Abstract
Psychiatric disorders represent a large class of medical disorders with unclear etiologies and limited effective treatments. Mitochondrial dysfunction is particularly interesting as it can manifest a wide variety of symptoms including abnormalities in central nervous system functioning with focal neurological, cognitive, behavioral, and psychiatric symptoms. Evidence has started to accumulate that mitochondrial dysfunction plays a role in schizophrenia, major depression, bipolar disorder, personality/mood disorders, Alzheimer’s disease, and autism spectrum disorders. The prevalence of mitochondrial dysfunction in these disorders is not clear. Studies reporting laboratory testing for the identification of mitochondrial dysfunction and treatments in these disorders are generally lacking. Notably, the identification of mitochondrial dysfunction in psychiatric conditions could lead to the development of better medications and other treatments for these conditions that target specific metabolic abnormalities. Although the evidence for mitochondrial dysfunction in psychiatric disorders is increasing, additional studies are warranted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autism Spectrum Disorder
- Autism Spectrum Disorder
- Psychiatric Disorder
- Bipolar Disorder
- Mitochondrial Dysfunction
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Background
Psychiatric disorders represent a large class of medical disorders with unclear etiologies and limited effective treatments. A simple single gene or chromosomal abnormality has not been found to explain most psychiatric disorders. Although linkage studies have identified many candidate regions of certain chromosomes that could be associated with many psychiatric disorders, these findings have been inconsistent across studies. For example, recent studies have identified genetic polymorphisms associated with increased susceptibility to psychiatric disorders such as schizophrenia, but most polymorphisms identified are in the noncoding regions of the genome, making the understanding of how these genetic changes contribute to psychiatric disorders opaque (Harrison and Weinberger 2005; Kleinman et al. 2011). Some research studies have started to investigate gene-environment interactions and epigenetic factors in psychiatric disorders, rather than fixed genetic defects. These studies may lead to a better understanding of how interactions between genetic polymorphisms and the environment contribute to the development of psychiatric disorders and also provide a deeper understanding of the pathophysiological mechanisms that cause these disorders. Other research studies examining the etiology of psychiatric disorders have embraced the study of pathophysiological mechanisms that could more directly result in cellular dysfunction and the subsequent development of psychiatric disorders. Pathophysiological mechanisms identified in some psychiatric disorders include immune dysregulation, inflammation, impaired detoxification, environmental toxicant exposures, redox regulation/oxidative stress, and mitochondrial dysfunction (Burke and Miller 2011; Dantzer et al. 2008; Ng et al. 2008; Shao et al. 2008). The focus of this chapter is on mitochondrial dysfunction in common psychiatric disorders.
2 Mitochondria and Their Physiological Function
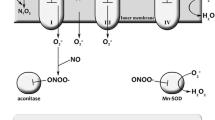
Mitochondria are distinct cellular organelles that generate adenosine triphosphate (ATP), the energy carrier in most mammalian cells, from adenosine diphosphate (ADP) by oxidizing glucose and fatty acids (Haas et al. 2007). Acetyl-CoA is a key intermediate generated from the oxidation of glucose and fatty acids that is further metabolized by the tricarboxylic acid (TCA) cycle. The TCA cycle produces flavin adenine dinucleotide (FADH2) and nicotinamide adenine dinucleotide (NADH). NADH and FADH2 transport energy to the mitochondrial electron transport chain (ETC), a series of reactions known as oxidative phosphorylation. Mitochondria contain two plasma membranes, an inner and an outer membrane. The ETC is located in the inner mitochondrial membrane and consists of five multi-subunit enzyme complexes (complexes I through V) and two electron carriers (ubiquinone, also known as coenzyme Q10, and cytochrome c) (Zeviani et al. 1996).
Mitochondria are the only organelle in mammalian cells with their own genome. The ETC is coded by both mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) (Zeviani et al. 1996). mtDNA contains 37 genes that code for 13 subunits of complexes I, III, IV, and V, as well as the machinery required to translate and transcribe the mtDNA genes into ETC complex subunits. The rest of the ETC complex subunits are coded by over 850 nDNA genes (Cotter et al. 2004). nDNA also codes for mitochondrial enzymes that participate in carbohydrate and fatty-acid oxidation. Thus, mutations in either genome can impair mitochondrial function and cause ETC complex deficiencies (DiMauro and Schon 2003).
The ETC complexes, particular complexes I and III, are the source as well as the major target of reactive oxygen species (ROS) (Fernandez-Checa et al. 1998; Trushina and McMurray 2007). The ETC is protected from damage caused by ROS by a mitochondrial specific superoxide dismutase and by antioxidants such as glutathione (GSH) (Fernandez-Checa et al. 1998) as well as by uncoupling proteins (Lambert and Brand 2004). Mitochondria lack the enzymes to synthesize GSH and therefore are dependent on cytosolic GSH production (Enns 2003; James et al. 2009b). The depletion of GSH in mitochondria makes cells more vulnerable to oxidative stress and damage from ROS originating from the mitochondria (Fernandez-Checa et al. 1997). Additionally, factors that increase ROS production (such as environmental toxicants, infections, and autoimmune disease) can directly and indirectly lead to impairments in ETC activity (Anderson et al. 2008; Calabrese et al. 2005; Munnich and Rustin 2001), deplete GSH (Calabrese et al. 2005), and activate mitochondrial- and non-mitochondrial-dependent biochemical cascades that result in programmed cell death (apoptosis) (Roberts et al. 2009).
