Abstract
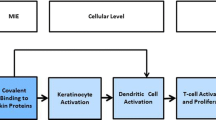
Advances in the understanding of the immunobiology of skin sensitization have led to the establishment of predictive in vivo tests which not only identify sensitizing hazards but also characterize their potency. Recently, appreciation of the underlying biology has also resulted in the development of mechanistically based in vitro alternatives which offer the prospect of the replacement of current in vivo methods. Assays under active validation include the Direct Peptide Reactivity Assay (DPRA), the human Cell Line Activation Test (h-CLAT), and KeratinoSens. None of the methods have a sufficient level of accuracy or freedom from applicability domain limitations to allow them to act as a standalone replacement. Consequently, it will be necessary to consider how to deploy these assays, perhaps in combination and/or in a structured assessment of skin sensitization hazard, to ensure at least the same level of predictive accuracy as the in vivo methods. However, a challenge remains: the capacity of these methods to provide potency information on skin-sensitizing chemicals has yet to be assessed. This is an essential requirement for future risk assessment without use of animal models if we are to retain the same level of human health protection that is currently delivered.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rustemeyer T, Van Hoogstraten IMW, Von Blomberg BMA, Gibbs S, Scheper RG (2011) Mechanisms of irritant and allergic contact dermatitis. In: Johansen JD, Frosch PJ, Lepoittevin J-P (eds) Textbook of contact dermatitis, 5th edn. Springer, Berlin, pp 43–90
Martin SF (2012) Contact dermatitis: from pathomechanisms to immunotoxicology. Exp Dermatol 21:382–389
McFadden JP, Puangpet P, Basketter DA, Dearman RJ, Kimber I (2013) Why does allergic contact dermatitis exist? Brit J Dermatol 168:692–699
McFadden JP, Basketter DA (2000) Contact allergy, irritancy and danger. Contact Dermatitis 42:123–127
Martin SF (2012) Allergic contact dermatitis: xenoinflammation of the skin. Curr Opin Immunol 24(6):720–729
Johansen JD, Frosch PJ, Lepoittevin J-P (2011) Contact dermatitis, 5th edn. Springer, Berlin
Rietschel RL, Fowler JF (2008) Fisher’s contact dermatitis, 6th edn. BC Decker, Hamilton
De Groot AC (2008) Patch testing, 3rd edn. Acdegroot Publishing, Wapserveen
Basketter DA (2008) Skin sensitisation: strategies for risk assessment and risk management. Brit J Dermatol 159:267–273
Andersen KE, Maibach HI (1985) Current problems in dermatology 14: contact allergy predictive tests in guinea pigs. Karger, Basel
Thyssen JP, Giménez-Arnau E, Lepoittevin JP, Menné T, Boman A, Schnuch A (2012) The critical review of methodologies and approaches to assess the inherent skin sensitization potential (skin allergies) of chemicals Part I. Contact Dermatitis 66(Suppl 1):11–24
Magnusson B, Kligman AM (1970) Allergic contact dermatitis in the guinea pig. Identification of contact allergens. Charles C Thomas, Springfield, IL
Buehler EV (1965) Delayed contact hypersensitivity in the guinea pig. Arch Dermatol 91:171–177
Wahlberg JE, Boman A (1985) Guinea pig maximization test. In: Andersen KE, Maibach HI (eds) Contact allergy: predictive test in guinea pigs. Current problems in dermatology. Karger, Basel, pp 59–106
Cronin MTD, Basketter DA (1994) Multivariate QSAR analysis of a skin sensitization database. SAR QSAR Environ Res 2:159–179
Basketter DA, Gerberick GF (1996) Interlaboratory evaluation of the Buehler test. Contact Dermatitis 35:146–151
Kimber I, Basketter DA (1992) The murine local lymph node assay; collaborative studies and new directions: a commentary. Food Chem Toxicol 30:165–169
