Abstract
The creatine kinase (CK) system is the final step in cardiac energy metabolism providing a direct link between energy production in the mitochondria and energy utilising ATPases. It acts as an energy storage and transport mechanism and maintains favourable local ATP/ADP ratios, thereby supporting further energy production and high levels of free energy from ATP hydrolysis. Down-regulation of CK activity and myocardial creatine levels is a universal finding in chronic heart failure, and the degree of impairment has been shown to be an excellent prognostic indicator in patients. However, it is unclear whether these changes represent epiphenomenon or contribute to disease pathophysiology. This chapter focuses on attempts over the past 20 years to address this question using genetic loss-of-function models in the mouse. Findings from these models have been equivocal and at times contradictory, however, recent evidence suggests that loss of creatine or CK is not detrimental in surgical models of chronic heart failure, providing the clearest evidence to date that such changes do not contribute to dysfunction. Despite this conclusion, over-expression of CK in mouse heart has been found to protect against heart failure and improve survival. In the setting of ischaemia-reperfusion injury, loss of creatine or CK impairs functional recovery and augmentation of either is cardioprotective. We are therefore entering an exciting new era of research in this field aimed at understanding the benefits of CK system augmentation and identifying new mechanisms to achieve this without genetic modification for possible future clinical translation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Creatine kinase
- Phosphagen systems
- Cardiac energetics
- Genetically-modified mice
- Heart failure
- Ischaemia
- Reperfusion injury
1 Introduction
1.1 The Phosphocreatine/Creatine Kinase System in the Healthy Heart
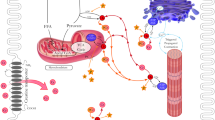
All vertebrates use creatine kinase as a phosphagen system in the heart, the role of which is to ensure continuity of energy supply in the face of fluctuating demands via the buffering and transport of high-energy phosphates. The utility of this system is based on the intracellular compartmentation of the reactants (Fig. 1) [1]. The forward reaction occurs at the mitochondrial membrane where sarcomeric mitochondrial-creatine kinase (Mt-CK) catalyses the transfer of a phosphoryl group from ATP onto creatine to form phosphocreatine (PCr), which accumulates to high levels within the cytosol. Compared to ATP, PCr is relatively small and less polar and therefore more readily diffusible. In this way the CK system acts as both a spatial and temporal energy buffer, with PCr providing a mobile form of high-energy phosphate storage and transport for near instantaneous regeneration of ATP at times of rapidly increasing demand. This reverse reaction is catalysed by cytosolic dimers of CK which are also found closely coupled to ATPases, e.g. the myosin ATPase [2]. In this way local metabolites are maintained at favourable levels, i.e. low [ATP/ADP] ratio at the mitochondria to stimulate oxidative phosphorylation, and relatively high (ATP/ADP) ratio at the ATPases to ensure maximal energy is available from the hydrolysis of ATP (i.e. ∆GATP is high) [3–5].
Schematic showing creatine synthesis pathway and the creatine kinase system in cardiomyocytes. Creatine can be biosynthesised via arginine:glycine amidinotransferase (AGAT), found principally in the kidneys, which catalyses the formation of guanidinoacetate from arginine and glycine. Guanidinoacetate is then methylated, predominately in the liver, by guanidinoacetate methyltransferase (GAMT) to form creatine. Creatine is taken up into the cardiomyocyte by a specific plasma membrane creatine transporter, where mitochondrial creatine kinase (Mito-CK) catalyses the transfer of a phosphoryl group from ATP to form phosphocreatine (PCr). PCr acts as an energy buffer and transport molecule with the reverse reaction catalysed by muscle-CK (MM-CK) to liberate ATP at times of high energy demand. Created using Servier Medical Art by Servier which is licensed under a Creative Commons Attribution 3.0 Unported License http://www.servier.com/slidekit
1.2 Origin of Creatine and CK in the Heart
The heart expresses four isoenzymes of CK, with Mt-CK and MM-CK by far the most abundant (35 % and 67 % of total CK activity in human heart, respectively) [6]. However, the brain isoform is also expressed and can form homo-dimers or dimerize with the muscle-isoform to give the low abundance cytosolic isoenzymes BB-CK and MB-CK.
