Abstract
Primary central nervous system lymphoma (PCNSL) is a rare extra-nodal B-cell non-Hodgkin lymphoma, which arises in the brain, spinal cord, meninges, or eyes. In individuals infected with HIV, PCNSL occurs with advanced immunosuppression and low CD4 T-cell count. It is considered an AIDS-defining malignancy. Unlike PCNSL in immune-competent hosts, AIDS-related PCNSL (AR-PCNSL) is almost exclusively caused by a cancer-causing herpesvirus, Epstein–Barr Virus (EBV, also known as human herpesvirus-4). While PCNSL in immune-competent patients and AR-PCNSL have pathologic and clinical overlap, AR-PCNSL is distinguished by its strong association with immunosuppression and viral etiology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebral Spinal Fluid
- Primary Central Nervous System Lymphoma
- Whole Brain Radiation Therapy
- Primary Central Nervous System Lymphoma Patient
- Systemic DLBCL
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Primary central nervous system lymphoma (PCNSL) is a rare extra-nodal non-Hodgkin lymphoma, which accounts for approximately 3–5 % of primary brain tumors. Prior to effective therapy for HIV, a large proportion of cases of PCNSL, especially in younger individuals, occurred in severely immune compromised patients with acquired immunodeficiency syndrome (AIDS) (Ziegler et al. 1984); therefore, PCNSL is considered an AIDS-defining malignancy (Raez et al. 1998). However, after the broad availability of highly activate antiretroviral therapy (HAART) in 1996 (Pipkin et al. 2011), the incidence of PCNSL in HIV-infected individuals has decreased by nearly 90 % (Wolf et al. 2005). Nonetheless, the incidence AIDS-related (AR)-PCNSL in the USA remains significantly elevated at an estimated 26 cases per 100,000 person-years among people with AIDS, and continues to affect mainly patients under the age of 50 (Shiels et al. 2011). In areas where HAART is available, most patients with AR-PCNSL are not taking HAART either because they are not aware they are HIV infected or because of poor adherence to HAART. At the same time that incidence of AR-PCNSL has decreased, there has been an increase in the incidence of PCNSL in immune-competent elderly patients, highlighting a changing epidemiology of PCNSL in the USA related to differences in the underlying pathogenic processes (Shiels et al. 2011).
2 Histopathology and Pathogenesis
PCNSL is a B-cell lymphoma with diffuse large cell morphology. The diagnosis should be distinguished from systemic lymphoma with CNS involvement, dural-based low-grade lymphomas, and other rare histologies presenting in the CNS (Rubenstein et al. 2008). PCNSL in HIV-infected and HIV-uninfected patients share some clinical and morphologic characteristics; however, pathogenesis differs in important ways.
Both AR-PCNSL and PCNSL in immune-competent hosts are multifocal angiocentric tumors that express pan-B-cell markers (CD20, CD19, CD22 and CD79a). These tumors are highly proliferative with high expression of Ki-67 (Braaten et al. 2003; Deckert et al. 2011; Lin et al. 2006). PCNSL generally has a post-germinal center phenotype. In immune-competent patients, immunohistochemistry suggests a majority of tumors have activated B-cell differentiation based on lack of CD10 and common expression of IRF4 (90 %), a transcription factor associated with lymphocyte activation. However the germinal center associated transcription factor BCL6 is also expressed in 60–80 % of cases (Larocca et al. 1998). In contrast, AR-PCNSL, the immunophenotype is generally BCL6 negative and IRF4 positive (Carbone et al. 1998a, b). This immunophenotype is also noted in post-transplant lymphoproliferative disorder (PTLD) (Abed et al. 2004). Additional characteristics of AR-PCNSL include expression of the plasma cell marker CD138, the activation marker CD30, and adhesion molecules CD11a and ICAM-1 (CD54) that may contribute to homing to cerebral blood vessels (Bashir et al. 1992).
There are several similarities between AR-PCNSL and PTLD. PTLD is a disorder of proliferating latently EBV-infected B-cells that may be clonal and sometimes presents with CNS-only manifestations. PTLD can occur either after solid organ (SOT) or hematopoietic stem cell transplants (HSCT) as a result of medical immunosuppression. In HSCT it usually occurs within 6 months post-transplant and the lymphoproliferation is of donor-cell origin prior to EBV-specific cytotoxic T-cell reconstitution, while in SOT >90 % is of recipient cell origin and may have a longer latency due to long-term T-cell suppression to prevent organ rejection (Heslop 2009). Both AR-PCNSL and PTLD often have immunoblastic features (Castellano-Sanchez et al. 2004). Like PTLD, AR-PCNSL tumor cells almost always have evidence of EBV-infection, as noted by staining for EBV-encoded small RNA (EBER1) transcripts (MacMahon et al. 1991). AR-PCNSL is usually clonal based on polymerase chain reaction (PCR) evaluation of the immunoglobulin heavy chain gene (IgH) (Schmitt-Graff et al. 1995).
Acquired T-cell immunosuppression is a common risk factor for both AR-PCNSL and PTLD. In patients with HIV, the majority of cases of AR-PCNSL occur in severely immune compromised patients with AIDS, and CD4 counts <50 cells/μL. AR-PCNSL patients have been shown to lack EBV-specific CD4+ T-cells, irrespective of absolute CD4 counts, supporting lack of immune regulation of EBV-infected B-cells as a critical mechanism of EBV-driven oncogenesis (Gasser et al. 2007). This is in contrast to PCNSL in immune-competent patients, in which evidence of EBV infection of the tumor cells is rare (MacMahon et al. 1991). A range of EBV viral proteins are expressed in both AR-PCNSL and PTLD, including the EBV-associated nuclear antigens (EBNAs) 1, 2, 3A, 3B, and 3C; latent membrane proteins (LMP) 1 and 2; and leader protein (LP). This pattern of EBV protein expression is referred to latency III, and is remarkable among EBV-associated tumors for the broadest expression of viral proteins. EBV-encoded genes may play an important role in lymphomagenesis, and at the same time, EBV latency III lymphoproliferations are also the most immunogenic EBV-associated tumors and responsive to immune-based therapies.
