Abstract
Proliferative vitreoretinopathy (PVR) refers to the proliferation of retinal pigment epithelial cells, hyalocytes, and glial cells at the vitreous and on the retinal surface in rhegmatogenous retinal detachment (RRD). Seen in 5–10 % of all cases of RRD [1] and found in 75 % of all recurrent retinal detachment cases [2], PVR is the most common reason for failure of retinal reattachment surgery. PVR results from the dispersion, migration, and proliferation of cells into the vitreous as well as the outer and inner surface of the retina. The cell types involved in the process of PVR formation include retinal pigment epithelial (RPE) cells, fibroblasts, macrophages, hyalocytes, and glial cells [3]. Cytokines such as fibronectin and platelet-derived growth factor (PDGF) have also been implicated in the process of PVR formation [4]. These cells and cytokines lead to membrane formation and contraction of the vitreous and retina resulting in recurrent retinal detachment [4].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitreous
- Rhegmatogenous retinal detachment
- Proliferative vitreoretinopathy
- Preretinal PVR membranes
- Subretinal fibrosis
- Traction retinal detachment
- Histopathology
- Pathophysiology
- Wound repair
- Cytokines
-
1.
Proliferative vitreoretinopathy is the undesired formation of fibrous contractile membranes at the vitreoretinal interface and on both surfaces of the retina that results from the proliferation of retinal pigment epithelial cells, hyalocytes, and glial cells in the vitreous and on the retinal surface following rhegmatogenous retinal detachment.
-
2.
At the time of retinal break formation, retinal pigment epithelial cells are released and migrate into the vitreous and onto the retinal surface along with fibroblasts, glial cells, and macrophage hyalocytes. Transforming growth factor-β released by retinal pigment epithelial cells stimulates production of collagen and fibronectin which in turn stimulates additional cell migration and proliferation, thus continuing the vicious cycle.
-
3.
Under the influence of cytokines, these cells form contractile membranes leading to recurrent retinal detachment. Much research is being conducted on the specific cytokines and cells involved in this complex pathophysiological process, and it is hoped that additional therapeutic and preventative options will be available in the future.
I. Introduction
Proliferative vitreoretinopathy (PVR) refers to the proliferation of retinal pigment epithelial cells, hyalocytes, and glial cells at the vitreous and on the retinal surface in rhegmatogenous retinal detachment (RRD). Seen in 5–10 % of all cases of RRD [1] and found in 75 % of all recurrent retinal detachment cases [2], PVR is the most common reason for failure of retinal reattachment surgery. PVR results from the dispersion, migration, and proliferation of cells into the vitreous as well as the outer and inner surface of the retina. The cell types involved in the process of PVR formation include retinal pigment epithelial (RPE) cells, fibroblasts, macrophages, hyalocytes, and glial cells [3]. Cytokines such as fibronectin and platelet-derived growth factor (PDGF) have also been implicated in the process of PVR formation [4]. These cells and cytokines lead to membrane formation and contraction of the vitreous and retina resulting in recurrent retinal detachment [4].
II. Clinical Features of PVR
A. Risk Factors of PVR
Multiple factors have been identified that increase the risk of developing PVR. Increased risk has been observed in patients with large retinal tears, multiple retinal tears, longer duration of retinal detachment, and high levels of vitreous protein such as occurs in vitreous hemorrhage and intraocular inflammation [5]. Presence of preoperative PVR, aphakia, and ocular trauma have also been associated with increased risk of PVR [5].
A model known as the PVR score has been used by many to determine the risk of developing PVR, where PVR score = 2.88 × (grade C PVR) + 1.85 × (grade B PVR) + 2.92 × (aphakia) + 1.77 × (anterior uveitis) + 1.23 × (quadrants of detachment) + 0.83 × (vitreous hemorrhage) + 23 × (previous cryotherapy) [6] with one added to the total score if retinal fibrosis is present. A patient is considered high risk if the score is greater than 6.33 [7].