Creatine is either obtained from the diet or via a two-step biosynthetic pathway (see Fig. 1) [2]. The proteins required for creatine biosynthesis are not expressed in cardiomyocytes; therefore creatine must be actively taken-up from the bloodstream against a large concentration gradient via a specific creatine transporter located in the plasma membrane (CrT: SLC6A8).
1.3 Scope of this Chapter: Lessons from the Genetic Era
Knockout mouse models have been described for all CK isoforms and also for loss-of-creatine models, CrT, GAMT and AGAT. In this review we seek to bring the story up-to-date by critical appraisal of what has been learnt from these mouse models, with particular reference to the role of CK system impairment as a putative causal factor in chronic heart failure and the potential for CK system augmentation as a novel therapeutic strategy for the treatment of cardiac disease.
2 Observations that Implicate a Role for the PCr/CK System in Heart Failure
The observation that myocardial creatine content and CK activity are significantly lower in the failing heart raises the possibility that such changes may have a direct role in disease progression. These findings occur regardless of disease aetiology or species, and the examples are numerous (see [7] for a summary). It should be noted that ATP is maintained at near normal levels in the failing heart due to the buffering capacity of PCr and only reduces significantly during end-stage disease as a result of cumulative loss of adenine nucleotides across the plasma membrane [3, 8]. Loss of creatine during heart failure is likely to occur secondary to reduced expression of the CrT [7, 9] (i.e. steady cellular loss but reduced uptake), although the signalling pathways governing this have yet to be established. Similarly, the mechanisms behind loss of CK activity are not yet known and would be a fruitful area for further research.
A significant relationship between maximal CK reaction velocity (the product of CK activity and [Cr]) and ventricular function has been observed in several models of heart failure (e.g. in mice [7] and Syrian cardiomyopathic hamsters [10]). The ratio of PCr to ATP measured by 31P-MRS is often used since absolute values are difficult to obtain and is a sensitive indicator of energetic status. In a murine model of transverse aortic constriction (TAC), low PCr/ATP measured at 3 weeks was predictive of chamber dilatation observed at 6 weeks [11], suggesting that energetic changes precede adverse LV remodelling and therefore could have a causal role. Particularly convincing is the correlative evidence from observations of PCr/ATP ratio in the clinical setting, where low PCr/ATP is associated with more severe heart failure in patients with dilated cardiomyopathy, correlating with New York Heart Association (NYHA) class [12], ejection fraction and wall thickness [13]. In a 2.5 year prospective follow-up study, PCr/ATP was found to be a better prognostic indicator of mortality than ejection fraction [14]. However, the question remains whether these are simply very good biomarkers, or whether loss of creatine and CK activity plays an active role in disease pathophysiology.
3 Loss-of-Function Models
Loss-of-function represent a standard experimental paradigm used to imply causality. For example, the importance of PCr/CK system down-regulation in the progression of heart failure would be inferred by the recapitulation of a failing phenotype when the system is ablated in otherwise normal animals.
3.1 Pharmacological Inhibition Studies
-
1.
A number of creatine analogues have been shown to compete with creatine for cellular uptake at the CrT, but are poor substrates for the CK reaction [2]. Of these, the most commonly used is β-guanidinopropionic acid (β-GPA), see [15] for a recent systematic review. β-GPA can be given in chow or water, however, loss of creatine from the myocardium is a slow process (~2 % of the total creatine pool per day [16]), therefore β-GPA has to be chronically dosed over a period of weeks, which may allow time for substantive compensatory adaptations to develop (for examples see [17, 18]). A further limitation is that creatine loss is incomplete (residual creatine 10–50 % of starting values). This is an important point since cardiac work is maintained over a wide-range of creatine levels and only drops significantly when total [Cr] falls below a threshold value of ~15 % of control, and even then, only under high workload conditions [19]. This may go some way to explain the variability in findings using this approach. Furthermore, there are potentially confounding systemic effects of creatine inhibition, for example, practically all studies using β-GPA report reduced body weight by ~10–15 %. Despite these limitations, there is general agreement that dysfunction becomes apparent or is exacerbated at higher workloads.
-
2.
