Abstract
There have been significant advancements in the field of transdermal drug delivery with the development of microneedles. Microneedles are capable of enhancing transdermal drug delivery by creating physical conduits in the stratum corneum of the epidermis. In this chapter, we discuss the various types of microneedles with a specific focus on out-of-plane microneedles and their modes of action. We further examine the safety and efficacy of microneedles along with their applications in the field of dermatology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The field of transdermal drug delivery has experienced significant innovative advancements [1]. Traditional modes of drug delivery include oral, parenteral, or cutaneous delivery via a hypodermic needle. Oral drug delivery is affected by the acidity of the stomach, poor intestinal absorption, and first-pass hepatic metabolism, which all contribute to lower bioavailability [2]. Hypodermic needles are painful and associated with needle phobia and apprehension [3]. Transdermal drug delivery is an alternative delivery method that introduces drugs by bypassing the skin barrier to allow for either systemic or local drug delivery.
The major barrier in transdermal drug delivery is the stratum corneum, the outermost layer of skin, ranging from 15 to 170 μm in thickness depending on the anatomical site [4, 5], and composed of keratinized dead cells. Transdermal drug delivery systems bypass the stratum corneum, which is usually the rate-limiting barrier. The stratum corneum is lipophilic in nature and is resistant to the passage of hydrophilic substances or the passage of molecules that are larger than a few hundred Daltons [6]. In general, multiple different approaches are taken to bypass the stratum corneum [1]. Microneedles enhance transdermal drug delivery through the creation of physical conduits or channels in the stratum corneum. Because microneedles typically penetrate over 200 μm, anatomical changes in the thickness of stratum corneum are unlikely to alter the efficacy of microneedle penetration past the stratum corneum. This field is growing as evidenced by the number of microneedle-based patents (Table 14.1). Several marketed microneedle devices are outlined in Table 14.2.
2 Microneedle Fabrication
Microneedles are miniature needles created using the lithographic techniques and are designed to penetrate the stratum corneum to enter the epidermis without piercing the underlying dermis. By doing so, they avoid nerve endings in the dermis to render painless insertions [7–11] . However, longer microneedles are available when dermal remodeling is desired such that the needles enter the dermis and thus are painful with application. The main feature of microneedles that contributes to pain is their length [12]. Shorter microneedles do not reach the dermis and do not come into contact with nerves or blood vessels; they either elicit little to no pain, thereby making short microneedles highly desirable for drug delivery and they are the focus of this chapter.
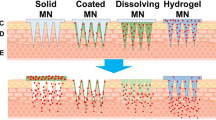
Microneedles have been developed in several different designs, including out-of-plane and in-plane microneedles [13]. Out-of-plane needles are designed such that the microneedle is perpendicular to the surface (Fig. 14.1), whereas in-plane microneedles are parallel to the surface but are more difficult to place into arrays [14]. As most of the research and applications have focused on the out-of-plane microneedle, this chapter will review their use in transdermal drug delivery. Out-of-plane microneedles are subdivided into solid and hollow microneedles . Solid microneedles are used to create temporary physical holes in the stratum corneum or are coated such that the drug is delivered from the surface of the microneedle upon insertion (Fig. 14.1). Hollow microneedles consist of a conduit (Fig. 14.1) allowing for either bolus or continuous infusion after the microneedle is inserted (Fig. 14.1). A variety of materials have been used to make microneedles including metal [15], titanium [16, 17], glass [18–20], polymers [19, 21–25], and sugars [8, 21, 26] .
Schematic of microneedle penetration of solid and hollow microneedles. a During insertion the microneedles penetrate to the epidermis. The partition coefficients k1 and k2 promote penetration into the epidermis and into the dermis, respectively. The penetration coefficient k3 promotes retention within the stratum corneum. b The microneedles leave behind conduits into the epidermis after they are removed. The conduits allow topically applied substances to have direct access to the epidermis. The hollow microneedles allow for intraepidermal injection as depicted
There are many factors that can impact the efficacy of microneedles including its design: e.g., radius of the microneedles, microneedle shape, the quantity of microneedles used, and microneedle thickness [27] .
