Abstract
This chapter will provide an overview of the major legislative and technology developments related to SCR for mobile applications. Regulatory initiatives are moving forward in all markets in the light-duty (LD), heavy-duty (HD), and non-road (NR) (agriculture and construction) sectors. NOx regulations are quite tight, forcing deNOx aftertreatment in all US LD diesel applications, and most HD and NR applications in the US, Europe, and Japan. CO2 regulations are also tightening worldwide, driving deNOx technologies on diesel engines. Knowledge of engine technologies will help define required deNOx aftertreatment needs. In the HD sector, because of the fuel consumption versus NOx trade-off, deNOx means deCO2. As such, 98 % selective catalytic reduction (SCR) deNOx to result in elimination of EGR (exhaust gas recirculation) and minimum operating cost and CO2 emissions is desired. The situation is similar on the LD side. Once SCR is added to the vehicle to meet the NOx regulations, it will likely be used to maximum efficiency in the context of consumer acceptance of ammonia consumption and replacement. On-board ammonia delivery systems for the SCR catalyst principally comprises liquid urea and gaseous ammonia types. Liquid ammonia systems are in their third generation of cost reduction and simplification. The gaseous ammonia system is attractive in LD applications due to the heavy weighting of cold start emissions in the regulations, and the ability to deliver ammonia at low exhaust temperatures. The field of catalysts, including oxidation catalysts, SCR catalysts, and ammonia slip catalysts is very dynamic. Oxidation catalysts are used to generate NO2 for better low-temperature performance of certain SCR catalysts (like Fe-zeolites). However, they can have undesirable N2O emissions, and NO2 production decreases with palladium content, when replacing platinum. Zeolite SCR catalysts are evolving beyond the copper and iron varieties into other formulations with better low-temperature and/or high-temperature performance. Vanadia catalysts have made recent impressive gains in high-temperature durability. High-efficient SCR systems generally are run with excess ammonia. Ammonia slip catalysts (ASC) are becoming more selective towards nitrogen, minimizing N2O, and NOx by-product formation. Finally, system design considerations are discussed, as well as emerging technologies. The author closes with comments on the future outlook and opportunities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Selective Catalytic Reduction
- Selective Catalytic Reduction Catalyst
- Diesel Oxidation Catalyst
- Selective Catalytic Reduction System
- Selective Catalytic Reduction Performance
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
NOx is formed when air is heated to very high temperatures, and is thus emitted from combustion and engines. The most prevalent NOx species from engines is NO. It will oxidize in the atmosphere to form NO2, and also react with many hydrocarbons (HCs) to form ozone; both ozone and NO2 are toxic and strong oxidants. Thus, NOx is a criteria pollutant and is regulated. NOx is very effectively controlled from gasoline engines with three-way catalysts (TWCs) (CO, HCs, NOx), but they only operate under stoichiometric conditions. For lean diesel conditions, selective catalytic reduction (SCR) is the leading method of remediation. The reductant, ammonia (NH3), which needs to be added to the exhaust, selectively reduces the NOx rather than being oxidized by the excess oxygen, as do the innate exhaust reductants, CO and HCs.
This chapter will set the stage for the other chapters in the book by providing a representative review of the regulations, general engine trends, and key developments in SCR catalyst technology. It is not intended to be all-encompassing and comprehensive. Representative papers and presentations were chosen here that provide examples of new, key developments, and direction. For a more detailed review of SCR technologies, and diesel emission control technology trends and developments in general, the reader is referred to Johnson [1–3].
2 Regulatory Overview
Although the first commercial lean deNOx system was a lean NOx trap (LNT) on the European Toyota Avensis in the early 2000s, and then on the US Dodge Ram truck (Cummins engine) in 2007, the first wide-scale use of deNOx was the implementation of SCR for heavy-duty (HD) truck applications in Europe in 2005. The US Tier 2 and California Low Emission Vehicle (LEVII) regulations were the first to force SCR on light-duty (LD) applications in 2007. SCR did not make its way into NR applications until 2011 in both the US and Europe.
Following is a general overview of the HD, LD, and NR regulations pertinent to understanding the main drivers for SCR systems.
2.1 Heavy-Duty Truck Regulations
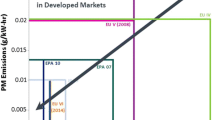
Figure 1.1 shows a summary of the key HD truck regulations in the world, along with estimates of the best commercially viable engine-out NOx and particulate matter (PM) capabilities, as measured on the European Steady-State Cycle (ESC). The first vehicle regulation in the world that was attained with SCR systems was the Japan 2005 HD truck regulation in October 2004, shortly followed by Euro IV in January 2005. Although Euro IV was only a 30 % NOx tightening from Euro III (2000), the PM regulation dropped ~80 %, and truck manufacturers generally elected to tune their engines for higher NOx and lower PM and fuel consumption, and then use SCR to drop the tailpipe levels to within the NOx (and PM) requirements. It is interesting to note that although the US2007 NOx regulations were 60 % lower than for Euro IV, and the PM regulations were about 35–55 % tighter (steady state and transient testing, respectively), the US manufacturers chose to meet the NOx regulations with engine technology (mainly exhaust gas recirculation (EGR)), and the PM regulations with diesel particulate filters (DPFs). The Japan 2005 regulation is intermediate between Europe and the US for both NOx and PM, and there was a split of approaches used in Japan, with trucks in high fuel consumption applications generally using a European SCR-only approach, and all others using a EGR + DPF approach.
In the 2009+ time frame, Japan 2009, US2010, and Euro VI (2013) all require both SCR and DPF solutions. These regulations range from 0.26 to 0.7 g NOx/kW-h and 0.010 to 0.013 g PM/kW-h.