Iodoacetamide (IA) is a rapid irreversible inhibitor of CK activity, affecting all CK isoenzymes to an equal extent [20]. It is an alkylating agent that prevents formation of disulphide bonds and is therefore likely to have diverse activity on multiple protein targets, as such, it is too toxic for chronic dosing and has been used in acute studies only (mostly perfused heart). Experimental findings have been highly variable, but, in general, the effects of CK ablation are mostly observed at high workloads. Of particular note is a study by Tian et al. who used different doses of IA in isolated perfused rat hearts to demonstrate dose-dependency between CK inhibition and dysfunction, establishing a role for the CK system in setting contractile reserve [21].
3.2 Evidence from CK Knockout Studies
Mice deficient in creatine kinase (CK) have been described for the myofibril-bound (M-CK−/−) [22], the sarcoplasmic mitochondrial isoenzyme (Mt-CK−/−) [23], and for the combined double knockout (M/Mt-CK−/−) [24]. There is broad agreement in the literature that M-CK−/− mice do not exhibit any discernible cardiac dysfunction or remodelling, either at baseline or at higher workloads, regardless of whether measured in isolated fibres, ex vivo perfused heart or in vivo [25–29].
Ex vivo—Numerous studies have failed to observe a functional deficit in CK knockout mice ex vivo (e.g. [25–27, 30–33]), and it may be that the workloads attainable are simply too low, even under maximally stimulated conditions. This is despite significantly impaired energetics e.g. lower PCr, reduced ATP synthesis rate [34], and low ΔGATP at high workloads [27].
In vivo findings have produced mixed results. Echocardiography showed impaired contractile reserve in M/Mt-CK−/− mice compared to C57BL/6 controls under one anaesthetic regime, but not another [35]. Lower systemic blood pressure has been reported in Mt-CK−/− when compared to either controls, M-CK−/−, or M/Mt-CK−/− [28]. However, subsequent measurements by aortic cannulation have not observed any differences in systolic or diastolic pressures [35, 36]. By 41 weeks of age both Mt-CK−/− and M/Mt-CK−/− mice were found to have significant LV dilatation, hypertrophy, and impaired perfusion using MRI, although with normal ejection fraction [28, 37].
All three CK−/− strains were generated on a mixed genetic background of C57BL/6 and 129/Sv, and it seems likely that much of the variability in the published literature is related to genetic drift combined with differences in gender and age. Our laboratory has compared mixed and pure genetic backgrounds at 1 year of age under identical experimental conditions. Male M/Mt-CK−/− mice with a mixed background had overt hypertrophy and congestive heart failure, whereas females only had LV dysfunction [29]. In comparison, M/Mt-CK−/− mice on a pure C57BL/6 background had normal LV dimensions, absence of LVH, and only mild functional impairment [29]. These results show that a primary defect in the PCr/CK system (i.e. loss of CK activity) is sufficient to drive the heart into failure. However, it also shows that this deterioration is progressive, occurring over a prolonged period of time, perhaps suggesting that initial adaptive mechanisms are not sustainable in the long run.
3.3 Evidence from “Cr Knockouts”
GAMT knockout mice lack the second essential enzyme in creatine biosynthesis and therefore have a whole body creatine deficiency. This has profound effects on body weight with GAMT−/− mice considerably lighter than controls despite normal activity and food intake [38]. In the initial description, GAMT−/− mice had creatine levels ~27 % of control values in the heart, which was later attributed to coprophagia of wild-type faeces [39]. When housed separately from wild-type mice, myocardial creatine and PCr levels are undetectable, while ATP is unaffected [40]. However, GAMT−/− mice accumulate the creatine pre-cursor guanidinoacetate, which is phosphorylated in the CK reaction to form phospho-guanidinoacetate (P-GA). This may partially compensate for the loss of PCr in an acute setting, since P-GA is apparently used up during ischaemia [39, 40], however, regeneration of P-GA is insufficient to aid recovery [40]. Saturation transfer experiments have since shown that the rate of phospho-transfer from ATP to P-GA is below the limits of detection suggesting that the kinetics of this reaction are too slow to be useful under most conditions [39, 41].