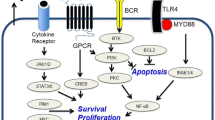
At the molecular level, a high level of aberrant somatic hypermutations have been detected both in PCNSL in immune-competent patients and in AR-PCNSL (Table 15.1) (Gaidano et al. 2003; Travi et al. 2012; Wolf et al. 2005). In AR-PCNSL, recurrent mutations have been noted in 5’ region non-coding region of BCL6, c-MYC, and TTF (Courts et al. 2008; Montesinos-Rongen et al. 2004). Additional insight into the molecular pathogenesis of PCNSL has mainly been evaluated in tumors from immune-competent subjects. In PCNSL not associated with AIDS, additional recurrent mutations have been identified (Table 15.1). Also, chromosomal abnormalities involving either IgH or BCL6 breakpoints detectable by fluorescent in situ hybridization (FISH) (Montesinos-Rongen et al. 2002) or gains and losses of genetic materials detectable by comparative genomic hybridization (CGH) (Schwindt et al. 2009) have been noted in PCNSL not associated with AIDS. In one study conducted in HIV-uninfected patients comparing immune-privileged sites (IP-testis and CNS) to systemic DLBCL identified a loss in 6p21.32-p35.3 in IP-DLBCL (Booman et al. 2008). Analysis of candidate genes encoded in that region identified two separate clusters: one involved in apoptosis, and a second involved in the immune response, including regulation of HLA expression (Booman et al. 2008). More recently, L265P gain of function point mutation in MYD88 (Montesinos-Rongen et al. 2011) has been noted to be a common recurrent mutation in PCNSL. This point mutation is also noted in a subset of activated B-cell diffuse large B-cell lymphomas (Ngo et al. 2011) and Waldenstrom’s macroglobinemia (Treon et al. 2012). Point mutations in the coiled region of CARD11 have also been noted. These later two genes are implicated in NFkB dysregulation in B-cell lymphomas.
Gene expression profiling in PCNSL has been limited due to relatively small sample sizes (n < 25), and has been mainly performed on tumors from HIV-uninfected patients. Three published studies (Montesinos-Rongen et al. 2008; Rubenstein et al. 2006; Tun et al. 2008) demonstrated a transcriptional signature that somewhat resembles systemic DLBCL. However, several genes of interest have been identified as being significantly upregulated in PCNSL compared to systemic DLBCL, including genes encoding adhesion-related proteins (CXCL13), extracellular matrix genes (SSP1, osteopontin), (Tun et al. 2008), and the oncogenes Pim-1, c-MYC, and Mina53. Interestingly, increased expression of the transcription factors XBP1 and ATF6, which are genes that regulate unfolded protein responses, was noted in one study. Transcriptional upregulation appears to be associated with by paracrine interactions with CNS vasculature, which is in part driven by tumor and endothelial IL-4 expression (Rubenstein et al. 2006).
The role of microRNAs in normal lymphoid development as well as lymphomagenesis is an area of active research. miR-155, which plays an important role in germinal center biology and is associated with B-cell proliferation and lymphomagenesis (Davidson-Moncada et al. 2010), appears to be even more highly expressed in PCNSL than systemic DLBCL. Additional microRNAs that are upregulated compared to systemic DLBCL include miR-17-5p and miR-20a, which target the c-MYC pathway, as well as those blocking B-cell differentiation (miR-9, miR-30b/c), while several putative tumor-suppressor miRNAs (miR-199a, miR-214, miR-193b, miR-145) may be downregulated (Fischer et al. 2011).
Further research is required to evaluate molecular similarities and differences between AR-PCNSL and PCNSL in immune-competent hosts. It remains unknown which of the genetic abnormalities seen in PCNSL also exist in AR-PCNSL, but it is likely that some molecular abnormalities in AR-PCNSL are due to interactions between EBV-encoded genes, microRNAs and lymphomagenic human signaling pathways. For example, latency membrane protein-1 (LMP1) is a viral oncogene that encodes a homologue to CD40 that is constitutively activated. Interactions between the C-terminal activating regions of LMP1 and tumor necrosis factor receptor associated factors (TRAFs) can lead to upregulation of NFkB (Kung and Raab-Traub 2010). LMP1 also upregulates of IRF4 (Xu et al. 2008), a hallmark of AR-PCNSL, while downregulating the transcription factor BLIMP1, which is required for plasma cell differentiation (Vrzalikova et al. 2011). LMP1 itself is upregulated by IL-4 (Kis et al. 2011), and therefore IL-4-dependent signaling appears to be important in both types of PCNSL. Interestingly, mouse models of lymphomas expressing LMP1 are notable for a high degree of tumor necrosis and vascular destruction and in some cases tumor regression, mirroring what is noted in AR-PCSNL. These findings may be mediated by upregulation of adhesion molecules and induction of chemokine anti-tumor responses (Cherney et al. 1998). Furthermore, miR-155, which is transcriptionally targeted by IRF4, is highly expressed in the Latency III pattern of EBV-infected B-cells as well as PTLD and is associated with cellular proliferation (Wang et al. 2011). Additional epigenetic mechanisms of lymphomagenesis attributable to dysregulated expression of EBV-encoded proteins and microRNAs in B-cells, as well as abnormal innate and acquired immune responses in AIDS patients are also likely.