2.2 Light-Duty Regulations
The leading LD diesel non-methane HC and NOx regulations are graphically shown in Fig. 1.2. Only the US (Federal and California LEVIII) has the test-cycle and limit value combination to force NOx aftertreatment. All require a DPF (regulations not shown). By 2013, perhaps a dozen diesel models will be on the US market. However, the majority of Euro 6 applications will have NOx aftertreatment to minimize NO2 emissions and fuel consumption.
One regulatory development that will drive SCR system design in Europe and elsewhere is emerging now in Europe: Real-World Driving Emissions (RDE). Investigators have found that NOx emissions from LD diesels can be 3–4X higher than the laboratory certification level. It is too early to note the details of these regulations, but they are likely to include portable emissions monitoring systems (PEMS) and require advance controls for cold start, high speed, and load conditions.
The regulatory trend in the US, Europe, and Japan is for very low-NOx emissions. All HD trucks in these markets will have SCR systems by early 2014. These regulations are migrating into the NR machine sector, wherein most engines >70 kW will have SCR systems in 2014+. For passenger cars, all but the smaller diesels will have SCR systems in the three markets. Tighter regulations are foreseen for the developing markets, with Brazil leading the way, followed by China and India.
3 Engine Developments
This section will summarize HD and LD diesel engine technologies. We may see lean-burn gasoline engines in the market, and SCR is a viable option for these engines, but this is beyond the scope of this summary.
3.1 Heavy-Duty Engines
HD engine technology is in development to meet the next round of OBD (onboard diagnostics) tightening in the US for 2013 and the new CO2 regulations in 2014. Concurrent with this, the Euro VI regulations come into play in 2013–2014.
Stanton [4] shows in Fig. 1.3 that the most effective engine means for reducing NOx, EGR is an efficient approach and can have fuel consumption benefits if SCR efficiency requires low engine-out NOx levels (<2.5–5 g/kW-h NOx) in low-load operating regimes. However, if increased SCR deNOx efficiency allows higher NOx levels, EGR results in a fuel penalty. Given this, Stanton estimates that if SCR can attain a 98 % cycle-average deNOx efficiency, EGR can be eliminated. Furthermore, as shown in Fig. 1.3, running at higher engine-out NOx can return substantial fuel consumption benefits. In the high-NOx regimes, about 1 % fuel can be saved for every 1.2–1.5 % urea consumed (relative to fuel) to drop the NOx. This is beneficial for both CO2 reductions and fluid cost savings (urea plus fuel).
EGR can have fuel consumption benefits at low engine-out NOx levels (<4 g/bhp-h or 5.2 g/kW-h), but at higher levels there is a fuel penalty versus SCR approaches with high deNOx efficiency [4]
Roberts [5] described some HD technologies for both high- and low-engine-out NOx approaches. A summary is shown in Fig. 1.4, wherein each point represents an engine hardware configuration that is optimized for low fuel consumption. As with previous such descriptions of advance engine technology packages [6], fuel consumption decreases as NOx increases, even out at >5 g NOx/kW-h. Roberts shows minimum fluid operating costs (top line) at 8–11 g/kW-h engine-out NOx. He assumes here that the urea (Diesel Exhaust Fluid (DEF)) is 65 % the cost of fuel. Emission control technologies (like SCR) would be needed to achieve at least 97–98 % efficiency to achieve this minimum fluid-consumption-cost calibration range, to meet the US 2010 regulations.
General advance HD engine technologies and the resultant urea (DEF) and fuel cost curves, assuming DEF costs $2.56 per gallon ($0.69/l) and diesel fuel cost $3.89/gallon ($1.05/l). Fluid costs are minimized at 8–11 g/kW-h NOx [5]
Zybell [7] also described some HD technology packages for meeting low emissions and fuel consumption, but mainly in the context of fuel injection technology. His slopes of fuel consumption versus NOx are not as steep as shown in Figs. 1.3 and 1.4, so his minimum cost range is in the 3–5 g/kW-h NOx range. However, when fuel injection pressure is increased from 1,800 to 2,400 bar, the fluid consumption drops about 0.6 % and the minimum calibration shifts to 2.5–4.0 g/kW-h NOx. Continuing the trend, if injection pressure is increased to 3,000 bar, fluid consumption drops another 0.1 % and the minimum point shifts to 2.0–3.0 g/kW-h NOx.
Kobayashi et al. [8] gave a detailed account of their attempt to drop engine-out NOx to 0.2 g/kW-h on a 10.5 l engine with the following features: 2,000 bar common rail fuel injection system, low-pressure (LP) and high-pressure (HP) EGR, variable valve actuation, 300 bar peak cylinder pressure, variable swirl, and advanced combustion chamber design. With a DPF, the engine achieved 0.8 g/kW-h NOx on the JE05 Japanese HD transient cycle. At 1,200 RPM and 8 bar BMEP, substituting about 40–70 % LP-EGR instead of HP-EGR results in similar NOx levels, despite 5–10 % higher total EGR rates, but with greatly reduced PM and fuel consumption. Also striving for high-efficiency and low-NOx, Ojeda [9] reported that a prototype 13-l engine with 2-stage EGR cooling, 2-stage turbocharging, a 2,200 bar injection system, and optimized combustion system achieved 45 % BTE at road loads, with a 0.5 g/bhp-h NOx (0.65 g/kW-h) NOx level. This is higher efficiency than some 2,010 engines running with SCR at much higher NOx levels. Although impressive, these studies show that achieving the engine-out NOx levels required to meet the emerging tailpipe regulations, without NOx aftertreatment is quite difficult and could be very expensive.