GAMT−/− mice do not develop cardiac hypertrophy and there were no differences in LV structure and function under baseline conditions with the exception of low LV systolic pressure. However, in general agreement with β-GPA feeding studies, contractile reserve was blunted in GAMT−/− mice [40]. Our laboratory has gone to considerable lengths to identify compensatory adaptations, but without success. For example, adenylate kinase, AMPK activation and mitochondrial organisation and respiration are all unaffected [41, 42]. We also took a non-biased approach using 2-D difference in-gel electrophoresis and quantitative mass spectrometry to compare proteomes of GAMT−/− and WT hearts. There were no significant differences in any of the 546 proteins detected [41]. It is not possible to prove the absence of adaptations, however these findings rule out the adaptations previously suggested for CK knockout mice and after β-GPA feeding [17].
AGAT KO—Mice with ablation of the first essential enzyme in creatine biosynthesis have the theoretical advantage that there is no accumulation of the potentially confounding creatine pre-cursor guanidinoacetate. These mice also have a low body fat phenotype and this was associated with protection against metabolic syndrome when mice were exposed to high-fat feeding [43]. The cardiac phenotype of AGAT−/− has yet to be described.
Comment on loss-of-function models: The current consensus view seems a reasonable one and is supported by the largest body of evidence, i.e. that loss or impairment of the CK system is generally not essential to support normal cardiac function under baseline and low workload conditions, and that the PCr/CK system is only truly important to cardiac function at times of high demand or stress.
4 Consequences of PCr/CK System Ablation During Pathological Stress
4.1 Ischaemia/Reperfusion
A fully-functioning CK system is vitally important for recovery of cardiac pump function following ischaemia. Rat hearts perfused with iodoacetamide prior to hypoxic challenge are incapable of regenerating PCr and subsequently fail to recover [20]. Identical results have been obtained in the genetic ablation models. Both GAMT−/− and M/Mt-CK−/− mice are more susceptible to ischaemia/reperfusion injury than wild-type controls [33, 40]. For example, in M/Mt-CK−/− mice, systolic recovery was prolonged, LVEDP increased, and there were more instances of contracture. This was associated with greater accumulation of diastolic calcium suggesting a role for CK in maintaining calcium homeostasis under stress conditions [33].
4.2 Myocardial Infarction
The results from β-GPA feeding are unequivocal, with two independent studies both suggesting that creatine is obligate for surviving acute MI. Following permanent ligation of the left coronary artery, mortality in creatine-depleted rats ranged from 93 % after 60 min [44] to 100 % mortality at 24 h [45]. In stark contrast, GAMT−/− mice, with zero myocardial creatine, have normal survival in the 24 h following myocardial infarction (~90 %) [41]. This is unlikely to be due to the presence of P-GA in GAMT−/− since β-GPA can also be phosphorylated by CK with similar reaction kinetics [46]. Another explanation might be off-target effects of β-GPA, but mice injected with β-GPA prior to infarction have normal survival [41]. It seems likely that these apparently contradictory results can be explained by species differences in arrhythmogenicity, with ventricular arrhythmia increased post-MI in the β-GPA fed rats [44]. Increased mortality in the β-GPA studies does not therefore necessarily reflect an energetic deficit.
4.3 Chronic Heart Failure
Rats fed β-GPA starting immediately after permanent coronary artery ligation had a trend for more substantial LV hypertrophy at 8 weeks, but cardiac function was indistinguishable from the untreated infarct group despite myocardial ATP levels 18 % lower [45]. A disadvantage of this approach is that creatine levels gradually decline throughout the experiment, reaching 13 % of control values by the end of the study. However, these findings have been recapitulated in the GAMT−/− mouse. Six weeks after MI, knockout mice were indistinguishable from wild-type infarct mice in terms of survival, LV remodelling and cardiac function [41]. Loss of CK activity is also well tolerated in the failing heart. M/Mt-CK−/− mice survive myocardial infarction and the extent of cardiac remodelling and dysfunction was found to be similar to infarcted wild-types when studied 4 weeks post-MI using MRI [28, 37].
Conclusion: Four studies taking three different approaches have all shown that reducing creatine or CK to very low levels in rodent models of heart failure does not have any consequences in terms of survival, LV function or remodelling. This constitutes strong (albeit surprising) evidence that the down-regulation of PCr/CK consistently observed in the failing heart does not make a significant contribution to disease progression.