Kimber I, Dearman RJ, Scholes EW, Basketter DA (1994) The local lymph node assay: developments and applications. Toxicology 93:13–31
Rovida C, Ryan C, Cinelli S, Basketter D, Dearman R, Kimber I (2012) The local lymph node assay (LLNA) (Chapter 20). Curr Protoc Toxicol (Suppl. 51): 20.7.1–20.7.14
Gerberick GF, Ryan CA, Kimber I, Dearman RJ, Lea LJ, Basketter DA (2000) Local lymph node assay: validation assessment for regulatory purposes. Am J Contact Derm 11:3–18
Dean JH, Twerdok LE, Tice RR, Sailstad DM, Hattan DG, Stokes WS (2001) ICCVAM evaluation of the murine local lymph node assay. II Conclusions and recommendations of an independent scientific peer review panel. Reg Toxicol Pharmacol 34:258–273
OECD (2002) Local lymph node assay. Test Guideline no 429. Organisation for Economic Cooperation and Development, Paris
OECD (2010) Organisation for Economic Cooperation and Development. Test Guideline 429. The Local Lymph Node Assay, France, Paris
OECD (2010b) Organisation for Economic Cooperation and Development. Guidelines for Test of Chemicals No 442a and 442b. Paris, France
Gerberick GF, Ryan CA, Kern PS, Schlatter H, Dearman RJ, Kimber I, Patlewicz GY, Basketter DA (2005) Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis 16:157–202
Kern PS, Gerberick GF, Ryan CA, Kimber I, Aptula A, Basketter DA (2010) Historical local lymph node data for the evaluation of skin sensitization alternatives: a second compilation. Dermatitis 21:8–32
Kimber I, Basketter DA (1997) Contact sensitization: a new approach to risk assessment. Hum Ecol Risk Assess 3:385–395
Basketter DA, Blaikie L, Dearman RJ, Kimber I, Ryan CA, Gerberick GF, Harvey P, Evans P, White IR, Rycroft RJG (2000) Use of the local lymph node assay for the estimation of relative contact allergenic potency. Contact Dermatitis 42:344–348
Ryan CA, Gerberick GF, Cruse LW, Basketter DA, Lea LJ, Blaikie L, Dearman RJ, Warbrick EV, Kimber I (2000) Activity of human contact allergens in the murine local lymph node assay. Contact Dermatitis 43:95–102
Basketter DA, Lea L, Cooper K, Dickens A, Briggs D, Pate I, Dearman RJ, Kimber I (1999) A comparison of statistical approaches to derivation of EC3 values from local lymph node assay dose responses. J Appl Toxicol 19:261–266
Gerberick GF, Robinson MK, Felter S, White I, Basketter DA (2001) Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis 45:333–340
Felter SP, Robinson MK, Basketter DA, Gerberick GF (2002) A review of the scientific basis for default uncertainty factors for use in quantitative risk assessment of the induction of allergic contact dermatitis. Contact Dermatitis 47:257–266
Api AM, Basketter DA, Cadby PA, Cano M-F, Ellis G, Gerberick GF, Griem P, McNamee PM, Ryan CA, Safford B (2008) Dermal sensitization quantitative risk assessment (QRA) for fragrance ingredients. Regul Toxicol Pharmacol 52:3–23
Basketter DA, Clapp CJ, Safford BJ, Jowsey IR, McNamee PM, Ryan CA, Gerberick GF (2008) Preservatives and skin sensitisation quantitative risk assessment: risk benefit considerations. Dermatitis 19:20–27
United Nations Nations (2011) Globally harmonized system of classification and labelling of chemicals (GHS). Part 3: Health Hazards. http://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/03e_part3.pdf. Accessed 22 Oct 2012
Lepoittevin J-P, Basketter DA, Goossens A, Karlberg A-T (1999) Allergic contact dermatitis: the molecular basis. Springer, Berlin
Roberts DW, Patlewicz G, Kern PS, Gerberick GF, Kimber I, Dearman RJ, Ryan CA, Basketter DA, Aptula AO (2007) Mechanistic applicability domain classification of a local lymph node assay dataset for skin sensitisation. Chem Res Toxicol 16:1019–1030