Improved thermal management is increasing in importance, especially as it pertains to reducing urban NOx from engines with SCR. The first evidence that this issue is being addressed on Euro VI engines was reported by Vermeulen et al. [10]. The 13-l prototype Scania engine had cooled-EGR to reduce low-load NOx and intake throttling for thermal management. NOx in-service conformity (ISC) was well below the 1.5X limit after allowable calibration adjustments, and NOx emissions generally vary from 0.35 to 0.76 g/kW-h for most trips and segments. The SCR system was fully functional after 500 s of operation after a cold start at 3 °C.
Finally, US HD engine manufacturers described their future approaches to meeting the US Department of Energy (DOE) goal of demonstrating 50 % BTE (break thermal efficiency) on a HD engine [1]. All four US HD truck engine manufacturers get much of their efficiency improvements from combustion (chamber design, control, mixing, etc.), reduction of friction and parasitic losses, and Rankine cycle waste heat recovery (WHR). Improved SCR performance is also mentioned commonly (for higher NOx calibrations).
Although HD NOx regulations might be met by further advancements in engine technology, the best balance of low fuel consumption and low tailpipe NOx emissions requires about 98+ % efficient SCR.
3.2 Light-Duty Diesel Engines
LD diesel engines are also improving to keep the efficiency advantage over gasoline. Pischinger [11] described future technologies for both diesel and gasoline engines to achieve 35 % CO2 reductions. Major improvements in both platforms include 25 % downsizing (7 % reductions), stop–start system (6 %), LP-EGR (3 %), and down-speeding (3 %). The approach can result in lower exhaust temperatures due to more turbocharging, and higher NOx as the result of the same fuel amount burned in a smaller cylinder. However, for gasoline vehicles to meet the 95 g/km fleet average CO2 emission requirement in Europe in 2020, Pischinger projects significant hybridization is needed. Diesel engines can meet the regulation with standard drivetrains.
In the US, to meet the tight LEVIII emissions, reduced cold start emissions are the key, requiring significant thermal management methods. Popuri et al. [12] use an intake throttle, bypass valves for the EGR, turbine, and LP-VGT (variable gate turbocharger), idle speed modulation, late cycle fuel injections, cylinder deactivation (fueling cut off), and an exhaust-manifold integrated diesel oxidation catalyst (DOC) to allow urea injection 125 s, earlier than for a baseline engine. Despite that the engine-out NOx increased 20 %, and fuel consumption increased 5–7 % when the methods are used, Federal Test Procedure (FTP) Bag 1 deNOx was an impressive 70 % and overall fuel efficiency increased 25 % compared to their baseline engine. A 4.5 l engine in a 5,000 pound (2,270 kg) vehicle achieved US Tier 2 Bin 5 standards at 25.5 MPG (9.1 l/100 km). Ruth [13] reported significant progress in the same program that reduces thermal management requirements and is now targeted to meet the LEVIII fleet average requirements (−70 % vs. Tier 2 Bin 5) by using a passive NOx adsorber (PNA) that adsorbs NOx at low temperature and releases it passively as temperature increases, a combination SCR + DPF (SCR catalyst coated onto a DPF), and gaseous NH3 injection. The progress shows how critical advanced SCR technology is to improve feasibility of clean LD diesel engines.
Another LEVIII approach was reported by Balland et al. [14]. To address the challenge SCR and other deNOx systems have in reducing high-load NOx, the investigators report that a standard DOC can efficiently remove NOx at stoichiometry, and thus run the engine in that mode during accelerations. The approach requires tight control of EGR, the turbocharger, and other engine parameters, and uses an “air-based control” approach similar to that of gasoline engines, rather than a fuel-based approach typical of diesel engines. Exhaust temperatures also increase substantially in the accelerations and the strategy is part of cold start thermal management.
Diesel engine costs have been a problem in competing with modern gasoline engines. Regner et al. [15] are updating the opposed-piston diesel engine, solving the historic problems using new materials and modern analytical techniques. Because it has no head or valve train, compared to a standard diesel engine, it has 40 % fewer parts, is 30 % lighter, and costs about 10 % less. Fuel consumption is 15–20 % lower than a state of the art 6.7 l diesel engine, but lube oil consumption and NOx emissions are about double.
Contrary to HD applications, wherein deNOx improvements are used to reduce fuel consumption and 98 % deNOx is desired, modern LD diesel engines do not have as strong a relationship between NOx and fuel consumption at the higher NOx levels. In the US, all of the available deNOx efficiency will be used to meet the LD NOx regulations. In Europe, deNOx efficiencies of 50–70 % are needed to meet the regulations in the most efficient means.
Lean NOx control (lean deNOx) technologies will be integral to meeting the emerging HD criteria pollutant regulations for diesel engines. Minimum removal efficiencies on the order of 85 % will be needed, but levels up to 97–98 % are desired to allow engines to operate in high-NOx low-fuel consumption regimes. For LD applications, the efficiency is as important in the US, but light-off or low-temperature performance characteristics are even more so.
4 SCR Technologies
4.1 SCR System Introduction
The TWC is the most effective NOx reduction system on vehicles but requires the absence of oxygen. It has been in production for more than 30 years and is removing more than 99 % of the NOx from modern engines. In this system, unburned CO and HCs are used to reduce NOx on a rhodium catalyst. The key to this technology is the gas mixture control. It is critical to have a near-stoichiometric mixture, wherein the mixture of air and fuel are near-ideal and there is very little excess oxygen. If oxygen is present in the exhaust, the CO and HCs will react with it rather than with the NOx. Diesel engines are lean-burn with plenty of excess oxygen. Practical and effective catalysts for selectively reducing NOx with CO or HCs in a lean environment are not yet available, but selective catalysts using ammonia as the reductant have been commercialized in the stationary sector for decades.