3 Microneedle Strategies for Drug Delivery
The strategy for drug delivery depends on the design of the microneedle. Solid microneedles cannot infuse drugs through the needle. Instead, they are used in three different strategies . The first strategy is to create micropores in the stratum through insertion and removal of the microneedle array. These pores remain open for as little as a few hours before closure when left uncovered to a range of 48 h when covered with a drug-delivered patch [11, 28]. Drug solutions can then be applied topically to traverse these transiently open channels to bypass the stratum corneum, and some studies have utilized iontophoresis to accelerate movement through these channels [29–31]. Because solid microneedle pretreatment of the skin create transient pores with direct access past the stratum corneum, this can enhance the penetration of a topical formulation without necessitating changes to the formulation.
Another strategy is to coat microneedles with a drug prior to insertion. The third strategy is to create dissolving microneedles that dissolve upon insertion to deliver a drug payload. The latter two strategies are limited to drugs that are stable enough to be coated onto or encapsulated during the microneedle fabrication process [32]. Hollow microneedles can be used to infuse drugs as a bolus [33, 34] or as a continuous infusion [35] after insertion .
Utilizing these various strategies, microneedles have been employed to deliver vaccines [36–38], insulin [15, 19, 35, 39, 40], erythropoietin [21], desmopressin [16], and methotrexate [41]. Testing in humans with in vivo studies have shown that microneedles can inject nicotinic acid derivatives through hollow silicon out-of-plane microneedles [33, 34] and insulin through a hollow glass micropipette tip [42] .
4 Transdermal Drug Delivery Applications
Microneedles are versatile and have numerous dermatologic applications. Short (70–80 µm) solid microneedle treatment has been shown to significantly increase the permeation of the small hydrophilic compound galathamine in vivo in mice [43]. Hollow 500 µm microneedles delivering a 2 % dose of lidocaine, have been shown to provide anesthesia as quickly and effectively as a hypodermic needle [44]. Solid microneedle generated micropores have been demonstrated to accelerate the delivery of topical dyclonine [45] and topical aminolevulinic acid [46]. A study in mice evaluated the use of a polymer-based microneedle roller to enhance the topical delivery of l-ascorbic acid for hair growth . This study employed microneedle rollers to create microchannels before topically applying l-ascorbic acid in vivo in mice. Pretreatment with the microneedle rollers significantly reduced skin pigmentation in melasma patients compared to depigmentation serums alone [47].
The applications of microneedles expand to enhancing photodynamic therapy (PDT) for the treatment of actinic keratoses. PDT requires the local application of a photosensitive drug combined with incident light to selectively cause cell death, presumably through locally generated singlet oxygen [48]. In one study, silicon microneedle patches were used to improve the efficiency of administering topical photosensitive drugs by creating transient microchannels in nude mice before delivering one of two preformed photosensitizer, either 5-ALA [49] or meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate [50]. A transdermal patch was used to deliver the photosensitizing drug over the skin perforated by a microneedle. The results revealed that the photosensitizing drug was delivered in greater quantities and in a shorter time in comparison to sites that were not perforated with the microneedle patch. The greater depth of penetration is likely the result of faster penetration past the stratum corneum, but this was not specifically studied. As such, one potential advantage of microneedle based therapy would be to reduce incubation times for PDT.
Indeed, clinical studies have demonstrated that channels produced by solid microneedles enhanced the delivery of both aminolevulinic acid (ALA) and methyl-aminolevulinic acid (mALA) [51]. Solid microneedle pretreatment decreased the requisite incubation time of 5-ALA from 60 to 20 min without affecting the efficacy of PDT treatment on actinic keratoses as measured by complete response rate [46]. Furthermore, combining mALA with microneedle pretreatment resulted in the effective treatment of actinic keratosis while preventing most reoccurrences [52, 53]. One clinical study evaluated the role of short microneedles with PDT for photorejuvenation and noted clinical improvement of photodamage [54]. Of note, this latter trial was limited by a lack of a control group.