3 Clinical Presentation, Diagnosis, and Baseline Evaluation
Neurologic disorders are prevalent in AIDS patients, and may be due to HIV-associated pathology, opportunistic infections, neoplasms, drug effects, and cerebrovascular disease. AR-PCNSL most often presents with neurologic symptoms such as headaches, lethargy, confusion, visual complaints, seizures, or focal neurologic symptoms such as cranial nerve dysfunction or hemiparesis. It occurs most of the time in patients with CD4 counts less than 50 cells/μL. CT-scans usually show ring-enhancing CNS mass lesions. The main differential diagnosis of such lesions in patients with AIDS includes toxoplasma encephalitis, the most common cause, followed by PCNSL. Other causes of mass lesions are cryptococcal mengioencephalitis and tuberculosis (Rosenblum et al. 1988). Before HAART, toxoplasmosis encephalitis occurred in 3–10 % of patients in the USA (Luft and Remington 1992). However, toxoplasmosis is much less frequent with HAART as well as medicines commonly used in patients with CD4 counts less than 200 to prevent pneumocystis pneumonia such as trimethoprim–sulfamethoxazole. In the HAART era, toxoplasmosis incidence is less than 1 %, but like AR-PCNSL, it is most commonly seen in medically underserved HIV-infected populations not on HAART.
AR-PCNSL occurs primarily in the brain parenchyma, and may disseminate to the leptomeninges. Spinal cord, ocular, and cranial nerve involvement is rare. Given advanced immunosuppression in this population, patients with AR-PCNSL are also at risk for concurrent opportunistic infections, including CNS infections such as toxoplasmosis, cryptococcus, or CMV (Bower et al. 2006). Preferred initial imaging in patients with HIV and neurologic symptoms include contrast-enhanced magnetic resonance imaging (MRI), although CT with contrast can be used if MRI is not available. In AR-PCNSL, these imaging studies can identify single or multiple CNS masses, which are generally ring-enhancing with central necrosis, but may also have diffuse or nodular enhancement (Fig. 15.1). Mass-associated edema is also common, and can be best noted on MRI fluid attenuated inversion recovery (FLAIR) sequence imaging. CT and MRI are useful in excluding significant mid-line shift that may make a lumbar puncture unsafe. Toxoplasmosis and AR-PCNSL have similar presentations and cannot be reliably distinguished based on imaging and clinical features.
Establishing a definitive diagnosis of cerebral mass lesions in patients with known or suspected HIV (Fig. 15.2) requires a medical history focusing on HIV and opportunistic infections, use of prophylactic antibiotics with anti-toxoplasmosis activity, and risks-factors for systemic malignancies, as well as physical exam including professional ophthalmology evaluation. Expedited evaluation should include the following studies: HIV ELISA and viral load, CD4 count, CNS and body imaging to evaluate for systemic infections or malignancies, toxoplasmosis serology, and lumbar puncture to evaluate cerebral spinal fluid (CSF) for leptomeningeal dissemination and/or CNS infection. In cases of suspected ocular involvement, vitrectomy is indicated (Abrey et al. 2005).
Lumbar puncture with CSF studies and nuclear imaging provide important diagnostic information to help differentiate between AR-PCNSL and CNS infection and identify concurrent pathologies. The discovery of an almost universal association of AR-PCNSL with EBV infection (Bashir et al. 1993; MacMahon et al. 1991) has led to use of PCR to detect EBV DNA in CSF in patients with suspected AR-PCNSL. Additional initial CSF studies to evaluate for leptomeningeal dissemination and/or infections include: opening pressure, cell count, protein, glucose, gram stain, cryptococcal Ag, cytopathology, flow cytometry and (PCR) evaluation for immunoglobulin heavy chain (IgH) clonal rearrangements, EBV (quantitative) viral load, JC virus, cytomegalovirus (CMV, also known as human herpesvirus-5), and toxoplasmosis (Fig. 15.1). Other microbiology studies may be indicated in some cases based on MRI findings and patient history.
Two nuclear imaging modalities have been evaluated in patients with AR-PCNSL, thallium-201 (Tl-201) single-photon emission CT (SPECT) and 18(F) fluorodeoxyglucose positron emission tomography (FDG-PET). With either nuclear imaging modality, infections usually appear as hypometabolic lesions, whereas PCNSL or other tumors are hypermetabolic (Fig. 15.1). In patients with HIV/AIDS and low CD4 count (generally less than 100 cells/μL), sensitivity and specificity of nuclear imaging range between 80 and 100 % (Kasamon and Ambinder 2005). Combining imaging findings with data on toxoplasmosis serology (Skiest et al. 2000) and CSF EBV viral load can further increase diagnostic accuracy. The combination of Tl-201 SPECT with CSF-based testing increased the specificity and positive predictive value to nearly 100 % in one study (Antinori et al. 1999). However, it should be noted that EBV can be detected frequently in the CSF of patients with HIV/AIDS with systemic non-Hodgkin lymphoma or other diseases, and is not specific for AR-PCNSL (Corcoran et al. 2008). Furthermore, anti-toxoplasmosis titers can be elevated in patients with AR-PCNSL, and do not exclude concurrent pathology. For these reasons, despite technical advances in minimally invasive diagnostic tools for evaluation intracranial masses in AIDS patients, diagnosis of AR-PCNSL is done by pathological examination in nearly all cases.