The key SCR catalyst reactions are shown as
Reaction (1.1) is generally the “standard SCR reaction”. As NO2 is always present in the exhaust to some extent (maybe 10 % of NOx), Reaction (1.2) is also pertinent, and is in fact the fastest and preferred NOx reduction reaction. To promote this “fast SCR reaction” a DOC is commonly used to form more NO2 over platinum by the following reaction:
If too much NO2 is produced in the DOC, more than 1:1 = NO:NO2, then Reaction (1.3) becomes operative. This is undesirable because the “excess” NO2 can yield N2O, which is a strong greenhouse gas:
If the reactants are not well-mixed, if excess ammonia is injected to obtain high deNOx efficiencies, or if ammonia stored on the SCR catalyst is released too fast, ammonia emissions can occur. To remediate this, an ASC is utilized:
SCR technology is entering its third or fourth generation since commercial introduction in Europe in 2003. Then, systems were removing upwards of 75 % NOx over the European HD Transient Cycle to meet Euro IV regulations. To meet the US2010 and emerging Euro VI regulations in 2013, cycle-average deNOx efficiencies approaching 95 % is realized. Work is continuing in the US to go even higher in efficiency to meet the current and emerging LD NOx regulations. In both HD and LD applications, targets of 98 % test-cycle deNOx efficiency are in the scope for future systems.
To achieve this high level of efficiency, all aspects of the system need to be optimized. An SCR system will generally comprise an ammonia delivery system, and the catalyst system itself comprises the DOC (and typically DPF), SCR catalyst, and the ammonia slip catalyst.
Casarella [16] shows the layout of a typical diesel emission control system incorporating the DPF, Fig. 1.5. In the US, the urea solution is referred to as DEF. In Europe, it is referred to as “AdBlue”.
Layout of a DPF + SCR system [16]
In addition to the SCR catalyst system (DOC, SCR, ASC, mixer) and the DPF, the other main components of the system are the urea delivery system, comprising the storage tank (DEF tank) and sensors, the heated delivery line, pump, and dosing module, including the injector and mixer; and the control system, comprising the sensors, dosing control module, engine control module, and controller area network (CAN) buses.
The following sections will provide more details on these subsystems.
4.2 Urea Delivery System
The urea tank, injector, controls, and mixer are significantly engineered systems. Ostertag [17] provides an example of the tank design alone. Figure 1.6 shows the heat-up features and basic designs. Urea is corrosive, so material selection is limited to stainless steel and plastic. Because urea solution (32.5 % urea, balance deionized water) will freeze at −11 °C, the design has to allow for the 7 % expansion upon freezing and for rapid thawing to enable prompt use of the system. Further, internal components like heaters, level and temperature sensors, and fill and extraction lines need to be designed to withstand impact by solids in partially thawed systems. The design shown in Fig. 1.6 has all these components integrated into one unit (in yellow). Finally, and especially for NR applications, the draw point for liquid urea needs to accommodate different vehicle angles of operation.
Basic features of the heating system of a urea storage tank [17]
The urea pump, dosing module, and injectors play a critical role. Designs have migrated from separate pumps and dosing modules to integrated designs [18]; from air-assisted to airless injection; and to systems with no return line. Injectors are designed to disperse fine droplets (20–100 μm mean size) into the exhaust, while minimizing contact with the exhaust pipe to minimize solid by-product formation.
Currently, good extended low-temperature (<200 °C) SCR performance is limited by urea injection issues (evaporation and hydrolysis). Much of this is dependent on good mixing. Improved mixers allow urea injections at temperatures as low as 180 °C, and can result in NOx reductions of ~30 % over the US cold HD transient cycle relative to no mixer [19]. Alano et al. [20], describe a compact mixer that needs only 75 mm of urea mixing length, compared to 350 mm in some commercial LD SCR systems, enabling the SCR catalyst to be placed closer to the engine for faster heat-up. The mixer achieves a urea mixing index of 0.95 (all cross-section NH3 measurements are within 5 % of one another) over a range of gas flows, with a maximum increase in back pressure of 0.4 kPa (4 mbar) during accelerations relative to a conventional system. In the closer position, the SCR catalyst got up to 25 °C hotter and achieved 67 % deNOx efficiency on the NEDC versus 37 % for a catalyst placed further back.
To accomplish the same objective, urea hydrolysis catalysts are emerging. Kröcher et al. [21] show that upwards of eight different decomposition products are emitted from urea upon heating, but with a titania decomposition catalyst, ammonia is produced at temperatures as low as 150–160 °C in model gas with no other unexpected decomposition products.
Urea injection control can be quite complex, but it is migrating from open-loop control based on engine operating parameters and engine-out NOx predictions, to closed-loop control based on NOx or urea and temperature sensors. Good urea control also needs to consider the ammonia stored on the catalyst. This will be discussed in detail later.