4.1 Hollow Microneedles
Only a few animal [35, 55] and human [33, 34, 42] studies of hollow microneedles have been performed. in vivo human experiments revealed that hollow microneedles significantly hastened penetration of methyl nicotinate compared to topical administration [7]. Hollow microneedles were shown to deliver insulin either through a transdermal patch that can actively release insulin in vivo in mice [35] or deliver a bolus of insulin in humans without the pain associated with hypodermic needles [42].
5 Safety of Microneedles
One of the initial concerns with microneedles was the biocompatibility of silicon or glass, since there are reports of silicon and glass related granulomas [56, 57]. Short microneedles are developed to only penetrate into the epidermis, and not into the dermis. Therefore, any remaining foreign body produced by fragments of the microneedle will likely be contained in the epidermis and discarded within the regular turnover time of 2–4 weeks. Silicon is expensive when considering mass production for commercialization, and manufacturing has moved away from silicon and toward polymer and sugar based synthesis. The polymers and sugars are biocompatible and many are designed to biodegrade. As microneedle technology continues to move toward biocompatibility, microneedle material related adverse events will likely become less of a concern.
The stratum corneum is also a formidable barrier against infection. Microneedles physically breach this barrier, raising the possibility for cutaneous infections. Channels created by solid microneedles are open transiently for a few hours or less [11], and microneedles create a lower bacterial burden than injection with traditional hypodermic needles [58]. Furthermore, facial transepidermal water loss (TEWL) after treatment with short microneedles of 150 and 250 µm recovered to near baseline levels after 8 h [59]. Considering that TEWL is a measurement of epidermal skin barrier function , this further suggests that the skin recovers quickly after a treatment to recover the protective skin barrier. Transient insertions of short solid microneedles of 70–80 µm did not affect the skin’s capability to resist Staphylococcus aureus infection despite incubation with the bacteria in vivo in mice [43]. However, the use of longer microneedles that reach the dermis increased the infection rates compared to the untreated control [43]. In comparison, the use of hollow microneedles for extended infusions may elevate the risk for infection. There are no studies of the infectious risk of long-term infusions with hollow microneedles and the risk of cutaneous infections will need to be further studied.
6 Pharmacokinetics and Pharmacodynamics
Comparative studies between oral and transdermal drug delivery have shown some similarities and differences. Studies in estrogen delivery have shown that transdermal delivery may mimic physiological levels more closely [60]. However, another study in subjects with Turner syndrome showed no difference when evaluating the metabolic effects of oral and transdermal estrogen [61]. However, few articles have compared microneedle based transdermal drug delivery to oral or intravenous based drug delivery .
Microneedle based delivery of desmopressin was found to have similar elimination kinetics compared to intravenous delivery in guinea pigs [16]. Short microneedle based drug delivery will require drugs to traverse the interstitial fluid of the epidermis and dermis prior to enter the blood stream but it is unknown how different or similar this may be in comparison to oral, intravenous, intramuscular, or subcutaneous delivery. Further studies in animals and in humans will be needed to better assess if microneedle-assisted delivery results in similar or different pharmacokinetic and pharmacodynamic profiles. These studies will need to take into account the 15 factors of transcutaneous penetration [62] .
7 Conclusion
Microneedle use in transdermal drug delivery is an innovative and practical technology with a bright future. Microneedles now make it possible to deliver agents through the stratum corneum that were previously impermeable. Microneedles enhance patient comfort by decreasing and often eliminating pain. The pharmacokinetics and pharmacodynamics will need to be further evaluated, especially when comparing one microneedle design against another. Collaboration between physicians and engineers will continue to drive the evolution and growth of microneedles and their use for cutaneous drug delivery.
References
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotech. 2008;26:1261–68.
Fasinu P, Pillay V, Ndesendo VMK, du Toit LC, Choonara YE. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm Drug Dispos. 2011;32:185–209.
Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg. 2003;68:341–4.
Koehler MJ, Vogel T, Elsner P, König K, Bückle R, et al. In vivo measurement of the human epidermal thickness in different localizations by multiphoton laser tomography. Skin Res Technol. 2010;16:259–64.
Egawa M, Hirao T, Takahashi M. In vivo estimation of stratum corneum thickness from water concentration profiles obtained with Raman spectroscopy. Acta Derm Venereol. 2007;87:4–8.
Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9.
Sivamani RK, Stoeber B, Wu GC, Zhai H, Liepmann D, et al. Clinical microneedle injection of methyl nicotinate: stratum corneum penetration. Skin Res Technol. 2005;11:152–6.
Miyano T, Tobinaga Y, Kanno T, Matsuzaki Y, Takeda H, et al. Sugar micro needles as transdermic drug delivery system. Biomed Microdevices. 2005;7:185–8.
Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, et al. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92:502–4.
Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Phar Sci. 2008;35:193–202.
Haq MI, Smith E, John DN, Kalavala M, Edwards C, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices 2009;11:35–47.
Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–94.
Sivamani RK, Liepmann D, Maibach HI. Microneedles and transdermal applications. Expert Opin Drug Deliv. 2007;4:19–25.
Reed ML, Lye WK. Microsystems for drug and gene delivery. Proc IEEE. 2004;92:56–75.
Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52:909–15.
Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–11.
Tsuchiya K, Nakanishi N, Uetsuji Y, Nakamachi E. Development of blood extraction system for health monitoring system. Biomed Microdevices. 2005;7:347–53.
Lee S, Jeong W, Beebe DJ. Microfluidic valve with cored glass microneedle for microinjection. Lab Chip. 2003;3:164–7.
McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100:13755–60.
Wang PM, Cornwell M, Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7:131–41.
Ito Y, Yoshimitsu J, Shiroyama K, Sugioka N, Takada K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J Drug Target. 2006;14:255–61.
Matsuda T, Mizutani M. Liquid acrylate-endcapped biodegradable poly(epsilon-caprolactone-co-trimethylene carbonate). II. Computer-aided stereolithographic microarchitectural surface photoconstructs. J Biomed Mater Res. 2002;62:395–403.
Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Controlled Release. 2005;104:51–66.
Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23:1008–19.
Han M, Hyun D-H, Park H-H, Lee SS, Kim C-H, et al. A novel fabrication process for out-of-plane microneedle sheets of biocompatible polymer. J Micromec Microeng. 2007;17:1184–91.
Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25:104–13.
Davidson A, Al-Qallaf B, Das DB. Transdermal drug delivery by coated microneedles: geometry effects on effective skin thickness and drug permeability. Chem Eng Res Des. 2008;86:1196–206.
Enfield J, O'Connell ML, Lawlor K, Jonathan E, O'Mahony C, et al. In-vivo dynamic characterization of microneedle skin penetration using optical coherence tomography. J Biomed Opt. 2010;15:046001.
Chen H, Zhu H, Zheng J, Mou D, Wan J, et al. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Control Release. 2009;139:63–72.
Katikaneni S, Badkar A, Nema S, Banga AK. Molecular charge mediated transport of a 13 kD protein across microporated skin. Int J Pharm. 2009;378:93–100.
Wu XM, Todo H, Sugibayashi K. Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis. J Control Release. 2007;118:189–95.
Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–80.
Sivamani RK, Stoeber B, Liepmann D, Maibach HI. Microneedle penetration and injection past the stratum corneum in humans. J Dermatol Treat. 2009;20:156–9.
Sivamani RK, Stoeber B, Wu GC, Zhai HB, Liepmann D, et al. Clinical microneedle injection of methyl nicotinate: stratum corneum penetration. Skin Res Technol. 2005;11:152–6.
Roxhed N, Samel B, Nordquist L, Griss P, Stemme G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans Biomed Eng. 2008;55:1063–71.
Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, et al. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27(49):6932–8.
Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968–73.
Ding Z, Verbaan FJ, Bivas-Benita M, Bungener L, Huckriede A, et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release. 2009;136:71–8.
Gardeniers H, Luttge R, Berenschot EJW, de Boer MJ, Yeshurun SY, et al. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst. 2003;12:855–62.
Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29:82–8.
Vemulapalli V, Yang Y, Friden PM, Banga AK. Synergistic effect of iontophoresis and soluble microneedles for transdermal delivery of methotrexate. J Pharm Pharmacol. 2008;60:27–33.
Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther. 2009;11:329–37.