CSF cytopathology as well as flow cytometry and IgH rearrangement studies may be useful in diagnosis of AR-PCNSL. The impetus for using CSF cytopathology as a diagnostic tool in PCNSL comes from studies utilizing this as adjunct in diagnosis of leptomeningeal involvement. CSF cytopathology can detect leptomeningeal spread in 15–40 % of untreated immune-competent PCNSL patients, and is associated with higher tumor burden (Balmaceda et al. 1995; Ferreri et al. 2003; Fischer et al. 2008). Multi-parameter flow cytometry is also useful for detecting leptomeningeal involvement of both systemic diffuse large B-cell lymphoma and PCNSL, and is more sensitive than cytopathology (Kraan et al. 2008; Schroers et al. 2010). IgH PCR studies of CSF can also detect clonal IgH rearrangements in patients with PCNSL, and provides important information that can be integrated with cytopathology and flow cytometric findings (Ekstein et al. 2006; Fischer et al. 2008). It should be noted that the utility of flow-cytometry and IgH PCR in AR-PCNSL remains to be fully defined, still it is reasonable to integrate these minimally invasive studies into the initial diagnostic evaluation of patients with AIDS and ring-enhancing CNS masses. In AIDS patients with ring-enhancing CNS masses, pathologic demonstration of leptomenigeal dissemination of lymphoma can establish a definitive diagnosis.
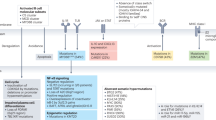
Despite these advances in minimally invasive diagnostic tools, histologic diagnosis remains the gold standard for diagnosis of AR-PCNSL, and biopsy is required in many cases for definitive diagnosis. As AR-PCNSL is a multifocal malignancy, craniotomy with resection of a lesion is generally not performed unless there is another indication such as decompression of mass effect or concern for an abscess. Stereotactic brain biopsy has a diagnostic yield of 85–90 % in evaluation of cerebral lesions in patients with AIDS, and is generally associated with low mortality, although the overall morbidity rate is approximately 8–10 % mainly due to intracranial bleeding (Davies et al. 1995; Iacoangeli et al. 1994; Luzzati et al. 1996; Skolasky et al. 1999). However, thalamic or basal ganglia lesions are associated with a significantly increased risk of morbidity, often from bleeding (McGirt et al. 2005). In AIDS patients with a CD4 count less than 100 cells/μL, positive EBV viral load in the CSF and lesions consistent with AR-PCNSL by nuclear imaging, biopsy of such high-risk lesions may be relatively contra-indicated. In such cases, diagnosis of AR-PCSNL can usually be established based on results of these established noninvasive studies (Fig. 15.2).
In 1998, the American Academy of Neurology issued recommendations to guide diagnostic evaluation of AIDS patients with ring-enhancing lesions (1998). However, this algorithm does not include more recent molecular diagnostics or flow cytometry, which are sensitive for detecting B-cell malignancies in the CSF (Kraan et al. 2008). Furthermore, this and other algorithms include a trial of empiric antibiotics for toxoplasmosis, which is no longer advisable given diagnostic advances and dramatically improved survival of patients with AIDS in the HAART era. An alternative algorithm for patients with AIDS and enhancing CNS masses that takes into account these new findings is proposed in Fig. 15.2. It should be noted that evaluation in some patients, for example those with inconclusive imaging and laboratory studies in which brain biopsy is felt to be dangerous can be challenging, and the best approach will require balancing various risks. Also, this algorithm should be considered a suggested guide, and readers should be alert for new developments or new official guidelines in this area.
AR-PCSNL and other CNS infections occurring in patients with AIDS can be associated with a rapid deterioration of functional and neurologic function. Patient performance status should be assessed using an Eastern Cooperative Oncology Group (ECOG) performance scale. Cognitive function should be monitored using serial scoring of Mini Mental Status Examination (MMSE). Given the rapid and sometimes irreversible decline in performance status in patients with untreated AR-PCNSL or untreated CNS infections and improved long-term outcomes in AIDS patients treated with HAART, expedited evaluation is crucial. Long-term neurologic function, quality-of-life, and survival are likely affected by the course of action taken during the initial diagnostic evaluation (Abrey et al. 2005).
4 Treatment and Prognosis
The advent of effective combination antiretroviral therapy, also known as HAART heralded a new era for HIV-infected patients. Control of HIV-replication and associated immune reconstitution lead to a decrease in deaths from opportunistic infections and dramatically improved overall survival for many AIDS patients. Studies of outcomes for HIV-related diseases are usually divided between the pre-HAART era (before 1996) and the era when HAART became broadly available in the USA and Europe (after 1996) (Ricard et al. 2012). Among populations with access to HAART, one of the great advances has been the prevention of malignancies occurring in the setting of very low CD4 counts, such as AR-PCNSL. However, despite improvement in overall survival attributable to HAART (Bayraktar et al. 2011; Bower et al. 2006; Diamond et al. 2006; Hoffmann et al. 2001; Newell et al. 2004; Skiest and Crosby 2003), patients who do develop AR-PCNSL still have an overall mortality close to 90 % at 2 years (Achenbach et al. 2011; Norden et al. 2011).
HAART is a necessary part of treatment of AR-PCNSL, and immune reconstitution is considered the main driver of this effect. Control of HIV viremia allows for T-cell immune reconstitution, and improved immunosurveillance against this immunogenic, virally associated malignancy. In rare cases, HAART alone has even been associated with complete resolution of PCNSL, (Aboulafia and Puswella 2007), presumably due to improved T-cell function.
