Abstract
Heart failure is a major cause of morbidity and mortality in the world. Cardiac energy metabolism, specifically fatty acid and glucose metabolism, is altered in heart failure and has been considered a contributing factor in the impaired heart function observed in patients with heart failure. Emerging evidence demonstrates that correcting these changes in energy metabolism by modulating mitochondrial oxidative metabolism may be effective treatment for heart failure. Promising strategies include the downregulation of fatty acid oxidation and increased coupling of glycolysis to glucose oxidation. Fatty acid β-oxidation enzymes carnitine palmitoyl transferase I and pyruvate dehydrogenase kinase are examples of metabolic targets for the treatment of heart failure. This article reviews the existing evidence for inhibition of fatty acid oxidation to treat heart failure. Further studies are needed to confirm the potential benefit of modulating these metabolic targets as an approach to treating heart failure in clinical settings.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
To preserve normal cardiac ejection, the myocardium requires more energy than do other tissues. Under the normal state, the major source of adenosine triphosphate (ATP) production is fatty acid oxidation (FAO), and others come from glucose and some amino acids. When heart failure occurs, the myocardial energy metabolism appears abnormal, which includes the downregulation of glucose and FAO, dependent more on glucose as its preferential substrate [1, 2]. In the early stage of heart failure, the abnormality may be inconspicuous; an animal experiment found that moderate or severe heart failure exhibited normal myocardial FAO [3]. This situation may be caused by elevated fatty acid concentration in heart failure. As failure progresses, mitochondrial oxidative metabolism is reduced and glycolysis is increased with the downregulation of glucose and FAO, and the shift of the preferential substrate is more significant. Considering that fatty acids are an inefficient energy substrate as compared with glucose, theoretically requiring 11–12 % more oxygen for identical ATP produced as carbohydrates [1], the change may be an important compensatory or protective mechanism for heart failure [4], which would improve “energy starvation”. The decrease in FAO and increase in glycolysis observed in pressure-overload hypertrophied hearts is accompanied by changes in expression and activity of metabolic enzymes involved in these pathways.
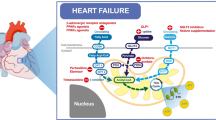
Because of insufficient energy metabolism in heart failure, the modulation of cardiac metabolism may be a new approach to the treatment of heart failure. One important strategy is inhibition of FAO, which modifies fatty acid β-oxidation, decreases levels of circulating fatty acids, and increases the utilization of glucose. The pathway of fatty acid metabolism includes many enzymes, with gene transcription perhaps controlled by peroxisome proliferator-activated receptor (PPAR) and regulated by the sympathetic nerve. So FAO can be inhibited in many ways and used to treat heart failure (Fig. 1).
Inhibition of fatty acid oxidation for the treatment of heart failure. β-Adrenoreceptor antagonists and peroxisome proliferator-activated receptor (PPAR) agonists decrease the level of circulating fatty acids. Carnitine palmitoyltransferase I (CPT-1) inhibitors decrease fatty acid β-oxidation and increase glucose oxidation by decreasing fatty acid transport into the mitochondria. Trimetazidine and ranolazine can directly inhibit fatty acid β-oxidation. Dichloroacetate (DCA) increases glucose oxidation by inhibiting pyruvate dehydrogenase kinase (PDK) activity, thereby stimulating pyruvate dehydrogenase (PDH)
2 Inhibitors of FA Beta-Oxidation
2.1 Trimetazidine
Trimetazidine (TMZ) is a piperazine derivative (1-(2,3,4-trimethoxybenzyl)-piperazine dihydrochloride) that optimizes energy metabolism presumably by partial inhibition of long-chain 3-ketoacyl CoA thiolase (3-KAT), with subsequent decrease in FAO and stimulation of glucose oxidation [5]. TMZ can relieve angina pectoris in patients with coronary artery disease [6–11], and these benefits do not depend on the change in heart rate, blood pressure [10], and rate-pressure product at rest, during submaximal and peak exercise [9, 11]. Some clinical trials have demonstrated the effect of TMZ in heart failure, including ischemia or nonischemia. One trial of 50 patients with ischemic cardiomyopathy indicated that in addition to conventional treatment, TMZ could improve exercise tolerance, reduce the plasma bone natriuretic protein (BNP) and cardiac troponin T levels without improving New York Heart Association (NYHA) class and left ventricular ejection fraction (LVEF) after 6-month follow-up [12]. Some other studies demonstrated that TMZ could improve NHYA class, LVEF, endothelium-dependent relaxation, and quality of life; prolong 6-min walk distance; increase cardiac phosphocreatine and adenosine triphosphate (PCr/ATP) ratio; and even reduce all-cause mortality and heart-failure hospitalization mortality [13–18]. In patients with diabetes and idiopathic dilated cardiomyopathy, TMZ could also significantly improve cardiac function and physical tolerance [19–21]. At the same time, in patients with TMZ, inflammatory response was decreased [21]. Especially, LVEF was greater in diabetic than nondiabetic patients [22]. Although some trials provided encouraging results, some controversies remained. To solve the problem of inconsistent results of different clinical trials, Gao et al. and Zhang et al. contributed to meta-analyses in 2010 and 2012, respectively [23, 24]. The two integrated analyses suggested that TMZ improves LVEF, improves subjective and objective measures of functional status in heart failure, simultaneously ameliorates LV remodeling, and reduces hospitalization. Zhang et al. noted a significant association of gender and LVEF improvement. Otherwise, there were some disputes on all-cause mortality.
