Abstract
Gas analysis in the environment is a broad field and includes emission and immission evaluation and control. Furthermore the determination of gases dissolved in water, such as oxygen and carbon dioxide, is a main task for environmental gas analysis. Depending on the matrix to be analysed, the gas concentration and the volume of gases which can be used for sampling and analysis have to be selected. The principles of gas sensors (high and normal temperature) are explained with many illustrative examples, such as lambda probe, Clark cell, fuel cell sensors and Severinghaus electrodes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Solid Electrolyte
- Limit Life Time
- Solid Electrolyte Sensor
- Mixed Potential Sensor
- Severinghaus Electrode
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 General Considerations
Gas analysis in the environment is a broad field and includes emission and immission evaluation and control. Furthermore, the determination of gases dissolved in water, such as oxygen and carbon dioxide, is a main task for environmental gas analysis. Depending on the matrix to be analysed, the gas concentration and the volume of gases which can be used for sampling and analysis have to be selected. In the environment the direct measurement in air or in water is preferred because sampling and transport of the gaseous analyte to the lab include a lot of possible sources of mistakes. For example, changes in temperature of the sampled gas must be avoided because water vapour can condense and gases can be dissolved in the water films. Additionally, the solubility of gases in water depends on the temperature. These mistakes caused by sampling cannot be compensated by the best analytical method. The majority of gas analyses are performed in special labs mostly by gas chromatography. For these analyses special knowledge is required. Depending on the amount of gas which is available for sampling either containers are used in flowing gas or small amounts of gas samples are taken by the so-called gas mouse. Electrochemical gas sensors offer an alternative concept especially due to the opportunity to measure directly in the matrix to be analysed, e.g. in field applications.
2 Principles of Electrochemical Gas Sensors
Therefore, electrochemical gas sensors are widely spread in many fields of environmental monitoring, for the investigation of metabolisms and the control of biological processes. They are well established to gain real-time information for process control by in situ measurements of the chemical composition without sampling. Electrochemical sensors are small and inexpensive and do not require a sophisticated knowledge in their application.1
Every electrochemical reaction, e.g. the reduction of oxygen, is connected with the flow of an ionic charge carrier and a turnover of mass. Electrochemical reactions can occur spontaneously only to a very small extent (10−12 mol). After the establishment of the electrochemical equilibrium an equilibrium voltage or the electromotive force (emf) can be measured. On the other hand, the reaction can be pushed by means of an external polarisation voltage. In that case a current is flowing due to the reaction turnover.
Depending on the used electrolyte and the working temperature, electrochemical gas sensors can be applied in real matrices at temperatures from −30 °C up to 1,600 °C. The so-called conventional electrochemical gas sensors, which work with aqueous or liquid electrolytes, are usually applied up to about 50 °C, whereas solid electrolyte-based sensors operate in the temperature range >500 °C. Both types of sensors work according to electrochemical measuring principles, like potentiometry, amperometry or impedimetry. Due to the cheap equipment also dynamic methods like cyclovoltammetry, linear sweep voltammetry and their differential modes are suited for field application. Sensitivity, selectivity and stability (reliability and durability) of sensors are mainly influenced by the measuring conditions with respect to temperature, pressure and chemical environment. In the low-temperature range, electrochemical gas sensors are used to measure O2, NO and CO in the gas phase as well as dissolved in water. NH3 and CO2 in gaseous as well as in aqueous matrices can be determined indirectly via pH changes of an inner electrolyte. For measurements of oxygen and combustibles like hydrogen, CO and hydrocarbons at high temperatures in exhausts (emission control) solid electrolyte sensors are commonly utilised. Sensors for the determination of free oxygen and equilibrium oxygen in reducing gases are also commercially available. The main advantage of high-temperature sensors is the very short response time in the ms range. Generally it is possible to measure most gases by normal temperature sensors as well as with high-temperature sensors. In Fig. 19.1 a general overview on the different electrochemical gas sensors und their operation mode is given. The preferably used gas sensor principles are marked in red.
3 High-Temperature Gas Sensors
Electrochemical cells with solid electrolytes are widely used as gas sensors for emission monitoring in exhausts and fermentation plants. As a solid electrolyte gas-tight sintered ceramics in form of tubes, discs, planar substrates or thick films consisting of stabilised zirconia (e.g. YSZ means the most common solid electrolyte yttria-stabilised zirconia) are utilised. With increasing temperature the electrolytical conductivity increases exponentially.