Pilot (Aboulafia et al. 2006; Jacomet et al. 1997) and retrospective studies (Hoffmann et al. 2001; Nagai et al. 2010; Newell et al. 2004; Pipkin et al. 2011) suggest further improvements in outcomes may be possible in this patient population with lymphoma directed therapeutics and appropriate supportive care that includes prophylaxis and treatment of opportunistic infections, and in some cases, short courses of steroids to manage neurologic symptoms. However, delayed diagnosis (Haldorsen et al. 2005), heterogeneous approaches to diagnosis (Cingolani et al. 1998; Davies et al. 1995; Kaufmann et al. 1996; Skiest et al. 2000; Skolasky et al. 1999), poor performance status (Raez et al. 1998), and infectious (Mahindra and Grossman 2003; Remick et al. 1990) or malignant (Ahsan and Neugut 1996) co-morbidities may be significant additional risk factors that effect overall survival. Unlike systemic non-Hodgkin lymphoma in patients with AIDS, for which dramatic improvements in overall survival have been achieved through prospective therapeutic studies, advances in AR-PCNSL has been limited by lack of prospective studies. As such, there is no consensus on how to treat AR-PCNSL.
The major definitive treatment modalities for PCNSL are whole brain radiation therapy (WBRT), chemotherapy, and the anti CD-20 monoclonal antibody, rituximab. However, there are no completed prospective lymphoma directed studies that have been performed in patients with AR-PCNSL in the HAART era. With the exception of HAART, which is indicated based on evidence showing improved overall survival, therapeutic interventions for AR-PCNSL are based on case series and expert opinion, or extrapolation from studies performed in immune-competent patients with PCNSL.
In AR-PCNSL, radiotherapy has been the predominant treatment modality since the beginning of the AIDS epidemic. Radiation has good activity against PCNSL. However, focal radiotherapy is associated with high recurrence rates and hence WBRT is recommended. Even WBRT is unlikely to treat leptomeningeal disease outside the radiation field. In retrospective studies performed in the pre-HAART era, median overall survival with WBRT alone was less than 6 months; death was generally from either other complications of AIDS or from progressive PCNSL. A more recent study performed in the HAART era has demonstrated 3-year survival of 64 % in patients with pathologically confirmed AR-PCNSL treated with HAART and WBRT (>30 Gy). Doses of WBRT (~40 Gy), recommended for better and more durable disease control, however, are associated with debilitating and at times life-threatening neurotoxicity (Correa et al. 2004; Nagai et al. 2010; Skiest and Crosby 2003; Wolf et al. 2005). Therefore, radiation-sparing approaches to AR-PCNSL are highly desirable.
High-dose methotrexate, either alone or in combination regimens, is the best-studied radiation-sparing approach to PCNSL in patients without HIV. However, many regimens evaluated in PCNSL in immune-competent patients also contain high-dose cytarabine, which adds substantial toxicity and may not be appropriate for patients with AIDS. A pilot study of high-dose methotrexate 3 g/m2 performed in ten patients with histologically confirmed AR-PCNSL in the pre-HAART era (Jacomet et al. 1997) demonstrated a complete response rate of 30 %; however, median overall survival was only 2 months. Further evaluation of radiation-sparing approaches is required in the HAART era.
Rituximab, a monoclonal antibody directed against CD20, is an important immunotherapy that has been evaluated in the treatment of PCNSL and PTLD, and is a rational agent in AR-PCNSL, in which 100 % of lymphoma cells express CD20. One challenge has been determining whether a therapeutic concentration rituximab can be obtained in the central nervous system. Pharmacokinetic studies have shown that CSF levels are approximately 0.1–1 % that of serum during peripheral administration (Larouche et al. 2011; Rubenstein et al. 2003). Though this may appear to be an insufficient concentration, it represents 1–10 times more than needed to saturate 80–95 % of CD20 positive cell surface (Chow et al. 2002). Furthermore, tumor-associated breakdown of the blood–brain barrier likely allows for increase accumulation at the site of the tumor. Indeed tumor accumulation of yttrium-90-labeled anti-CD20 antibodies can be demonstrated in a majority of patients with PCNSL (Maza et al. 2009).
Clinically, a retrospective study examined the addition of rituximab to high-dose methotrexate and ifosfamide on outcomes in newly diagnosed PCNSL in HIV-negative patients. The addition of rituximab increased the CR rate (100 vs. 68.4 %; p = 0.02) and 6-month progression-free survival (94.1 vs. 63.2 %; p = 0.04) (Birnbaum et al. 2012). A subsequent prospective multicenter study evaluating rituximab in combination with high-dose methotrexate and temozolomide had a 63 % complete response rate. Patients went on to receive consolidation therapy with high-dose cytarabine and etoposide, and mature results from this study are forthcoming.
Overall, the results of trials in HIV-uninfected patients with PCNSL suggest that radiation-sparing modalities can achieve good results. While patients with AR-PCNSL may be more susceptible to toxicities from such regimens because of their severe underlying acquired immunodeficiency, such approaches are appropriate to consider at the HAART era. However, there are no completed studies of radiation-sparing approaches with HAART in AR-PCNSL. Curative-intent, radiation-sparing treatment of AR-PCNSL is currently under investigation in a prospective study evaluating of rituximab, high-dose methotrexate, and HAAART in patients with AR-PCNSL (NCT00267865).
5 Conclusion
AR-PCNSL is a rare AIDS-defining malignancy with a high mortality. The advent of the widespread distribution of HAART has led to a substantial decline in incidence and a modest improvement in overall survival. For AIDS patients who develop AR-PCNSL, diagnostic as well as therapeutic challenges exist. A streamlined evaluation of CNS masses (Fig. 15.2) may lead to earlier diagnoses and treatments, and improved outcomes. All patients should be started on HAART, and given the lack of a standard therapy, should also receive curative intent lymphoma directed therapy, ideally within a clinical trial.