TMZ may contribute to the shift of energy production from free fatty acids (FFAs) to glucose oxidation, preservation of intracellular levels of phosphocreatine and ATP; reduction of cell acidosis, calcium overload and free radical-induced injury, and cardiac fibrosis; and improvement of endothelial function, which benefit heart failure. However, direct measurement of cardiac FAO in patients with chronic non-ischemic heart failure revealed no changes in FFA uptake and only a 10 % decrease in FAO with TMZ. This finding challenges the concept that the beneficial effect of TMZ is mediated primarily by FAO inhibition [25]. Otherwise, researchers demonstrated that TMZ did not inhibit any component of β-oxidation in an isolated human cardiomyocyte cell line in 2003 [26]. Recently, some evidence indicated that the benefits of TMZ also came from its effect on the whole metabolism modulation. According to Fragasso and Tuunanen [18, 19], TMZ reduced the whole-body resting energy expenditure, regardless of the etiology and diabetic status of heart failure, reduced the cardiac FFA oxidation and improved whole-body insulin sensitivity in heart failure without diabetes. These data suggest that the metabolic effect of TMZ may also take place in other organs and tissues, to reduce the whole-body energy demand. Then, the improvement in insulin resistance, which contributes to the development of LV dysfunction by reducing cardiac efficiency through a metabolic shift toward fatty acid utilization would also decrease whole-body FFA oxidation.
An increasing number of studies have indicated that the cardioprotective effect of TMZ may occur via different mechanisms possibly involving regulation of mitochondrial function. In hypertrophied hearts, TMZ normalized post-ischemic function and fractional glucose oxidation via inhibiting glycolysis, possibly in response to reduced energy reserve and/or low rates of FAO. In ischemia-reperfusion, TMZ could decrease the infarct size of myocardium as with ischemia preconditioning, whose cardioprotective effect was represented by inhibiting mitochondria permeability transition pore (mPTP) opening, a critical event leading to cell death [27]. In myocytes of the failing heart, mPTP opening could also be antagonized by TMZ, which benefitted heart failure related to reduced complex II- and uncoupled oxidative stress mediated by mitochondrial nitric oxide synthase [25]. In a rat model of ex vivo perfusion with glucose, TMZ had a positive impact on mitochondrial homeostasis, which significantly increased the respiration control rate and respiratory chain complex I activity, thus leading to decreased reactive oxygen species (ROS) production, maintained mitochondrial electrical potential, and improved mitochondrial membrane integrity [28]. These effects may be attributed to the antioxidation of TMZ. When acute ischemia occurs, the positive effects of TMZ occur by increasing complex I activity, with decreased futile O2 consumption and reduced ROS production, rather than depending on an increase in ATP production.
2.2 Ranolazine
Ranolazine is similar to TMZ, a piperazine derivative, ((±)-N-(2,6-dimethyl-phenyl-4-[2-hydroxy-3-(2-methoxy-phenoxy)-propyl]-1-pi-perazine acetamide; RS-43285)) [37]. It is an anti-ischemia drug and approved in the United States and some European countries for treatment, combined with amlodipine, beta-blockers or nitrates, of chronic stable angina in patients [29]. Some clinical evidence has demonstrated that ranolazine could improve ischemia symptoms and quality of life of patients with chronic stable or severe angina; it increased the exercise capacity, time to angina and time to ≥1-mm ST-segment depression, and reduced angina frequency without clinically meaningful hemodynamic effects [30–34]. In non–ST-elevated acute coronary syndrome, ranolazine could increase exercise duration, reduce worsening angina and the incidence of arrhythmias, and thus decrease the risk of cardiovascular death, myocardial infarction and recurrent ischemia with elevated BNP accompanied by acute coronary syndrome [34–39]. Ranolazine has benefits in anti-ischemia, but the mechanism is not completely clear. One proposed mechanism is metabolic modulation. In vitro studies suggested that ranolazine inhibits FAO in skeletal muscle, and in isolated working rat hearts, ranolazine stimulated glucose oxidation, which may be a primary effect and concomitantly decrease FAO. This metabolic modulation would have some advantages for cells exposed to conditions of oxygen limitation or heart failure, including increased efficiency of ATP production, reduced production of lactate and H+, and reduction of other adverse effects from increased fatty acid metabolites. In guinea-pig hearts during low-flow ischaemia, ranolazine could improve pyruvate dehydrogenase activity, which was inhibited by global low-flow ischaemia [40]. Subsequent studies indicated no effects of ranolazine on pyruvate dehydrogenase kinase or phosphatase or on pyruvate dehydrogenase catalytic activity, which suggests that ranolazine activates pyruvate dehydrogenase indirectly [41]. Some evidence supported ranolazine attenuating calcium overload and ROS generation, thus preserving mitochondrial function [42, 43]. Therefore, ranolazine should partially inhibit β-oxidation, but the main mechanism does not appear to be through inhibition of myocardial FAO.