Potentiometric sensors (Fig. 19.2, left) are able to measure free oxygen and oxygen in an established thermodynamically equilibrium, e.g. the ratio of partial pressures of burnt and non-burnt components.2,3 They are oxygen concentration cells which can be symbolised by
Basic principles of gas sensors based on solid electrolytes2
Oxygen cannot penetrate the gas-tight ceramic. Under electrochemical equilibrium it takes up two electrons from the metal (platinum) and is incorporated in the solid electrolyte as an oxide ion O2− (cathodic reaction, arrow in the right direction). In the opposite direction oxide ions are removed from the solid electrolytes, electrons remain in the platinum and oxygen is formed (anodic reaction, left direction). The cell reaction is the electrochemical transfer of oxygen from the side of higher partial pressure to the other with lower one. According to the Nernst’s equation the open-circuit voltage depends logarithmically on the gas partial pressure \( {p}_{O_2} \) on both sides:
If the total pressure on both sides is equal and nearly 1 bar the partial pressure can be expressed by the volume concentration \( {\varphi}_{O_2} \)
with R = 8.314 V A s/(mol K) and F = 96,485 A s/mol, on one side air with 50 % r. h. the following results (quantity equation is used):
In amperometric sensors (Fig. 19.2 right)1,4 one electrode is covered by a diffusion-limiting layer (also a chamber with small holes can be used) so that the transport of oxygen to the electrode is the rate-limiting step. This is schematically shown in Fig. 19.3. When the electrode is polarised by an external voltage, the current increases as long as enough electrochemically active species are present.
In the plateau phase the electrochemical turnover only depends on the transport rate (diffusion rate) of active species. In case of an oxygen electrode all oxygen that reaches the electrode is reduced electrochemically. The rate-limiting current is proportional to the gas concentration. Therefore these sensors exhibit a linear sensor function and can be calibrated with air.
With the area A and the length L of the hole and the diffusion coefficient of oxygen \( {D}_{O_2} \) the current measured is proportional to the oxygen partial pressure:
For the measurement of gas components like hydrocarbons (HC) or nitric oxides (NO x ) in non-equilibrated gas phases kinetically determined sensors are used (Fig. 19.2 middle).5,6 Depending on the electrode material, the gas components do not equilibrate on the measuring electrode at temperatures <700 °C. Thus gas components which are not thermodynamically stable are electrochemically active. In an HC- and O2-containing gas, for example, at least two electrode reactions can take place: the electrochemical reduction of oxygen and the electrochemical oxidation of hydrocarbons. The measured open-circuit voltage does not obey the Nernst equation. Therefore such electrode behaviour is often referred to non-Nernstian electrodes (or mixed potential sensors). The cell voltage depends logarithmically on the concentrations of the hydrocarbons:
The mixed potential of such solid electrolyte electrodes is, in contrary to that of electrodes in aqueous solution, very stable and reproducible.
Sensors based on solid electrolytes are also known for CO2.7 The general setup of a thick film sensor is given in Fig. 19.4. As a solid electrolyte sodium ion conductors like ß-alumina or Nasicon together with sodium carbonate are used. The electrochemical reactions take place at temperatures between 350 and 550 °C. The electrode reactions of the measuring and reference electrode are
In the cell reaction sodium silicate and CO2 react to sodium carbonate and silica:
If the solid components are pure the cell emf depends only on the CO2 partial pressure:
Such sensors show long-term stability without cross sensitivity vs. water vapour. Organic compounds are oxidised by oxygen on the surface of hot electrodes.