OECD (2012c) The OECD QSAR toolbox. Version 3.0. http://www.oecd.org/chemicalsafety/assessmentofchemicals/theoecdqsartoolbox.htm. Accessed 21 Oct 2012
Patlewicz G, Aptula AO, Roberts DW, Kern PS, Gerberick GF, Kimber I, Dearman RJ, Ryan CA, Basketter DA (2007) An evaluation of selected global (Q)SARs/expert systems for the prediction of skin sensitisation potential. SAR QSAR Environ Res 18:515–541
Roberts DW, Aptula AO, Patlewicz GY (2011) Chemistry-based risk assessment for skin sensitization: quantitative mechanistic modeling for the S (N) Ar domain. Chem Res Toxicol 24(7):1003–1011
Vandebriel RJ, van Loveren H (2010) Non-animal sensitization testing: state-of-the-art. Crit Rev Toxicol 40:389–404
Kimber I, Basketter DA, Dearman RJ (2013) Dendritic cells and the assessment in vitro of skin sensitizing potential. Cut Ocul Toxicol 32:54–59
Vocanson M, Nicolas J-F, Basketter DA (2013) In vitro approaches to the identification and characterization of skin sensitisers. Exp Rev Dermatol 8:395–405
Rovida C, Martin SF, Vivier M, Weltzien HU, Roggen E (2013) Advanced tests for skin and respiratory sensitization assessment. ALTEX 30(2):231–252
OECD (2012) The adverse outcome pathway for skin sensitization. Organisation for Economic Cooperation and Development, Paris
Basketter D, Angers-Loustau A, Casati S, ECVAM (2012) ECVAM: progressing skin sensitisation alternatives for hazard identification. Contact Dermatitis 66(Suppl 2):24
Gerberick GF, Vassallo JD, Bailey RE, Chaney JG, Morrall SW, Lepoittevin JP (2004) Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci 81: 332–343
Gerberick GF, Vassallo JD, Foertsch LM, Price BB, Chaney JG, Lepoittevin JP (2007) Quantification of chemical peptide reactivity for screening contact allergens: a classification tree model approach. Toxicol Sci 97:417–427
Natsch A, Emter R (2008) Skin sensitizers induce antioxidant response element dependent genes: application to the in vitro testing of the sensitization potential of chemicals. Toxicol Sci 102:110–119
Emter R, Ellis G, Natsch A (2010) Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol Appl Pharmacol 245:281–290
Ashikaga T, Yoshida Y, Hirota M, Yoneyama K, Itagaki H, Sakaguchi H, Miyazawa M, Ito Y, Suzuki H, Toyoda H (2006) Development of an in vitro skin sensitization test using human cell lines: the human cell line activation test (h-CLAT). I. Optimization of the h-CLAT protocol. Toxicol In Vitro 20:767–773
Sakaguchi H, Ashikaga T, Miyazawa M, Yoshida Y, Ito Y, Yoneyame K, Hirota M, Itagaki H, Toyoda H, Suzuki H (2006) Development of an in vitro skin sensitization test using human cell lines; human cell line activation test (h-CLAT). II. An international study of the h-CLAT. Toxicol In Vitro 20:774–784
Ashikaga T, Sakaguchi H, Sono S, Kosaka N, Ishikawa M, Nukada Y, Miyazawa M, Ito Y, Nishiyama M, Itagaki H (2010) A comparative evaluation of in vitro skin sensitisation tests: the human cell-line activation test (h-CLAT) versus the local lymph node assay (LLNA). ATLA 38:275–284
Divkovic M, Pease CM, Gerberick GF, Basketter DA (2005) Hapten-protein binding: from theory to practical application in the in vitro prediction of skin sensitisation. Contact Dermatitis 53:189–200
Aleksic M, Thain E, Roger D, Saib O, Davies M, Li J, Aptula A, Zazzeroni R (2009) Reactivity profiling: covalent modification of single nucleophile peptides for skin sensitization risk assessment. Toxicol Sci 108(2):401–411
Natsch A (2010) The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers: functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci 113:284–292