References
Abed N, Casper JT, Camitta BM, Margolis D, Trost B, Orentas R, et al. Evaluation of histogenesis of B-lymphocytes in pediatric EBV-related post-transplant lymphoproliferative disorders. Bone Marrow Transplant. 2004;33(3):321–7.
Aboulafia DM, Puswella AL. Highly active antiretroviral therapy as the sole treatment for AIDS-related primary central nervous system lymphoma: a case report with implications for treatment. AIDS Patient Care STDS. 2007;21(12):900–7.
Aboulafia DM, Ratner L, Miles SA, Harrington Jr WJ. Antiviral and immunomodulatory treatment for AIDS-related primary central nervous system lymphoma: AIDS Malignancies Consortium pilot study 019. Clin Lymphoma Myeloma. 2006;6(5):399–402.
Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–43.
Achenbach CJ, Cole SR, Kitahata MM, Casper C, Willig JH, Mugavero MJ, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25(5):691–700.
Ahsan H, Neugut AI. High risk of Kaposi’s sarcoma and central nervous system lymphoma in the same individuals: a finding related to acquired immunodeficiency syndrome. Int J Cancer. 1996;66(2):176–8.
Antinori A, De Rossi G, Ammassari A, Cingolani A, Murri R, Di Giuda D, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17(2):554–60.
Balmaceda C, Gaynor JJ, Sun M, Gluck JT, DeAngelis LM. Leptomeningeal tumor in primary central nervous system lymphoma: recognition, significance, and implications. Ann Neurol. 1995;38(2):202–9.
Bashir R, Coakham H, Hochberg F. Expression of LFA-1/ICAM-1 in CNS lymphomas: possible mechanism for lymphoma homing into the brain. J Neurooncol. 1992;12(2):103–10.
Bashir R, Luka J, Cheloha K, Chamberlain M, Hochberg F. Expression of Epstein-Barr virus proteins in primary CNS lymphoma in AIDS patients. Neurology. 1993;43(11):2358–62.
Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011;101(2):257–65.
Birnbaum T, Stadler EA, von Baumgarten L, Straube A. Rituximab significantly improves complete response rate in patients with primary CNS lymphoma. J Neurooncol. 2012;109(2):285–91.
Booman M, Szuhai K, Rosenwald A, Hartmann E, Kluin-Nelemans H, de Jong D, et al. Genomic alterations and gene expression in primary diffuse large B-cell lymphomas of immune-privileged sites: the importance of apoptosis and immunomodulatory pathways. J Pathol. 2008;216(2):209–17.
Bower M, Powles T, Nelson M, Mandalia S, Gazzard B, Stebbing J. Highly active antiretroviral therapy and human immunodeficiency virus-associated primary cerebral lymphoma. J Natl Cancer Inst. 2006;98(15):1088–91.
Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9(3):1063–9.
Carbone A, Gaidano G, Gloghini A, Larocca LM, Capello D, Canzonieri V, et al. Differential expression of BCL-6, CD138/syndecan-1, and Epstein-Barr virus-encoded latent membrane protein-1 identifies distinct histogenetic subsets of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood. 1998a;91(3):747–55.
Carbone A, Gloghini A, Gaidano G, Franceschi S, Capello D, Drexler HG, et al. Expression status of BCL-6 and syndecan-1 identifies distinct histogenetic subtypes of Hodgkin’s disease. Blood. 1998b;92(7):2220–8.
Castellano-Sanchez AA, Li S, Qian J, Lagoo A, Weir E, Brat DJ. Primary central nervous system posttransplant lymphoproliferative disorders. Am J Clin Pathol. 2004;121(2):246–53.
Cherney BW, Sgadari C, Kanegane C, Wang F, Tosato G. Expression of the Epstein-Barr virus protein LMP1 mediates tumor regression in vivo. Blood. 1998;91(7):2491–500.
Chow KU, Sommerlad WD, Boehrer S, Schneider B, Seipelt G, Rummel MJ, et al. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: role of cytokines, complement, and caspases. Haematologica. 2002;87(1):33–43.
Cingolani A, De Luca A, Larocca LM, Ammassari A, Scerrati M, Antinori A, et al. Minimally invasive diagnosis of acquired immunodeficiency syndrome-related primary central nervous system lymphoma. J Natl Cancer Inst. 1998;90(5):364–9.
Corcoran C, Rebe K, van der Plas H, Myer L, Hardie DR. The predictive value of cerebrospinal fluid Epstein-Barr viral load as a marker of primary central nervous system lymphoma in HIV-infected persons. J Clin Virol. 2008;42(4):433–6.
Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62(4):548–55.
Courts C, Montesinos-Rongen M, Brunn A, Bug S, Siemer D, Hans V, et al. Recurrent inactivation of the PRDM1 gene in primary central nervous system lymphoma. J Neuropathol Exp Neurol. 2008;67(7):720–7.
Davidson-Moncada J, Papavasiliou FN, Tam W. MicroRNAs of the immune system: roles in inflammation and cancer. Ann N Y Acad Sci. 2010;1183:183–94.
Davies MA, Pell MF, Brew BJ. Stereotactic biopsy of cerebral lesions in acquired immunodeficiency syndrome. J Clin Neurosci. 1995;2(1):40–4.
Deckert M, Engert A, Bruck W, Ferreri AJ, Finke J, Illerhaus G, et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia. 2011;25(12):1797–807.
Diamond C, Taylor TH, Im T, Miradi M, Wallace M, Anton-Culver H. Highly active antiretroviral therapy is associated with improved survival among patients with AIDS-related primary central nervous system non-Hodgkin’s lymphoma. Curr HIV Res. 2006;4(3):375–8.