The number of mitochondria in each cell depends on the cellular energy demands. For example, low-energy cells, such as skin cells, have fewer mitochondria, while cells that require high energy demands, such as muscle, liver, brain, cerebrovascular endothelium, and GI cells, have many mitochondria. Neural synapses are areas of high energy consumption (Ames 2000) and are therefore especially dependent on mitochondrial function (Mattson and Liu 2002). Mitochondria are concentrated in the dendritic and axonal termini where they play an important role in ATP production, calcium homeostasis, synaptic plasticity (Chen and Chan 2009; Li et al. 2004), as well as neurotransmitter release (Vos et al. 2010). Mitochondria help to regulate neuroplasticity, and abnormalities in mitochondrial function can play a role in psychiatric and neurodegenerative disorders (Mattson 2007). Therefore, central nervous system (CNS) manifestations are common in patients with mitochondrial disorders (MD) (Finsterer 2006).
Studies of healthy individuals have revealed a decrease in brain mitochondrial function associated with healthy aging (Forester et al. 2010) and brain mtDNA mutations are generally more common in elderly subjects compared to younger individuals (Lin et al. 2002). Mitochondrial dysfunction is particularly interesting to study as it has been implicated in a wide variety of diseases including psychiatric disorders (Anglin et al. 2012b; Jou et al. 2009; Manji et al. 2012; Marazziti et al. 2011, 2012; Rezin et al. 2009; Scaglia 2010; Shao et al. 2008) such as schizophrenia (Clay et al. 2011; Kato et al. 2011; Scaglia 2010; Verge et al. 2011), bipolar disorder (Clay et al. 2011; Kato et al. 2011; Scaglia 2010), depression (Kato et al. 2010; Scaglia 2010), and autism spectrum disorder (Frye and Rossignol 2011; Rossignol and Bradstreet 2008; Rossignol and Frye 2011) as well as neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (Federico et al. 2012). Mitochondrial dysfunction has also been reported in genetic syndromes associated with neurodevelopmental delays such as Rett syndrome (Condie et al. 2010; Gibson et al. 2010; Grosser et al. 2012), PTEN abnormalities (Napoli et al. 2012), Phelan-McDermid syndrome (Frye 2012a), 15q11-q13 duplication syndrome (Filipek et al. 2003; Frye 2009), Angelman syndrome (Su et al. 2011), Septo-optic dysplasia (Schuelke et al. 2002), and Down syndrome (Pagano and Castello 2012; Pallardo et al. 2010) along with a wide variety of medical disorders such as persistent systemic inflammation (Cox 2012), cardiac disease (Dai et al. 2012), and diabetes (Naudi et al. 2012). Mitochondria are also intimately involved in programmed cell death (apoptosis), calcium homeostasis, synaptic plasticity, and neurotransmitter release (Anderson et al. 2008; Roberts et al. 2009). In fact, mitochondrial dysfunction may be one of the common pathways in the development of pathology associated with a wide variety of diseases, especially since mitochondrial dysfunction can cause profound dysfunction in many organ systems, particularly high-energy organs such as the nervous and immune systems and the gastrointestinal tract (Rossignol and Frye 2011). The identification of mitochondrial dysfunction in psychiatric conditions could lead to the development of better medications for these conditions (Manji et al. 2012). Given the importance of mitochondria in CNS function, we review the involvement of mitochondria and MD in patients with psychiatric disorders.
3 Psychiatric Disorders in Patients with Mitochondrial Disease
Psychiatric disorders appear to be relatively common in patients with MD. For example, in a study of 36 adults with MD, 54 % had major depression, 17 % had bipolar disorder, and 11 % had a panic disorder (Fattal et al. 2007). In one study of 24 Italian patients with MD, psychiatric conditions were more common (60 %) than in the general population and included agoraphobia, panic disorder, anxiety disorders, and psychotic syndromes (Mancuso et al. 2013). As a group, fourteen adolescents and young adults with MD self-reported significant depression and anxiety on the Behavior Assessment System for Children (Schreiber 2012) and in another study, 14 % of children with MD developed symptoms of major depression before the MD diagnosis (Koene et al. 2009). Dementia has also been reported in multiple types of MD (Finsterer 2009).