B. Clinical Signs and Staging of PVR
The earliest signs of PVR are the presence of flare, haze, and pigment within the vitreous. As PVR evolves, wrinkling of the inner retinal surface can be seen with vessel tortuosity and rolled edges of retinal breaks. Later states include fixed retinal folds (Figure III.J-1), macular pucker (Figure III.J-2), star folds of the retina (Figure III.J-3), and subretinal fibrotic bands (Figure III.J-4). As the membranes contract, localized detachments can also be seen (Figure III.J-5), which typically take on a concave shape versus the convex shape of a rhegmatogenous retinal detachment. Other associated but nonspecific signs include flare in the anterior chamber, neovascularization of the iris from ischemia, and hypotony resulting from total retinal detachment (Figures III.J-6 and III.J-7 top) [3], as well as traction by the anterior loop of the vitreous case upon the ciliary body [see chapter III.H. Peripheral vitreo-retinal pathology].
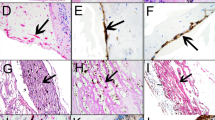
This 26-year-old man with a history of a rhegmatogenous retinal detachment and proliferative vitreoretinopathy treated with scleral buckle, vitrectomy, lensectomy, and silicone oil presented 4 months following surgery with inferior proliferative vitreoretinopathy and recurrent inferior retinal detachment under silicone oil
Anterior PVR occurs in the collapsed vitreous base, demonstrated by arrows in gross (a) and microscopic (b) sections. Traction from the vitreous base may lead to incorporation and later proliferation of elements from the peripheral retina and ciliary body, thus eventually resulting in hypotony and phthisis (b. H&E, 2×)
Two main classification systems have been utilized in the staging of PVR. The first, more widely used classification system, was initially proposed by the Retina Society in 1983 and is organized based on severity and location of ocular findings (Table III.J-1) [8]. Grade A is presence of haze and pigmented cells within the vitreous. Grade B is wrinkling seen at the edge of the retinal tear or at the inner retinal surface, and grade C is the presence of fixed retinal folds. Three subtypes of grade C PVR exist depending on the location of the PVR: posterior, anterior, or both. The second classification system was proposed by the Silicone Study Group, which categorizes based on type of contraction and location (Table III.J-2) [6].
C. Histopathology of PVR
D. Schwartz et al. studied transmission electron microscopy characteristics of PVR membranes removed from 20 human eyes and noted membranes were composed of RPE cells, fibrous astrocytes, fibrocytes, macrophages (some probably hyalocytes), and cells with myoblastic differentiation [9]. In addition, extracellular matrix consisting of collagen and fibrin was also found in the PVR membranes. We have found a similar composition of PVR membranes at our institution (Figure III.J-8).
Proliferative vitreoretinopathy (PVR) is composed of fibrocellular tissue (arrow, top left) on the inner surface of the retina. There may be up to five cell types in the tissue as shown, including RPE, fibrous astrocytes containing intermediate filaments and patches of basement membrane (arrows, top right), fibrocytes including rough endoplasmic reticulum and centrioles (between brackets in the lower left hand image), myofibroblasts containing intracytoplasmic fusiform densities (arrows), and macrophages with intracytoplasmic inclusions of varying electron density (arrows, lower right) (top left, H&E 63×; other images uranyl acetate lead citrate 2,000× with insets 25,000×)
D. Pathophysiology of PVR
An understanding of the pathogenesis of PVR is critical in the prevention and treatment of this disease. The development and progression of PVR is a complex interaction between the different cells, inflammatory cytokines, and growth factors leading to fibrous membrane formation [10]. The cycle begins with the dispersion of retinal pigment epithelial cells at the time of retinal break formation [11]. Scleral depression and cryotherapy can cause further release of RPE cells into the vitreous cavity [12]. In addition, RPE cells can migrate through the retinal break into the vitreous and onto the retinal surface, along with other cell types such as fibroblasts, glial cells, and macrophages [13]. The presence of cytokines such as fibronectin and platelet-derived growth factor can stimulate further cell migration and proliferation [14]. Cytokines may be present from vitreous hemorrhage, intraocular inflammation, or breakdown of the blood-retinal barrier as occurs following cryotherapy [12]. RPE cells can release transforming growth factor-β which can stimulate production of collagen and fibronectin which in turn stimulates additional cell migration and proliferation thus continuing this vicious cycle [15]. Hyalocytes are mononuclear phagocytes (macrophages) that reside in the posterior vitreous cortex [see chapter II.D. Hyalocytes] that would be exposed to all these cytokine stimuli. Under the influence of the cytokine stimuli, all these cells migrate, proliferate, and, because they form membranes at the vitreoretinal interface, can contract and pull on the dense collagen matrix of the posterior vitreous cortex [see chapter II.E. Vitreo-retinal interface and ILM]. Eventually, this results in contraction of the PVR membranes in the posterior vitreous that mediates traction upon the retina leading to recurrent retinal detachment.
