Abstract
For women living with HIV, there are several issues to consider in attending to their reproductive health and contraceptive needs. In this chapter we review common medical comorbidities, pregnancy-associated risks, and reproductive health concerns that present among HIV-infected women. Although most contraceptive methods are safe for women with HIV, careful consideration of other comorbidities and current medications are important in helping the individual select an ideal contraceptive option. While controversies exist regarding the potential for certain hormonal contraceptives to contribute to increased HIV transmission and progression, the current research is inconclusive and does not influence current contraceptive recommendations. As several antiretroviral drugs may interact with hormonal contraceptives to reduce effectiveness, careful patient counseling during method selection and encouragement of dual protection with condoms are important.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Human Immunodeficiency Virus
- Human Immunodeficiency Virus Infection
- Sexually Transmitted Infection
- Bacterial Vaginosis
- Contraceptive Method
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Background on the HIV Epidemic: Globally and in the USA
Human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), survives via inhabiting and killing the immune cells that fight infections. The first cases of AIDS were recognized in the early 1980s in the USA, with the first published case series from the US Centers for Disease Control and Prevention (CDC) in 1981 of five homosexual men [1]. Since then, global recognition of the virus has led to great advances in our understanding of the disease, transmission, pathogenesis, prognosis, and treatment. Although there is no cure for HIV infection at this time, with the development of highly active antiretroviral therapy (HAART), individuals with HIV infection can live longer and transmission can be reduced. While the incidence of new HIV infections and HIV-related mortality continues to decline due to improvements in care and treatment, the prevalence of HIV infections globally continues to rise, and HIV infection remains a leading cause of death among women of childbearing age worldwide [2, 3].
The Global Epidemic
As of 2012, an estimated 35.3 million people were living with HIV worldwide [4]. Sub-Saharan Africa contains two-thirds of the world’s HIV-infected population. In this region, HIV disproportionately affects women, who make up 58 % of those infected. In the Caribbean region, with the second highest prevalence of HIV in the world, 1 in 100 people live with HIV infection [4]. It should be noted that a large proportion of the data on women with HIV infection stems from international research efforts particularly in sub-Saharan Africa. Furthermore, given immigration to the USA, it is important that health care providers, especially those who serve low-income and migrant populations, are aware of the global context of the HIV/AIDS epidemic.
United States
An estimated 1.1 million people in America are living with HIV, and nearly 1 in 5 are unaware of their infection [5]. Approximately 50,000 new people are additionally infected each year [5, 6]. The proportion of HIV/AIDS cases diagnosed among women in the USA has grown annually from 8 % in 1985 to an estimated 25 % in 2010. HIV disproportionately affects black women in the USA. In 2010, heterosexual transmission among black women made up one of the most common routes of new infections, with greater than 5,000 new infections transmitted in this way. Of the total number of new HIV infections among women in the USA in 2010, 64 % occurred in black women, 18 % were in white women, and 15 % were in Hispanic women. Young women ages 25–44 years accounted for the majority of new infections in women in 2010. Fortunately, among all racial groups, the rate of new infections in women has been slowly declining [7].
Why Are Women at Risk?
Due to a complex array of factors, women are particularly vulnerable to acquiring HIV. Women may not be aware of their partner’s risk factors for HIV and may not be able to successfully negotiate consistent condom use or mutual monogamy with their partners. The vast majority of these new infections come from heterosexual contact with men who have risk factors that are unknown to their female partners (e.g., men who have sex with other men or inject illicit drugs) [8]. Challenges that many women face, such as domestic violence, discrimination, stigma, substance abuse, mental health disorders, and poverty, increase their HIV susceptibility. Additionally, women are more susceptible to acquiring HIV during unprotected vaginal sex than men, with even higher risk during unprotected anal sex, due to a variety of factors, including the concentration of virus in semen, delicacy of vaginal tissues, and cervical ectropion.
For women who are aware of their partner’s HIV status, they often lack the ability to advocate for the use of strategies to reduce HIV transmission risk, such as condoms and male circumcision. HIV pre-exposure prophylaxis, a strategy in which antiretroviral drugs are used orally or topically by HIV-uninfected persons before potential HIV exposure, has recently shown promise in HIV prevention. The daily use of oral fixed-dose combination tablets containing tenofovir, disoproxil, fumarate, and emtricitabine is approved by the Food and Drug Administration for use among sexually active adults at risk for HIV infection. Additionally, a tenofovir-containing vaginal microbicide has shown promise for HIV prevention in at-risk women and is being explored further [9].
Unfortunately, it is estimated that in the USA almost 1 in 5 women who are HIV-infected are unaware of their status. This highlights the importance of routine HIV testing in women, as recommended by CDC guidelines, to improve women’s health and prevent HIV transmission. Linking HIV-infected women into appropriate medical care, retaining them in care, and optimizing HIV therapy for affected women are crucial to maintain health, improve survival, and reduce HIV transmission in the community [10, 11]. Relative to men, women living with HIV infection in the USA have been shown to be more vulnerable with regard to health care resource utilization [12, 13], potentially due to challenges such as transportation, childcare, insurance, substance abuse, and stigma. Key health indicators such as rates of clinic visits, antiretroviral treatment adherence, virologic suppression, and mean CD4 T-cell counts are lower for the HIV-infected women of racial/ethnic minority backgrounds [14, 15]. Since many of these women are the sole providers of care for their children, illness and death ultimately threaten the stability and welfare of families in their communities. These statistics underscore the urgent need for interventions aimed at women for the prevention and effective treatment of HIV infection. Family planning clinics provide an important venue for women of reproductive age who are living with or at risk for HIV to access the health care system, and serve as a crucial step for women to receive HIV testing and for HIV-infected women to be linked into appropriate medical care.
Living with HIV: HIV Care over the Past 30 Years
Primary HIV infection is often asymptomatic and if symptoms do occur they can be nonspecific and flu-like in nature. After the primary infection, many months or years may pass before the person is diagnosed. Symptoms that may prompt evaluation include opportunistic infections, or minor skin or constitutional symptoms. Women may present at a later stage of the disease or may discover their HIV-positive status during routine prenatal testing.
The natural history of HIV infection has changed over the past few decades with the use of HAART [16]. Mortality from HIV infection has declined and opportunistic infections are becoming less common, with more than half of the deaths among individuals with HIV infection now related to conditions other than AIDS. The paradigm of care in HIV infection in the era of HAART has shifted to that of chronic disease management. Despite this, some women still are diagnosed or present to care late in their disease course with conditions related to AIDS. In caring for HIV-infected women, care providers must therefore consider their overall health in relation to chronic non-AIDS complications such as cardiovascular disease (CVD), bone disease, renal disease, liver disease, and malignancies whose prevalence are significantly high, particularly among women (relative to men) living with HIV/AIDS [17], as well as possible immune suppression if AIDS-related conditions are present.
AIDS-Related Complications
Compared to women without HIV infection, HIV-infected women are at risk for recurrent candida vulvovaginitis, recurrent or complicated pelvic inflammatory disease, persistent or recurrent bacterial vaginosis, severe and prolonged genital herpes infections, cervical dysplasia and cancer, and abnormal uterine bleeding. The presence of any of these conditions should trigger HIV testing in a woman who does not have a diagnosis of HIV, and women with HIV should be screened regularly and managed aggressively for these conditions as several of them can increase their risk of transmitting HIV to their partner (see the section “Reproductive Health Care for Women with HIV”).
Rarely, AIDS-defining conditions may involve the female reproductive tract, and women with such conditions may present to a reproductive care provider. These conditions include invasive cervical cancer, pelvic or genital tract tuberculosis infection, genital tract lymphoma, or endometritis due to uncommon pathogens. Women with these conditions should be carefully evaluated for signs of systemic infection and referred to a specialist for appropriate management.
Non-AIDS Complications
Early recognition and effective management of certain non-AIDS conditions could have implications on the reproductive and contraceptive choices available to women living with HIV/AIDS. HIV infection confers a heightened risk of CVD beyond that accounted for by traditional risk factors. The risk of CVD is up to 50 % higher for HIV-infected individuals relative to the general population [18–26]; this HIV-related risk may be more pronounced in women [22]. HIV infection precipitates premature CVD at an average age of 44 years, 10–15 years earlier than in the uninfected population [27, 28]. Finally, rates and the severity of CVD complications, such as ischemic cardiomyopathy and acute myocardial infarction, are aggravated by HIV infection [29–32]. Certain risk factors for CVD, such as diabetes mellitus and hyperlipidemia, may be more common in HIV-infected women, particularly in the setting of exposure to certain antiretroviral drugs.
Chronic liver disease due to chronic viral hepatitis, alcohol use, or fatty liver disease is also one of the leading causes of hospitalization and death in HIV-infected persons [17]. Similarly, HIV-infected patients are increasingly affected by kidney disease due to either traditional risk factors, such as diabetes and hypertension, HIV itself, drug toxicities, or other comorbidities, such as hepatitis [33]. The risk of venous thromboembolism may also be higher in HIV-infected patients [34]. Additionally, osteopenia and osteoporosis are seen in up to 70 % and 15 %, respectively, of HIV-infected patients in the USA [35–37], a risk that is over six and three times that of HIV-negative persons. Consequently, fracture rates several-fold higher than the general population are being reported in persons with HIV infection [38–42]. While the risk of certain non-AIDS-defining cancers, such as anal, liver, and lung cancer, have been shown to be higher in HIV-infected patients compared to the general population, the risk of breast cancer appears to be similar between HIV-infected and HIV-uninfected women [43]. Finally, HIV-infected women may also be at particular risk for neurocognitive disease [44], depression [45], and intimate partner violence [46].