Some patients with mtDNA mutations have been reported to have psychiatric symptoms. For example, one study reported that 19 adults with mtDNA mutations had more depressive symptoms and mood disorders compared to 10 controls (Inczedy-Farkas et al. 2012). Progressive psychiatric disturbance and dementia along with neurological disturbances have been reported in a 57-year-old woman (Young et al. 2010) and a 27-year-old man (Salsano et al. 2011) with mitochondrial transfer RNA mutations. One study reported on a family with multiple deletions in mtDNA; this family contained multiple generations of psychiatric problems, including bipolar disorder, schizophrenia, and depression (Mancuso et al. 2008). Dementia (Hopkins et al. 2010) and psychiatric problems (Komulainen et al. 2010) have also been reported in the polymerase gamma-1 (POLG1) mutation. In one study of 50 patients with MD, of whom 52 % had mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS), the most common psychiatric diagnoses were mood disorders, psychosis, cognitive deterioration, and anxiety (Anglin et al. 2012a). Confusion, aggressive behaviors, hallucinations, and paranoid delusions can also occur along with nonconvulsive status epilepticus and recurrent complex partial seizures in MELAS (Kaufman et al. 2010). In addition, MELAS has been associated with depression (Ju Seok et al. 2009) and obsessive-compulsive disorder (Lacey and Salzberg 2008).
Biomarkers of MD have been found to be abnormal in some patients with psychiatric disorders. For example, some hospitalized psychiatric patients have been reported to have low carnitine levels (Cuturic et al. 2011). Treatment with vitamins and minerals which improve mitochondrial function might help with certain psychiatric symptoms. One case report revealed that coenzyme Q10 improved psychiatric symptoms in a patient with MELAS (Shinkai et al. 2000). Another case report demonstrated that riboflavin treatment normalized behavior in a patient with MD and psychiatric illness (Triggs et al. 1992).
4 Schizophrenia
Several studies have reported genetic findings that might affect mitochondrial function in patients with schizophrenia. For example, one study reported that postmortem analysis of brains in patients with schizophrenia revealed a global downregulation of genes encoding mitochondrial elements, such as the electron transport chain (Iwamoto et al. 2005). Another study reported that postmortem brain samples from the dorsolateral prefrontal cortex revealed base pair substitutions in the mtDNA genome that was more common in individuals with schizophrenia compared to controls (Rollins et al. 2009). Finally, one study of 100 patients with schizophrenia found evidence of mtDNA inheritance (Verge et al. 2012).
Several studies have examined mitochondrial function in patients with schizophrenia. One postmortem study reported a 63 % reduction in complex IV activity in the nucleus caudatus and a 43 % reduction in the cortex gyrus frontalis in patients with schizophrenia compared to controls (Cavelier et al. 1995). Decreased hippocampal neuron gene expression affecting mitochondrial function was reported in 22 patients with schizophrenia compared to 24 controls (Altar et al. 2005). One study reported a patient with MELAS who had paranoid delusions, confusion, hallucinations, and aggressive behaviors (Kaufman et al. 2010). Finally, one study reported improvements in 15 patients with schizophrenia using normobaric hyperoxia (40 % inspired oxygen) compared to room air; the investigators suggested that the increased oxygen may have improved mitochondrial function by augmenting oxygen delivery to mitochondria (Bloch et al. 2012).
5 Major Depression
One case reported discussed a 17-year-old girl with MELAS and reported depressed mood, loss of interest, and catatonia that improved with medication (Ju Seok et al. 2009). Another study reported on 35 children with MD; five of these children had major depression prior to the diagnosis of MD (Koene et al. 2009). Finally, one study reported symptoms of depression and anxiety in students with MD (Schreiber 2012).
6 Bipolar Disorder
Several studies have reported genetic findings in individuals with MD that might contribute to bipolar disorder. For example, one study reported that postmortem analysis of brains in patients with bipolar disorder revealed a global downregulation of genes encoding mitochondrial elements, such as the electron transport chain (Iwamoto et al. 2005). Another study reported that 2 of 35 patients had a mitochondrial DNA deletion which was not found in any of 29 normal controls (Kato and Takahashi 1996). Finally, one study reported an increased level of common deletions in mtDNA in the dorsolateral prefrontal cortex in patients with bipolar disorder compared to controls (Sequeira et al. 2012).
Some studies have also reported depressed mitochondrial function in patients with bipolar disorder. In one postmortem study of 15 patients with bipolar disorder, complex I activity in the prefrontal cortex was significantly depressed compared to 15 patients with depression and 15 patients with schizophrenia (Andreazza et al. 2010). In another study, 32 patients with bipolar disorder exhibited gray matter increases in lactic acid levels on brain spectroscopy imaging compared to controls, suggesting mitochondrial dysfunction (Dager et al. 2004). Finally, one study of 10 older adults with bipolar disorder reported a decrease in depressive symptoms with coenzyme Q10 (400–1,200 mg/day) in an open-label study (Forester et al. 2012).
7 Personality/Mood Disorders
One study of 238 healthy Japanese volunteers reported that a mitochondrial DNA polymorphism (C5178A) was associated with increased extraversion compared to those with the 5178C polymorphism (Kato et al. 2004). Another study reported two individuals with obsessive-compulsive disorder who also had MELAS; the response to standard treatments was relatively poor (Lacey and Salzberg 2008). Finally, one study reported that genetic variants in mitochondrial proteins were associated with oxidative stress in patients with obsessive-compulsive disorder (OCD) (Orhan et al. 2012).