A few reports have described the effects of ranolazine on human metabolism. In the MERLIN TIMI-36 trial, ranolazine improved hyperglycemia control, with lower haemoglobin A1c level and fasting plasma glucose in diabetic patients [44, 45]. A few registered clinical trials have studied the effect of ranolazine in heart-failure patients, but most are not completed, and the results of the complete RALI-DHF trial have not been published [46]. However, animal experiments have given some inspiring results. Studies of acute intravenous infusion of ranolazine previously indicated improved LVEF, LV end-diastolic pressure (LVEDP), LV end-systolic volume, stroke volume, and cardiac output without an increase in myocardial oxygen consumption and thus improve mechanical efficiency in dogs with chronic heart failure [47, 48]. Another study of long-term ranolazine monotherapy in dogs with heart failure demonstrated significantly decreased LVEDP, accompanied by increased LVEF, stroke volume, and cardiac index [49].
2.3 Dichloroacetate
Dichloroacetate (DCA) inhibits pyruvate dehydrogenase (PDH) kinase activity, thereby stimulating PDH and carbohydrate oxidation, the rate-limiting enzyme of glucose oxidation. DCA treatment could increase glucose uptake and cardiac energy reserves and ameliorate chronic heart failure [50]. When DCA was administered intravenously for 30 min, myocardial lactate consumption and forward stroke volume were elevated, myocardial oxygen consumption was reduced, and LV mechanical efficiency was improved in heart failure patients with NYHA functional class III–IV [51]. However, another clinical trial demonstrated that intravenous infusion of DCA with the same dose over 15 min did not significantly protect heart-failure patients with LVEF ≤ 40 %, which increased LV diastolic and systolic volumes and did not increase LVEF and stroke volume significantly [52]. Recently, some data suggested that DCA may be useful for treating pulmonary hypertension by increasing the mitochondria-dependent apoptosis of pulmonary artery smooth muscle cells and right ventricular failure.
3 Carnitine Palmitoyltransferase/Carnitine System Inhibitors
The rate-limiting enzyme for fatty acid β-oxidation and thus a potential drug target for regulating mitochondrial fatty acid uptake is carnitine palmitoyltransferase (CPT). Drugs targeting CPT-1 include etomoxir, an irreversible CPT-1 inhibitor; perhexiline; oxfenicine; and mildronate.
3.1 Etomoxir
Carnitine palmitoyltransferase 1(CPT-1) is the first limiting-rate enzyme for mitochondrial β-oxidation of long-chain fatty acids, which is located on the outer membrane of mitochondria. Etomoxir is a CPT-1 inhibitor.. This inhibition of mitochondrial CPT-1 is common to a number of oxirane carboxylic acid derivatives and is both irreversible and stereospecific. Etomoxir has been developed for treating diabetes mellitus type 2 but has been rarely explored for heart failure. A small clinical trial of heart-failure patients with NYHA class II-III indicated that etomoxir had neither a positive inotropic effect nor vasodilatory properties in acute studies; however, long-term treatment with etomoxir reduced resting heart rate, elevated LVEF, with increased CO and stroke volume during exercise [53]. However, in the ERGO study, etomoxir induced unacceptably high liver transaminase levels, which caused premature study termination. Then, according to the ERGO data, etomoxir produced no improvement of 6-min corridor walk test or echocardiographical values [54]. Long-term etomoxir treatment improved the performance of the hypertrophied ventricle in rats with ascending aorta constriction, including increased maximal developed pressure, LV pressure-volume area, and ±dP/dt(max) [55]. Yet, in a similar animal experiment with shorter treatment time and smaller drug dose, etomoxir could not affect cardiac function in vivo but improved function associated with a substrate switch in the isolated heart [56]. This evidence suggested that the effects of etomoxir on heart failure were still indistinct, and the effectiveness and safety required further investigation. In isolated working hearts with ischemia-reperfusion, low-dose etomoxir decreased long-chain acylcarnitine and long-chain acyl-coenzyme A (CoA) levels but did not prevent depressed function. In contrast, a high dose of etomoxir prevented the palmitate-induced depression of function but did not decrease myocardial long-chain acylcarnitine or long-chain acyl-CoA levels, accompanied by decreased oxygen consumption per unit work during reperfusion recovery and increased ATP and creatine-phosphate levels. Thus, the potential protection of etomoxir was unrelated to changes in levels of long-chain acylcarnitines but might be due to increased glucose use by the reperfused heart. Moreover, this CPT-1 inhibitor increased the sarcoplasmic reticulum (SR) Ca2+ uptake rate. In rats with hypertrophied hearts by aortic constriction, etomoxir could prevent the reduced SR Ca2+-ATPase (SERCA2) gene expression and thus may prevent the transition of cardiac hypertrophy into heart failure [57, 58].