Ekstein D, Ben-Yehuda D, Slyusarevsky E, Lossos A, Linetsky E, Siegal T. CSF analysis of IgH gene rearrangement in CNS lymphoma: relationship to the disease course. J Neurol Sci. 2006;247(1):39–46.
Evaluation and management of intracranial mass lesions in AIDS. Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 1998;50(1):21–6.
Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–72.
Fischer L, Martus P, Weller M, Klasen HA, Rohden B, Roth A, et al. Meningeal dissemination in primary CNS lymphoma: prospective evaluation of 282 patients. Neurology. 2008;71(14): 1102–8.
Fischer L, Hummel M, Korfel A, Lenze D, Joehrens K, Thiel E. Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro-oncology. 2011;13(10): 1090–8.
Gaidano G, Pasqualucci L, Capello D, Berra E, Deambrogi C, Rossi D, et al. Aberrant somatic hypermutation in multiple subtypes of AIDS-associated non-Hodgkin lymphoma. Blood. 2003;102(5):1833–41.
Gasser O, Bihl FK, Wolbers M, Loggi E, Steffen I, Hirsch HH, et al. HIV patients developing primary CNS lymphoma lack EBV-specific CD4+ T cell function irrespective of absolute CD4+ T cell counts. PLoS Med. 2007;4(3):e96.
Haldorsen IS, Espeland A, Larsen JL, Mella O. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44(7):728–34.
Heslop HE. How I, treat EBV lymphoproliferation. Blood. 2009;114(19):4002–8.
Hoffmann C, Tabrizian S, Wolf E, Eggers C, Stoehr A, Plettenberg A, et al. Survival of AIDS patients with primary central nervous system lymphoma is dramatically improved by HAART-induced immune recovery. AIDS. 2001;15(16):2119–27.
Iacoangeli M, Roselli R, Antinori A, Ammassari A, Murri R, Pompucci A, et al. Experience with brain biopsy in acquired immune deficiency syndrome-related focal lesions of the central nervous system. Br J Surg. 1994;81(10):1508–11.
Jacomet C, Girard PM, Lebrette MG, Farese VL, Monfort L, Rozenbaum W. Intravenous methotrexate for primary central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS. 1997;11(14):1725–30.
Kasamon YL, Ambinder RF. AIDS-related primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2005;19(4):665–87, vi–vii.
Kaufmann T, Nisce LZ, Coleman M. A comparison of survival of patients treated for AIDS-related central nervous system lymphoma with and without tissue diagnosis. Int J Radiat Oncol Biol Phys. 1996;36(2):429–32.
Kis LL, Gerasimcik N, Salamon D, Persson EK, Nagy N, Klein G, et al. STAT6 signaling pathway activated by the cytokines IL-4 and IL-13 induces expression of the Epstein-Barr virus-encoded protein LMP-1 in absence of EBNA-2: implications for the type II EBV latent gene expression in Hodgkin lymphoma. Blood. 2011;117(1):165–74.
Kraan J, Gratama JW, Haioun C, Orfao A, Plonquet A, Porwit A, et al. Flow cytometric immunophenotyping of cerebrospinal fluid. Current protocols in cytometry/editorial board, J Paul Robinson, managing editor [et al]. 2008;Chapter 6:Unit 6, 25.
Kung CP, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 modulates distinctive NF- kappaB pathways through C-terminus-activating region 1 to regulate epidermal growth factor receptor expression. J Virol. 2010;84(13):6605–14.
Larocca LM, Capello D, Rinelli A, Nori S, Antinori A, Gloghini A, et al. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood. 1998;92(3):1011–9.
Larouche J-F, Bergeron M, Hampson G, Illidge T, Delage R. Rituximab Cerebrospinal Fluid Levels in Patients with Primary Central Nervous System Lymphoma Treated with Intravenous High Dose Rituximab. ASH Annual Meeting Abstracts. 2011;118(21):1644.
Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12(4): 1152–6.
Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15(2):211–22.
Luzzati R, Ferrari S, Nicolato A, Piovan E, Malena M, Merighi M, et al. Stereotactic brain biopsy in human immunodeficiency virus-infected patients. Arch Intern Med. 1996;156(5):565–8.
MacMahon EM, Glass JD, Hayward SD, Mann RB, Becker PS, Charache P, et al. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338(8773): 969–73.
Mahindra AK, Grossman SA. Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors. J Neurooncol. 2003;63(3):263–70.
Maza S, Kiewe P, Munz DL, Korfel A, Hamm B, Jahnke K, et al. First report on a prospective trial with yttrium-90-labeled ibritumomab tiuxetan (Zevalin) in primary CNS lymphoma. Neuro-oncology. 2009;11(4):423–9.
McGirt MJ, Woodworth GF, Coon AL, Frazier JM, Amundson E, Garonzik I, et al. Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg. 2005;102(5):897–901.
Montesinos-Rongen M, Zuhlke-Jenisch R, Gesk S, Martin-Subero JI, Schaller C, Van Roost D, et al. Interphase cytogenetic analysis of lymphoma-associated chromosomal breakpoints in primary diffuse large B-cell lymphomas of the central nervous system. J Neuropathol Exp Neurol. 2002;61(10):926–33.
Montesinos-Rongen M, Van Roost D, Schaller C, Wiestler OD, Deckert M. Primary diffuse large B-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation. Blood. 2004;103(5):1869–75.
Montesinos-Rongen M, Brunn A, Bentink S, Basso K, Lim WK, Klapper W, et al. Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia. 2008;22(2):400–5.
Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122(6):791–2.