8 Alzheimer’s Disease
In an animal model of Alzheimer’s disease, decreased activity of complex IV was observed compared to control mice and was related to the production of beta-amyloid (Manczak et al. 2006). Two studies reported changes in TCA cycle enzyme activities in postmortem brain samples in patients with Alzheimer’s disease which were consistent with mitochondrial dysfunction (Bubber et al. 2005, 2011). A study of 17 patients with Alzheimer’s disease reported increased free radical production and decreased ATP production in brain samples compared to controls (Lin et al. 2002). Beta-amyloid production was associated with changes in mitochondrial structure including fragmentation in another study (Wang et al. 2008). Finally, in one study, relatives of patients who had Alzheimer’s disease and who were at increased risk for Alzheimer’s disease had evidence of reduced cerebral metabolism (Small et al. 1995).
9 Autism
Several review articles have reported mitochondrial dysfunction in individuals with autism (Frye and Rossignol 2011; Haas 2010; Rossignol and Frye 2011). Autism spectrum disorder (ASD) has also been reported in 120 cases of MD in 21 studies (Castro-Gago et al. 2008; Chauhan et al. 2010; Correia et al. 2006; Ezugha et al. 2010; Filiano et al. 2002; Filipek et al. 2003; Frye 2012a, b; Frye and Naviaux 2011; Gargus and Imtiaz 2008; Graf et al. 2000; Laszlo et al. 1994; Marin-Garcia et al. 1999; Nissenkorn et al. 2000; Oliveira et al. 2005, 2007; Pancrudo et al. 2007; Poling et al. 2006; Pons et al. 2004; Scaglia et al. 2009; Shoffner et al. 2010; Tsao and Mendell 2007; Weissman et al. 2008). One article reported that out of 153 studies examining various aspects of mitochondrial dysfunction in individuals with autism, 145 (95 %) implicated mitochondrial dysfunction in ASD (Rossignol and Frye 2012).
Several studies have suggested that treatment with mitochondrial cofactor supplementation, including antioxidants, coenzyme Q10, carnitine, and B vitamins, may improve mitochondrial function and behavior in some children with ASD (Rossignol and Frye 2011). l-carnitine may be particularly helpful in children with ASD since carnitine deficiency has been implicated in ASD (Filipek et al. 2004; Mostafa et al. 2005) and some studies have reported improvements with the use of l-carnitine in ASD (Ezugha et al. 2010; Filipek et al. 2003; Gargus and Imtiaz 2008; Gargus and Lerner 1997; Pastural et al. 2009; Poling et al. 2006). One double-blind, placebo-controlled study reported improvements in children with ASD using l-carnitine (50 mg/kg/day), including hand muscle strength and cognition (Geier et al. 2011). A second double-blind, placebo-controlled study of l-carnitine (100 mg/kg/day) reported significant improvements over 6 months of treatment in ASD symptoms compared to placebo (Fahmy et al. 2013). Two double-blind, placebo-controlled studies using a multivitamin containing B vitamins, antioxidants, vitamin E, and coenzyme Q10 reported various improvements in ASD symptoms compared to placebo (Adams et al. 2011; Adams and Holloway 2004). Treatments for oxidative stress have also been shown to be beneficial for some children with ASD. For example, methylcobalamin and folinic acid have been reported to significantly increase glutathione concentrations in children with ASD and appear to improve certain autistic behaviors (James et al. 2004, 2009a). A recent study has demonstrated that N-acetylcysteine (NAC) improves irritability in children with ASD compared to placebo (Hardan et al. 2012). Several other antioxidants (Rossignol 2009), including vitamin C (Dolske et al. 1993) and carnosine (Chez et al. 2002), have also been reported to significantly improve autistic behaviors. Finally, one study reported improvements in ASD symptoms using NADH and d-ribose (Freedenfeld et al. 2011).
10 Conclusions
Evidence has started to accumulate that mitochondrial dysfunction plays a role in the development of many psychiatric disorders. The number of studies published to date which have examined mitochondrial function in these disorders is small. Additional studies are needed to evaluate mitochondrial function in psychiatric disorder in order to identify the burden that mitochondrial dysfunction plays. Studies examining prevalence, severity, laboratory testing, and treatments of mitochondrial dysfunction in psychiatric disorders are warranted.