1. Cell Types in PVR
The main cell types involved in PVR formation are RPE cells, macrophages (including hyalocytes), fibroblasts, and glial cells [13], all noted to migrate into the vitreous and along the vitreoretinal interface following breakdown of the blood-retinal barrier (Figure III.J-9). Their response, typically seen in wound healing, leads to matrix and membrane formation, which leads to increasing traction on the retina and detachment.
a. Retinal Pigment Epithelial (RPE) Cells
RPE cells are postmitotic cells and serve the role of supporting photoreceptor cells. Following a retinal break, RPE cells migrate into the vitreous and form undifferentiated cells within the extracellular matrix (ECM) at the vitreoretinal interface. The RPE neural retinal adhesions and ECM adhesions are lost in the transformation process, and the RPE cells can undergo epithelial-mesenchymal transition and migrate into the vitreous, and also undergo metaplastic transformation [16]. This change of the RPE cells is influenced by the change in environment and the rich abundance of growth factors and cytokines within the vitreous, such as PDGF, FGF, and IGF-1 produced by other cells such as Muller cells, macrophages, and fibroblasts which enter the vitreous via the retina break [17]. The transformed RPE cells lay down fibrotic membranes that form a thick contractile membrane, which leads to contraction and traction of the retina and to a recurrent retinal detachment (Figure III.J-10) [18]. Membrane peeling is usually needed in order to relieve traction and successfully reattach the retina [see chapter V.B.5. Surgical management of retinal detachment with PVR]. Furthermore, the loss of RPE cells also causes loss of support for the photoreceptors, leading to photoreceptor destruction [19].
Retinal pigment epithelium (RPE) is present on the surface of this PVR membrane. The RPE contains intracytoplasmic lancet-shaped melanin pigment granules and exhibits surface microvillous processes (black arrowhead). There are fibrocytes and collagen in the sub-RPE tissue (uranyl acetate, lead citrate 2,000×)
b. Macrophages/Hyalocytes
Macrophages, including hyalocytes, have also been found to play a key role in the development of PVR (Figure III.J-11) [20]. Studies in rabbits have found that the injection of macrophages into the eyes of rabbits led to an inflammatory response similar to that seen in PVR and also the formation of fibrous membranes [20]. Macrophages are thought to play a multifactorial role, both through the secretion of cytokines and differentiation into fibroblast-like cells [20, 21]. Hyalocytes elicit monocyte migration from the circulation, which is augmented by the breakdown in the blood-ocular barrier that is well documented in PVR, further increasing the cell population that forms PVR membranes. The cytokines produced such as platelet-derived growth factor (PDGF) cause a change in the overall vitreous structure, leading to proteolysis and development of fibrous membranes [14]. The transformation of macrophages into fibroblast-like cells leads to further fibrosis and inflammatory changes within the vitreous.
c. Glial Cells
The glial cells mentioned above are actually activated Mueller cells. The initial retinal break which triggers the proliferative and inflammatory response also activates Muller cells, the supporting cells of the retina. Activated Muller cells migrate into the vitreous and undergo proliferation and transformation at the vitreoretinal interface, causing an increase in glial fibrillary acid protein (GFAP) and vimentin [22]. Activated Muller cells also have an increase in PDGFR-α production, a key cytokine in the pathogenesis process that will be discussed later. Muller cells have the plasticity and capacity to transform phenotypes, and this dedifferentiation and transformation helps to contribute to a change to the vitreous’ overall environment and structure.
d. Myofibroblasts
Myofibroblasts also migrate into the vitreous following a break in the blood-retinal barrier. Possessing both contractile and fibroblast properties, myofibroblasts lead to an increase in the expression of α smooth muscle actin, imparting great contractile properties [23]. Myofibroblasts are also able to produce and secrete an extracellular matrix, especially collagen, that mediates the contractile properties within the proliferative membranes to the retina.
e. Immune Cells
There has, as it is basically an inflammatory response typically seen in wound repair also been speculation as to whether the immune system plays a role in PVR following injury anywhere in the body. However, studies using mouse models have found that PVR develops in mice in the absence of B or T cell immunity [24]. Thus, the immune system is not essential for the development of PVR, but the related cells and inflammatory/immune cytokines play essential roles.