3.2 Perhexiline
Perhexiline is an antianginal agent that inhibits rat cardiac mitochondrial CPT-1 and CPT-2 levels [59]. Perhexiline inhibits FAO by reducing mitochondrial fatty acid uptake. However, in working rat hearts, perhexiline increased the efficiency of myocardial oxygen utilization by approximately 30 % without significant effects on palmitate oxidation, which suggested that the mechanism of perhexiline with improvement of myocardial efficiency was largely or entirely independent of its effects on CPT [60]. The use of this agent decreased because of the acute and chronic potential toxicity. Recently, some investigators conducted a re-evaluation of perhexiline. They collated the retrospective clinical data for patients with chronic heart failure and/or refractory angina and found that perhexiline therapy offered symptom relief for most patients, with only a small minority showing any side effects or abnormal liver function test results, and patients with refractory angina were more likely to be responders [61]. With careful plasma drug level monitoring for dose titration, perhexiline was effective and safe, with admirable results in chronic heart failure and especially refractory angina. A short-term, placebo-controlled clinical study of 56 patients with ischemic or nonischemic heart failure reported that perhexiline improved the quality of life, peak exercise oxygen consumption and LVEF [62]. However, perhexiline therapy in LV dysfunction after myocardial infarction failed to demonstrate an improvement in echocardiographical parameters and function of viable segments by dobutamine echocardiography [63]. Another clinical trial indicated that in symptomatic patients with hypertrophic cardiomyopathy, perhexiline improved diastolic dysfunction and exercise capacity and increased the myocardial ratio of phosphocreatine to ATP as measured by nuclear magnetic resonance, which is consistent with a metabolic mechanism of action [64]. Although these small clinical studies suggest that perhexiline should have beneficial effects in heart failure patients, more large-scale pivotal trials should be conducted in patients with heart failure or hypertrophic cardiomyopathy to further confirm the findings.
3.3 Oxfenicine
Oxfenicine (S-4-hydroxyphenyl glycine) is a selective inhibitor of cardiac mitochondrial CPT-1. It has not been used in human clinical studies, but some animal experiments have explored its effects on metabolism, myocardiac ischemia and heart failure. Oxfenicine could increase glucose metabolism and insulin sensitivity in mice with diet-induced obesity and insulin resistance via reduced FAO [65]. As well, early and sustained treatment with this CPTI inhibitor prevented by almost 1 week LV chamber dilation, wall thinning and delayed onset of decompensation in dogs with pacing-induced heart failure [66]. This finding is consistent with the drug’s prevention of various changes in protein phenotype, especially metalloproteinase-2 and -9. In rats with diet-induced obesity, chronic oxfenicine treatment improved post-ischemic cardiac function and reduced myocardial infarct size by approximately 40 % after ischemia-reperfusion [67]. However, oxfenicine also affected myocardial hypertrophy in dogs and rats after 1 or 2 years, which might be caused by inhibition of FAO. However, 8-week administration of oxfenicine did not result in cardiac hypertrophy or contractile dysfunction in normal rats, even combined with a high-fat diet [68]. Otherwise, oxfenicine significantly blocked cell death induced by the combination of palmitate and carnitine, which suggests that oxfenicine could reduce palmitate-induced cardiac apoptosis [69].