Overview of the Importance of Balanced Family Planning for Women with HIV
Fertility Intentions
Several studies have explored the impact of HIV infection on fertility decisions and pregnancy rates [47–53]. Evidence suggests that sociocultural factors play a large role in fertility decision-making and that there is a rich and complex range of factors, including HIV status and HAART use, which influence reproductive decisions [53, 54]. Previous studies in Malawi [55] and Uganda [56] suggested that desire for children was lower among HIV-infected women in comparison to their uninfected peers. Among HIV-infected women in Côte d'Ivoire and Kenya, more education [57] and marriage [58] were associated with increased contraceptive use while in Uganda previous discussions of family planning with a partner and a current marital relationship increased the likelihood of contraceptive use [59]. Among HIV-infected women, contraceptive use might change over time on HAART [60, 61], possibly due to improved health, changing desires for family size, or concerns about interactions of contraceptives and HAART [62]. Previous studies have shown that among HIV-infected women in Rwanda, despite high initial contraceptive uptake after counseling, contraceptive use declined over time [60, 61]. Although these studies noted changes in fertility intentions and contraceptive use among those with HIV infection, the role of HAART on these decisions remains unclear.
There are few studies evaluating fertility intentions among women with HIV infection in the USA. A 2001 study showed that nearly 70 % of HIV-infected women (about one quarter of whom had no children) surveyed did not desire future fertility, and 31 % said if they became pregnant, they “definitely would” have an abortion [47]. Desires and expectations for future fertility in HIV-infected women were less than HIV-negative women; however, notably with the increase in HAART use, this difference may not be as sizable.
Importance of Family Planning
Prevention of unintended pregnancy among women with HIV infection is critical to prevent the unnecessary morbidity and mortality associated with pregnancy, and to prevent vertical transmission of HIV. The World Health Organization’s (WHO’s) 4-Component Strategy for prevention of maternal to child transmission includes: (1) prevention of HIV infection in women, especially young women; (2) prevention of unintended pregnancies in HIV-infected women; (3) prevention of transmission from HIV-infected women to their infants; and (4) support for HIV-infected women, their infants, and their families [63].
Health Risks Among HIV-Infected Women During Pregnancy
HIV infection contributes to the global maternal mortality with an estimated 56,100 pregnancy-related deaths attributed to HIV infection in 2011 [64]. HIV-infected pregnant women are at two- to tenfold higher risk of death compared to HIV-negative pregnant women [65–67]; thus the CDC considers HIV/AIDS a condition that is associated with increased risk of adverse health events as a result of unintended pregnancy [68]. Infectious etiologies such as tuberculosis (TB), meningitis, and pneumonia are large contributors to this increased risk; however, puerperal sepsis, largely related to cesarean section and abortion, is also a major contributor [67]. Furthermore, there is higher morbidity in pregnancy among women with HIV infection with higher risks of prematurity [69] and low birth weight [70] compared to HIV-negative women. One should note however that it is challenging to distinguish the impact of HIV infection itself from the effects of poverty, addiction, or poor generalized health on pregnancy outcomes in this population. Interestingly, HAART appears to modify this risk, reducing the incidence of maternal morbidity and mortality [71, 72]. It should be noted that a vast majority of the data on health outcomes in pregnancy have been generated from studies in low-income countries where the maternal health infrastructure may be fragile. Data from the US and other high-income countries show better outcomes, though maternal morbidity and mortality in the setting of HIV infection are compromised even in this setting [73]. In general, most women in the USA with HIV infection who choose to have a child have uncomplicated pregnancies with favorable outcomes.
Transmission of HIV to the Child: Prevention of Maternal to Child Transmission (PMTCT)
In the late 1980s and early 1990s, perinatal transmission and progression of perinatally transmitted neonatal infection was a major health concern in the pediatric population. At that time, the risk of perinatal transmission of HIV was as high as 20–30 %, and factors predicting which women were more or less likely to transmit the virus were mostly unknown. Diagnosis of infection in the first year of life was still very difficult, and it was thought that infected babies faced an imminent risk of early childhood death. In these early days of the epidemic, the CDC recommended that HIV-infected women delay or defer childbirth until more was known about the virus [8].
However, since that time, the standard of intrapartum care in the setting of HIV infection has dramatically changed globally. Most treatment guidelines now recommend HAART for women during pregnancy regardless of CD4 T-cell counts or plasma HIV-RNA PCR (viral loads) [74, 75]. Those with viral loads greater than 400 copies/mL should receive zidovudine before vaginal or cesarean delivery, those with viral loads greater than 1,000 are recommended to undergo cesarean section before the onset of labor to reduce the risk of perinatal transmission, and all infants should be referred for prophylaxis after birth. With these recommendations, the risk of perinatal HIV transmission has dropped significantly in most countries, and in the USA, it is now lower than 3 % [6]. Public health authorities in many countries including the World Health Organization (WHO), U.S. Preventive Services Task Force (USPSTF), and the CDC have since revised their reproductive policy recommendations for HIV-infected women to a policy of non-directed reproductive counseling that is supportive of the patient’s reproductive desires [76].
These recommendations notwithstanding, in 2010, 390,000 children became infected with the HIV globally, 90 % of which were acquired through mother-to-child transmission (MTCT) during pregnancy, labor and delivery, or breastfeeding, and nearly all of them were born in sub-Saharan Africa [77]. Global prevention of pediatric HIV infection therefore remains a sexual and reproductive health priority and is highlighted in 4 of the 8 United Nations Millennium Development Goals—promoting gender equality and empowering women, reducing child mortality, improving maternal health, and combating HIV/AIDS, malaria, and other diseases [78].
Transmission of HIV to the Partner
An additional issue of public health importance is that several studies have suggested that the risk of acquiring HIV or transmitting HIV to an uninfected partner may be higher during pregnancy [79, 80]. Although not all studies have supported this finding [81], this only adds to the imperative of effectively preventing unintended pregnancy as a HIV prevention effort. Condom use promotion and effective use of antiretroviral therapy is paramount to the prevention of transmission. In addition, as previously mentioned, the daily oral fixed-dose combination tablet containing tenofovir, disoproxil, fumarate, and emtricitabine is approved for use for HIV prevention among sexually active adults at risk for HIV infection and serves as an additional prevention tool for certain individuals.
Contraceptive Care: Setting and Counseling
Integration of Family Planning and HIV Care
In regions of the USA and globally with a high prevalence of HIV and sexually transmitted infections (STI) in heterosexual populations, target audiences for HIV/STI and family planning services overlap broadly and can benefit from, and in fact prefer, joint services [82–87]. Barriers to integration have roots in historical, philosophical, and structural differences in the areas of family planning and HIV prevention [88], which has resulted in disjointed services in many regions. Clinic staff often view family planning and HIV prevention as mutually independent services and are not trained to administer them together [89]. Service delivery in family planning clinics tends to be an instructive and fact-giving approach, while HIV-testing service delivery is often a client-centered, counseling approach [90]. Dual-method use is not widely promoted; family planning programs often emphasize condom use rather than dual-method use despite the high failure rate of condoms for prevention of pregnancy [88]. Given the importance of dual-method use, it is therefore encouraged that HIV prevention and family planning programs provide integrated services mutually reinforcing HIV prevention and family planning goals [84, 88, 91].
Barriers to Contraceptive Use
Common factors influencing nonuse of contraception are lack of female decision-making power [62], poor economic resources [92], low quality care of family planning services, and desire for large families. The influence of HIV infection and HAART on these factors is poorly understood. Fear of side effects from contraception may be amplified among HIV-infected individuals who are often sensitive to their health status [62].
Fertility-Based Contraceptive Counseling
As with all women, it is important to develop a reproductive health plan that allows them to decide whether and when to have children. For women with HIV infection, it is especially important to consider the status of their disease in this counseling. As noted, when HIV infection is well controlled, the risks of maternal complications and transmission potential to the infant are significantly reduced. It is also important to recognize that HIV infection is not in itself a reason to assume that a woman does not desire to have children or to encourage women to not have children. One recent study among women attending an HIV clinic in Atlanta reported that the most common form of contraception was a tubal ligation, but many of these women regretted this decision and desired future fertility [93]. This same study reported that only about half of the women who were sexually active had discussed their contraceptive plans with their provider within the past year. This observation highlights the fact that although many women with HIV infection may access care, their care may not include a discussion of their fertility intentions or contraceptive needs.
For a woman who desires fertility, it is important to counsel her or refer her to a practitioner who can counsel her regarding optimization of her HIV status, potential health risks of pregnancy, the risk of transmission to her child, choice of antiretroviral medications that are safer to use during pregnancy, and methods to conceive that will reduce exposure and transmission to an HIV-negative partner. Women with HIV can successfully have a healthy pregnancy and, with correct use of effective antiretroviral therapy, have a very low risk of transmission of HIV to the child. While outside the scope of this chapter, this topic is extremely important for the health of the mother and her child and is the subject of US Department of Health and Human Services (DHHS) guidelines [76].