Li WZ, Huo MR, Zhou JP, Zhou YQ, Hao BH, et al. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389:122–9.
Gupta J, Denson DD, Felner EI, Prausnitz MR. Rapid local anesthesia in humans using minimally invasive microneedles. Clin J Pain. 2012;28:129–35.
Li X, Zhao R, Qin Z, Zhang J, Zhai S, et al. Microneedle pretreatment improves efficacy of cutaneous topical anesthesia. Am J Emerg Med. 2010;28:130–4.
Lev-Tov H, Larsen L, Zackria R, Chahal H, Eisen D, et al. Microneedle pretreatment may reduce incubation time in photodynamic therapy; 2013; International Investigative Dermatology Meeting Edinburgh, UK.
Fabbrocini G, De Vita V, Fardella N, Pastore F, Annunziata MC, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma. Plast Surg Int. 2011;2011:158241.
Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B 1990;6:143–8.
Donnelly RF, Morrow DI, McCarron PA, Woolfson AD, Morrissey A, et al. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: potential for enhanced topical photodynamic therapy. J Control Release. 2008;129:154–62.
Donnelly RF, Morrow DI, McCarron PA, David Woolfson A, Morrissey A, et al. Microneedle arrays permit enhanced intradermal delivery of a preformed photosensitizer. Photochem Photobiol. 2009;85:195–204.
Mikolajewska P, Donnelly RF, Garland MJ, Morrow DI, Singh TR, et al. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm Res. 2010;27:2213–20.
Bencini PL, Galimberti MG, Pellacani G, Longo C. Application of photodynamic therapy combined with pre-illumination microneedling in the treatment of actinic keratosis in organ transplant recipients. Br J Dermatol. 2012;167:1193–94.
Clementoni MT, M BR, Munavalli GS. Photodynamic photorejuvenation of the face with a combination of microneedling, red light, and broadband pulsed light. Lasers Surg Med. 2010;42:150–9.
Clementoni MT, B-Roscher M, Munavalli GS. Photodynamic photorejuvenation of the face with a combination of microneedling, red light, and broadband pulsed light. Lasers Surg Med. 2010;42:150–9.
Hafeli UO, Mokhtari A, Liepmann D, Stoeber B. In vivo evaluation of a microneedle-based miniature syringe for intradermal drug delivery. Biomed Microdevices. 2009;11(5):943–50.
Millard DR Jr., Maisels DO. Silicon granuloma of the skin and subcutaneous tissues. Am J Surg. 1966;112:119–23.
Finley J, Knabb J. Cutaneous silica granuloma. Plast Reconstr Surg. 1982;69:340–3.
Donnelly R, Singh T, Tunney M, Morrow D, McCarron P, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–22.
Han TY, Park KY, Ahn JY, Kim SW, Jung HJ, et al. Facial skin barrier function recovery after microneedle transdermal delivery treatment. Dermatol Surg. 2012;38:1816–22.
Taboada M, Santen R, Lima J, Hossain J, Singh R, et al. Pharmacokinetics and pharmacodynamics of oral and transdermal 17beta estradiol in girls with Turner syndrome. J Clin Endocrinol Metab. 2011;96:3502–10.
Mauras N, Shulman D, Hsiang HY, Balagopal P, Welch S. Metabolic effects of oral versus transdermal estrogen in growth hormone-treated girls with turner syndrome. J Clin Endocrinol Metab. 2007;92:4154–60.
Ngo MA, Maibach HI. 15 Factors of percutaneous penetration of pesticides. Parameters for pesticide QSAR and PBPK/PD models for human risk assessment. American chemical society: Washington, DC; 2012. p. 67–86.
Singh TR, Dunne NJ, Cunningham E, Donnelly RF. Review of patents on microneedle applicators. Recent Pat Drug Deliv Formul. 2011;5:11–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Leo, M., Lev-Tov, H., Kamangar, F., Maibach, H., Sivamani, R. (2014). Efficacy and Toxicity of Microneedle-Based Devices. In: Shah, V., Maibach, H., Jenner, J. (eds) Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1289-6_14
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1289-6_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1288-9
Online ISBN: 978-1-4939-1289-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)