3.4 Mildronate
Mildronate (3-(2,2,2-trimethylhydrazine)) propionate, also named THP and MET-88, could inhibit the uptake of long-chain fatty acids into mitochondria by reducing the level of carnitine biosynthesis, which is required by CPT-1, and inhibit FAO ultimately. In the mouse, long-term mildronate treatment significantly decreased carnitine concentration in plasma and heart tissue and increased the rate of insulin-stimulated glucose uptake by 35 %, accompanied by upregulated expression of related genes, including glucose transporter 4, hexokinase II, and insulin receptor proteins. These findings suggested that mildronate has benefits by decreasing FAO but also increasing glucose oxidation, prompting the shift of substrate preference. Mildronate has benefits on cardioprotection. In ischemia-reperfusion, mildronate could reduce the infarct size significantly, improve the recovery of cardiac function and decrease the incidence of ventricular fibrillation, thus preventing the accumulation of long-chain acylcarnitine induced by ischemia [70, 71]. Interestingly, mildronate did not affect cardiac function and energy metabolism without ischemia. In addition, long-term mildronate treatment attenuated the development of atherosclerosis in apoE/low-density-lipoprotein receptor double-deficient mice [72]. Mildronate prolonged survival with a median 50 % survival of 103 days as compared with 79 days for rats with heart failure following myocardial infarction as compared with control rats and attenuated the dilatation of the left ventricle and decreased ATP level, thus preventing the increase in right atrial pressure [73]. The mildronate improvement in cardiac function in rats with heart failure induced by coronary artery ligation was attributed to increased SR Ca2+ pump activity. In LV myocardial homogenates, SERCA2 protein content was 32 % lower in the myocardial infarction group than the sham-operated group. However, in the mildronate group with myocardial infarction, SERCA2 content was the same as in the sham-operated group [74]. In another similar model, mildronate significantly prevented the decrease in Vmax for SR Ca2+ uptake activity and improved myocardial high-energy phosphate [75]. Mildronate prevented the cardiopathologic changes and reduced the increased expression of nuclear factor kBp65 induced by azidothymidine. Subsequent experiments indicated that mildronate acted at the level of complex I, mainly by reducing H2O2 to protect mitochondria in liver [76]. However, mildronate pre-treatment of rats at 100 or 200 mg/kg/day for 1 or 2 weeks did not prevent ischemia-reperfusion–induced mitochondrial dysfunction and liver injury [77]. Recently, mildronate was found to have beneficial effects in diabetes and neuroprotective properties. In conclusion, mildronate has greater protective action than inhibition of carnitine biosynthesis and thus would be an extremely potential drug.
4 Beta-Blocker
When heart failure occurs, β-adrenergic overdrive increases the level of circulating fatty acids, caused by enhanced fatty acid mobilization, inhibited myocardial uptake of glucose, and promoted onset of insulin resistance. Then, excess plasma fatty acid and insulin resistance could result in abnormalities of myocardial function. Therefore, an indirect therapeutic approach to inhibit FAO in the failing heart would be to reduce the circulating levels of fatty acids via a β-blocker. Long-term therapy with b-adrenergic receptor antagonists (metoprolol and carvedilol) can improve cardiac performance and survival in patients with heart failure. In cultured mouse C2C12 cells, carvedilol could inhibite palmitate oxidation and increase glycolysis by nearly 50 %. A few small clinical trials assessed the metabolic modulation of β-blockers. Carvedilol or bisoprolol treatment in heart failure patients could improve NYHA class and LVEF and increase mean cardiac phosphocreatine and ATP (PCr/ATP) ratio by 33 % on in vivo 31P-magnetic resonance spectroscopy [78]. Carvedilol treatment for 6 months for heart failure also reduced total body resting energy production rate and increased glucose oxidation. In another trial of nine patients with ischemic cardiomyopathy, carvedilol treatment in patients with heart failure resulted in a 57 % decrease in myocardial FFA use without a significant change in glucose use [79]. Metoprolol also caused a significant decrease in basal plasma FFA levels in patients with heart failure and decreased CPTI activity and increase in triglyceride content in dogs with coronary microembolism-induced heart failure [80]. A model of dogs with pacing-induced dilated cardiomyopathy was used for evaluating the effects of carvedilol and metoprolol on myocardial metabolism. Short-term treatment with carvedilol had superior hemodynamic and metabolic effects as compared with metoprolol and included increasing plasma insulin levels and suppressing nonesterified fatty acids and glucagon levels [81]. These findings suggested that carvedilol had a more pronounced ability of shifting the substrate preference from FFAs to glucose. Not unsurprisingly, carvedilol treatment caused a 20 % reduction in myocardial FFA extraction, whereas metoprolol had a neutral effect in patients with chronic heart failure after 4 months of therapy [82]. Meanwhile, carvedilol treatment tended to increase cardiac lactate extraction, so it caused a shift from FFA utilization to lactate utilization in heart failure. The differences in these two agents on cardiac sympathetic activity and energy metabolism may be related to the differential effects of these drugs on clinical outcomes.
5 Conclusions
Although some small clinical trials showed surprisingly efficacious effects of inhibition of fatty acid metabolism on heart failure, some other studies did not support the approach for heart failure. For example, in a review published in 1994, cardiomyopathy often develops in children with genetic defects in FAO enzymes, which forces the heart to rely on glucose. This finding suggested that FAO may be indispensable for myocytes. Acipimox, a nicotinic-acid derivative that could decrease the FFA level of plasma, was used for heart failure, without profound benefits in cardiac efficiency [83]. The peroxisome proliferator-activated receptor alpha (PPARα) agonist, which would prevent changes in myocardial substrate metabolism in the failing heart treated with fenofibrate in pigs with pacing-induced heart failure, increased the expression of PPARα-regulated genes, prevented LV hypertrophy, and delayed the development of LV dilation and dysfunction [84]. In addition, new evidence supports that metabolic abnormalities in the failing heart post-infarction revealed that the heart was not energetically starved but rather inefficient in energy utilization for mechanical function [85]. Thus, we regret that we cannot state clearly that inhibition of FAO is an adaptive or a maladaptive process based on existing evidence. In conclusion, although energy metabolic modulation is an important and effective approach in heart failure, some questions remain but are worth more efforts to search for evidence to verify further mechanisms and clinical effects.