Nagai H, Odawara T, Ajisawa A, Tanuma J, Hagiwara S, Watanabe T, et al. Whole brain radiation alone produces favourable outcomes for AIDS-related primary central nervous system lymphoma in the HAART era. Eur J Haematol. 2010;84(6):499–505.
Newell ME, Hoy JF, Cooper SG, DeGraaff B, Grulich AE, Bryant M, et al. Human immunodeficiency virus-related primary central nervous system lymphoma: factors influencing survival in 111 patients. Cancer. 2004;100(12):2627–36.
Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–9.
Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primary central nervous system lymphoma, 1973–2004. J Neurooncol. 2011;101(3):487–93.
Pipkin S, Scheer S, Okeigwe I, Schwarcz S, Harris DH, Hessol NA. The effect of HAART and calendar period on Kaposi’s sarcoma and non-Hodgkin lymphoma: results of a match between an AIDS and cancer registry. AIDS. 2011;25(4):463–71.
Raez LE, Patel P, Feun L, Restrepo A, Raub Jr WA, Cassileth PA. Natural history and prognostic factors for survival in patients with acquired immune deficiency syndrome (AIDS)-related primary central nervous system lymphoma (PCNSL). Crit Rev Oncog. 1998;9(3–4):199–208.
Remick SC, Diamond C, Migliozzi JA, Solis O, Wagner Jr H, Haase RF, et al. Primary central nervous system lymphoma in patients with and without the acquired immune deficiency syndrome. A retrospective analysis and review of the literature. Medicine (Baltimore). 1990;69(6):345–60.
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–96.
Rosenblum ML, Levy RM, Bredesen DE, So YT, Wara W, Ziegler JL. Primary central nervous system lymphomas in patients with AIDS. Ann Neurol. 1988;23(Suppl):S13–6.
Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466–8.
Rubenstein JL, Fridlyand J, Shen A, Aldape K, Ginzinger D, Batchelor T, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107(9):3716–23.
Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma. 2008;49 Suppl 1:43–51.
Schmitt-Graff A, Hummel M, Anagnostopoulos I, Stoltenburg G, Stein H. Primary brain lymphoma in acquired immunodeficiency syndrome. Immunophenotype and molecular pathologic characterization in stereotactic biopsy, autopsy and cerebrospinal fluid cytology. Pathologe. 1995;16(1):75–80.
Schroers R, Baraniskin A, Heute C, Vorgerd M, Brunn A, Kuhnhenn J, et al. Diagnosis of leptomeningeal disease in diffuse large B-cell lymphomas of the central nervous system by flow cytometry and cytopathology. Eur J Haematol. 2010;85(6):520–8.
Schwindt H, Vater I, Kreuz M, Montesinos-Rongen M, Brunn A, Richter J, et al. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia. 2009;23(10):1875–84.
Shiels MS, Pfeiffer RM, Hall HI, Li J, Goedert JJ, Morton LM, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA. 2011;305(14):1450–9.
Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17(12):1787–93.
Skiest DJ, Erdman W, Chang WE, Oz OK, Ware A, Fleckenstein J. SPECT thallium-201 combined with Toxoplasma serology for the presumptive diagnosis of focal central nervous system mass lesions in patients with AIDS. J Infect. 2000;40(3):274–81.
Skolasky RL, Dal Pan GJ, Olivi A, Lenz FA, Abrams RA, McArthur JC. HIV-associated primary CNS lymorbidity and utility of brain biopsy. J Neurol Sci. 1999;163(1):32–8.
Travi G, Ferreri AJ, Cinque P, Gerevini S, Ponzoni M, Terreni MR, et al. Long-term remission of HIV-associated primary CNS lymphoma achieved with highly active antiretroviral therapy alone. J Clin Oncol. 2012;30(10):e119–21.
Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367(9):826–33.
Tun HW, Personett D, Baskerville KA, Menke DM, Jaeckle KA, Kreinest P, et al. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111(6):3200–10.
Vrzalikova K, Vockerodt M, Leonard S, Bell A, Wei W, Schrader A, et al. Down-regulation of BLIMP1alpha by the EBV oncogene, LMP-1, disrupts the plasma cell differentiation program and prevents viral replication in B cells: implications for the pathogenesis of EBV-associated B-cell lymphomas. Blood. 2011;117(22):5907–17.
Wang L, Toomey NL, Diaz LA, Walker G, Ramos JC, Barber GN, et al. Oncogenic IRFs provide a survival advantage for Epstein-Barr virus- or human T-cell leukemia virus type 1-transformed cells through induction of BIC expression. J Virol. 2011;85(16):8328–37.
Wolf T, Brodt HR, Fichtlscherer S, Mantzsch K, Hoelzer D, Helm EB, et al. Changing incidence and prognostic factors of survival in AIDS-related non-Hodgkin’s lymphoma in the era of highly active antiretroviral therapy (HAART). Leuk Lymphoma. 2005;46(2):207–15.
Xu D, Zhao L, Del Valle L, Miklossy J, Zhang L. Interferon regulatory factor 4 is involved in Epstein-Barr virus-mediated transformation of human B lymphocytes. J Virol. 2008;82(13): 6251–8.
Ziegler JL, Beckstead JA, Volberding PA, Abrams DI, Levine AM, Lukes RJ, et al. Non-Hodgkin’s lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med. 1984;311(9):565–70.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Davidson-Moncada, J., Uldrick, T.S. (2014). AIDS-Related Primary Central Nervous System Lymphoma. In: Yarchoan, R. (eds) Cancers in People with HIV and AIDS. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0859-2_15
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0859-2_15
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0858-5
Online ISBN: 978-1-4939-0859-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)