Reproductive Health Care for Women with HIV
As encounters for contraception are frequently combined with encounters for general and/or gynecologic wellness, a few practical considerations should be kept in mind when providing gynecologic care for women with HIV. Beyond the increased health risks associated with HIV infection discussed previously, women with HIV may be at greater risk of STIs, more frequent outbreaks from herpes simplex virus (HSV) and condyloma, vaginitis with candida and bacterial vaginosis, irregular menses, and early menopause. Furthermore, HPV-related vulvar, vaginal, and cervical dysplasias may occur more commonly among HIV-infected women, who have higher rates of HPV persistence and progression to cancer. Based on these increased risks, when providing reproductive health care the following are recommended.
Screening for STIs
The CDC recommends yearly screening for syphilis, gonorrhea, and chlamydia in women with HIV [94]. Although the prevalence of these infections tends to be the same as the prevalence in HIV-negative women, co-infection with STIs other than HIV can increase the transmission of HIV. Diagnosis and treatment of syphilis, trichomonas, gonorrhea, and chlamydial infections are the same as in HIV-negative women. Pelvic inflammatory disease may be more severe or complicated in HIV-infected women, but management does not differ overall from HIV-negative women [95].
HIV-infected women are more likely to suffer from recurrent HSV outbreaks that are extremely painful and may take longer to resolve. Some women require suppressive therapy to reduce the frequency of outbreaks. Because there is evidence that HIV transmission may be increased among women with genital HSV even without active lesions and that the treatment of HSV can reduce plasma and genital HIV viral load [96–98], some providers support suppressive therapy for HIV-infected women with HSV seropositivity. This is not a universal recommendation, however, as the most recent clinical trials have not shown a reduction in HIV or HSV transmission risk [99, 100]. Active and suppressive treatment of HSV for women with HIV infection typically requires higher doses and longer durations of appropriate antiviral agents. Current recommendations are available from the CDC (http://www.cdc.gov/std/treatment/2010/genital-ulcers.htm#hsv). Importantly, if women fail to respond to treatment, a viral culture should be obtained with sensitivity testing done for evaluation of resistant infections. Similar to HSV, chancroid (ulcers that occur following infection with H. ducreyi) requires close monitoring and typically longer therapy for resolution among HIV-infected women and should be treated per CDC guidelines.
Cervical Dysplasia and Cervical Cancer Screening
Current cervical cancer screening recommendations for HIV-infected women call for screening twice in the first year after diagnosis of HIV infection and then annually if the first two test results are normal. Although the incidence of cervical dysplasia is increased in women with HIV, women who undergo the recommended screening for and treatment of cervical dysplasia are not at increased risk of cervical cancer [95]. The role of HPV testing in the screening of HIV-infected women for cervical dysplasia has not been established but is likely to be the subject of future guidelines.
Vulvar, Vaginal, and Rectal Dysplasia, and Cancer
Although there are no well-established guidelines for screening for vulvar or vaginal dysplasia, a careful vulvar and vaginal examination should be done whenever a pelvic examination is performed. HIV-infected women should, therefore, undergo a pelvic examination annually at a minimum, based on cervical cancer screening guidelines. There are no guidelines for rectal pap smears among HIV-infected women; thus at this time a comprehensive evaluation of risk factors and symptoms in combination with an annual external examination at the time of a pelvic examination is recommended. Clinical manifestations of vulvar intraepithelial neoplasia (VIN) and vaginal intraepithelial neoplasia (VAIN) in HIV-infected women are similar as for women without HIV infection. Approximately 50 % of women with VIN are asymptomatic. In symptomatic women, the most common complaint is vulvar pruritus; other presentations include perineal pain or burning, dysuria, a visible lesion, or a palpable abnormality.
Human Papillomavirus (HPV) Vaccination
Efficacy of HPV vaccination in HIV-infected women is currently unknown and studies addressing this question are in progress [101, 102]. Many believe that, similar to the general population, HPV vaccination would offer some benefit to women with HIV infection, and since it is not contraindicated with immunosuppression, is recommended by some guidelines for HIV-infected males and females ages 13–26 years [75]. Both quadrivalent (for HPV types 6, 11, 16, 18) and bivalent (for HPV types 16 and 18) formulations are available, but the quadrivalent vaccine offers the additional potential benefit of prevention of anogenital warts associated with HPV types 6 and 11, which can be extensive in immunosuppressed patients.
Vulvovaginal Candidiasis (VVC) and Bacterial Vaginosis (BV)
The incidence of VVC is higher among HIV-infected women compared to uninfected women and correlates to the severity of immunodeficiency. Further, these infections may recur frequently especially among women with poorly controlled HIV infection. Treatment for VVC should not differ for HIV-infected women from that of uninfected women with the added importance of optimizing their HIV control [94, 103]. Recurrent infections, defined as four or more episodes each year, should be treated as complicated infections and treated with prolonged therapy. Women with CD4 counts less than 200 may have more persistent BV infections than those with well-controlled HIV. Similar to VVC, the treatment for BV in HIV positive women is the same as in uninfected women [94, 95].
Menstrual Problems
Evaluation of abnormal uterine bleeding among HIV-infected women should follow the same principles as among uninfected women. Evaluation should include the same endocrinologic evaluations (such as thyroid stimulating hormone [TSH] and prolactin levels), infection evaluation (such as gonorrhea and Chlamydia testing), imaging studies, and endometrial biopsy when indicated. Treatment to improve bleeding should consider the future fertility goals and expectations of the patient in terms of desired bleeding pattern.
Menopause
Menopause may occur earlier in HIV-positive women than HIV-negative women for reasons that remain poorly understood. Early menopause and the associated hypoestrogenemia may further heighten CVD and fragility bone disease risks for women living with HIV/AIDS [104]. As with HIV-negative women, hormone therapy may be considered for management of bothersome symptoms. Little has been studied about the interaction of hormone therapy with antiretroviral medications. A transdermal route may avoid first-pass metabolism and decrease drug interactions [105].
Starting a Birth Control Method
Taking a History
Prior to starting a contraceptive method for any woman, clinicians should obtain a directed history. This will include a medical history, contraceptive history, psychosocial and sexual history, as well as an assessment of her beliefs, possible misconceptions and fears, and her expectations with regard to use of contraception.
For women with HIV, specific history questions should include the following:
-
1
Do you have any medical problems? Do you have high blood pressure or diabetes? Do you get frequent headaches or migraines? Have you ever had a blood clot? Do you have active liver disease? Have you ever had cancer? Do you smoke?
Medical conditions or behaviors that limit the use of certain contraceptives need to be assessed among all women, including women with HIV. Other medical comorbidities such as hypertension, diabetes, breast cancer, or vascular disease will be important to consider when choosing a contraceptive method. Important potential contraindications will be present from conditions that may impact their vascular risk and increase their risk of blood clots including stroke associated with combined hormonal contraception. For example, it is important to probe about headaches to determine if a patient has migraines with aura that would be a contraindication to combined hormonal contraceptive methods. Some chronic medical conditions may impact women with HIV infection more commonly than those who do not have HIV infection. For example, liver diseases occur more frequently among HIV-infected women and are considered contraindicated with some contraceptives (see Chap. 18). After attaining a thorough history, referring to the CDC Medical Eligibility Criteria for Contraceptive Use (MEC) will be helpful in considering if any current medical comorbidities will limit the use of a specific contraceptive method [106].
-
2.
What Medications Are You Currently Taking?
There are several specific medications that have drug interactions with different contraceptives. To review potential medication contraindications, the use of checklists for this purpose is encouraged as often asking directly without probing questions may overlook important issues to consider. Antiretroviral medications that are important to consider are certain protease inhibitors such as ritonavir and other pharmacologic boosters such as cobicistat that interact with the cytochrome p450 pathway and may impact the efficacy of both the contraceptive and antiretroviral drug. Antibiotics and antifungal medications are generally safe to use with any of the contraceptive methods. As new medications are developed and integrated into clinical care, it is important to evaluate for potential interactions prior to initiation. For details regarding drug interactions, including those with antibiotics and antifungals, see Chap. 20. Drug interactions with antiretroviral drugs and contraceptives are reviewed later in this chapter.
-
3.
Have You Had Tuberculosis (TB) or Are You Currently Taking Medication for TB?
Tuberculosis is more common among HIV-infected individuals due to their immunosuppression. Although uncommon in the USA, pelvic TB is one of the rare conditions where an intrauterine device (IUD) is not recommended. Per the CDC MEC, pelvic TB is considered a category 4 (method should not be used) for initiation of an IUD and a category 3 (risks usually outweigh benefits) for continuation of an IUD [106]. Thus in most cases, IUDs should not be placed and should be removed for women with pelvic TB. History of successfully treated pelvic TB, however, should not preclude IUD use.
Rifampin and rifabutin, which are commonly used for treatment of TB, are considered a category 3 for combined hormonal contraceptives, progestin-only pills, and contraceptive implants due to drug–drug interactions. For details regarding drug interactions, see Chap. 20.
-
4.
Have You Recently Been Ill? Were You Recently Started on HAART? What Is Your Most Recent CD4 Count?
Accessing the individuals’ current HIV status is important before initiating a contraceptive method. HIV is not a contraindication for initiating any contraceptive, but caution should be exercised in placing an IUD in a woman with late-stage AIDS who is not receiving antiretroviral medications. Although IUD use over time is not associated with an increase in pelvic infections and may actually reduce the risk of pelvic infections, there is a slightly increased risk in pelvic infection over the first few weeks after placement. In women with AIDS who are severely immunocompromised with low CD4 T-cell counts or an active opportunistic infection, it may be prudent to delay an IUD placement until active opportunistic infections are controlled and/or immune status is improved with HAART (risks for these individuals typically outweigh the benefits in a CDC category 3 recommendation). Of note, these women are still at risk for pregnancy, and it is particularly important to protect from unplanned pregnancy given the increased risk of poor maternal and fetal outcomes. For these women, initiating an effective contraceptive immediately while concurrently stabilizing their HIV infection is a priority. Once their HIV is controlled with HAART, an IUD may be placed and may remain without increased risk of pelvic infection even if clinical status declines.