References
Lionetti V, Stanley WC, Recchia FA (2011) Modulating fatty acid oxidation in heart failure. Cardiovasc Res 90:202–209
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129
Chandler MP, Kerner J, Huang H et al (2004) Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol 287:H1538–H1543
Fillmore N, Lopaschuk GD (2013) Targeting mitochondrial oxidative metabolism as an approach to treat heart failure. Biochim Biophys Acta 1833:857–865
Kantor PF, Lucien A, Kozak R et al (2000) The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 86:580–588
Cesar LA, Mathias W Jr, Armaganijan D et al (2007) Trimetazidine to reverse ischemia in patients with class I or II angina: a randomized, double-blind, placebo-controlled dobutamine-atropine stress echocardiography study. Coron Artery Dis 18:259–263
Ruzyllo W, Szwed H, Sadowski Z et al (2004) Efficacy of trimetazidine in patients with recurrent angina: a subgroup analysis of the TRIMPOL II study. Curr Med Res Opin 20:1447–1454
Koylan N, Bilge AK, Adalet K et al (2004) Comparison of the effects of trimetazidine and diltiazem on exercise performance in patients with coronary heart disease. The Turkish trimetazidine study (TTS). Acta Cardiol 59:644–650
Chazov EI, Lepakchin VK, Zharova EA et al (2005) Trimetazidine in Angina Combination Therapy–the TACT study: trimetazidine versus conventional treatment in patients with stable angina pectoris in a randomized, placebo-controlled, multicenter study. Am J Ther 12:35–42
Vitale C, Wajngaten M, Sposato B et al (2004) Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J 25:1814–1821
Kolbel F, Bada V (2003) Trimetazidine in geriatric patients with stable angina pectoris: the tiger study. Int J Clin Pract 57:867–870
Di Napoli P, Di Giovanni P, Gaeta MA et al (2007) Beneficial effects of trimetazidine treatment on exercise tolerance and B-type natriuretic peptide and troponin T plasma levels in patients with stable ischemic cardiomyopathy. Am Heart J 154(602):e601–e605
Fragasso G, Palloshi A, Puccetti P et al (2006) A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 48:992–998
Belardinelli R, Solenghi M, Volpe L et al (2007) Trimetazidine improves endothelial dysfunction in chronic heart failure: an antioxidant effect. Eur Heart J 28:1102–1108
Fragasso G, Perseghin G, De Cobelli F et al (2006) Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J 27:942–948
Fragasso G, Rosano G, Baek SH et al (2013) Effect of partial fatty acid oxidation inhibition with trimetazidine on mortality and morbidity in heart failure: results from an international multicentre retrospective cohort study. Int J Cardiol 163:320–325
Marazzi G, Gebara O, Vitale C et al (2009) Effect of trimetazidine on quality of life in elderly patients with ischemic dilated cardiomyopathy. Adv Ther 26:455–461
Fragasso G, Salerno A, Lattuada G et al (2011) Effect of partial inhibition of fatty acid oxidation by trimetazidine on whole body energy metabolism in patients with chronic heart failure. Heart 97:1495–1500
Tuunanen H, Engblom E, Naum A et al (2008) Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 118:1250–1258
Fragasso G, Piatti Md PM, Monti L et al (2003) Short- and long-term beneficial effects of trimetazidine in patients with diabetes and ischemic cardiomyopathy. Am Heart J 146:E18
Zhao P, Zhang J, Yin XG et al (2013) The effect of trimetazidine on cardiac function in diabetic patients with idiopathic dilated cardiomyopathy. Life Sci 92:633–638
Gunes Y, Guntekin U, Tuncer M et al (2009) Improved left and right ventricular functions with trimetazidine in patients with heart failure: a tissue Doppler study. Heart Vessels 24:277–282
Gao D, Ning N, Niu X et al (2011) Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart 97:278–286
Zhang L, Lu Y, Jiang H et al (2012) Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol 59:913–922
Dedkova EN, Seidlmayer LK, Blatter LA (2013) Mitochondria-mediated cardioprotection by trimetazidine in rabbit heart failure. J Mol Cell Cardiol 59:41–54
MacInnes A, Fairman DA, Binding P et al (2003) The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 93:e26–e32
Argaud L, Gomez L, Gateau-Roesch O et al (2005) Trimetazidine inhibits mitochondrial permeability transition pore opening and prevents lethal ischemia-reperfusion injury. J Mol Cell Cardiol 39:893–899
Monteiro P, Duarte AI, Goncalves LM et al (2004) Protective effect of trimetazidine on myocardial mitochondrial function in an ex-vivo model of global myocardial ischemia. Eur J Pharmacol 503:123–128
McCormack JG, Barr RL, Wolff AA et al (1996) Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation 93:135–142
Chaitman BR, Skettino SL, Parker JO et al (2004) Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 43:1375–1382