2. Cytokines of PVR
The inciting event of the retinal break in PVR causes the retinal cell layer to come into contact with the vitreous, triggering RPE proliferation and migration into the vitreous. The transformation allows the activated RPE cells to secrete numerous proteins, components of the extracellular matrix, and cytokines. This different cytokines that are released into the vitreous and their roles in creating an inflammatory and fibrotic environment within the vitreous are discussed herein.
a. Tumor Necrosis Factor-α (TNF-α)
TNF-α is one of the cytokines released by the activated RPE cells and macrophages. Typically a marker in inflammation and wound healing, it activates the endothelial cells in the retina to produce leukocyte adhesion molecules, facilitating the influx of cells and the inflammatory response. Study has shown that TNF-α was found in the preretinal membrane of 22 out of 26 patients with PVR, and staining detected its presence within the extracellular matrix, showing its prominence and thus represents a potential subject for further research [25].
b. Transforming Growth Factor-β (TFG-β)
TFG-β is another cytokine that is found in elevated levels in the vitreous after retinal detachment. The second isoform TFG-β2 is the most predominant form present and can elevate up to three times the normal amount in PVR. It is secreted into the vitreous by numerous cells, such as the ciliary body, macrophages, migrated RPE cells, and Muller cells [26]. TFG-β2 helps to induce the epithelial-mesenchymal transformation of RPE cells into fibroblastic cells and also induces collagen synthesis in the transformed RPE cells. In addition, it increases RPE-mediated contraction leading to increase in fibrosis [15]. As discussed earlier, RPE cells play a crucial part in initiating fibrosis and formation of vitreoretinal membranes, and TFG-β2 helps to induce RPE cells to take on fibroblastic characteristics and induce fibrosis.
c. Platelet-Derived Growth Factor Receptor-α (PDGFR-α)
PDGFR-α is garnering much attention in the field, since PDGFR-α is a key cytokine that is triggered in ocular injury. A chemoattractant and mitogen, it acts as a mediator of cellular contraction for RPE cells. There are 4 isoforms, with PDGFR-αC being the most predominant [27]. It is activated by PDGF but also by non-PDGR molecules such as insulin, epidermal growth factor (EGF), and hepatocyte growth factor (HGF). The activation of the receptor leads to suppression of P53, a key tumor suppressor gene, and increased proliferation, membrane formation, contraction, and survival. Research has shown that PDGFR-α is found in large quantities in rabbit models of PVR and that RPE and Muller cell interaction lead to a further upregulation of PDGFR-α and increased pathogenicity of Muller cells [28]. In addition, recent research has demonstrated that inhibition of PDGFR-α prevented PVR formation in a rabbit model [29]. Thus, targeting inhibition of PDGFR-α may lead to potential therapeutic agents in the future.
d. Miscellaneous Cytokines
Various other cytokines have also been shown to have roles in the proliferative and inflammatory processes. Activated RPE cells express hepatocyte growth factor (HGF), which stimulates RPE migration [30]. Significantly elevated levels of osteoponin, high-mobility group box-1 (HMBGB1), and connective tissue growth factor (CTGF) have also been found in PVR [31]. CTGF has been shown to be a potent stimulator of hyalocyte activity, particularly the induction of membrane contraction [see chapter II.D. Hyalocytes]. HMBGB1 has been shown to have angiogenic and fibrogenic effects. In vitro studies have shown that HMGB1 stimulated proliferation and migration of fibroblasts [32, 33]. CTGF is a downstream mediator of TGF-β and is a mitogen and stimulated the formation of extracellular matrix. Potential therapeutic agents targeting this cytokine have also been found [34, 35]. Elevated levels of pigment epithelium-derived factor (PEDF) have also been found in the vitreous of patients with PVR compared to patients without PVR [31]. PEDF inhibits migration of endothelial cells which may account for the avascular nature of PVR membranes [31], a phenomenon observed in the human fetal vitreous during regression of the hyaloid vasculature [see chapter I.D. Vitreous proteomics and regression of the fetal hyaloid vasculature].