-
5.
What Have You Used Before? What Have You Been Told? What Are You Looking for with a Contraceptive Method?
Many women have had prior experience with different birth control methods. This experience may impact their willingness to use a method, both favorably and unfavorably. Added on to their prior experience, many women have been told that they cannot use specific methods of contraception. The information that they have received may not always be accurate. It is therefore important to start out discussing what their knowledge and perceptions are regarding contraception as this will direct your discussion and may impact contraceptive selection and continuation.
Lastly, what are their expectations with their contraceptive? Are they looking for something that is easy to use or something that makes their periods lighter? Choice of contraceptive should be directed toward addressing the patients’ specific goals. For example, women who are seeking to continue to have regular predictable cycles should consider combined hormonal contraceptives or the copper IUD. A discussion additionally should focus on the tiers of contraceptive efficacy. For example, tubal ligation, IUDs, and implants are the most effective methods to prevent pregnancy. For women desiring a highly effective long-term option, long-acting reversible contraceptives (LARCs) methods should be encouraged.
Choosing a Method
In choosing a contraceptive, you must work with the patient to identify her key goals, review the information, and help her find a method that will be best for her. Having HIV itself is not a contraindication to the use of any contraceptive method (Table 6.1); however, there are some issues to consider with each method.
For the most part, LARCs are ideal first choice methods to consider for this population given their superior effectiveness and safety profile for most women. LARC methods are user independent, meaning they do not require action by the patient to maintain effectiveness, such as the need to take pills daily or injections every 3 months. These methods are extremely safe with low risk of complications from use and are easily reversible with rapid return of fertility should the woman desire pregnancy. Furthermore, although the initial investment is high, LARCs are the most cost-effective methods for use over time as there is no additional cost accrued for the duration of contraceptive use until replaced. Further, these methods can be safely placed immediately postpartum and postabortion.
Levonorgestrel IUD (Mirena)
This LARC method is an ideal choice for many women with HIV, given that it is in the highest tier for effectiveness, with protection from pregnancy for 5 years with some evidence of off-label efficacy for up to 7 years [108], high continuation rates, and an excellent safety and tolerability profile. Typical use and perfect use pregnancy rates with use of the levonorgestrel IUD (LNG-IUD) are equivalent since it is a user independent method, with 0.2 unplanned pregnancies per 100 women in the first year of use [109, 110]. As mentioned above, if a woman is clinically unstable with late-stage AIDS and/or active opportunistic infections, placement may need to be delayed. However, once placed, it is an ideal method for continued use irrespective of clinical status. Further, there is some evidence to suggest that the LNG-IUD may reduce the risk of pelvic inflammatory disease (PID) as its mechanism of action is to thicken the cervical mucus, providing a barrier to semen and ascending infections [111]. Additional benefits include less bleeding, which would reduce transmission risk with the handling of infectious sanitary products. Further, the LNG-IUD offers protection of the endometrium that is important for women with anovulatory cycles or other risk factors for endometrial cancer. That said, women must know prior to placement that their bleeding pattern will change, they may have irregular bleeding, lighter menses, or no bleeding. Placement of an IUD is easily accomplished during a clinic visit with mild patient discomfort of short duration. Patient counseling should focus on initial irregular bleeding with the LNG-IUD that typically improves with continued use over the first 3–6 months. Women who are at high risk for HIV are likely at high risk for other pelvic infections as well. Keep in mind that a diagnosis of cervicitis or PID in a woman with an IUD does not necessitate removal of the IUD and women should be encouraged to keep it in place unless PID persists or worsens despite appropriate treatment.
Copper IUD (Paragard)
Another LARC method that should be considered among the best methods for women with HIV infection is the copper IUD. With exceptional protection from pregnancy lasting for 10 years with some evidence supporting efficacy for up to 12 years [112, 113], the copper IUD can be safely placed in most women with very few restrictions. Unintended pregnancy rates with copper IUD use are low with 0.6 and 0.8 pregnancies per 100 women in the first year of use for perfect and typical use, respectively [110]. This is an ideal method for women who desire to see a regular cycle every month and who are willing to tolerate potential increases in bleeding or cramping during their cycles. There are few contraindications to placement; similar to the LNG-IUD, as long as a woman is clinically stable with regard to her HIV, the IUD can safely be placed.
Contraceptive Implants
The contraceptive implant is a LARC method similarly ideal for HIV-infected women with few exceptions that would limit its safe initiation and use. Nexplanon (Merck, Whitehouse Station, NJ, USA), an etonogestrel implant, is a single rod device that once placed can last for up to 3 years. Other implants that are not currently available in the USA but are widely used in Africa include the levonorgestrel implants Jadelle and Sino-implant, which each last for 4–5 years with high typical and perfect use effectiveness (0.05 unintended pregnancies per 100 women in the first year of use for both typical and perfect use [110]). There have been case reports of increased pregnancy rates when etonogestrel implants are used in patients taking the antiretroviral drug efavirenz, a non-nucleotide reverse transcriptase inhibitor, but data are limited to make conclusive recommendations regarding the use of the implant for women on efavirenz [114] (see the section “Drug Interactions with Antiretroviral Regimens”).
Injectable Contraceptives
Depo-medroxyprogesterone acetate (DMPA) is marketed as Depo-Provera (Pfizer Inc., New York NY, USA) in the USA. Other progestin-only contraceptive injections are available in other countries. DMPA is highly convenient, requiring injections only every 3 months. With perfect use, defined as receiving an intramuscular injection every 11–13 weeks, there is a 0.2 % pregnancy rate in the first year, but a 6 % pregnancy rate with typical use [110] The most common reason for discontinuing the method is changes in bleeding pattern, but discontinuation rates are much lower among women who are adequately counseled on the possibility of irregular or heavy bleeding, or cessation of menstrual bleeding. DMPA may remain equally effective up to 15 weeks after the injection, which allows for some forgiveness in the “three month” rule [115]. However, this comes hand in hand with the disadvantage that return to fertility after discontinuation may be 9–10 months after the last injection. DMPA may cause increased weight gain in comparison to other hormonal contraceptive methods (see Chap. 10).
DMPA and other injectable progestins are widely available and affordable around the world, but some studies in high-risk populations outside the USA have raised concerns about the possibility that DMPA increases transmissibility of HIV from HIV-infected women to their uninfected partners, and increases susceptibility of HIV-negative women to the virus. However, the US Medical Eligibility Criteria continue to recommend DMPA as a “category 1,” indicating that the method can be used without restrictions, although with a clarification statement that describes the inconclusive nature of the evidence and instruction to recommend condoms for prevention of HIV in these populations [107]. This topic is discussed further in the section “Controversies and Research Gaps.”
Combined Hormonal Contraceptives
Combined hormonal contraceptives containing both progestin and estrogen to achieve their contraceptive benefit can be delivered via pills (many brands marketed in the USA), the transdermal patch (only available as Ortho Evra [Ortho-McNeil Pharmaceutical Inc., Raritan, NJ, USA] in the USA), and hormone-eluting vaginal ring (NuvaRing, Merck, Whitehouse Station, NJ, USA). Oral contraceptives are still the most popular hormonal contraceptive method in the USA. These methods have the benefits of being private and patient-controlled, and not requiring active involvement of medical providers after the initial prescription is given. They can be used in a cyclic way that simulates a natural monthly menstrual cycle for women who prefer that. On the other hand, they can also be used in a continuous manner to suppress menses when that is desired. With perfect use, combined contraceptive methods have a 0.3 % failure rate but a 9 % typical use pregnancy rate in the first year of use [110]. While combined contraceptive methods are recommended as safe in women with HIV or those at high risk of HIV (designated a “category 1” denoting a method that can be used without restriction in the CDC MEC), they may be contraindicated or used with caution in women who are using certain antiretroviral drugs (see the section “Drug Interactions with Antiretroviral Regimens”). As in HIV-negative women, estrogen-containing contraceptives are contraindicated in women with a history of venous thromboembolism (VTE), hypercoagulability, cardiovascular disease, migraine with aura, or smoking over the age of 35, due to increased risk of VTE or stroke in these women. Therefore, it is important to refer to the CDC MEC for guidance on contraceptive method selection for all women with complex medical conditions [106].
Progestin-Only Pills
Progestin-only pills (POPs) formulated in the USA contain 0.35 mg norethindrone. They carry the same rate of unintended pregnancy as combined contraceptives, 0.3 % with perfect use and 9 % with typical use [110]. Patients must be counseled that they should take the pill at the same time each day to provide effective contraception as the therapeutic level of each pill lasts only 25 h. The ideal candidate for this contraceptive is one who is highly reliable and has a regular daily schedule. Unlike estrogen-containing contraceptives, progestin-only pills are not contraindicated in women at risk for hypercoagulability. However, like combined contraceptives, progestin-only pills may alter and may be altered when combined with certain antiretroviral drugs (see the section “Drug Interactions with Antiretroviral Regimens”).