Rousseau MF, Pouleur H, Cocco G et al (2005) Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol 95:311–316
Chaitman BR, Pepine CJ, Parker JO et al (2004) Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 291:309–316
Sendon JL, Lee S, Cheng ML et al (2012) Effects of ranolazine on exercise tolerance and angina frequency in patients with severe chronic angina receiving maximally-tolerated background therapy: analysis from the Combination Assessment of Ranolazine In Stable Angina (CARISA) randomized trial. Eur J Prev Cardiol 19:952–959
Stone PH, Gratsiansky NA, Blokhin A et al (2006) Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 48:566–575
Scirica BM, Sabatine MS, Jarolim P et al (2011) Assessment of multiple cardiac biomarkers in non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 trial. Eur Heart J 32:697–705
Morrow DA, Scirica BM, Sabatine MS et al (2010) B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol 55:1189–1196
Wilson SR, Scirica BM, Braunwald E et al (2009) Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol 53:1510–1516
Scirica BM, Morrow DA, Hod H et al (2007) Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation 116:1647–1652
Morrow DA, Scirica BM, Karwatowska-Prokopczuk E et al (2007) Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA 297:1775–1783
Clarke B, Spedding M, Patmore L et al (1993) Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br J Pharmacol 109:748–750
Clarke B, Wyatt KM, McCormack JG (1996) Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J Mol Cell Cardiol 28:341–350
Aldakkak M, Camara AK, Heisner JS et al (2011) Ranolazine reduces Ca2+ overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts. Pharmacol Res 64:381–392
Dehina L, Descotes J, Chevalier P et al (2013) Protective effects of ranolazine and propranolol, alone or combined, on the structural and functional alterations of cardiomyocyte mitochondria in a pig model of ischemia/reperfusion. Pharmacol, Fundam Clin
Chisholm JW, Goldfine AB, Dhalla AK et al (2010) Effect of ranolazine on A1C and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care 33:1163–1168
Morrow DA, Scirica BM, Chaitman BR et al (2009) Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 119:2032–2039
Jacobshagen C, Belardinelli L, Hasenfuss G et al (2011) Ranolazine for the treatment of heart failure with preserved ejection fraction: background, aims, and design of the RALI-DHF study. Clin Cardiol 34:426–432
Chandler MP, Stanley WC, Morita H et al (2002) Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res 91:278–280
Sabbah HN, Chandler MP, Mishima T et al (2002) Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail 8:416–422
Rastogi S, Sharov VG, Mishra S et al (2008) Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol 295:H2149–H2155
Kato T, Niizuma S, Inuzuka Y et al (2010) Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3:420–430
Bersin RM, Wolfe C, Kwasman M et al (1994) Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol 23:1617–1624
Lewis JF, DaCosta M, Wargowich T et al (1998) Effects of dichloroacetate in patients with congestive heart failure. Clin Cardiol 21:888–892
Schmidt-Schweda S, Holubarsch C (2000) First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 99:27–35
Holubarsch CJ, Rohrbach M, Karrasch M et al (2007) A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 113:205–212
Turcani M, Rupp H (1997) Etomoxir improves left ventricular performance of pressure-overloaded rat heart. Circulation 96:3681–3686
Schwarzer M, Faerber G, Rueckauer T et al (2009) The metabolic modulators, Etomoxir and NVP-LAB121, fail to reverse pressure overload induced heart failure in vivo. Basic Res Cardiol 104:547–557
Rupp H, Vetter R (2000) Sarcoplasmic reticulum function and carnitine palmitoyltransferase-1 inhibition during progression of heart failure. Br J Pharmacol 131:1748–1756
Zarain-Herzberg A, Rupp H, Elimban V et al (1996) Modification of sarcoplasmic reticulum gene expression in pressure overload cardiac hypertrophy by etomoxir. FASEB J 10:1303–1309
Kennedy JA, Kiosoglous AJ, Murphy GA et al (2000) Effect of perhexiline and oxfenicine on myocardial function and metabolism during low-flow ischemia/reperfusion in the isolated rat heart. J Cardiovasc Pharmacol 36:794–801
Unger SA, Kennedy JA, McFadden-Lewis K et al (2005) Dissociation between metabolic and efficiency effects of perhexiline in normoxic rat myocardium. J Cardiovasc Pharmacol 46:849–855
Phan TT, Shivu GN, Choudhury A et al (2009) Multi-centre experience on the use of perhexiline in chronic heart failure and refractory angina: old drug, new hope. Eur J Heart Fail 11:881–886