Monocyte chemotactic protein-1 (MCP-1) has also been found at elevated levels in patients with PVR compared to no PVR [36–38]. Experimental retinal detachment induces increased MCP-1 expression in Müller cells and increased numbers of macrophages and microglial in detached retina [39]. MCP-1 also stimulates human RPE cell migration in vitro [40]. Mice with gene deficiencies in MCP-1 or the use of a MCP-1 blocking antibody greatly reduces macrophage and microglial response as well as photoreceptor apoptosis from the retinal detachment [39].
III. Treatment of PVR
A. Surgery of PVR
Currently the mainstay treatment for retinal detachment from PVR is surgery [see chapter V.B.5. Surgical management of retinal detachment with PVR]. Although successful reattachment occurs in 60–80 % of all cases, re-detachment is very common. [41] The main principles of treating recurrent retinal detachment from PVR include closing all retinal breaks, decreasing retinal traction, reattaching the retina, and minimizing recurrent traction. Decreasing traction on the retina can be achieved in several ways by membrane peeling, scleral buckling, debulking of the vitreous base, relaxing retinectomies [see chapter V.B.6. Retinectomy in recalcitrant retinal detachments], and internal tamponade. Often times, successful retinal reattachment requires a combination of the above techniques [see chapter V.B.5. Surgical management of retinal detachment with PVR].
B. Pharmacotherapy of PVR
Several studies are underway to identify patients at high risk for developing PVR so as to prevent PVR. Biomarkers are currently being utilized to study a myriad of disease processes. Studies have demonstrated that the use of a biomarker panel has the potential to predict PVR and having a favorable versus unfavorable outcome [42]. The panel of biomarkers CCL22, IL-3, and MIF have been shown to have great potential in identifying patients at high risk for PVR with unfavorable outcomes [42]. Identifying high-risk groups is important, as early application of the agents discussed below to high-risk eyes may in fact prevent the development of PVR and subsequent need for multiple surgeries and visual compromise.
Several pharmacologic agents are being investigated in efforts to minimize recurrent retinal traction [see chapter IV.F. Pharmacotherapy of PVR]. Current research is being conducted to study potential targets in the inflammatory pathway, and different medications are being tested in animal trials and small human trials. Antibodies targeting the PDGFR pathway have been used in rabbit models, but yielding mixed results, as PDGF has been shown to be inhibited but not PVR [43]. However, n-acetylcysteine (NAC), an antioxidant against reactive oxygen species, has been shown to prevent the activation of PDGFR in rabbit models and reduced the overall inflammatory response seen in prPVR [1]. Overall, results showed that NAC suppressed PDGFR receptor activation and retinal detachment, making it a potential candidate for pharmacological therapy.
Existing medications have also been studied for their potential in preventing or decreasing PVR. 5-FU (5-fluorouracil), a chemotherapy agent, has been shown to inhibit fibroblast formation. Human trials using 5-FU for PVR have yielded mixed results thus far [44]. The efficacy of low-molecular-weight heparin (LMWH) has also been studied [45]. LMWH has been shown to bind to fibronectin, FGF, and PDFGR. One study of 174 high-risk patients found the combination of 5-FU and LMWH reduced the incidence of PVR and reoperation rate, but had no change in overall visual acuity [44]. Although these results are very promising, the combination is currently not widely used in the clinical setting.
13-cis-retinoic acid is another agent currently being studied as a potential agent against PVR. In vitro studies have shown that 13-cis-retinoic acid is capable of inhibiting RPE proliferation [46]. A recent clinical trial of 35 patients showed that the use of retinoic acid in the treatment group resulted in significantly lower rates of macular traction and pucker and better visual acuity compared to the control group [47].
Nutlin 3 is another agent studied. This molecule is known to inhibit the MDM2/p53 interaction and thus preventing P53 decline [48]. Studies have shown that the application of nutlin to cells isolated from the PVR membranes of patients prevented cell contraction [29]. Suppression of P53 has been found to be required in the PDGFR-α activation, which results in contraction of cells and inflammatory changes. Thus, P53 is thought to be a checkpoint in PVR and is becoming an area of active research within the field.
Recent studies have also focused on new routes of delivery of medications and antimetabolites into the retina for prevention of PVR. A study using porcine eyes showed that the use of aerosolized nanoparticles during vitrectomy is a potential method for delivering antimetabolites to the retina in the future [49].