4.3 Alternative Sources for Ammonia and Systems
Although the urea infrastructure is well developed in Europe, Japan, and the US, finding alternative sources for ammonia is still of significant interest to enable SCR catalysts to function better at low exhaust temperatures, decrease the size and cost of the system, and to enable use of the system at very low ambient temperatures. Johannessen [22] updated the developments on a gaseous ammonia system using chloride-based adsorbents. Both HD and LD systems were described showing 100X dosing ranges within 5 % accuracy and <1.5 % deviation in set-point, under a range of exhaust conditions. Start-up units initially draw 550 W in HD, and 250 W in LD applications, but go down to the 100 W range during normal operation. Safety and durability issues appear addressed, and system optimization through testing and simulation is continuing. A European consortia of automotive companies was recently formed to begin standardizing the system and exploring gaps [23]. In tests on the system in challenging low-load urban driving conditions, gaseous ammonia injections started at 100 °C, and 25–83 % deNOx efficiency was obtained, depending on the amount of ammonia stored in the catalyst. In other reagent studies, Thomas and Highfield [24] describe some early performance data with an ammonium formate and urea system containing 54 % water, versus 67.5 % for standard urea solutions. Advantages include reduced freezing point (−30 °C), better high-temperature storage stability, lower hydrolysis temperature, no polymerization like with urea (fewer or no deposits), and they demonstrated full “drop-in” capability in a urea system on a new diesel pickup truck with an SCR system.
4.4 DOC Overview
DOCs play two primary roles in commercial emission control systems:
-
(1)
Oxidize HCs and CO, either to reduce emissions coming from the engine, or to create exothermic heat used to regenerate a DPF.
-
(2)
Oxidize NO to NO2 (Reaction (1.4), above), which is used to continuously oxidize soot on a DPF, and for enhancing the fast SCR deNOx reactions (Reaction 1.2), particularly at low temperatures.
This section will focus on the role of DOCs in SCR systems.
Henry et al. [25] looked at the interplay of the above two functions by using a series of iterative reaction-decoupling experiments to explain interactions between HC and NO oxidation. They show that NO oxidation is inhibited on Pt/Pd due to the reduction reaction with NO2 by HCs. Long chain alkanes had a more adverse effect than short chain alkenes due to slower oxidation rate with oxygen. Decreasing space velocity was shown to help NO2 formation in the presence of HCs. Prestoring HCs on the DOC improved NO oxidation up to 300 °C. The interplay of CO and HC removal and NO2 generation takes another tack as well. Spurk et al. [26] investigated NO2 coming from a catalyzed DPF for use in a downstream SCR system. Surprisingly, they found the NO2 coming out of the DOC and going into the DPF is not as important as the HCs coming from the DOC. Essentially, the HCs going into the DPF can interfere with the NO2 formation in the DPF. The total DPF platinum loading is more important to NO2 formation than total precious metal loading on the DPF.
Jen et al. [27] showed that platinum can migrate from DOCs (or presumable DPFs) to SCR catalysts, if they are exposed to temperature greater than 670 °C for extended periods of time (16 h). SCR efficiencies can decrease, especially if the DOC is exposed to temperatures greater than 750 °C, as even minute quantities of platinum can cause oxidation of ammonia. These temperatures might be experienced during DPF thermal regenerations. Later, Cavataio et al. [28] showed that if palladium replaces some of the platinum in the DOC, less migration can occur. Although the 2:1 Pt:Pd mixture shows some deterioration in SCR deNOx efficiency, it is much worse for the Pt-only formulation. Washcoat formulation and/or processing can make a difference, and NO, HC, or CO oxidation is unaffected or enhanced with the Pd additions.
Kim et al. [29] did a systematic study on the effects of varying the Pt:Pd ratio on DOC HC and NO oxidation and durability in a variety of conditions. All bimetallic Pt–Pd catalysts show better HC light-off activity and thermal stability than the Pt- or Pd-only catalyst. NO oxidation to NO2 was found to always depend directly on platinum content, with similar durability trends as with HCs. Figure 1.7 shows a schematic representation of these findings. They found that HC–CO mixtures synergistically have ~20 °C lower light-off temperatures than either one alone.
Effect of increasing Pd mass fraction respect to Pt content in DOCs. Low Pd % does not significantly affect NO oxidation, but improves catalyst durability and HC oxidation (adapted from Kim et al. [29])
Going further in catalyst cost reduction, Wang et al. [30] developed an NO oxidation catalyst that does not use any precious metal. The manganite-based catalyst drops the optimum NO:NO2 = 1:1 oxidation temperature about 50–250 °C versus a platinum catalyst. Other manganite catalysts also exhibit CO and HC oxidation characteristics [31].
DOCs can be a major source of the strong greenhouse gas N2O. Glover and coworkers [32] also did a study on Pt:Pd effects on DOC properties, adding N2O formation and looking more at fundamentals. CO plays a key role on the overall catalyst performance by its positive effect on propylene oxidation which, in turn, is responsible for NO reduction to N2O and the onset of NO2 formation. On the Pt:Pd = 4:1 catalyst, propylene partially reduces NO to form N2O at about 200 °C, but this temperature shifts to 250 °C when CO is added. The effect of higher Pd concentration on NO conversion is detrimental for NO oxidation to NO2, but is positive for producing less N2O, especially at high oxygen concentrations. NOx storage and release may play an important role in NO2 formation over the lightly loaded full Pt DOC formulation studied. A 40 g/ft3 (1.4 g/l) bimetal formulation (Pt:Pd = 4:1) is comparable on CO and HC light-off to a 113 g/ft3 Pt formulation. Closing on N2O formation, Kamasamudram et al. [33] show propylene forms much more N2O than dodecane (C12H26).
4.5 SCR Catalysts
The heart of the SCR system is the SCR catalyst. Three general families of SCR catalysts are in commercial use today: Vanadia ,tungsten, copper zeolite, and iron zeolite. Walker [34] recently summarized the main characteristics of each of these catalysts.
Copper zeolite has the best low-temperature performance and its steady-state performance shows very little sensitivity to NO2 concentration. However, it is susceptible to sulfur poisoning and requires an occasional high-temperature cleaning step (>500 °C; Tang et al. [35]) to thermally remove the contaminates. Copper zeolites have markedly improved in the last couple of years, making them the preferred catalyst for high-performing systems.