Lee L, Campbell R, Scheuermann-Freestone M et al (2005) Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation 112:3280–3288
Bansal M, Chan J, Leano R et al (2010) Effects of perhexiline on myocardial deformation in patients with ischaemic left ventricular dysfunction. Int J Cardiol 139:107–112
Abozguia K, Elliott P, McKenna W et al (2010) Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 122:1562–1569
Keung W, Ussher JR, Jaswal JS et al (2013) Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes 62:711–720
Lionetti V, Linke A, Chandler MP et al (2005) Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res 66:454–461
Maarman G, Marais E, Lochner A et al (2012) Effect of chronic CPT-1 inhibition on myocardial ischemia-reperfusion injury (I/R) in a model of diet-induced obesity. Cardiovasc Drugs Ther 26:205–216
Okere IC, Chandler MP, McElfresh TA et al (2007) Carnitine palmitoyl transferase-I inhibition is not associated with cardiac hypertrophy in rats fed a high-fat diet. Clin Exp Pharmacol Physiol 34:113–119
Kong JY, Rabkin SW (2002) Palmitate-induced cardiac apoptosis is mediated through CPT-1 but not influenced by glucose and insulin. Am J Physiol Heart Circ Physiol 282:H717–H725
Vilskersts R, Liepinsh E, Kuka J et al (2009) Myocardial infarct size-limiting and anti-arrhythmic effects of mildronate orotate in the rat heart. Cardiovasc Drugs Ther 23:281–288
Hayashi Y, Tajima K, Kirimoto T et al (2000) Cardioprotective effects of MET-88, a gamma-butyrobetaine hydroxylase inhibitor, on cardiac dysfunction induced by ischemia/reperfusion in isolated rat hearts. Pharmacology 61:238–243
Vilskersts R, Liepinsh E, Mateuszuk L et al (2009) Mildronate, a regulator of energy metabolism, reduces atherosclerosis in apoE/LDLR−/− mice. Pharmacology 83:287–293
Hayashi Y, Kirimoto T, Asaka N et al (2000) Beneficial effects of MET-88, a gamma-butyrobetaine hydroxylase inhibitor in rats with heart failure following myocardial infarction. Eur J Pharmacol 395:217–224
Yonekura K, Eto Y, Yokoyama I et al (2000) Inhibition of carnitine synthesis modulates protein contents of the cardiac sarcoplasmic reticulum Ca2+-ATPase and hexokinase type I in rat hearts with myocardial infarction. Basic Res Cardiol 95:343–348
Hayashi Y, Ishida H, Hoshiai M et al (2000) MET-88, a gamma-butyrobetaine hydroxylase inhibitor, improves cardiac SR Ca2+ uptake activity in rats with congestive heart failure following myocardial infarction. Mol Cell Biochem 209:39–46
Pupure J, Fernandes MA, Santos MS et al (2008) Mitochondria as the target for mildronate's protective effects in azidothymidine (AZT)-induced toxicity of isolated rat liver mitochondria. Cell Biochem Funct 26:620–631
Trumbeckaite S, Kincius M, Preidis A et al (2009) Effects of ischemia-reperfusion and pretreatment with mildronate on rat liver mitochondrial function. Pharmacol Rep 61:859–869
Spoladore R, Fragasso G, Perseghin G et al (2013) Beneficial effects of beta-blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam Clin Pharmacol 27:455–464
Wallhaus TR, Taylor M, DeGrado TR et al (2001) Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 103:2441–2446
Panchal AR, Stanley WC, Kerner J et al (1998) Beta-receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Card Fail 4:121–126
Nikolaidis LA, Poornima I, Parikh P et al (2006) The effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathy. J Am Coll Cardiol 47:1871–1881
Al-Hesayen A, Azevedo ER, Floras JS et al (2005) Selective versus nonselective beta-adrenergic receptor blockade in chronic heart failure: differential effects on myocardial energy substrate utilization. Eur J Heart Fail 7:618–623
Halbirk M, Norrelund H, Moller N et al (2010) Suppression of circulating free fatty acids with acipimox in chronic heart failure patients changes whole body metabolism but does not affect cardiac function. Am J Physiol Heart Circ Physiol 299:H1220–H1225
Brigadeau F, Gele P, Wibaux M et al (2007) The PPARalpha activator fenofibrate slows down the progression of the left ventricular dysfunction in porcine tachycardia-induced cardiomyopathy. J Cardiovasc Pharmacol 49:408–415
Masoud WG, Ussher JR, Wang W et al (2014) Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc Res 101:30–38
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Yan, R., Wei, J., Gao, D. (2014). Inhibition of Fatty Acid Oxidation to Treat Heart Failure in Patients. In: Lopaschuk, G., Dhalla, N. (eds) Cardiac Energy Metabolism in Health and Disease. Advances in Biochemistry in Health and Disease, vol 11. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1227-8_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1227-8_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1226-1
Online ISBN: 978-1-4939-1227-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)