Male and Female Condoms
Traditional male condoms have a typical use failure rate of 18 % [110], and thus are not a highly reliable single choice for contraception. However, no contraceptive method other than condoms can prevent the transmission of HIV or STIs. The simultaneous use of highly effective contraception and condom use, termed dual protection, should be strongly encouraged in women with HIV and those women at high risk of acquiring HIV in order to avoid STI transmission and acquisition. Although not many high-quality studies comparing male to female condoms exist, available evidence suggests that female condoms prevent STIs including HIV as well as male condoms [116, 117]. Every contraceptive method should be presented in conjunction with condoms as a dual preventative strategy. In discussing condom use, a discussion of both the male and female condom is essential, reviewing strategies to increase use in the context of the individual’s sexual relationships, how to obtain and how to use these types of condoms correctly.
Spermicides
Spermicides are frequently used with other barrier methods of contraception such as condoms, sponges, and diaphragms. When used alone, perfect use has an 18 % failure rate, and typical use a 29 % failure rate [109]. Nonoxynol-9 is the most common active ingredient in spermicides in the USA. Besides having a high failure rate, it also may cause irritation and epithelial erosions of the vagina with repeated use. This may increase the opportunity for HIV transmission. In one study of high-risk women, those who used nonoxynol-9 more than three times a day had increased rates of HIV transmission [118]. For this reason, spermicides are labeled as a “4” in the CDC MEC—method is not to be used due to unacceptable health risks—in women with HIV and women at high risk for acquiring HIV [106].
Microbicides
Studies are currently ongoing to test vaginal microbicides that can help prevent HIV in high-risk populations. While the spermicide nonoxynol-9 is effective in vitro at killing HIV virions, as previously mentioned, in vivo it was associated with a higher risk of HIV transmission, likely due to disruption in the vaginal mucosa [118]. Clinical trials involving a topical gel containing the antiretroviral drug tenofovir have shown some promise in reducing the risk of HIV transmission. Other exciting technologies in development are microbicide-eluting vaginal rings, which eventually may be able to be combined with contraceptive methods to prevent both pregnancy and HIV in a single system. At this time, no effective microbicide is commercially available [119].
Lactational Amenorrhea
For HIV-infected mothers, recommendations in the USA are to bottle-feed infants to reduce the risk of postnatal HIV transmission via breast milk. This recommendation is different in developing countries where clean water, milk, or formula availability may limit adequate and safe nutrition for the baby. HIV transmission risk from breast milk can vary based upon maternal viral load, antiretroviral medications, and exclusivity of feeding. However, in countries where supplemental access to enriched formula is available, it is currently believed that the risks of breastfeeding in terms of HIV transmission to the child outweigh the potential benefits to the mother and the baby.
Cervical Cap or Diaphragm
Cervical caps and diaphragms are barrier contraceptives that are not commonly used. These methods have low levels of typical use effectiveness [110] and studies have shown that they do not reduce the risk of HIV transmission [120].
Emergency Contraceptives
Currently available emergency contraceptives include both the emergency contraceptive pills and the copper IUD. For pills, there are currently two drugs available for use, levonorgestrel-based regimens and ulipristal acetate. Ulipristal is more effective than levonorgestrel-based emergency contraceptives from 4 to 5 days after unprotected intercourse [121], and neither is recommended as reliable pregnancy prevention more than 5 days after unprotected intercourse. There is little data on the influence of HAART regimens on the effectiveness of these drug regimens; however there is some suggestion that the effectiveness will be reduced when taken with drugs that impact cytochrome p450 (see the section “Drug Interactions with Antiretroviral Regimens”). That said, as an “emergency” regimen, it is a last effort to reduce the risk of pregnancy when more effective contraceptive methods were not employed or a condom slipped or broke. For women using condoms as a primary birth control method, information about emergency contraception is imperative. Although some formulations are available over the counter, a prescription may be most affordable for certain patients and should be provided when appropriate. As the copper IUD is the most effective form of emergency contraceptive, it avoids all potential challenges associated with drug interactions, and offers long-term protection from pregnancy, this is an ideal method to promote in the setting when emergency contraception is needed.
Continuation of Contraception
For many contraceptive methods, anticipatory guidance can help avoid discontinuation of contraception. Many studies show that counseling on changes in bleeding patterns anticipated by contraceptive methods, such as progestin-only methods, helps reduce discontinuation of these methods. Many women will be reassured that although progestin-only methods cause irregular bleeding, for most women, the overall quantity of bleeding will be reduced and may completely stop after 1 year of use. Frequent visits when initiating a new method of contraception can help alleviate concerns. Women with HIV may also have frequent changes in their health status—e.g., changes in HAART regimens or development of liver disease—that may necessitate frequent reevaluation of the appropriateness of their contraceptive plan.
Drug Interactions with Antiretroviral Regimens
Antiretroviral drugs may affect the level of steroid hormones in the blood and vice versa, owing to shared metabolic pathways utilizing hepatic cytochrome P450 [68, 75, 76]. This could potentially change the effectiveness and/or safety of either the contraceptive method or the antiretroviral drug. Several antiretroviral drugs (ARVs) have interactions with combined oral contraceptives that either decrease or increase blood levels of ethinyl estradiol or the progestin component, which could potentially decrease contraceptive effectiveness or increase estrogen- or progestin-related adverse effects, respectively. In particular, ritonavir-boosted protease inhibitors may substantially decrease the bioavailable steroid hormone [122, 123] in combined oral contraceptives, which may lead to contraceptive failure. The use of oral contraceptives, both combined and progestin-only, in women on ritonavir-boosted protease inhibitors is considered a category 3 per the CDC MEC [68], meaning that the risks of use typically outweigh the benefits of use. The newer pharmacologic booster cobicistat also affects the cytochrome P450 system and may result in increased progestin levels. The effects of this are not yet known and current guidelines for the USMEC do not specify specific guidelines to avoid these combined regimens. However, whenever possible, alternative contraceptives should be considered. Neither DMPA nor LNG-IUDs have been demonstrated to significantly interact with antiretroviral regimens [124]. The action of the copper IUD is independent of drug metabolism mechanisms and carries no theoretical or actual interactions with antiretroviral therapy.
No interactions have been reported between nucleoside reverse transcription inhibitor (NRTI) drugs and contraceptives. The clinical significance of smaller alterations in hormonal bioavailability from non-nucleoside reverse transcription inhibitor (NNRTI) drugs is unclear. The NIH Guidelines for Antiretroviral Use recommend the use of alternative or additional contraceptive methods for the use of certain NNRTIs [125]; however the CDC MEC currently considers use of progestin-only pills, hormonal implants, and all combined hormonal methods to be a category 2, meaning that the benefits typically outweigh the risks [106]. Overall, data are relatively limited and the clinical implications of these findings are unclear. Recommendations regarding other combined hormonal contraceptive methods are based on combined oral contraceptive pill use, as very little evidence is available regarding the effects of ARVs on bioavailable hormone from transdermal patches or vaginal rings [126]. Small studies of HIV-infected women receiving DMPA while on ART showed no significant interactions between DMPA and efavirenz, nevirapine, nelfinavir, or NRTI drugs [110, 127–129].
Although the data on efavirenz and birth defects are limited, due to potential teratogenic effects, it is often not used as a first-line option in women of childbearing age. If it is used, it should be accompanied by a very reliable contraceptive plan. Unfortunately, efavirenz can lead to decreases in circulating progestins [114, 130]. For progestin-containing methods, the degree of reduction in effectiveness of these methods may vary based on the method. For example, DMPA has very high circulating levels of progestin, so small reductions in the progestin concentration will likely not impact its effectiveness. Etonogestrel implants, on the other hand, have lower circulating progestin levels such that small reductions could impact the method effectiveness. The use of efavirenz has also been associated with increased risk of failure of progestin implants in case reports [114]. However, given the limited data, the current CDC MEC [68] recommendations consider the use of efavirenz a category 2 for use. There are no data at this time regarding the use of efavirenz and levonorgestrel-containing IUDs.
A summary of recommendations regarding specific antiretroviral drugs, their effect on hormone levels, and current USMEC guidelines is given in Table 6.2. Notably, current recommendations [75] support that concerns about pharmacokinetic interactions between oral and implant hormonal contraceptives and ARVs should not prevent clinicians from prescribing hormonal contraceptives for women on ART if that is their preferred contraceptive method. If a woman chooses to use hormonal contraceptives and drug interactions with ARVs are known or potential, then additional or alternative contraceptive methods may be recommended. Particularly, consistent use of condoms to prevent transmission of HIV and protect against other sexually transmitted diseases is recommended for all HIV-infected women and their partners, regardless of contraceptive use.
Controversies and Research Gaps
Concerns Regarding Increased HIV Acquisition, Transmission, and Disease Progression
Some observational studies have raised concerns about an increased risk of acquiring HIV in HIV-negative women and shedding HIV in HIV-positive women using hormonal contraception, particularly DMPA [148, 149]. Theoretically, progestins could increase susceptibility to and acquisition of HIV by thinning the vaginal epithelium, increasing the frequency of target cells, and modulating the systemic immune system [150]. However, the available population-based studies are inconsistent, underpowered, and often flawed [151]. The literature is even weaker on a possible association between oral contraceptive pills and HIV risk. No studies have examined the association of HIV susceptibility with progestin-containing implants or intrauterine devices, hormonal patches, or hormonal rings [151]. Because of the heterogeneous outcomes and low quality of the studies available on hormonal contraception and HIV risk, both the WHO and the revised USMEC put no restrictions (category 1) on contraceptive use in women at high risk for acquiring HIV or HIV-positive women. At the same time, both MECs make strong recommendations that because of the unclear information, women with HIV or those at risk for HIV should always use condoms to prevent HIV transmission [106, 107].