5 Augmenting the Cr/CK System in Heart Failure
The phenotype of knockout mice may be confounded by physiological redundancy and/or metabolic flexibility, which obscures the full significance of the protein being studied. However, the more clinically interesting question is whether CK system augmentation has promise as a therapeutic intervention, and this requires testing in relevant disease models.
5.1 Elevating [Cr] in Heart Failure
Our laboratory has produced mice constitutively over-expressing the creatine transporter (CrT-OE) resulting in mice with variable levels of creatine and phosphocreatine in the heart [47]. Very high levels of total creatine (>2-fold above wild-type) were found to be detrimental resulting in hypertrophy, dilatation and progressive dysfunction [47]. This was due to an inability to keep the enlarged creatine pool adequately phosphorylated, which adversely affects the energy available from ATP hydrolysis [47]; and reduced expression of enolase resulting in impaired glycolytic capacity [48]. However, levels of creatine up to twofold (<140 nmol/mg protein) are well tolerated even in ageing mice [49], and, using in vivo 1H-MRS [50], we therefore pre-selected mice within this range before subjecting them to permanent coronary artery ligation. Six weeks later both WT and CrT-OE groups developed LV remodelling and chronic heart failure, but there was no benefit associated with maintaining elevated creatine levels throughout. While creatine alone was not beneficial in heart failure, in silico modelling suggests that combination with other metabolic approaches could yet prove energetically favourable [51].
5.2 Elevating CK in Heart Failure
Mice overexpressing M-CK have been created using a “tet-off” conditional expression approach (M-CK-OE). With the transgene switched on, total CK activity and flux was significantly increased without altering PCr and ATP concentrations or baseline cardiac function [52]. Following transverse aortic constriction (TAC), in vivo CK flux remained elevated in M-CK mice throughout development of heart failure and PCr/ATP ratio was better preserved. This was associated with higher indices of contractile function and significantly improved survival. In an elegant demonstration of cause and effect, the protective effect was lost when the transgene was switched off [52]. This represents the first direct evidence that augmentation of the CK system can be therapeutically beneficial in the failing heart.
6 Augmenting Cr/CK System in Ischaemia/Reperfusion
6.1 Moderately Increasing Myocardial Creatine is Beneficial
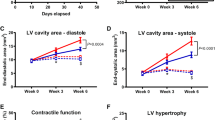
Our laboratory tested CrT-OE mice using an in vivo model of 45 min ischaemia and 24 h reperfusion. CrT-OE mice had 27 % less myocardial injury than control mice correlating with [Cr] levels suggesting a “dose-dependent” protective effect. CrT-OE mice also exhibited significantly improved functional recovery following 20 min global ischaemia ex vivo (Fig. 2) [49]. We have identified the key mechanisms behind this beneficial effect [49]: (1) Baseline myocardial PCr levels were 49 % higher in CrT-OE mice, thereby delaying ischaemic onset, and PCr recovery was more rapid and complete at reperfusion. (2) CrT-OE mice maintained a more favourable ATP/ADP ratio resulting in higher ΔGATP. (3) CrT-OE mice had baseline levels of myocardial glycogen 2.8-fold above WT, which may in itself be protective [53]. (4) Creatine reduced the opening probability of the mitochondrial permeability transition pore (mPTP) when tested in HL1 cells.
Creatine transporter overexpressing mice (CrT-OE) are protected from ischaemia-reperfusion injury. In vivo: (a) Mice with elevated myocardial creatine subjected to 45 min ischaemia and 24 h reperfusion had 27 % smaller infarct size compared to control mice, a reduction comparable in effect to ischaemic post-conditioning (IPC). (b) There was a significant inverse correlation between infarct size and myocardial creatine levels. Ex vivo: (c) Isolated perfused CrT-OE hearts had elevated phosphocreatine at baseline and during reperfusion, which resulted in greatly improved functional recovery (d) compared to wild-type (WT). RPP is rate pressure product, i.e. heart rate x developed pressure. Reproduced from [49] with permission from Oxford University Press on behalf of the European Society of Cardiology
6.2 Over-Expression of M-CK is Beneficial
A similar beneficial effect has recently been described in M-CK-OE [54]. Isolated perfused hearts were subjected to 25 min global no-flow ischaemia and 40 min reperfusion. M-CK-OE hearts had significantly better functional recovery (65 % of baseline vs. 14 %) and reduced LDH release indicating less cellular damage. The rate of ATP synthesis via CK was higher in transgenic mice, both at baseline and during recovery, resulting in reduced acidosis during ischaemia, and greatly improved re-synthesis of PCr.