Iron zeolite has the best HT temperature performance, but NO2 management of the inlet gas is needed for improved LT performance. As such, system precious metal usage on the DOC is higher. Fe-zeolites show no sulfur poisoning but moderate HC poisoning is observed, minimizing the HT cleaning step.
Vanadia is the cheapest of the catalysts, but has poor HT durability (deteriorates at 550–600 °C), and thus cannot be utilized in systems that have a DPF that requires active regeneration (T > 650 °C). As with iron zeolites, the LT performance strongly depends on NO2 availability.
Figure 1.8 shows the sensitivity of the three types of catalysts to the inlet NO2/NOx ratio at 200 °C, wherein copper zeolite shows superior LT deNOx performance with no or little NO2 in the gas. However, in a standard system with a DOC for HC control that will also oxidize NO to NO2 for optimum performance, vanadia performs similarly to copper zeolite in the temperature range of 225–275 °C, but iron zeolite is inferior. Copper zeolite almost as well as Fe zeolite are at temperatures higher than this.
Sensitivity of SCR catalyst deNOx performance inlet NO2 content at 200 °C [34]
SCR catalyst formulations and design are improving both low- and high-temperature performance through better dispersion of the cation in the zeolite and with much more durable zeolite structures. HC and sulfur poisoning effects are becoming better understood and controlled, for example, through better modeling of deterioration and more restrictive zeolite cages that keep most HCs from entering the catalyst structure.
Vanadia SCR catalysts are used in Europe, the emerging markets, and in some agricultural applications in the US, but not in Japan due to durability issues related to thermal exposure when DPFs are regenerated. Advances are now reported [36] on vanadate SCR catalysts that have no volatility up to 750 °C or higher, versus 550–600 °C for some commercial catalysts, giving them similar HT durability to zeolites. DeNOx performance at 250 and 350 °C is 5–10 points better after HT aging (>700 °C) than for a benchmarked commercial catalyst, but less severely aged catalysts have lower efficiencies than the base catalyst. Walker [37] reports that new Cu-zeolite formulations now sustain aging to 900 °C and form less N2O. Narula et al. [38] show that it is possible to modify zeolite structures systematically to influence the electron density at metal centers, and to provide ammonia bonding sites in the vicinity of the metal centers. They replaced alumina in the structure with several trivalent cations. In another contribution [39], they showed that chemical mixtures of copper and iron zeolites can improve LT performance over than copper alone, and when lanthanum is added to the binary formulation performance is improved further.
HC poisoning is an issue for both Cu- and Fe-zeolites. HCs can accumulate at the lower temperatures, and then ignite at the higher temperatures, causing thermal deterioration of the catalyst. Prikhodko et al. [40] quantified HC adsorption for state of the art Cu- and Fe-zeolites. They show that Fe-zeolite adsorb upwards of 5–10 times more HCs than Cu-zeolite, but the Cu-zeolite oxidizes a higher percentage upon release. The authors show that both types adsorb more HCs in PCCI combustion mode (premixed charge compression ignition) than in conventional diesel combustion mode due to changes in HC speciation. Cu-zeolites are more susceptible to both HC uptake and generation of oxidation exotherms. This is more significant for LD applications wherein PCCI mode is used more, and more time is spent at lower temperatures. Luo et al. [41] studied the effects of propylene (C3H6) and dodecane (n-C12H26) exposure on the SCR performance of two Cu-exchanged zeolite catalysts, one was a state of the art Cu-zeolite with relatively small pores (unspecified type), and the other was a standard Cu-BEA zeolite with somewhat larger pores. The small-pored sample was completely unaffected by dodecane at temperatures lower than 300 °C, and only slightly inhibited (less than 5 % conversion loss) by propylene. With the standard catalyst at 150 °C, no propylene inhibition was noted due to oxidation of the HC; but at 300 °C, both oxidation intermediates and coke formation led to deactivation. Dodecane inhibition was observed over the whole temperature range by strong HC adsorption blocking of pores and active catalyst sites. The small pores in the state of the art sample do not allow the diffusion of large HC molecules into the pores that hinder adsorption onto active sites. Han et al. [42] showed that low-temperature performance and reduced HC effects can be achieved if a ceria oxygen storage catalyst is layered on top of an iron zeolite catalyst. The catalyst helps urea decomposition, thus improving the low-temperature deNOx capability from 32 to 58 % at 200 °C an LD steady-state test. After 8 h of exposure to high HC levels from a burner, the layered catalyst maintained a deNOx efficiency of 80 % at 240 °C, while the original version was only at 60 % under the same conditions due to HC poisoning.
On sulfur poisoning, Tang et al. [35], used SO2 exposure levels equal to those obtained with ultra-low sulfur diesel fuel (<15 ppm sulfur), and show that copper zeolite catalyst started losing deNOx efficiency after about 400 h of operation at temperatures of 200–300 °C. These results are shown in Fig. 1.9. Through 1,300 h of operation, the catalyst had deteriorated continuously from 98 % deNOx efficiency to 60 % efficiency. Also, the NO2:NOx ratio from the filter deteriorated from 0.60 to 0.30 during the first 600 h, but then remained the same. They found that most of the sulfur was in the top layer of washcoat in the first third of the catalyst. Most of the poisoning was attributed to ammonium sulfate, which comes off at 400–500 °C, and to a much lesser extent, copper sulfate, which comes off at 500–850 °C. When heated to 500 °C, the SCR catalyst performance recovered, and this was done every 700 h of operation at the lower temperatures.