- 5-FU:
-
5-fluorouracil
- CCL22:
-
Chemokine cluster locus 22
- CTGF:
-
Connective tissue growth factor
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- FGF:
-
Fibroblastic growth factor
- GFAP:
-
Glial fibrillary acidic protein
- HGF:
-
Hepatocyte growth factor
- HMBGB1:
-
High-mobility group box-1
- IGF1:
-
Insulin-like growth factor 1
- IL-3:
-
Interleukin 3
- LMWH:
-
Low-molecular-weight heparin
- MCP1:
-
Monocyte chemotactic protein-1
- MDM2:
-
Mouse doubling minute homologue 2
- NAC:
-
n-acetylcysteine
- PDGF:
-
Platelet-derived growth factor
- PDGFR-α:
-
Platelet-derived growth factor alpha
- PEDF:
-
Pigment epithelium-derived factor
- PVR:
-
Proliferative vitreoretinopathy
- RPE:
-
Retinal pigment epithelium
- RRD:
-
Recurrent retinal detachment
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
References
Lei H, Velez G, Cui J, et al. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am J Pathol. 2010;177(1):132–40.
Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998;43(1):3–18.
Aylward W. Ophthalmology, chapter 6.41. In: Proliferative vitreoretinopathy. 3rd ed. New York, NY: Elsevier Inc.;2009.
Smiddy W, Michels R, Green R. Morphology, pathology, and surgery of idiopathic vitreoretinal macular disorders. Retina. 1990;10(4):288–96.
Cowley M, Conway BP, Campochiro PA, et al. Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol. 1989;107(8):1147–51.
Lean JS, Stern WH, Irvine AR, et al. Classification of proliferative vitreoretinopathy used in the silicone study. The Silicone Study Group. Ophthalmology. 1989;96(6):765–71.
Asaria RH, Kon CH, Bunce C, et al. How to predict proliferative vitreoretinopathy: a prospective study. Ophthalmology. 2001;108(7):1184–6.
The Classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983;90(2):121–25.
Schwartz D, De La Cruz ZC, Green WR, Michels RG. Proliferative vitreoretinopathy: ultrastructural study of 20 retroretinal membranes removed by vitreous surgery. Retina. 1988;8:275–81.
Sadaka A, Giuliari GP. Proliferative vitreoretinopathy: current and emerging treatments. Clin Ophthalmol. 2012;6:1325–31.
Machemer R, Aaberg TM, Freeman HM, et al. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112(2):159–65.
Campochiaro P, Kaden I, Vidaurri-Leal J, Glaser B. Cryotherapy enhanced intravitreal dispersion of viable retinal pigment epithelial cells. Arch Ophthalmol. 1985;103(3):434–6.
Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron- immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 1990;31(1):14–28.
Muhar HS, Pollock RA, Wang C, et al. PDGF and its receptors in the developing rodent retina and optic nerve. Development. 1993;118(2):539–52.
Yokoyama K, Kimoto K, Itoh Y, et al. The PI3K/Akt pathway mediates the expression of type I collagen induced by TGF-bet2 in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):15–23.
Du YH, Hirooka K, Miyamoto O, et al. Retinoic Acid suppresses the adhesion and migration of human retinal pigment epithelial cells. Exp Eye Res. 2013;109:22–30.
Wiedemann P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol. 1992;36(5):373–84.
Umazume K, Barak Y, McDonald K, et al. Proliferative Vitreoretinopathy in the Swine- a new model. Invest Ophthalmol Vis Sci. 2012;53(8):4910–6.
Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33(4):295–317.
Lin ML, Li YP, Li ZR, et al. Macrophages acquire fibroblast characteristics in a rat model of proliferative vitreoretinopathy. Ophthalmic Res. 2011;45(4):180–90.
Kirchhof B, Sorgente N. Pathogenesis of proliferative vitreoretinopathy. Modulation of retinal pigment epithelial cell functions by vitreous and macrophages. Dev Ophthalmol. 1989;16:1–53.
Okada M, Matsumura M, Ogino N, Honda Y. Muller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefes Arch Clin Exp Ophthalmol. 1990;228(5):467–74.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210.
Zhang W, Tan J, Liu Y, et al. Assessment of the innate and adaptive immune system in proliferative vitreoretinopathy. Eye. 2010;26(6):872–81.
Limb GA, Alam A, Earley O, et al. Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res. 1994;13(11):791–8.