Abbreviations
- ADP:
-
Adenosine diphosphate
- ASD:
-
Autism spectrum disorder
- ATP:
-
Adenosine triphosphate
- CNS:
-
Central nervous system
- ETC:
-
Electron transport chain
- FADH2 :
-
Flavin adenine dinucleotide
- GSH:
-
Glutathione
- MD:
-
Mitochondrial disorder
- MELAS:
-
Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes
- mtDNA:
-
Mitochondrial DNA
- NAC:
-
N-acetylcysteine
- NADH:
-
Nicotinamide adenine dinucleotide
- nDNA:
-
Nuclear DNA
- OCD:
-
Obsessive-compulsive disorder
- POLG1:
-
Polymerase gamma-1
- ROS:
-
Reactive oxygen species
- TCA:
-
Tricarboxylic acid
References
Adams JB, Holloway C (2004) Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J Altern Complement Med 10(6):1033–1039
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E et al (2011) Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr 11:111
Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y et al (2005) Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry 58(2):85–96
Ames A 3rd (2000) CNS energy metabolism as related to function. Brain Res Brain Res Rev 34(1–2):42–68
Anderson MP, Hooker BS, Herbert MR (2008) Bridging from cells to cognition in autism pathophysiology: biological pathways to defective brain function and plasticity. Am J Biochem Biotechnol 4(2):167–176
Andreazza AC, Shao L, Wang JF, Young LT (2010) Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry 67(4):360–368
Anglin RE, Garside SL, Tarnopolsky MA, Mazurek MF, Rosebush PI (2012a) The psychiatric manifestations of mitochondrial disorders: a case and review of the literature. J Clin Psychiatry 73(4):506–512
Anglin RE, Mazurek MF, Tarnopolsky MA, Rosebush PI (2012b) The mitochondrial genome and psychiatric illness. Am J Med Genet B Neuropsychiatr Genet 159B(7):749–759
Bloch Y, Applebaum J, Osher Y, Amar S, Azab AN, Agam G et al (2012) Normobaric hyperoxia treatment of schizophrenia. J Clin Psychopharmacol 32(4):525–530
Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE (2005) Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol 57(5):695–703
Bubber P, Hartounian V, Gibson GE, Blass JP (2011) Abnormalities in the tricarboxylic acid (TCA) cycle in the brains of schizophrenia patients. Eur Neuropsychopharmacol 21(3):254–260
Burke MG, Miller MD (2011) Practical guidelines for evaluating lead exposure in children with mental health conditions: molecular effects and clinical implications. Postgrad Med 123(1):160–168
Calabrese V, Lodi R, Tonon C, D’Agata V, Sapienza M, Scapagnini G et al (2005) Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci 233(1–2):145–162
Castro-Gago M, Blanco-Barca O, Gomez-Lado C, Pintos-Martinez E, Campos-Gonzalez Y, Eiris-Punal J (2008) Association between autistic spectrum and mitochondrial pathology. Rev Neurol 47(1):52–53
Cavelier L, Jazin EE, Eriksson I, Prince J, Bave U, Oreland L et al (1995) Decreased cytochrome-c oxidase activity and lack of age-related accumulation of mitochondrial DNA deletions in the brains of schizophrenics. Genomics 29(1):217–224
Chauhan A, Chauhan V, Brown WT (eds) (2010) Autism: oxidative stress, inflammation, and immune abnormalities. CRC Press, Boca Raton
Chen H, Chan DC (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum Mol Genet 18(R2):R169–R176
Chez MG, Buchanan CP, Aimonovitch MC, Becker M, Schaefer K, Black C et al (2002) Double-blind, placebo-controlled study of l-carnosine supplementation in children with autistic spectrum disorders. J Child Neurol 17(11):833–837
Clay HB, Sillivan S, Konradi C (2011) Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci 29(3):311–324
Condie J, Goldstein J, Wainwright MS (2010) Acquired microcephaly, regression of milestones, mitochondrial dysfunction, and episodic rigidity in a 46, XY male with a de novo MECP2 gene mutation. J Child Neurol 25(5):633–636
Correia C, Coutinho AM, Diogo L, Grazina M, Marques C, Miguel T et al (2006) Brief report: high frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord 36(8):1137–1140
Cotter D, Guda P, Fahy E, Subramaniam S (2004) MitoProteome: mitochondrial protein sequence database and annotation system. Nucleic Acids Res 32(Database issue):D463–D467
Cox CE (2012) Persistent systemic inflammation in chronic critical illness. Respir Care 57(6):859–864, discussion 864–856
Cuturic M, Abramson RK, Moran RR, Hardin JW (2011) Carnitine and metabolic correlates in hospitalized psychiatric patients: a follow-through report. J Psychiatr Pract 17(1):35–40
Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK et al (2004) Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry 61(5):450–458
Dai DF, Rabinovitch PS, Ungvari Z (2012) Mitochondria and cardiovascular aging. Circ Res 110(8):1109–1124
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9(1):46–56
DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348(26):2656–2668
Dolske MC, Spollen J, McKay S, Lancashire E, Tolbert L (1993) A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog Neuropsychopharmacol Biol Psychiatry 17(5):765–774
Enns GM (2003) The contribution of mitochondria to common disorders. Mol Genet Metab 80(1–2):11–26
Ezugha H, Goldenthal M, Valencia I, Anderson CE, Legido A, Marks H (2010) 5q14.3 deletion manifesting as mitochondrial disease and autism: case report. J Child Neurol 25(10):1232–1235