DOC + CSF begins deteriorating after about 250 h of operation in “deratd FTP” testing (200–350 °C) simulating fuel with 15 ppm sulfur. Four g/l sulfur is roughly 400 h of operation in the simulation that uses 35 ppm SO2. Cu-zeolite deteriorates after 400 h [35]
Regarding the SCR catalyst substrate support, today, SCR substrates generally have 300 and 400 cells/square inch (300- and 400-csi). Heibel [43] showed that in the mass transfer controlled regime (230–350 °C), 600-csi substrates react 35 % faster than 400-csi catalysts. Substrates with higher cell densities are being evaluated in advanced programs for improved deNOx performance.
SCR catalyst systems will age, and this needs to be understood for good performance over the full useful life of the catalysts. Bartley et al. [44] describe NH3 storage capacity measurement data as a function of SCR catalyst aging time and temperature. The researchers modeled the aging using first principle Langmuir adsorption isotherms. These data can be used in model-based control algorithms to calculate the current NH3 storage capacity of an SCR catalyst operating in the field, based on time and temperature history.
The US Environmental Protection Agency (EPA) capped nitrous oxide (N2O) emissions in both the LD and HD greenhouse gas rules, so much work is being done to understand its sources. Kamasamudram et al. [33] show that N2O is very stable, and forms by three mechanisms in an SCR catalyst:
-
LT (T < 250 °C) decomposition of ammonium nitrate by the reaction:
$$ {\text{NH}}_{ 4} {\text{NO}}_{ 3} \to {\text{N}}_{ 2} {\text{O}} + 2 {\text{H}}_{ 2} {\text{O}} $$(1.7) -
HT oxidation of ammonia by copper zeolites by the reaction:
$$ 2 {\text{NH}}_{ 3} + {\text{ 2O}}_{ 2} \to {\text{ N}}_{ 2} {\text{O}} + 3 {\text{H}}_{ 2} {\text{O}} $$(1.8) -
Reaction of excess NO2 (>50 % of NOx) to form ammonium nitrate by the reaction:
Ammonium nitrate then immediately decomposes by Reaction (1.7) at temperatures greater than 200 °C.
Kamasamudram et al. [33] show that increasing copper loading in the Cu-zeolite SCR catalyst can decrease N2O formation by the first two mechanisms, and better DOC design and control can prevent the third mechanism. The investigators show that it is possible to reduce N2O to nitrogen, but these reactions occur at much higher temperatures than those at which they are formed.
4.6 Ammonia Slip Catalysts
In high performing SCR systems, excess ammonia is injected to make sure ammonia and NOx are present together at the catalyst for high conversion efficiency. Some unreacted ammonia may pass through the catalyst, so ammonia slip catalysts are needed. The catalysts can have good selectivity to nitrogen, but these catalysts can also form N2O. Matsui et al. [45] show in Fig. 1.10 that upto 80 % of the ammonia going into the slip catalyst can convert into N2O if there is also a relatively high amount of NO (2X vs. NH3); the reaction is,
Nitrous oxide formation in ammonia slip catalysts is promoted by high NO:NH3 ratios coming out of the SCR catalyst [45]
A high NO:NH3 ratio coming out of the SCR catalyst can occur, for example, if there is poor urea mixing prior to entering the SCR catalyst, and urea is injected at less than stoichiometric requirements, or if ammonia is partially oxidized (by Cu-zeolite, for example) to form NO. Kamasamudram et al. [33] show that slip catalysts with lower precious metal content minimize N2O formation.
Ammonia slip catalysts are improving in terms of cost and selectivity to nitrogen. Figure 1.11 from Walker [34] shows modern catalysts have similar performance to the first generation catalysts, but with only 20 % of the platinum loading. The latest catalyst has much better selectivity to nitrogen with less undesirable by-products, yet with half the precious metal of its predecessor. Formation of NO and NO2 is still an issue but improving.
Generational improvements in ammonia slip catalysts. Early improvements dropped cost with minor compromise in selectivity. The latest generation recovers the performance with greatly reduced precious metal loading [34]
5 SCR System Design
Many new SCR systems designs are emerging to meet the tightening cold start and deNOx efficiency requirements under low-load urban driving conditions.
For LD applications, Holderbaum and Kwee [46] evaluated the placement of the SCR relative to the DPF. Considering the added fuel needed to heat the SCR system for cold start, and to regenerate the DPF with different frequencies due to changes in passive NO2 regeneration, the authors conclude that for an 1,800 kg car with a 2-l engine, if cold starts occur more frequently than once every 60 km, it is better to place the SCR in front of the DPF. Figure 1.12 shows some results. Note that the 60 km threshold is greater than the distance used in certification cycles, wherein placing the SCR behind the DPF incurs a 2 % fuel penalty versus a front placement. The forward SCR placement aids European certification for both CO2 and NOx emissions.
Considering fuel consumption for heating and regenerating the system, placing the SCR in front of the DPF in light-duty applications is beneficial if there is less than 60 km between cold starts [46]
For US LD diesels, removing cold start NOx emissions are key to meeting the tailpipe emissions regulations. A new combination NOx adsorber and SCR catalyst configuration was shown by Henry et al. [47, 48]. Figure 1.13 shows some performance characteristics and the concept when coupled with an SCR catalyst. The system consists of an upstream PNA that might capture 65 % of the NOx at temperatures less than 150 °C, and then passively releases it at temperatures greater the 150 °C. At these temperatures, a copper zeolite is just becoming active and can reduce some of this released NOx. The technology enables NOx reductions of about 15 mg/mile (24 mg/km) on the US LD FTP cycle. Walker [34] reported on improvements in the PNA wherein stored NOx is held up to 250–300 °C.
