the atmosphere, and this often requires the use of radiolabelled compounds due to the complexity of extraction and quantification of the compound in the matrices used to trap the chemical. Indeed, it is a regulatory requirement for many classes of chemicals under OECD test guidelines to fully define the distribution of the volatile compounds above the skin, within the skin, and in the receptor fluid [25]. This is now routinely undertaken in regulatory dermal absorption studies and devices have been developed for in vitro diffusion cells (Fig. 5.1). This is a key element of the mass balance and in situations where this has not been quantified by trapping the test substance in the void above the skin surface, the study is compromised and the ‘missing’ fraction of the dose would therefore be assumed to have been absorbed, under a precautionary principle. The usual procedure for studies with volatile test substances is to place a charcoal filter above the donor chamber (in vitro) or skin device (in vivo), and to extract the compound from the matrix at the end of the exposure period. The use of such filters allows the skin surface to remain essentially unoccluded because an occluding trapping device would not allow the evaporation of the vehicle thus, ultimately enhancing skin penetration. An adaptation of the standard static diffusion cell to incorporate charcoal filters is shown in the Fig. 5.1.
5.2 Solids and Powders
Glass diffusion cells are very useful for studying the dermal absorption of actives from solid applications. For example, studies in humans using solids or powders containing an active ingredient are challenging and are actually best undertaken in vitro using chamber devices. A distinct advantage here is that the application stays where it was on a measured area of the skin. In humans, the application is prone to move or even come away from the site of application. In animals, the skin site also needs to be protected from grooming by the animal and also oral ingestion. Therefore, this is one area where the diffusion chamber could be perceived as a better model compared with the in vivo situation. Indeed, the large static type cell allows good spreading of material, which can be applied to a fixed area of skin.
In the pharmaceutical sector, there are few drugs that are applied in solid form to the skin as spreading and skin contact are important aspects for delivering the intended dose. Hence, gels, ointments, lotions and creams are the conventional types of platforms used in dermatological products. There are some obscure dusting treatments for foot infections, but use of powders poses other safety issues such as inhalation exposure and also the fact that effective dermal penetration normally relies on other adjuvants present in the product. The fact that the dermal absorption of test substances (or formulated products containing the test substance) that are in a solid form at the temperature of the skin is poor and normally requires a vehicle or carrier to allow it to penetrate into the stratum corneum; and has been exploited by manufacturers of pesticides and other potentially toxic chemicals as dermal exposure is generally lower for the same compounds in solid form.
One area that does impact on the pharmaceutical industry when it comes to solids is during the manufacture of the drug active and any intermediates in the synthetic process that are solid at room temperature. Another scenario is the dermal exposure during veterinary use of powders containing an active drug. Studies on the dermal absorption for solid or powder materials using in vitro chamber systems can therefore be useful in measuring absorption and setting exposure limits and indicating the correct protective equipment to be used. Dry solid large particles or granules are generally very poor platforms for dermal absorption. Fine particles and dusts (depending on the actual particle size) will absorb any surrounding moisture on the skin surface and will therefore have more direct surface contact and a greater ability to penetrate into the skin. When in vitro or in vivo dermal absorption studies are designed to assess the risk from contact with solids, it is important to examine the actual real life exposure scenario. This includes a number of aspects such as the particle size and the potential exposure period. For example, if the material at the top of a container is a lumpy granule but is a fine dust at the bottom of the same container due to settling, it is prudent to test the finer dust material as it is likely to have a greater opportunity to release and deliver the active onto and into the skin. The OECD test guidelines suggest that moistening of the solid should be undertaken for in vitro and in vivo dermal absorption studies [25]. In order to better simulate typical exposure, it would be more useful and relevant to compare the neat material with a simulated sweat type of application. This would involve preparing a ground down version of the solid in this sweat medium that is then applied to the skin as a paste. This would provide a conservative assessment of dermal absorption relative to the neat solid, and represent a worst case scenario that may occur during handling of the chemical or product.
6 Bioequivalence Testing
An important area that utilizes the in vitro chamber approach is the area of dermatological product development . The chamber method provides a rapid and consistent method of selection and optimization of the release, and ultimately the dermal absorption and skin distribution of drugs. With so many generic products containing the same active (but often different adjuvants and other ingredients) the diffusion cell method provides data to confirm equivalence, or otherwise, for newly introduced products. There are several approaches here, depending on the specific question being addressed. In some cases, it is the release of a drug from its formulation that is important. Here, there is a guidance for the type of artificial membranes and the protocols that are acceptable in diffusion chamber experiments [36]. Standard static diffusion cells can normally be used with these artificial membranes . In other situations, where the question relates to predicting systemic exposure of the drug, human skin is used in conventional chamber systems to study the flux or proportion of a drug that is absorbed over a specific time course. An example of this in the literature is the bioequivalence of the drug aciclovir in a study using a wide range of dermatological preparations that were compared using the static diffusion cell chamber system and human dermatomed skin [38]. This is a key area in pharmaceutics that is not only ethically acceptable, as it minimizes the number of in vivo investigations, but it also allows a systematic assessment of the impact of even minor formulation components in addition to the effect of changing the drug loading concentration. The in vitro chamber method can also provide important information on the time course kinetics for release into and through the skin, and also the stability of the drug under simulated physiological conditions .