Fahmy SF, El-hamamsy MH, Zaki OK, Badary OA (2013) l-Carnitine supplementation improves the behavioral symptoms in autistic children. Res Autism Spectr Disord 7(1):159–166
Fattal O, Link J, Quinn K, Cohen BH, Franco K (2007) Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr 12(6):429–438
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322(1–2):254–262
Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M et al (1997) GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol 273(1 Pt 1):G7–G17
Fernandez-Checa JC, Garcia-Ruiz C, Colell A, Morales A, Mari M, Miranda M et al (1998) Oxidative stress: role of mitochondria and protection by glutathione. Biofactors 8(1–2):7–11
Filiano JJ, Goldenthal MJ, Rhodes CH, Marin-Garcia J (2002) Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol 17(6):435–439
Filipek PA, Juranek J, Smith M, Mays LZ, Ramos ER, Bocian M et al (2003) Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol 53(6):801–804
Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ (2004) Relative carnitine deficiency in autism. J Autism Dev Disord 34(6):615–623
Finsterer J (2006) Central nervous system manifestations of mitochondrial disorders. Acta Neurol Scand 114(4):217–238
Finsterer J (2009) Mitochondrial disorders, cognitive impairment and dementia. J Neurol Sci 283(1–2):143–148
Forester BP, Berlow YA, Harper DG, Jensen JE, Lange N, Froimowitz MP et al (2010) Age-related changes in brain energetics and phospholipid metabolism. NMR Biomed 23(3):242–250
Forester BP, Zuo CS, Ravichandran C, Harper DG, Du F, Kim S et al (2012) Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. J Geriatr Psychiatry Neurol 25(1):43–50
Freedenfeld SH, Hamada K, Audhya T, Adams JB (2011) Biochemical effects of ribose and NADH therapy in children with autism. Autism Insights 3:3–13
Frye RE (2009) 15q11.2-13 duplication, mitochondrial dysfunction, and developmental disorders. J Child Neurol 24(10):1316–1320
Frye RE (2012a) Mitochondrial disease in 22q13 duplication syndrome. J Child Neurol 27(7):942–949
Frye RE (2012b) Novel mitochondrial Cytochrome b gene polymorphisms associated with autism. J Pediatr Neurol 10:35–40
Frye RE, Naviaux RK (2011) Complex IV overactivity in regressive autism. J Pediatr Neurol 9:427–434
Frye RE, Rossignol DA (2011) Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res 69((5 Pt 2)):41R–47R
Gargus JJ, Imtiaz F (2008) Mitochondrial energy-deficient endophenotype in autism. Am J Biochem Biotech 4(2):198–207
Gargus JJ, Lerner MA (1997) Familial autism with primary carnitine deficiency, sudden death, hypotonia and hypochromic anemia. Am J Human Genet 61:A98
Geier DA, Kern JK, Davis G, King PG, Adams JB, Young JL et al (2011) A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med Sci Monit 17(6):PI15–PI23
Gibson JH, Slobedman B, Harikrishnan KN, Williamson SL, Minchenko D, El-Osta A et al (2010) Downstream targets of methyl CpG binding protein 2 and their abnormal expression in the frontal cortex of the human Rett syndrome brain. BMC Neurosci 11:53
Graf WD, Marin-Garcia J, Gao HG, Pizzo S, Naviaux RK, Markusic D et al (2000) Autism associated with the mitochondrial DNA G8363A transfer RNA(Lys) mutation. J Child Neurol 15(6):357–361
Grosser E, Hirt U, Janc OA, Menzfeld C, Fischer M, Kempkes B et al (2012) Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiol Dis 48(1):102–114
Haas RH (2010) Autism and mitochondrial disease. Dev Disabil Res Rev 16(2):144–153
Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N et al (2007) Mitochondrial disease: a practical approach for primary care physicians. Pediatrics 120(6):1326–1333
Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, Herzenberg LA et al (2012) A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry 71(11):956–961
Harrison PJ, Weinberger DR (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10(1):40–68; image 45
Hopkins SE, Somoza A, Gilbert DL (2010) Rare autosomal dominant POLG1 mutation in a family with metabolic strokes, posterior column spinal degeneration, and multi-endocrine disease. J Child Neurol 25(6):752–756
Inczedy-Farkas G, Remenyi V, Gal A, Varga Z, Balla P, Udvardy-Meszaros A et al (2012) Psychiatric symptoms of patients with primary mitochondrial DNA disorders. Behav Brain Funct 8:9
Iwamoto K, Bundo M, Kato T (2005) Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet 14(2):241–253
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW et al (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80(6):1611–1617
James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O et al (2009a) Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr 89(1):425–430
James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O et al (2009b) Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J 23(8):2374–2383
Jou SH, Chiu NY, Liu CS (2009) Mitochondrial dysfunction and psychiatric disorders. Chang Gung Med J 32(4):370–379
Ju Seok R, Sook Joung L, In Young S, Tae Sung K, Han Ik Y (2009) Depressive episode with catatonic features in a case of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). J Child Neurol 24(10):1307–1309
Kato T, Takahashi Y (1996) Deletion of leukocyte mitochondrial DNA in bipolar disorder. J Affect Disord 37(2–3):67–73
Kato C, Umekage T, Tochigi M, Otowa T, Hibino H, Ohtani T et al (2004) Mitochondrial DNA polymorphisms and extraversion. Am J Med Genet B Neuropsychiatr Genet 128B(1):76–79