Tanihara H, Yoshida M, Matsumoto M, Yoshimura N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1993;34(2):413–9.
Moysidis SN, Thanos A, Vavvas DG. Mechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedside. Mediators Inflamm. 2012;2012:815937. doi:10.1155/2012/815937. Epub 2012 Sep 25.
Velez G, Weingarden A, Tucker B, et al. Retinal pigment epithelium and muller progenitor cell interaction increase muller progenitor cell expression of PDGFR alpha and ability to induce proliferative vitreoretinopathy in a rabbit model. Stem Cells Int. 2012;2012:106486. doi:10.1155/2012/106486.
Lei H, Rheaume M, Cui J, et al. A novel function of P53, a gatekeeper of retinal detachment. Am J Pathol. 2012;181(3):866–74.
He P, He S, Garner J, et al. Retinal pigment epithelial cells secrete and respond to hepatocyte growth factor. Biochem Biophys Res Commun. 1998;249(1):253–7.
El-Asrar AM, Nawaz MI, Kangave D, et al. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediators Inflamm. 2010;2012:493043.
Yoshizaki A, Komura K, Iwata Y, et al. Clinical Significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: association with disease severity. J Clin Immunol. 2009;29(2):180–9.
Ranzato E, Patrone M, Pedrazzi M, Burlando B. HMGB1 Promotes Wound healing of 3 T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys. 2010;57(1):9–17.
Blom I, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21(6):473–82.
Shimo T, Nakanishi T, Nishida T, et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126(1):137–45.
Elner SG, Elner VM, Jaffe GJ, et al. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14(11):1045–53.
El-Asrar AM, Van Damme J, Put W, et al. Monocyte chemotactic protein-1 in proliferative Vitreoretinal disorders. Am J Ophthalmol. 1997;123(5):599–606.
Capeans C, De Rojas MV, Lojo S, et al. C-C chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina. 1998;18(6):546–50.
Nakazawa T, Hisotomi T, Nakazawa C, et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci U S A. 2007;104(7):2425–30.
Han QH, Hui YN, Du HJ, et al. Migration of retinal pigment epithelial cells in vitro modulated by monocyte chemotactic protein-1: enhancement and inhibition. Graefes Arch Clin Exp Ophthalmol. 2001;239(7):531–8.
Capeans C, Lorenzo J, Santos L, et al. Comparative study of incomplete posterior vitreous detachment as a risk factor for proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1998;236(7):481–5.
Ricker LJ, Kessels AG, de Jager W, et al. Prediction of proliferative vitreoretinopathy after retinal detachment surgery: potential of biomarker profiling. Am J Ophthalmol. 2012;154(2):347–54.
Lei H, Velez G, Hovland P, et al. Growth factors outside the PDGF family drive experimental PVR. Invest Ophthalmol Vis Sci. 2009;50(7):3394–403.
Asaria R, Kon C, Bunce C, et al. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy. Ophthalmology. 2001;108(7):1179–83.
Kumar A, Nainiwal S, Sreenivas B. Intravitreal low molecular weight heparin in PVR surgery. Indian J Ophthalmol. 2003;51(1):67–70.
Wu W, Hu D, Mehta S, Chang Y. Effects of retinoic acid on retinal pigment epithelium from excised membranes from proliferative vitreoretinopathy. J Ocul Pharmacol Ther. 2005;21(1):44–54.
Chang Y, Hu D, Wu W. Effect of oral 13-cis-retinoic acid treatment on postoperative clinical outcome of eyes with proliferative vitreoretinopathy. Am J Ophthalmol. 2008;146(3):440–6.
Prives C. Signaling to P53: breaking the MDM2-p53 circuit. Cell. 1998;95(1):5–8.
Zhang G, Feng X, Wabner K, et al. Intraocular nanoparticle drug delivery: a pilot study using an aerosol during pars plana vitrectomy. Invest Ophthalmol Vis Sci. 2007;48(11):5243–9.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mehta, S., Zhang, R., Grossniklaus, H.E. (2014). III.J. Cell Proliferation at the Vitreoretinal Interface in Proliferative Vitreoretinopathy and Related Disorders. In: Sebag, J. (eds) Vitreous. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1086-1_22
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1086-1_22
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1085-4
Online ISBN: 978-1-4939-1086-1
eBook Packages: MedicineMedicine (R0)