Bauch C, Kolle SN, Fabian E, Pachel C, Ramirez T, Wiench B, Wruck CJ, van Ravenzwaay B, Landsiedel R (2011) Intralaboratory validation of four in vitro assays for the prediction of the skin sensitizing potential of chemicals. Toxicol In Vitro 25:1162–1168
Python F, Goebel C, Aeby P (2007) Assessment of the U937 cell line for the detection of contact allergens. Toxicol Appl Pharmacol 220(2):113–124
EURL ECVAM Skin Sensitisation: http://ihcp.jrc.ec.europa.eu/our_labs/eurl-ecvam/validation-regulatory-acceptance/topical-toxicity/skin-sensitisation#2-alternative-test-methods. Accessed 28 Jul 2013
Jeong YH, An S, Shin K, Lee TR (2013) Peptide reactivity assay using spectrophotometric method for high-throughput screening of skin sensitization potential of chemical haptens. Toxicol In Vitro 27(1):264–271
Roberts DW, Aptula AO, Patlewicz G, Pease C (2008) Chemical reactivity indices and mechanism-based read-across for non-animal based assessment of skin sensitisation potential. J Appl Toxicol 28(4):443–454
Gerberick GF, Troutman JA, Foertsch LM, Vassallo JD, Quijano M, Dobson RL, Goebel C, Lepoittevin JP (2009) Investigation of peptide reactivity of pro-hapten skin sensitizers using a peroxidase-peroxide oxidation system. Toxicol Sci 112:164–174
Troutman JA, Foertsch LM, Kern PS, Dai HJ, Quijano M, Dobson RL, Lalko JF, Lepoittevin JP, Gerberick GF (2011) The incorporation of lysine into the peroxidase peptide reactivity assay for skin sensitization assessments. Toxicol Sci 122:422–436
Bauch C, Kolle SN, Ramirez T, Eltze T, Fabian E, Mehling A, Teubner W, van Ravenzwaay B, Landsiedel R (2012) Putting the parts together: combining in vitro methods to test for skin sensitizing potentials. Regul Toxicol Pharmacol 63:489–504
McKim JM Jr, Keller DJ III, Gorski JR (2010) A new in vitro method for identifying chemical sensitizers combining peptide binding with ARE/EpRE-mediated gene expression in human skin cells. Cutan Ocul Toxicol 29(3): 171–192
Johansson H, Albrekt AS, Borrebaeck CA, Lindstedt M (2013) The GARD assay for assessment of chemical skin sensitizers. Toxicol In Vitro 27(3):1163–1169
McKim JM Jr, Keller DJ III, Gorski JR (2012) An in vitro method for detecting chemical sensitization using human reconstructed skin models and its applicability to cosmetic, pharmaceutical, and medical device safety testing. Cutan Ocul Toxicol 31(4):292–305
dos Santos GG, Spiekstra SW, Sampat-Sardjoepersad SC, Reinders J, Scheper RJ, Gibbs S (2011) A potential in vitro epidermal equivalent assay to determine sensitizer potency. Toxicol In Vitro 25(1):347–357
Corsini E, Galbiati V, Mitjans M, Galli CL, Marinovich M (2013) NCTC 2544 and IL-18 production: a tool for the identification of contact allergens. Toxicol In Vitro 27(3): 1127–1134
Teunis M, Corsini E, Smits M, Madsen CB, Eltze T, Ezendam J, Galbiati V, Gremmer E, Krul C, Landin A, Landsiedel R, Pieters R, Rasmussen TF, Reinders J, Roggen E, Spiekstra S, Gibbs S (2013) Transfer of a two-tiered keratinocyte assay: IL-18 production by NCTC2544 to determine the skin sensitizing capacity and epidermal equivalent assay to determine sensitizer potency. Toxicol In Vitro 27(3):1135–1150
Gibbs S, Corsini E, Spiekstra SW, Galbiati V, Fuchs HW, Degeorge G, Troese M, Hayden P, Deng W, Roggen E (2013) An epidermal equivalent assay for identification and ranking potency of contact sensitizers. Toxicol Appl Pharmacol 272:529–541
Landsteiner K, Jacobs J (1936) Studies on the sensitisation of animals with simple chemical compounds. J Exp Med 64:625–639
Baer R (1954) Cross sensitization phenomena. In: McKenna RMB (ed) Modern trends in dermatology. Butterworth and Co., London, pp 232–258, 1955