Theoretical concerns together with one randomized controlled trial have also called attention to the possibility of accelerated HIV/AIDS disease progression in HIV-positive women using hormonal contraception. A study by Stringer et al. [152] randomized HIV positive postpartum women to receive copper IUD or hormonal contraception (including DMPA, combined oral contraceptives, and progestin-only pills), showing an increased risk of HIV progression to CD4 count below 200 cells/mL in the hormonal contraception users. However, this study is flawed by differential losses to follow up, high rates of switching of methods, and a substandard control group (copper IUD, for which implications on HIV progression have not been studied) [153]. Other observational studies published on hormonal contraception and HIV progression show no association [154, 155].
Conclusion: Key Points in Providing Care
-
Effective contraception can reduce maternal and pediatric morbidity and mortality from unintended pregnancies complicated by HIV.
-
Desires for planned pregnancy should not be overlooked in women with HIV. Addressing fertility intentions can help optimize health status before pregnancy occurs.
-
Family planning and STI prevention services should be integrated whenever possible as they share common goals.
-
Regular STI and cervical cancer screening are critical parts of routine reproductive health care in women with HIV. Recommendations for screening may differ from those for HIV-negative women.
-
Long-acting reversible contraceptives (LARCs) are highly effective and ideal for most women with HIV.
-
Spermicides should be avoided in women with HIV and women at high risk for acquiring HIV.
-
Interactions exist between antiretroviral drugs and oral contraceptives that may limit contraceptive effectiveness.
-
All women, especially HIV-positive women and those women at high risk of acquiring HIV, should be encouraged to use “dual protection” by combining a highly effective contraceptive with condoms during sex to maximally prevent pregnancy and STI infection.
References
Centers for Disease Control and Prevention (CDC). Pneumocystis pneumonia – Los Angeles. MMWR. 1981;30:250–2.
Ribeiro PS, Jacobsen KH, Mathers CD, Garcia-Moreno C. Priorities for women's health from the Global Burden of Disease study. Int J Gynaecol Obstet. 2008;102(1):82–90. Epub 2008/04/05.
World Health Organization. Women and health: today's evidence, tomorrow's agenda. Geneva: World Health Organization; 2009.
Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic; 2013.
Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data – United States and 6 U.S. dependent areas – 2010. HIV Surveill Suppl Rep. 2012;17(3). Epub Jun 2012.
Centers for Disease Control and Prevention (CDC). Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. HIV Surveill Suppl Rep. 2012;17(4). Epub Dec 2012.
Centers for Disease Control and Prevention (CDC). Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. HIV Surveill Suppl Rep. 2012;17(4).
Centers for Disease Control and Prevention (CDC). Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR. 1985;34(48):721–6. 31–2. Epub 1985/12/06.
Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. Epub 2010/07/21.
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. Epub 2011/07/20.
Reynolds SJ, Makumbi F, Nakigozi G, Kagaayi J, Gray RH, Wawer M, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25(4):473–7. Epub 2010/12/17.
Cunningham WE, Andersen RM, Katz MH, Stein MD, Turner BJ, Crystal S, et al. The impact of competing subsistence needs and barriers on access to medical care for persons with human immunodeficiency virus receiving care in the United States. Med Care. 1999;37(12):1270–81. Epub 1999/12/22.
Fleishman JA, Gebo KA, Reilly ED, Conviser R, Christopher Mathews W, Todd Korthuis P, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000–2002. Med Care. 2005;43(9 Suppl):III40–52. Epub 2005/08/24.
Ribaudo H, Smith K, Robbins G, Flexner C, Haubrich R, Chen Y, et al. Race differences in the efficacy of initial ART on HIV infection in randomized trials undertaken by ACTG. 18th Conference on retroviruses and opportunistic infections, Boston, MA, 27 Feb to 2 Mar 2011.
Murphy DA, Greenwell L, Hoffman D. Factors associated with antiretroviral adherence among HIV-infected women with children. Women Health. 2002;36(1):97–111. Epub 2002/09/07.
Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–9. Epub 2008/07/03.
Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. Epub 2006/08/16.
Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33(4):506–12. Epub 2003/07/19.
Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24(8):1228–30. Epub 2010/04/20.
Klein D, Hurley LB, Quesenberry Jr CP, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30(5):471–7. Epub 2002/08/03.
Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205 Suppl 3:S355–61. Epub 2012/05/18.
Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. Epub 2007/04/26.
Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. Epub 2013/03/06.
Freiberg MS, Chang CC, Skanderson M, McGinnis K, Kuller LH, Kraemer KL, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4(4):425–32. Epub 2011/06/30.
Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr. 2011;57(3):245–53. Epub 2011/04/19.
Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44(12):1625–31. Epub 2007/05/23.
Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61(5):511–23. Epub 2013/02/02.
WWHOT report: WWHOTwh. 2012. http://www.who.int/gho/publications/world_health_statistics/2012/en/. Accessed 8 Aug 2013
Pearce D, Ani C, Espinosa-Silva Y, Clark R, Fatima K, Rahman M, et al. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol. 2012;110(8):1078–84. Epub 2012/07/06.
Boccara F, Mary-Krause M, Teiger E, Lang S, Lim P, Wahbi K, et al. Acute coronary syndrome in human immunodeficiency virus-infected patients: characteristics and 1 year prognosis. Eur Heart J. 2011;32(1):41–50. Epub 2010/10/23.
Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H, et al. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127(17):1767–74. Epub 2013/04/02.
Klein D, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, et al. Contribution of immunodeficiency to CHD: cohort study of HIV+ and HIV− Kaiser permanent members. Program and abstracts of the 18th conference on retroviruses and opportunitistic infections, Boston, MA; 2011.
Fine DM, Atta MG. Kidney disease in the HIV-infected patient. AIDS Patient Care STDS. 2007;21(11):813–24. Epub 2008/02/05.
Crum-Cianflone NF, Weekes J, Bavaro M. Review: thromboses among HIV-infected patients during the highly active antiretroviral therapy era. AIDS Patient Care STDS. 2008;22(10):771–8. Epub 2008/09/12.
Madeddu G, Spanu A, Solinas P, Calia GM, Lovigu C, Chessa F, et al. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging. 2004;48(1):39–48. Epub 2004/06/15.
Vescini F, Borderi M, Buffa A, Sinicropi G, Tampellini L, Chiodo F, et al. Bone mass in HIV-infected patients: focus on the role of therapy and sex. J Acquir Immune Defic Syndr. 2003;33(3):405–7. Epub 2003/07/05.
Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–74. Epub 2006/11/07.
Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. health care system. J Clin Endocrinol Metab. 2008;93(9):3499–504.
Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52(8):1061–8. Epub 2011/03/15.
Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6(2):e17217. Epub 2011/03/02.
Prior J, Burdge D, Maan E, Milner R, Hankins C, Klein M, et al. Fragility fractures and bone mineral density in HIV positive women: a case-control population-based study. Osteoporos Int. 2007;18(10):1345–53. Epub 2007/08/01.
Guerri-Fernandez R, Vestergaard P, Carbonell C, Knobel H, Aviles FF, Castro AS, et al. HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J Bone Miner Res. 2013;28(6):1259–63. Epub 2013/01/31.
Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728–36. Epub 2008/05/21.
Maki PM, Martin-Thormeyer E. HIV, cognition and women. Neuropsychol Rev. 2009;19(2):204–14. Epub 2009/05/12.
Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. Epub 2001/05/01.
Siemieniuk RA, Krentz HB, Gill MJ. Intimate partner violence and HIV: a review. Curr HIV AIDS Rep. 2013;10(4):380–9.
Chen JL, Philips KA, Kanouse DE, Collins RL, Miu A. Fertility desires and intentions of HIV-positive men and women. Fam Plan Perspect. 2001;33(4):144–52, 65. Epub 2001/08/11.
Panozzo L, Battegay M, Friedl A, Vernazza PL. High risk behaviour and fertility desires among heterosexual HIV-positive patients with a serodiscordant partner – two challenging issues. Swiss Med Wkly. 2003;133(7–8):124–7. Epub 2003/03/20.
Ross A, Van der Paal L, Lubega R, Mayanja BN, Shafer LA, Whitworth J. HIV-1 disease progression and fertility: the incidence of recognized pregnancy and pregnancy outcome in Uganda. AIDS. 2004;18(5):799–804. Epub 2004/04/13.
Sherr L, Barry N. Fatherhood and HIV-positive heterosexual men. HIV Med. 2004;5(4):258–63. Epub 2004/07/09.
Stephenson JM, Griffioen A. The effect of HIV diagnosis on reproductive experience. Study Group for the Medical Research Council Collaborative Study of Women with HIV. AIDS. 1996;10(14):1683–7. Epub 1996/12/01.
Thackway SV, Furner V, Mijch A, Cooper DA, Holland D, Martinez P, et al. Fertility and reproductive choice in women with HIV-1 infection. AIDS. 1997;11(5):663–7. Epub 1997/04/01.
Cooper D, Moodley J, Zweigenthal V, Bekker LG, Shah I, Myer L. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav. 2009;13 Suppl 1:38–46. Epub 2009/04/04.