4 Normal-Temperature Gas Sensors
4.1 Amperometric Gas Sensors
Amperometric gas sensors are the second most important group of electrochemical gas sensors. The development of these sensors can be traced back to the introduction of the Clark-electrode in the mid-1950s, which is well known for the determination of dissolved oxygen. Amperometric gas sensors consist of a working electrode mostly covered by a membrane, a counter and a reference electrode which are in connection with a liquid electrolyte solution. These sensors have been designed in different forms and are significant also in commercial terms. The schematic setup is shown in Fig. 19.5.8
The electrolyte phase has to carry the cell current by enabling the transport of charge carriers in the form of ions and often it has to provide co-reactants (usually water, protons or hydroxide ions) to the electrode as well as to allow the removal of ionic products from the reaction site. That is the main reason for aging and limiting life time. Counter and reference electrodes may be combined into a single electrode for non-critical applications. For gas sensors the most important challenge consists in the development of a working electrode which is accessible for the sample gas while still being in contact with the usually liquid internal electrolyte solution. Either, the gas must be dissolved in the electrolyte solution before coming in contact with the electrode, or the so-called triple points are required where the gas, liquid and solid electrode phases meet. The electrochemical cell is separated from the medium to be analysed by a gas-permeable membrane. This is pressed against a flat electrode leaving only a thin layer of electrolyte solution between electrode and membrane. The membrane has the important role of eliminating the interference of redox-active substances other than the measured gas which might be present in the sample and to avoid fouling of the working electrode. The membrane also allows the internal use of a high concentration of an electrolyte in the electrochemical cell without having to modify the sample itself, thus eliminating errors due to the i · R drop which would otherwise be present. Chloride ions in the electrolyte solution are involved in the counter electrode mechanism and also establish a constant reference potential. Modern Clark electrodes are equipped with a porous poly(tetrafluoroethylene) (PTFE) membrane. Due to the hydrophobic behaviour of the material the pores (typical diameter 10 μm) are not wetted but allow the transport of dissolved gases. As the mass-transfer rate of the analyte is slow, the faradaic current is controlled by diffusion rather than the kinetics of the electrode reaction and this assures a linear dependence of the current on the concentration of the dissolved oxygen (see Fig. 19.3). The layer of the electrolyte solution between membrane and electrode is kept thin in order not to compromise sensitivity and response time.
The selectivity to a certain gas species can be ensured not only by the kind of membrane but also by the polarisation voltage which is necessary to promote a certain electrochemical reaction. The voltage which has to be applied between the working and the reference electrode must be higher than the equilibrium emf.
In Table 19.1 electrode reactions in which gases are involved are listed.
The cell reaction consists of an oxidation of the gas which is to be measured and the reduction of molecular oxygen. Such sensors can be applied at temperatures from −20 to +50 °C, with a usual recommendation between 5 and 25 °C. According to the special shape and the size of the electrodes these sensors have typically a sensitivity of 0.1 μA/ppm ± 0.02 μA/ppm. These sensors are available on the market from several companies, e.g. City Technology, London, UK; EnviteC Wismar, Germany; and IT Dr. Gambert, Wismar, Germany. Depending on the gas, its concentration and the expected life time the response time is between 5 and 25 s and the lifetime in air <3 years. Generally, these sensors are not so robust and the lifetime is limited, but they have a higher selectivity as the resistive semiconductor sensors.
The determination of ozone can be carried out directly in an irreversible reaction at +2.07 V which leads to a poisoning of the electrode surface and a shortening of the lifetime. The better way is to use an indirect reaction via a redox mediator. Ozone reacts with the reduced mediator and the oxidised mediator in turn is reduced on the working electrode (Fig. 19.6). The flowing current is proportional to the ozone concentration. As mediators inorganic redox couples like Br−, OBr−; AsO3 3−, AsO3 3−; and Fe2+, Fe3+ are used. Amperometric ozone sensors are broadly available on the market as well for measuring in air as for monitoring of cleaning processes.
4.2 Fuel Cell Principle
In a more simple design the fuel cell principle without an external voltage is used to measure oxygen (Fig. 19.7). The voltage is generated between the oxygen cathode and an anode made of lead. According to the oxygen concentration the cathode may be metallic or covered by a polymer membrane. At present such bare electrodes are only rarely used; an exception is the determination of oxygen in sewage and in beverages with electrodes which are regularly abraded mechanically for cleaning.
Oxygen is reduced in alkaline solution at a voltage of +0.401 V according to the cathodic reaction
As an anode reaction lead is oxidised:
For the overall cell reaction results,
Therefore the cell voltage is 0.981 V. The current which is proportional to the oxygen partial pressure is measured by means of a 100 Ω resistor.
4.3 Severinghaus Sensors (Electrodes)
Unlike other gases such as oxygen the determination of dissolved CO2 is more difficult due to its chemical reactions with water. This is partly true for measuring CO2 in ambient air which contains more or less water especially under condensing conditions. CO2 cannot be reduced electrochemically in aqueous solution so that a simple sensor setup like for the determination of oxygen is not possible. This is important for interpreting analytical results as well as for understanding the measurement principle applied, as for example in the Severinghaus electrode. The concentration of CO2 is only reasonable with the knowledge of the pH of the analysed medium.9
In Fig. 19.8 the interaction of CO2 and water is shown in terms of the pH dependence of the equilibrium concentration of the formed components. At pH values below 4.3 nearly the total carbonate exists as free dissolved CO2 in the solution. It should be mentioned that the values depend slightly on the temperature and on the alkalinity and salinity of the solution. The same arguments apply to the determination of ammonia (NH3).