Kato M, Nakamura M, Ichiba M, Tomiyasu A, Shimo H, Higuchi I, Ueno S, Sano A (2011) Mitochondrial DNA deletion mutations in patients with neuropsychiatric symptoms. Neurosci Res 69(4):331–336
Kaufman KR, Zuber N, Rueda-Lara MA, Tobia A (2010) MELAS with recurrent complex partial seizures, nonconvulsive status epilepticus, psychosis, and behavioral disturbances: case analysis with literature review. Epilepsy Behav 18(4):494–497
Kleinman JE, Law AJ, Lipska BK, Hyde TM, Ellis JK, Harrison PJ et al (2011) Genetic neuropathology of schizophrenia: new approaches to an old question and new uses for postmortem human brains. Biol Psychiatry 69(2):140–145
Koene S, Kozicz TL, Rodenburg RJ, Verhaak CM, de Vries MC, Wortmann S et al (2009) Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J Affect Disord 114(1–3):327–332
Komulainen T, Hinttala R, Karppa M, Pajunen L, Finnila S, Tuominen H et al (2010) POLG1 p.R722H mutation associated with multiple mtDNA deletions and a neurological phenotype. BMC Neurol 10:29
Lacey CJ, Salzberg MR (2008) Obsessive-compulsive disorder with mitochondrial disease. Psychosomatics 49(6):540–542
Lambert AJ, Brand MD (2004) Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382(Pt 2):511–517
Laszlo A, Horvath E, Eck E, Fekete M (1994) Serum serotonin, lactate and pyruvate levels in infantile autistic children. Clin Chim Acta 229(1–2):205–207
Li Z, Okamoto K, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119(6):873–887
Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF (2002) High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet 11(2):133–145
Mancuso M, Ricci G, Choub A, Filosto M, DiMauro S, Davidzon G et al (2008) Autosomal dominant psychiatric disorders and mitochondrial DNA multiple deletions: report of a family. J Affect Disord 106(1–2):173–177
Mancuso M, Orsucci D, Ienco EC, Pini E, Choub A, Siciliano G (2013) Psychiatric involvement in adult patients with mitochondrial disease. Neurol Sci 34(1):71–74
Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH (2006) Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15(9):1437–1449
Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M et al (2012) Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci 13(5):293–307
Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E et al (2011) Mitochondrial alterations and neuropsychiatric disorders. Curr Med Chem 18(30):4715–4721
Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E et al (2012) Psychiatric disorders and mitochondrial dysfunctions. Eur Rev Med Pharmacol Sci 16(2):270–275
Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ, Filiano JJ, Sarnat HB (1999) Skeletal muscle mitochondrial defects in nonspecific neurologic disorders. Pediatr Neurol 21(2):538–542
Mattson MP (2007) Mitochondrial regulation of neuronal plasticity. Neurochem Res 32(4–5):707–715
Mattson MP, Liu D (2002) Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med 2(2):215–231
Mostafa GA, El-Gamal HA, El-Wakkad ASE, El-Shorbagy OE, Hamza MM (2005) Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatr 2(2):179–188
Munnich A, Rustin P (2001) Clinical spectrum and diagnosis of mitochondrial disorders. Am J Med Genet 106(1):4–17
Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, Sakaguchi D et al (2012) Mitochondrial dysfunction in Pten Haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PLoS One 7(8):e42504
Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H et al (2012) Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res 2012:696215
Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11(6):851–876
Nissenkorn A, Zeharia A, Lev D, Watemberg N, Fattal-Valevski A, Barash V et al (2000) Neurologic presentations of mitochondrial disorders. J Child Neurol 15(1):44–48
Oliveira G, Diogo L, Grazina M, Garcia P, Ataide A, Marques C et al (2005) Mitochondrial dysfunction in autism spectrum disorders: a population-based study. Dev Med Child Neurol 47(3):185–189
Oliveira G, Ataide A, Marques C, Miguel TS, Coutinho AM, Mota-Vieira L et al (2007) Epidemiology of autism spectrum disorder in Portugal: prevalence, clinical characterization, and medical conditions. Dev Med Child Neurol 49(10):726–733
Orhan N, Kucukali CI, Cakir U, Seker N, Aydin M (2012) Genetic variants in nuclear-encoded mitochondrial proteins are associated with oxidative stress in obsessive compulsive disorders. J Psychiatr Res 46(2):212–218
Pagano G, Castello G (2012) Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol 724:291–299
Pallardo FV, Lloret A, Lebel M, d’Ischia M, Cogger VC, Le Couteur DG et al (2010) Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology 11(4):401–419
Pancrudo J, Shanske S, Coku J, Lu J, Mardach R, Akman O et al (2007) Mitochondrial myopathy associated with a novel mutation in mtDNA. Neuromuscul Disord 17(8):651–654
Pastural E, Ritchie S, Lu Y, Jin W, Kavianpour A, Khine Su-Myat K et al (2009) Novel plasma phospholipid biomarkers of autism: mitochondrial dysfunction as a putative causative mechanism. Prostaglandins Leukot Essent Fatty Acids 81(4):253–264
Poling JS, Frye RE, Shoffner J, Zimmerman AW (2006) Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol 21(2):170–172