Dupuis G, Benezra C (1982) Allergic contact dermatitis to simple chemicals: a molecular approach. Marcel Dekker, New York
Smith CK, Hotchkiss SAM (2001) Allergic contact dermatitis: chemical and metabolic mechanisms. Taylor & Francis Ltd., London
Barratt MD, Basketter DA, Chamberlain M, Payne MP, Admans GD, Langowski JJ (1994) Development of an expert system rulebase for identifying contact allergens. Toxicol In Vitro 8(4):837–839
Langton K, Patlewicz GY, Long A, Marchant CA, Basketter DA (2006) Structure-activity relationships for skin sensitization: recent improvements to Derek for Windows. Contact Dermatitis 55:342–347
Barratt MD, Langowski JJ (1999) Validation and subsequent development of the DEREK skin sensitization rulebase by analysis of the BgVV list of contact allergens. J Chem Inf Comput Sci 39(2):294–298
Patlewicz G, Dimitrov SD, Low LK, Kern PS, Dimitrova GD, Comber MI, Aptula AO, Phillips RD, Niemelä J, Madsen C, Wedebye EB, Roberts DW, Bailey PT, Mekenyan OG (2007) TIMES-SS: a promising tool for the assessment of skin sensitization hazard. A characterization with respect to the OECD validation principles for (Q) SARs and an external evaluation for predictivity. Regul Toxicol Pharmacol 48:225–239
Roberts DW, Patlewicz G, Dimitrov SD, Low LK, Aptula AO, Kern PS, Dimitrova GD, Comber MI, Phillips RD, Niemelä J, Madsen C, Wedebye EB, Bailey PT, Mekenyan OG (2007) TIMES-SS: a mechanistic evaluation of an external validation study using reaction chemistry principles. Chem Res Toxicol 20: 1321–1330
OECD (2013) The OECD QSAR toolbox. Version 3.1. Paris. http://www.oecd.org/env/ehs/risk-assessment/theoecdqsartoolbox.htm. Accessed 28 Jul 2013
Nukada Y, Ashikaga T, Miyazawa M, Hirota M, Sakaguchi H, Sasa H, Nishiyama N (2012) Prediction of skin sensitization potency of chemicals by human cell line activation test (h-CLAT) and an attempt at classifying skin sensitization potency. Toxicol In Vitro 26(7): 1150–1160
OECD (2013) Paris
Natsch A, Emter R, Ellis G (2009) Filling the concept with data: integrating data from different in vitro and in silico assays on skin sensitizers to explore the battery approach for animal-free skin sensitization testing. Toxicol Sci 107:106–121
Nukada Y, Miyazawa M, Kazutoshi S, Sakaguchi H, Nishiyama N (2013) Data integration of non-animal tests for the development of a test battery to predict the skin sensitizing potential and potency of chemicals. Toxicol In Vitro 27(2):609–618
Jaworska J, Dancik Y, Kern P, Gerberick F, Natsch A (2013) Bayesian integrated testing strategy to assess skin sensitization potency: from theory to practice. J Appl Toxicol
Basketter DA, Alepee N, Ashikaga T, Barroso J, Gilmour N, Goebel C, Hibatallah J, Hoffmann S, Kern P, Martinozzi-Teissier S, Maxwell G, Millet M, Reisinger K, Sakaguchi H, Schepky A, Tialhardat M, Templier M (2013) Categorisation of chemicals according to their relative human skin sensitizing potency. Dermatitis 25:11–21
Natsch A, Ryan CA, Foertsch L, Emter R, Jaworska J, Gerberick F, Kern P (2013) A dataset on 145 chemicals tested in alternative assays for skin sensitization undergoing prevalidation. J Appl Toxicol. doi:10.1002/jat.2868. [Epub ahead of print]
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this protocol
Cite this protocol
Basketter, D., Casati, S. (2014). Dermal Toxicity: Skin Sensitization. In: Bal-Price, A., Jennings, P. (eds) In Vitro Toxicology Systems. Methods in Pharmacology and Toxicology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-0521-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0521-8_10
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-0520-1
Online ISBN: 978-1-4939-0521-8
eBook Packages: Springer Protocols