During the early stages of drug development of a new dermatological product, various adaptations of the in vitro chamber model have been utilized. For example, pig skin is used as a surrogate for human skin, and is recognized as the closest animal species to human skin for dermal absorption investigation. The key element is the barrier properties of pig skin either used as heat-separated epidermal membranes prepared from pig ears, or as flank skin prepared with a dermatome. The functional properties of the barrier, as assessed by chemical permeation, electrical resistance or transepidermal water loss, are all quite similar between pig and human [4], making pig skin a very useful model to compare the dermal absorption of drugs from different formulations. Another advantage of pig skin over, say rodent skin, is the density and depth of hair follicles. Porcine skin is very similar to human skin and this therefore allows the stratum corneum to be tape-stripped using an in vitro procedure with resected human skin that was developed and validated with human volunteers in a side-by-side investigation [37] . Such a procedure allows the profiling of a drug through individual strips of the stratum corneum, thus providing key information on the penetration into the skin barrier. This technique, another adaptation of the glass chamber methodology, is now widely used to study drug delivery and is particularly powerful when the tape stripping investigation is substantiated by visualization of the drug in the different layers of the stratum corneum using techniques such as time-of-flight mass spectrometry [22] .
References
Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies IV: the flow-through diffusion cell. J Pharm Sci. 1985;74:64–7.
Burch CE, Winsor T. Diffusion of water through dead plantar, palmar and dorsal human skin and through toe nails. Arch Dermatol Syphilol. 1942;53:39–41.
Clowes HM, Scott RC, Heylings JR. Skin absorption: flow-through or static diffusion cells. Toxicol In Vitro. 1994;8:827–30.
Davies DJ, Ward RJ, Heylings JR. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol In Vitro. 2004;18:351–8.
Dick IP, Scott RC. Pig ear skin as an in vitro model for human skin permeability. J Pharm Pharmacol. 1992;44:640–5.
Dugard PH. Absorption through skin: theory, in vitro techniques and their applications. Food Chem Toxicol. 1986;24:749–53.
E.C: Guidance document on dermal absorption. Eur Comm, SANCO/222/2000 rev. 2004;7:1–15.
ECETOC: Percutaneous absorption. European centre for ecotoxicology and toxicology of chemicals monograph no. 20, 1993; Brussels.
EFSA: European food safety authority. Guidance on dermal absorption. EFSA J. 2012;10(4):2665.
Feldmann RJ, Maibach HI. Absorption of some organic compounds through the skin in man. J Invest Dermatol. 1970;54:399–404.
Feldmann RJ, Maibach HI. Percutaneous penetration of some pesticides and herbicides in man. Toxicol Appl Pharmacol. 1974;28:399–404.
Flynn GL, Yalkowsky SH. Correlation and prediction of mass transport across membranes. I. Influence of alkyl chain length on flux-determining properties for barrier and diffusant. J Pharm Sci. 1972;61:838–52.
Franz TJ. Percutaneous absorption. On the relevance of in vitro data. J Invest Dermatol. 1975;64:190–5.
Franz TJ. The finite dose technique as a valid in vitro model for the study of percutaneous absorption in man. Curr Problems Dermatol. 1978;7:58–68.
Heylings JR. Risk assessment. In Benson HAE, Watkinson AC, editors. Transdermal and topical drug delivery: principles and practice. 1st. ed. Hoboken: Wiley; 2012. pp. 183–99.
Heylings JR, Garner A. Regulation of HCO3 - transport by luminal acid in the frog in vitro. Am J Physiol. 1984;246:G235–42.
Heylings JR, Esdaile DJ. Percutaneous absorption of pesticides. In: Roberts MS, Walters KA, editors. Dermal absorption and toxicity assessment. 2nd. ed. New York: Informa Healthcare; 2007.pp. 575–91.
Heylings JR, Clowes HM, Hughes L. Comparison of tissue sources for the skin integrity function test (SIFT). Toxicol In Vitro. 2001;15:597–600.