Pons R, Andreu AL, Checcarelli N, Vila MR, Engelstad K, Sue CM et al (2004) Mitochondrial DNA abnormalities and autistic spectrum disorders. J Pediatr 144(1):81–85
Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL (2009) Mitochondrial dysfunction and psychiatric disorders. Neurochem Res 34(6):1021–1029
Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EM, Doorn JA et al (2009) Nitrative and oxidative stress in toxicology and disease. Toxicol Sci 112(1):4–16
Rollins B, Martin MV, Sequeira PA, Moon EA, Morgan LZ, Watson SJ et al (2009) Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS One 4(3):e4913
Rossignol DA (2009) Novel and emerging treatments for autism spectrum disorders: a systematic review. Ann Clin Psychiatry 21(4):213–236
Rossignol DA, Bradstreet JJ (2008) Evidence of mitochondrial dysfunction in autism and implications for treatment. Am J Biochem Biotech 4(2):208–217
Rossignol DA, Frye RE (2011) Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry 17(3):290–314
Rossignol DA, Frye RE (2012) A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry 17(4):389–401
Salsano E, Giovagnoli AR, Morandi L, Maccagnano C, Lamantea E, Marchesi C et al (2011) Mitochondrial dementia: a sporadic case of progressive cognitive and behavioral decline with hearing loss due to the rare m.3291 T > C MELAS mutation. J Neurol Sci 300(1–2):165–168
Scaglia F (2010) The role of mitochondrial dysfunction in psychiatric disease. Dev Disabil Res Rev 16(2):136–143
Scaglia F, Zhang S, Tan Z, Fouladi N, Schmitt E, Wong L-J (2009) Prevalence of autism spectrum disorders in subjects with definite diagnosis of mitochondrial cytopathies. In: The American Society of Human Genetics 59th annual meeting. Honolulu, p 22
Schreiber H (2012) Pilot study on executive function and adaptive skills in adolescents and young adults with mitochondrial disease. J Child Neurol 27(12):1506–1516
Schuelke M, Krude H, Finckh B, Mayatepek E, Janssen A, Schmelz M et al (2002) Septo-optic dysplasia associated with a new mitochondrial cytochrome b mutation. Ann Neurol 51(3):388–392
Sequeira A, Martin MV, Rollins B, Moon EA, Bunney WE, Macciardi F et al (2012) Mitochondrial mutations and polymorphisms in psychiatric disorders. Front Genet 3:103
Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM et al (2008) Mitochondrial involvement in psychiatric disorders. Ann Med 40(4):281–295
Shinkai T, Nakashima M, Ohmori O, Terao T, Nakamura J, Hiramatsu N et al (2000) Coenzyme Q10 improves psychiatric symptoms in adult-onset mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes: a case report. Aust N Z J Psychiatry 34(6):1034–1035
Shoffner J, Hyams L, Langley GN, Cossette S, Mylacraine L, Dale J et al (2010) Fever plus mitochondrial disease could be risk factors for autistic regression. J Child Neurol 25(4):429–434
Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA et al (1995) Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 273(12):942–947
Su H, Fan W, Coskun PE, Vesa J, Gold JA, Jiang YH et al (2011) Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neurosci Lett 487(2):129–133
Triggs WJ, Roe CR, Rhead WJ, Hanson SK, Lin SN, Willmore LJ (1992) Neuropsychiatric manifestations of defect in mitochondrial beta oxidation response to riboflavin. J Neurol Neurosurg Psychiatry 55(3):209–211
Trushina E, McMurray CT (2007) Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 145(4):1233–1248
Tsao CY, Mendell JR (2007) Autistic disorder in 2 children with mitochondrial disorders. J Child Neurol 22(9):1121–1123
Verge B, Alonso Y, Valero J, Miralles C, Vilella E, Martorell L (2011) Mitochondrial DNA (mtDNA) and schizophrenia. Eur Psychiatry 26(1):45–56
Verge B, Alonso Y, Miralles C, Valero J, Vilella E, Boles RG et al (2012) New evidence for the involvement of mitochondrial inheritance in schizophrenia: results from a cross-sectional study evaluating the risk of illness in relatives of schizophrenia patients. J Clin Psychiatry 73(5):684–690
Vos M, Lauwers E, Verstreken P (2010) Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci 2:139
Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y et al (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105(49):19318–19323
Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL et al (2008) Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS One 3(11):e3815
Young TM, Blakely EL, Swalwell H, Carter JE, Kartsounis LD, O’Donovan DG et al (2010) Mitochondrial transfer RNA(Phe) mutation associated with a progressive neurodegenerative disorder characterized by psychiatric disturbance, dementia, and akinesia-rigidity. Arch Neurol 67(11):1399–1402
Zeviani M, Bertagnolio B, Uziel G (1996) Neurological presentations of mitochondrial diseases. J Inherit Metab Dis 19(4):504–520
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Rossignol, D.A., Frye, R.E. (2015). Mitochondrial Dysfunction in Psychiatric Disorders. In: Dietrich-Muszalska, A., Chauhan, V., Grignon, S. (eds) Studies on Psychiatric Disorders. Oxidative Stress in Applied Basic Research and Clinical Practice. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-0440-2_12
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0440-2_12
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-0439-6
Online ISBN: 978-1-4939-0440-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)