Comment: Both augmentation of substrate (creatine) and enzyme (M-CK) have now independently shown promise as cardio-protective agents in I/R. Much additional work remains to be done in this area. For example, studies need to be repeated under more clinically relevant conditions, e.g. in older animals with co-morbidities. There are other obvious related targets such as testing the combination of elevated substrate + enzyme and over-expression of other CK isoenzymes, e.g. Mt-CK and B-CK. Mt-CK is of particular interest in I/R injury since creatine has an inhibitory effect on mPTP opening, with mito-CK localisation within the inter-mitochondrial membrane obligate for this effect [55].
Clinical translation remains a long way off and the lack of pharmacological tools for activating CrT and CK activity is a major limiting factor. This will require a much better understanding of the normal physiological control of creatine levels and CK activity in the heart, and non-biased approaches may be particularly useful in this regard. For example, we recently used gene-expression profiling to identify thioredoxin interacting protein (Txnip) as an endogenous inhibitor of CrT activity, which suggests a potential role for redox regulation [56].
7 Conclusions
Observational studies in animal models and patients clearly suggest a causal role for the CK system in the development of heart failure. However, evidence from knockout mice is equivocal in this regard and difficult to interpret. CK system deficiency probably limits contractile reserve, but on the whole, does not readily recapitulate a heart failure phenotype. The one exception is the M/Mt-CK−/− mouse, but only in ageing male mice with permissive genetic backgrounds. Since equivalent creatine loss is much better tolerated, we should be open to the possibility that the CK proteins may have other, as yet undefined, functions within the cell. The strongest evidence against causality is that loss of CK activity or creatine does not affect outcome in chronic heart failure models. It could be argued that this simply reflects physiological redundancy or the development of compensatory adaptations, but in that case, why don’t similar adaptations occur in the failing heart. Ultimately, the argument over causality has been side-lined by the findings that CK or creatine augmentation can be cardio-protective in ischaemia/reperfusion injury and that M-CK overexpression is beneficial in heart failure. This heralds a new and exciting phase of research with the opportunity to focus on translational aspects of creatine and CK augmentation in the heart.
References
Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 1762:164–180
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Ingwall JS, Weiss RG (2004) Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 95:135–145
Neubauer S (2007) The failing heart–an engine out of fuel. N Engl J Med 356:1140–1151
Wallimann T, Wyss M, Brdiczka D et al (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281(Pt 1):21–40
Sylven C, Lin L, Kallner A et al (1991) Dynamics of creatine kinase shuttle enzymes in the human heart. Eur J Clin Invest 21:350–354
Lygate CA, Fischer A, Sebag-Montefiore L et al (2007) The creatine kinase energy transport system in the failing mouse heart. J Mol Cell Cardiol 42:1129–1136
Shen W, Asai K, Uechi M et al (1999) Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs: a compensatory role for the parallel loss of creatine. Circulation 100:2113–2118
Neubauer S, Remkes H, Spindler M et al (1999) Downregulation of the Na(+)-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation 100:1847–1850
Tian R, Nascimben L, Kaddurah-Daouk R et al (1996) Depletion of energy reserve via the creatine kinase reaction during the evolution of heart failure in cardiomyopathic hamsters. J Mol Cell Cardiol 28:755–765
Maslov MY, Chacko VP, Stuber M et al (2007) Altered high-energy phosphate metabolism predicts contractile dysfunction and subsequent ventricular remodeling in pressure-overload hypertrophy mice. Am J Physiol Heart Circ Physiol 292:H387–H391