Nattabi B, Li J, Thompson SC, Orach CG, Earnest J. A systematic review of factors influencing fertility desires and intentions among people living with HIV/AIDS: implications for policy and service delivery. AIDS Behav. 2009;13(5):949–68. Epub 2009/03/31.
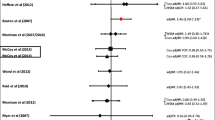
Hoffman IF, Martinson FE, Powers KA, Chilongozi DA, Msiska ED, Kachipapa EI, et al. The year-long effect of HIV-positive test results on pregnancy intentions, contraceptive use, and pregnancy incidence among Malawian women. J Acquir Immune Defic Syndr. 2008;47(4):477–83. Epub 2008/01/23.
Heys J, Kipp W, Jhangri GS, Alibhai A, Rubaale T. Fertility desires and infection with the HIV: results from a survey in rural Uganda. AIDS. 2009;23 Suppl 1:S37–45. Epub 2010/02/02.
Desgrées-Du-Loû A, Msellati P, Viho I, Yao A, Yapi D, Kassi P, et al. Contraceptive use, protected sexual intercourse and incidence of pregnancies among African HIV-infected women. DITRAME ANRS 049 Project, Abidjan 1995–2000. Int J STD AIDS. 2002;13(7):462–8.
Balkus J, Bosire R, John-Stewart G, Mbori-Ngacha D, Schiff MA, Wamalwa D, et al. High uptake of postpartum hormonal contraception among HIV-1-seropositive women in Kenya. Sex Transm Dis. 2007;34:25–9.
Polis CB, Gray RH, Lutalo T, Nalugoda F, Kagaayi J, Kigozi G, et al. Trends and correlates of hormonal contraceptive use among HIV-infected women in Rakai, Uganda, 1994–2006. Contraception. 2011;83:549–55.
King R, Estey J, Allen S, Kegeles S, Wolf W, Valentine C, et al. A family planning intervention to reduce vertical transmission of HIV in Rwanda. AIDS. 1995;9 Suppl 1:S45–51. Epub 1995/07/01.
Rutenberg N, Baek C. Field experiences integrating family planning into programs to prevent mother-to-child transmission of HIV. Stud Fam Plann. 2005;36(3):235–45. Epub 2005/10/08.
Grabbe K, Stephenson R, Vwalika B, Ahmed Y, Vwalika C, Chomba E, et al. Knowledge, use, and concerns about contraceptive methods among sero-discordant couples in Rwanda and Zambia. J Womens Health (Larchmt). 2009;18(9):1449–56. Epub 2009/08/28.
Pitter C. PMTCT implementation and the importance of male involvement. Washington, DC: Elizabeth Glaser Pediatric AIDS Foundation; 2010.
Lozano R, Wang H, Foreman KJ, Rajaratnam JK, Naghavi M, Marcus JR, et al. Progress towards millennium development goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet. 2011;378(9797):1139–65. Epub 2011/09/23.
van Dillen J, Meguid T, van Roosmalen J. Maternal mortality audit in a hospital in Northern Namibia: the impact of HIV/AIDS. Acta Obstet Gynecol Scand. 2006;85(4):499–500. Epub 2006/04/14.
Wandabwa JN, Doyle P, Longo-Mbenza B, Kiondo P, Khainza B, Othieno E, et al. Human immunodeficiency virus and AIDS and other important predictors of maternal mortality in Mulago Hospital Complex Kampala Uganda. BMC Public Health. 2011;11:565. Epub 2011/07/16.
Moran NF, Moodley J. The effect of HIV infection on maternal health and mortality. Int J Gynaecol Obstet. 2012;119 Suppl 1:S26–9. Epub 2012/08/15.
Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use. MMWR. 2010;59(RR-4):1–86.
Mirpuri J, Jain L. Issues of prematurity and HIV infection. Clin Perinatol. 2010;37(4):887–905, xi. Epub 2010/11/17.
Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105(8):836–48. Epub 1998/09/24.
Kuhn L, Semrau K, Ramachandran S, Sinkala M, Scott N, Kasonde P, et al. Mortality and virologic outcomes after access to antiretroviral therapy among a cohort of HIV-infected women who received single-dose nevirapine in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009;52(1):132–6. Epub 2009/06/10.
Marazzi MC, Palombi L, Nielsen-Saines K, Haswell J, Zimba I, Magid NA, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS. 2011;25(13):1611–8. Epub 2011/06/16.
Parikh L, Timofeev J, Singh J, Sullivan S, Huang CC, Landy HJ, et al. Racial disparities in maternal and neonatal outcomes in HIV-1 positive mothers. Am J Perinatol. 2013. Epub 2013/09/04.
World Health Organization. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Programmatic update. Geneva: World Health Organization; 2012.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Contract No.: Nov 12, 2013.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2013 [updated 7/31/12]. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 14 Nov 2013.
UNICEF. Childinfo: monitoring the situation of children and women: statistics by area/HIV/AIDS. 2012.
UN. The millennium development goals report 2012. New York, NY: 2012. Contract No.: 12-24532.
Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–8. Epub 2005/10/04.
Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–95. Epub 2011/07/26.
Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21(8):1027–34. Epub 2007/04/26.
Stover J, editor. Reducing the costs of effective AIDS control programmes through appropriate targeting of interventions. International AIDS conference, 1996, Vancouver, Canada; 1996.
Peterman TA, Todd KA, Mupanduki I. Opportunities for targeting publicly funded human immunodeficiency virus counseling and testing. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(1):69–74.
Sweeney C. 1 of 3 Pregnancies unwanted. Popline. 1992;14:1. Epub 1992/01/01.
Kell PD, Barton SE, Boag FC. Incorporating patients' views in planning services for women with HIV infection. Genitourin Med. 1992;68(4):233–4.
McCarthy GA, Cockell AP, Kell PD, Beevor AS, Boag FC. A women-only clinic for HIV, genitourinary medicine and substance misuse. Genitourin Med. 1992;68(6):386–9.
Carlin EM, Russell JM, Sibley K, Boag FC. Evaluating a designated family planning clinic within a genitourinary medicine clinic [see comments]. Genitourin Med. 1995;71(2):106–8.
Cates Jr W, Stone K. Family planning, sexually transmitted disease, and contraceptive choice: a literature update. Part II. Fam Plann Perspect. 1992;24(3):122–8.
Daley D. Reproductive health and AIDS-related services for women: how well are they integrated? Fam Plann Perspect. 1994;26(6):264–9. Epub 1994/11/01.
Becker J, Ureno M. How HIV has helped family planning: sexuality, the essential link. International conference on AIDS, Vancover, BC, 7–12 Jul 1996
Chandhok K, Sankaran S, Peters S, Mathews G, Manoharan S, Thomas S, editors. Developing comprehensive intervention programs for women in the reproductive age group. International AIDS conference, Vancouver, BC, Canada; 1996.
Hamid S, Stephenson R. Provider and health facility influences on contraceptive adoption in urban Pakistan. Int Fam Plan Perspect. 2006;32(2):71–8. Epub 2006/07/14.
Badell ML, Lathrop E, Haddad LB, Goedken P, Nguyen ML, Cwiak CA. Reproductive health care needs and desires in a cohort of HIV-positive women. Infect Dis Obstet Gynecol. 2012;2012:107878. Epub 2012/07/05.
Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR 2010;59(RR-12). Epub 17 Dec 2010.
ACOG Practice Bulletin No. 117: gynecologic care for women with human immunodeficiency virus. Obstet Gynecol. 2010;116(6):1492–509. Epub 26 Nov 2010.
Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196(10):1500–8. Epub 2007/11/17.
Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198(12):1804–8. Epub 2008/10/22.
Dunne EF, Whitehead S, Sternberg M, Thepamnuay S, Leelawiwat W, McNicholl JM, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49(1):77–83. Epub 2008/08/01.
Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–39. Epub 2010/01/22.
Mujugira A, Magaret AS, Celum C, Baeten JM, Lingappa JR, Morrow RA, et al. Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis. 2013;208(9):1366–74. Epub 2013/08/01.
Kojic E, Cu-Uvin S. Safety of and immune response to the human papillomavirus (HPV) vaccine in HIV-infected women. National Library of Medicine; 2013. http://clinicaltrials.gov/ct2/show/NCT00604175. Accessed 14 Nov 2013
Kahn J, Squires K. Impact of an HPV vaccine in HIV-infected young women. National Library of Medicine; 2013. http://clinicaltrials.gov/ct2/show/NCT00604175. Accessed 14 Nov 2013
Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010 MMWR recommendations and reports. MMWR. 2010;59(RR-12):1–110. Epub 2010/12/17.
Conde DM, Silva ET, Amaral WN, Finotti MF, Ferreira RG, Costa-Paiva L, et al. HIV, reproductive aging, and health implications in women: a literature review. Menopause. 2009;16(1):199–213. Epub 2008/09/19.
Kanapathipillai R, Hickey M, Giles M. Human immunodeficiency virus and menopause. Menopause. 2013;20(9):983–90. Epub 2013/03/28.
Centers for Disease Control. U.S. medical eligibility criteria for contraceptive use, 2010. MMWR recommendations and reports: morbidity and mortality weekly report recommendations and reports. MMWR. 2010;59(RR-4):1–86. Epub 2010/06/19.
CDC. Update to CDC's US medical eligibility criteria for contraceptive use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR. 2012;61(24):449–52. Epub 2012/06/22.
Sivin I, Stern J, Coutinho E, Mattos CE, el Mahgoub S, Diaz S, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the Copper T380 Ag IUDS. Contraception. 1991;44(5):473–80. Epub 1991/11/01.