Heylings JR, Diot S, Esdaile DJ, Fasano WJ, Manning LA, Owen HM. A prevalidation study on the in vitro skin irritation function test (SIFT) for prediction of acute skin irritation in vivo: results and evaluation of ECVAM phase III. Toxicol In Vitro 2003;17:123–38.
Howes D, Guy R, Hadgraft J, Heylings JR, Hoeck U, Kemper F, Maibach H, Marty J-P, Merk H, Parra J, Rekkas D, Rondelli I, Schaefer H, Tauber U, Verbiese N. Methods for assessing percutaneous absorption, report and recommendations of ECVAM workshop report 13. Altern Lab Animals. 1996;24:81–106.
IPCS: Dermal Absorption. World Health Organization, International Programme on Chemical Safety. Environmental Health Criteria No. 235. 2006.
Judd AM, Scurr DJ, Heylings JR, Wan K-W, Moss GP. Distribution and visualization of chlorhexidine within the skin using ToF-SIMS: a potential platform for the design of more efficacious skin antiseptic formulations. Pharm Res. 2013;30:1896–905.
Malkinson FD. Studies on the percutaneous absorption of 14C labelled steroids by use of the gas flow cell. J Invest Dermatol. 1958;31:19–28.
OECD: Guideline 427 for the testing of chemicals. Skin absorption: in vivo method. Paris: Organization for economic cooperation and development; 13 April 2004.
OECD: Guideline 428 for the testing of chemicals. Skin absorption: in vitro method. Paris: Organization for economic cooperation and development; 13 April 2004.
OECD: Guidance document number 28. The conduct of skin absorption studies. Paris: Organization for economic cooperation and development; 5 March 2005.
OECD: Guidance notes on dermal absorption. Paris: Organization for Economic Cooperation and Development; 18 August 2011.
Ramsey JD, Woollen BH, Auton TR, Batten PL, Leeser JE. Pharmacokinetics of fluazifop-butyl in human volunteers II: dermal dosing. Hum Exp Toxicol. 1992;11:247–54.
Ramsey JD, Woollen BH, Auton T R, Scott, RC. The predictive accuracy of in vitro measurements for the dermal absorption of a lipophilic penetrant (Fluazifop-Butyl) through rat and human skin. Fundam Appl Toxicol. 1994;23:230–6.
SCCS: Scientific committee on consumer safety. Basic criteria for the in vitro assessment of dermal absorption of cosmetic ingredients. SCCS/1358/10. 2010.
Scheuplein RJ. Mechanisms of percutaneous absorption. I. routes of penetration and the influence of solubility. J Invest Dermatol. 1965;45:334–46.
Scott RC, Dugard PH. The properties of skin as a diffusion barrier and route for absorption. In: Greaves MW, Schuster S, editors. Handbook of experimental pharmacology, Vol 87/II. Pharmacology of the skin II. Berlin: Springer; 1989.
Scott RC, Clowes HM. In vitro percutaneous absorption experiments: a guide to the technique for use in toxicology assessments. Toxicol Methods. 1992;2:113–23.
Scott RC, Batten PL, Clowes HM, Jones BK, Ramsey JD. Further validation of an in vitro method to reduce the need for in vivo studies for measuring the absorption of chemicals through rat skin. Fundam Appl Toxicol. 1992;19:484–92.
Scott RC, Carmichael NG, Huckle KR, Needham D, Savage T. Methods for measuring dermal penetration of pesticides. Food Chem Toxicol. 1993;31:523–9.
SUPAC-SS: Guidance for Industry, Non-Sterile Semi-solid Dosage Forms, Scale-Up and Post-approval Changes: chemistry, Manufacturing and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation, USDHHS, FDA, CDER, May 1997. In Vitro Release Test; 1997. pp. 19–24.
Trebilcock KL, Heylings JR, Wilks MF. In vitro tape stripping as a model for in vivo skin stripping. Toxicol In Vitro. 1994;8:665–7.
Trottet L, Owen H, Holme P, Heylings JR, Collin IP, Breen AP, Siyad MN, Nandra RS, Davis AF. Are all acyclovir cream formulations bioequivalent? Int J Pharm. 2005;304:63–71.
U.S. EPA: Health Effects Test Guidelines. U.S. Environmental Protection Agency, 1998, OPPTS 870.7600. Dermal Penetration 1–12. 1998.
U.S. EPA: Proposed test rule for in vitro dermal absorption rate testing of certain chemicals of interest to occupational safety and health administration; proposed rule. Fed Regis. 1999;64(110):31073–90.
Ussing HH. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951;23:110–27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Heylings, J. (2014). Diffusion Cell Design. In: Shah, V., Maibach, H., Jenner, J. (eds) Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1289-6_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1289-6_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1288-9
Online ISBN: 978-1-4939-1289-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)