Neubauer S, Krahe T, Schindler R et al (1992) 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86:1810–1818
Neubauer S, Horn M, Pabst T et al (1995) Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J 16:115–118
Neubauer S, Horn M, Cramer M et al (1997) Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96:2190–2196
Oudman I, Clark JF, Brewster LM (2013) The effect of the creatine analogue beta-guanidinopropionic acid on energy metabolism: a systematic review. PLoS One 8:e52879
Bloch K, Schoenheimer R, Rittenberg D (1941) Rate of formation and disappearance of body creatine in normal animals. J Biol Chem 138:155–166
Wiesner RJ, Hornung TV, Garman JD et al (1999) Stimulation of mitochondrial gene expression and proliferation of mitochondria following impairment of cellular energy transfer by inhibition of the phosphocreatine circuit in rat hearts. J Bioenerg Biomembr 31:559–567
Mekhfi H, Hoerter J, Lauer C et al (1990) Myocardial adaptation to creatine deficiency in rats fed with beta-guanidinopropionic acid, a creatine analogue. Am J Physiol Heart Circ Physiol 258:H1151–H1158
Kapelko VI, Kupriyanov VV, Novikova NA et al (1988) The cardiac contractile failure induced by chronic creatine and phosphocreatine deficiency. J Mol Cell Cardiol 20:465–479
Hamman BL, Bittl JA, Jacobus WE et al (1995) Inhibition of the creatine kinase reaction decreases the contractile reserve of isolated rat hearts. Am J Physiol Heart Circ Physiol 269:H1030–H1036
Tian R, Ingwall JS (1996) Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Physiol 270:H1207–H1216
van Deursen J, Heerschap A, Oerlemans F et al (1993) Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 74:621–631
Steeghs K, Heerschap A, de Haan A et al (1997) Use of gene targeting for compromising energy homeostasis in neuro-muscular tissues: the role of sarcomeric mitochondrial creatine kinase. J Neurosci Methods 71:29–41
Steeghs K, Benders A, Oerlemans F et al (1997) Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell 89:93–103
Ventura-Clapier R, Kuznetsov AV, d’Albis A et al (1995) Muscle creatine kinase-deficient mice. I. Alterations in myofibrillar function. J Biol Chem 270:19914–19920
Van Dorsten FA, Nederhoff MG, Nicolay K et al (1998) 31P NMR studies of creatine kinase flux in M-creatine kinase-deficient mouse heart. Am J Physiol Heart Circ Physiol 275:H1191–H1199
Saupe KW, Spindler M, Tian R et al (1998) Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res 82:898–907
Nahrendorf M, Spindler M, Hu K et al (2005) Creatine kinase knockout mice show left ventricular hypertrophy and dilatation, but unaltered remodeling post-myocardial infarction. Cardiovasc Res 65:419–427
Lygate CA, Medway DJ, Ostrowski PJ et al (2012) Chronic creatine kinase deficiency eventually leads to congestive heart failure, but severity is dependent on genetic background, gender and age. Basic Res Cardiol 107:276
Spindler M, Niebler R, Remkes H et al (2002) Mitochondrial creatine kinase is critically necessary for normal myocardial high-energy phosphate metabolism. Am J Physiol Heart Circ Physiol 283:H680–H687
Bonz AW, Kniesch S, Hofmann U et al (2002) Functional properties and Ca(2+). (i) metabolism of creatine kinase–KO mice myocardium. Biochem Biophys Res Commun 298:163–168
Gustafson LA, Van Beek JH (2002) Activation time of myocardial oxidative phosphorylation in creatine kinase and adenylate kinase knockout mice. Am J Physiol Heart Circ Physiol 282:H2259–H2264
Spindler M, Meyer K, Stromer H et al (2004) Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol Heart Circ Physiol 287:H1039–H1045
Saupe KW, Spindler M, Hopkins JC et al (2000) Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. Reaction kinetics of the creatine kinase isoenzymes in the intact heart. J Biol Chem 275:19742–19746
Crozatier B, Badoual T, Boehm E et al (2002) Role of creatine kinase in cardiac excitation-contraction coupling: studies in creatine kinase-deficient mice. FASEB J 16:653–660
Lygate CA, Hunyor I, Medway D et al (2009) Cardiac phenotype of mitochondrial creatine kinase knockout mice is modified on a pure C57BL/6 genetic background. J Mol Cell Cardiol 46:93–99