Trussell J. Contraceptive efficacy. Arch Dermatol. 1995;131(9):1064–8. Epub 1995/09/01.
Hatcher RA, Trussell J, Stewart FH, Nelson AL, Cates W, Guest F, et al. Contraceptive technology, 20th rev ed. New York: Ardent Media, Inc.; 2011. xxviii, 871 p., [7] p. of plates p.
Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years' comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77(2):261–4. Epub 1991/02/01.
Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–52. Epub 1998/03/12.
Hatcher R, Trussell J, Nelson A, Cates W, Stewart F, Kowel D. Contraceptive technology. 20th ed. New York: Ardent Media; 2011.
Leticee N, Viard JP, Yamgnane A, Karmochkine M, Benachi A. Contraceptive failure of etonogestrel implant in patients treated with antiretrovirals including efavirenz. Contraception. 2012;85(4):425–7. Epub 2011/11/01.
CDC. U.S. selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Centers Dis Control. 2013;62(RR-05):1–60. Epub 2013/06/21.
Trussell J, Sturgen K, Strickler J, Dominik R. Comparative contraceptive efficacy of the female condom and other barrier methods. Fam Plann Perspect. 1994;26(2):66–72. Epub 1994/03/01.
Gallo MF, Kilbourne-Brook M, Coffey PS. A review of the effectiveness and acceptability of the female condom for dual protection. Sex Health. 2012;9(1):18–26. Epub 2012/02/22.
Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–7. Epub 2002/10/18.
Gupta SK. Nutan. Clinical use of vaginal or rectally applied microbicides in patients suffering from HIV/AIDS. HIV. HIV AIDS (Auckl). 2013;5:295–307.
Padian NS, van der Straten A, Ramjee G, Chipato T, de Bruyn G, Blanchard K, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370(9583):251–61. Epub 2007/07/17.
Brache V, Cochon L, Deniaud M, Croxatto HB. Ulipristal acetate prevents ovulation more effectively than levonorgestrel: analysis of pooled data from three randomized trials of emergency contraception regimens. Contraception. 2013;88(5):611–8. Epub 2013/07/03.
German P, Wang M, Warren D, Kearney B, editors. Pharmacokinetic interaction between norgestimate/ethinyl estradiol and EVG/COBI/FTC/TDF single tablet regimen. 12th International workshop on clinical pharmacology of HIV therapy, Miami, FL, Apr 2011.
Carten M, Kiser J, Kwara A, Ma Whinney S, S. C-U, editors. Pharmacokinetic (PK) interactions between the hormonal emergency contraception Plan B (levonorgestrel) and efavirenz (EFV). 17th Conference on retroviruses and opportunistic infection, San Francisco, Feb 2010
Robinson JA, Jamshidi R, Burke AE. Contraception for the HIV-positive woman: a review of interactions between hormonal contraception and antiretroviral therapy. Infect Dis Obstet Gynecol. 2012;2012:890160. Epub 2012/08/29.
Adolescents PoAGfAa. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2013. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 2 Nov 2013.
Centers for Disease Control and Prevention (CDC). Update to CDC's US medical eligibility criteria for contraceptive use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR. 2012;61(24):449–52. Epub 22 Jun 2012.
Nanda K, Amaral E, Hays M, Viscola MA, Mehta N, Bahamondes L. Pharmacokinetic interactions between depot medroxyprogesterone acetate and combination antiretroviral therapy. Fertil Steril. 2008;90(4):965–71. Epub 2007/09/21.
Watts DH, Park JG, Cohn SE, Yu S, Hitti J, Stek A, et al. Safety and tolerability of depot medroxyprogesterone acetate among HIV-infected women on antiretroviral therapy: ACTG A5093. Contraception. 2008;77(2):84–90. Epub 2008/01/30.
Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, et al. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clin Pharmacol Therapy. 2007;81(2):222–7. Epub 2006/12/29.
Vogler MA, Patterson K, Kamemoto L, Park JG, Watts H, Aweeka F, et al. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: pharmacokinetic results of ACTG trial A5188. J Acquir Immune Defic Syndr. 2010;55(4):473–82. Epub 2010/09/16.
Kearney B, Isaacson E, Sayre J, Cheng A, editors. Tenofovir DF and oral contraceptives: lack of a pharmacokinetic drug interaction. 43rd Interscience conference on antimicrobial agents and chemotherapy, Chicago, IL; Washington, DC, 14–17 Sep 2003.
Aweeka FT, Rosenkranz SL, Segal Y, Coombs RW, Bardeguez A, Thevanayagam L, et al. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS. 2006;20(14):1833–41. Epub 2006/09/07.
Joshi A, Fiske W, Benedek I, White SJ, Joseph JL, Kornhauser DM. Lack of a pharmacokinetic interaction between efavirenz (DMP 266) and ethinyl estradiol in healthy female volunteers. 5th Conference on retroviruses and opportunistic infections, Chicago, IL, 1–5 Feb 1998.
Sevinsky H, Eley T, He B, et al. Effect of efavirenz on the pharmacokinetics of ethinyl estradiol and norgestimate in healthy female subjects [Abstract A958]. In: Program and abstracts of the 48th interscience conference on antimicrobial agents and chemotherapy, Washington, DC, 25–28 Oct 2008. Washington, DC: American Society for Microbiology; 2008.
Scholler-Gyure M, Debroye C, Aharchi F, et al. No clinically relevant effect of TMC125 on the pharmacokinetics of oral contraceptives. 8th International congress on drug therapy in HIV infection, Glasgow, 12–16 Nov 2006.
Mildvan D, Yarrish R, Marshak A, Hutman HW, McDonough M, Lamson M, et al. Pharmacokinetic interaction between nevirapine and ethinyl estradiol/norethindrone when administered concurrently to HIV-infected women. J Acquir Immune Defic Syndr. 2002;29(5):471–7. Epub 2002/05/01.
Tibotec Therapeutics Inc. Edurant [package insert]. Raritan, NJ; 2011.
Bristol-Meyers Squibb. Reyataz (atazanavir) package label. 2010.
Sekar V, Lefebvre E, Seal S. Pharmacokinetic interaction between nevirapine and ethinyl estradiol, norethindrone, and TMC114, a new protease inhibitor [Abstract A-368]. San Francisco, CA: American Society for Microbiology, 27–30 Sep 2006. Report No.; 2006
Glaxo Smith Kline. Prescription medicines. Lexiva (fosamprenavir calcium). 2009.
Glaxo Smith Kline. Study APV10020. A phase I, open label, two period, single-sequence, drug–drug interaction study comparing steady-state plasma ethinyl estradiol and norethisterone pharmacokinetics following administration of brevinor for 21 days with and without fosamprenavir 700 mg twice daily (BID) and ritonavir 100 mg (BID) for 21 days in healthy adult female subjects. 2009.
Abbott Laboratories. Lopinavir and ritonavir prescribing information. North Chicago, IL: Abbott Laboratories; 2009.
Boehringer Ingelheim Pharmaceuticals I. Aptivus [package insert]. Ridgefield, CT: Nov 2005. Report No.; 2005.
Food and Drug Administration. Highlights of prescribing information. Aptivus (Tipranavir) Capsules. USFDA; 2009.
Glaxo Wellcome Inc. Agenerase [package insert]. Research Triangle Park, NC; 2004.
Agouron Pharmaceuticals. Viracept (Nelfinavir mesylate) prescribing information. San Diego, CA: Agouron Pharmaceuticals; 2008.
Anderson MS, Hanley WD, Moreau AR, Jin B, Bieberdorf FA, Kost JT, et al. Effect of raltegravir on estradiol and norgestimate plasma pharmacokinetics following oral contraceptive administration in healthy women. Br J Clin Pharmacol. 2011;71(4):616–20. Epub 2011/03/15.
Baeten JM, Benki S, Chohan V, Lavreys L, McClelland RS, Mandaliya K, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21(13):1771–7. Epub 2007/08/11.
Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. Epub 2011/10/07.
Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31(1):79–97. Epub 2009/11/12.
Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13(9):797–808. Epub 2013/07/23.
Stringer EM, Kaseba C, Levy J, Sinkala M, Goldenberg RL, Chi BH, et al. A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. Am J Obstet Gynecol. 2007;197(2):144e1-8. Epub 2007/08/11.
Phillips SJ, Curtis KM, Polis CB. Effect of hormonal contraceptive methods on HIV disease progression: a systematic review. AIDS. 2013;27(5):787–94. Epub 2012/11/09.
Cejtin HE, Jacobson L, Springer G, Watts DH, Levine A, Greenblatt R, et al. Effect of hormonal contraceptive use on plasma HIV-1-RNA levels among HIV-infected women. AIDS. 2003;17(11):1702–4. Epub 2003/07/11.
Richardson BA, Otieno PA, Mbori-Ngacha D, Overbaugh J, Farquhar C, John-Stewart GC. Hormonal contraception and HIV-1 disease progression among postpartum Kenyan women. AIDS. 2007;21(6):749–53. Epub 2007/04/07.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Haddad, L.B., Tarleton, J., Sheth, A.N., Ofotokun, I. (2014). Contraception for Women Living with HIV. In: Allen, R., Cwiak, C. (eds) Contraception for the Medically Challenging Patient. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1233-9_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1233-9_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1232-2
Online ISBN: 978-1-4939-1233-9
eBook Packages: MedicineMedicine (R0)