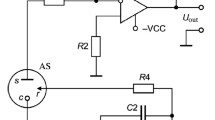
Figure 19.9 shows schematically the so-called Severinghaus carbon dioxide sensor (sometimes also called electrode) and illustrates its mode of operation. Main constituents of the sensor are a thin polymer membrane, the hydrogen carbonate containing electrolyte solution, a thin hydrophilic spacer sheet soaked with the electrolyte solution and a pH sensor. CO2 permeates from the gaseous or liquid specimen through the membrane into the electrolyte film in the spacer until equilibrium between the CO2 partial pressure on both sides of the membrane has been established. During measurement virtually no CO2 is consumed.
Whereas CO2 sensors for measurements in gases can simply be calibrated by using commercially available test gases, the calibration of electrochemical CO2 sensors for measurements in liquids is not trivial. Contrary to standardised, long-term stable buffer solutions with defined pH values, which are generally used in pH measuring technique, solutions with defined CO2 content are not long-term stable and therefore commercially not available. For this reason, calibration solutions with defined CO2 concentration must be prepared immediately before starting the calibration procedure.
Since the sensitivity of electrochemical CO2 sensors changes only slightly in the course of time, it is sufficient to carry out regularly one-point calibrations in order to correct the zero-point drift. Two-point calibrations are only necessary in longer time intervals or after the sensor had been out of use for a longer period. If the sensor is continuously in use, as a rule, one-point calibrations at least once per week and two-point calibrations at least once per month are recommended. For two-point calibrations two calibration solutions with different CO2 concentrations are needed.
4.4 Application
As discussed in the former paragraphs there are high- and low-temperature gas sensors (Table 19.2). Which sensor for which application can be recommended is a question of expected information, response time and lifetime. This depends on the matrix to be analysed and on the temperature. For long-term applications at high as well as low temperatures with a short response time high-temperature sensors based on solid electrolytes are preferred. They can be used for the most part maintenance free. On the other hand, the price of those sensors is mostly much higher than that of normal-temperature sensors. Normal-temperature sensors need calibrations from time to time.
References
Gründler P (2004) Chemische Sensoren (in German). Springer, Berlin
Guth U (2012) Gas sensors. In: Bard A, Inzelt G, Scholtz F (eds) Electrochemical dictionary. Springer, Heidelberg, pp 400–406, 595–596, 2nd extended edition
Kleitz M, Siebert E, Fabry P, Fouletier J (1992) Solid-state electrochemical sensors. In: Göpel W, Hesse J, Zemel JN (eds) Sensors. A comprehensive survey, vol 2. Weinheim, Wiley-VCH, p 413
Moos R, Sahner K, Fleischer M, Guth U, Barsan N, Weimar U (2009) Solid state gas sensor research in Germany - a status report. Sensors 9:4323–4365. doi:10.3390/s90604323
Guth U, Zosel J (2004) Electrochemical solid electrolyte gas sensors – hydrocarbon and NOx analysis in exhaust gases. Ionics 10:366–377
Fergus JW (2007) Solid electrolyte based sensors for the measurement of CO and hydrocarbon gases. Sensors Actuators B 122:683–693
Fergus JW (2008) A review of electrolyte and electrode materials for high temperature electrochemical CO2 and SO2 gas sensors. Sensors Actuators B 134:1034–1041
Guth U, Vonau W, Zosel J (2009) Recent developments in electrochemical sensor application and technology - a review. Meas Sci Technol 20: 1–14, 042002
Zosel J, Oelßner W, Decker M, Gerlach G, Guth U (2011) The measurement of dissolved and gaseous carbon dioxide concentration. Meas Sci Technol 22, 072001, doi: 10.1088/0957-0233/22/7/072001
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Guth, U., Vonau, W., Oelßner, W. (2014). Gas Sensors. In: Moretto, L., Kalcher, K. (eds) Environmental Analysis by Electrochemical Sensors and Biosensors. Nanostructure Science and Technology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0676-5_19
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0676-5_19
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0675-8
Online ISBN: 978-1-4939-0676-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)