Nahrendorf M, Streif JU, Hiller KH et al (2006) Multimodal functional cardiac MR imaging in creatine kinase deficient mice reveals subtle abnormalities in myocardial perfusion and mechanics. Am J Physiol Heart Circ Physiol 290:H2516–H2521
Schmidt A, Marescau B, Boehm EA et al (2004) Severely altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency. Hum Mol Genet 13:905–921
Kan HE, Renema WK, Isbrandt D et al (2004) Phosphorylated guanidinoacetate partly compensates for the lack of phosphocreatine in skeletal muscle of mice lacking guanidinoacetate methyltransferase. J Physiol 560:219–229
ten Hove M, Lygate CA, Fischer A et al (2005) Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation 111:2477–2485
Lygate CA, Aksentijevic D, Dawson D et al (2013) Living without creatine: unchanged exercise capacity and response to chronic myocardial infarction in creatine-deficient mice. Circ Res 112:945–955
Branovets J, Sepp M, Kotlyarova S et al (2013) Unchanged mitochondrial organization and compartmentation of high-energy phosphates in creatine-deficient GAMT−/− mouse hearts. Am J Physiol Heart Circ Physiol 305:H506–H520
Choe C-u, Nabuurs C, Stockebrand MC et al (2013) l-arginine:glycine amidinotransferase deficiency protects from metabolic syndrome. Hum Mol Genet 22:110–123
Lorentzon M, Ramunddal T, Bollano E et al (2007) In vivo effects of myocardial creatine depletion on left ventricular function, morphology, and energy metabolism–consequences in acute myocardial infarction. J Card Fail 13:230–237
Horn M, Remkes H, Stromer H et al (2001) Chronic phosphocreatine depletion by the creatine analogue beta-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation 104:1844–1849
Boehm EA, Radda GK, Tomlin H et al (1996) The utilisation of creatine and its analogues by cytosolic and mitochondrial creatine kinase. Biochim Biophys Acta 1274:119–128
Wallis J, Lygate CA, Fischer A et al (2005) Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure: insights from creatine transporter-overexpressing transgenic mice. Circulation 112:3131–3139
Phillips D, ten Hove M, Schneider JE et al (2010) Mice over-expressing the myocardial creatine transporter develop progressive heart failure and show decreased glycolytic capacity. J Mol Cell Cardiol 48:582–590
Lygate CA, Bohl S, ten Hove M et al (2012) Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc Res 96:466–475
Schneider JE, Tyler DJ, ten Hove M et al (2004) In vivo cardiac 1H-MRS in the mouse. Magn Reson Med 52:1029–1035
Wu F, Zhang J, Beard DA (2009) Experimentally observed phenomena on cardiac energetics in heart failure emerge from simulations of cardiac metabolism. Proc Natl Acad Sci U S A 106:7143–7148
Gupta A, Akki A, Wang Y et al (2012) Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest 122:291–302
Vanoverschelde JL, Janier MF, Bakke JE et al (1994) Rate of glycolysis during ischemia determines extent of ischemic injury and functional recovery after reperfusion. Am J Physiol Heart Circ Physiol 267:H1785–H1794
Akki A, Su J, Yano T et al (2012) Creatine kinase over-expression improves ATP kinetics and contractile function in post-ischemic myocardium. Am J Physiol Heart Circ Physiol 303:H844–H852
Dolder M, Walzel B, Speer O et al (2003) Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem 278:17760–17766
Zervou S, Ray T, Sahgal N et al (2013) A role for thioredoxin-interacting protein (Txnip) in cellular creatine homeostasis. Am J Physiol Endocrinol Metab 305:E263–E270
Acknowledgements
Work in the authors’ laboratory is funded by the British Heart Foundation (Programme grant RG/13/8/30266).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lygate, C.A., Neubauer, S. (2014). The Myocardial Creatine Kinase System in the Normal, Ischaemic and Failing Heart. In: Lopaschuk, G., Dhalla, N. (eds) Cardiac Energy Metabolism in Health and Disease. Advances in Biochemistry in Health and Disease, vol 11. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1227-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1227-8_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1226-1
Online ISBN: 978-1-4939-1227-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)