An upstream passive NOx adsorber (PNA) captures NOx generated at T < 150 °C. A LT urea-SCR catalyst can then convert this NOx upon release at T > 150 °C [47]
Work is continuing on the combination SCR + DPF system, wherein SCR catalyst is coated onto the DPF. This allows SCR catalyst to be placed on the vehicle without using an added component, and can get the SCR catalyst closer to the engine for faster light-off. Numerous reports dating to 2008 show that total NOx removal efficiency is thus improved, with little to no compromise in DPF regeneration. Walker [34] and Folic and Johansen [49] show that passive DPF regeneration with NO2 is adversely impacted by adding SCR catalyst to the DPF, but still occurs and can be managed. Tan et al. [50] found that DPF regeneration calibration needs to be adjusted to longer times or higher temperatures to get the same cleaning performance as the base DPF system. They also showed a new issue when soot is accumulated on the DPF + SCR: Ammonia storage capacity decreases for fresh samples at all temperatures and soot loadings tested (200–350 °C, 1.0–2.5 g/l), but is not affected by soot loading for aged samples (except at 200 °C). Loss of ammonia storage capacity impacts SCR performance at 200 °C, but not at 300 °C at a soot load of 2 g/l. Schrade et al. [51] demonstrated the opposite effect of soot on ammonia storage capacity, so this is still an open question. They also report that soot on the DPF can help deNOx functionality if the NO2 content is higher than optimal, but hinders it if the NO2 is lower. The soot will be oxidized by NO2, resulting in lower levels getting to the underlying SCR catalyst. Folic and Johansen [49] also looked at the relationship between filter porosity, SCR catalyst deNOx efficiency, and back pressure. With all filters, there was an optimum back pressure versus deNOx performance, wherein generally they both increase together, but at higher catalyst loadings, the deNOx efficiency can decrease with increasing back pressure. Filters with >60–65 % porosity have better deNOx performance at a given back pressure than filters with <60 % porosity.
Urea injection parameters are determined by NOx quantity in the exhaust (concentration and flow rate), temperature, and the amount of ammonia stored in the catalyst. There is normally closed-loop feedback control using an NOx sensor at the SCR exit, and in many applications, an NOx sensor is used upstream to determine inlet NOx levels.
As urea generally cannot be injected at temperatures less than 180 °C due to evaporation and hydrolysis kinetics, it is important to properly manage ammonia storage in the catalyst for low-load applications. Murata et al. [52] show that SCR efficiency at temperatures <265 °C is strongly dependent on the amount of ammonia that is stored in the catalyst. They developed an algorithm that kept stored urea within control limits, resulting in improved deNOx efficiency, from nominally 50–75 % in the Japanese HD transient cycle with an average temperature of only 160 °C. The concept is shown in Fig. 1.14.
Key controlling parameters in algorithm for the use of stored ammonia. An adsorption model is used to define a desired range of stored ammonia. Ammonia release is conveniently predicted for SCR NOx conversion (Adapted from Murata et al. [52])
Another approach to managing the stored ammonia for improved low-temperature performance is described by Yasui et al. [53] and illustrated in Fig. 1.15. They use two Fe-zeolite SCR catalysts placed downstream from the DPF system. An ammonia sensor is placed between the two SCR catalysts, and ammonia is generously injected to keep the first catalyst loaded at all times, as conditions allow. This accomplishes two goals. First, the efficiency of the SCR system is improved as there is plentiful ammonia present in the system. More importantly, the strategy helps cold start management. In traditional cold start thermal management, the SCR catalyst is heated as fast as possible to get it . Here, the catalyst is always loaded with ammonia, and the catalyst is heated slowly to prevent rapid release of ammonia during this period.
Layout of a new LD SCR system incorporating two SCR catalysts with an ammonia sensor in between. The first catalyst is kept loaded with ammonia, as indicated by the sensor [53]
6 Onboard Generation of Ammonia Using Lean NOx Traps
LNT is a very different deNOx concept from SCR, and is a favored approach for smaller vehicles. But it is also being used in conjunction with SCR catalysts, so a few comments are warranted here. The LNT adsorbs NOx as an alkaline earth nitrate (baria, potassia) during lean operation. Eventually the capacity to efficiently adsorb NOx is reached, and the LNT is regenerated with a periodic migration to a rich exhaust gas. The nitrate decomposes, and the released NOx is reduced by CO and HCs on a rhodium catalyst in the absence of oxygen, as in a three-way catalyst. The lean-rich periods are nominally 30–90 s lean and 2–6 s rich, but this can vary tremendously depending on driving conditions. During the brief rich period, ammonia is formed and can be captured in a downstream SCR catalyst, and used for additional NOx reduction during the lean period.
The first LD diesel sold in the US to meet recent tailpipe regulations had the BlueTec™ 1 emission control system, utilizing an LNT followed by an SCR catalyst. The unique system used the rich cycle of the LNT to generate ammonia, which was captured and used by the downstream SCR for lean NOx reduction. Weibel et al. [54] reported that ammonia selectivity increases with aging and rich period, and decreases with increasing the air/fuel ratio (λ). Under conditions of λ = 0.88 and rich periods of 5 s (180 s lean), ammonia selectivity is greater than 70 % in the temperature range of 225–350 °C for all aging tests temperatures greater than 600 °C and 50,000 miles. The SCR adds about 20 % deNOx efficiency over an LNT-only configuration.
Theis et al. [55] reported on an interesting study whereby they alternated LNT and SCR slices in one can to check the effect of NOx, ammonia, and HC distribution on deNOx performance. The system performance improved as the number of alternating slices of the LNT and SCR increased, keeping the total volume constant. The deNOx efficiency for the eight segment system (four pairs of LNT and SCR catalysts) was 81 % in a reference test at 275 °C, versus 78 % for four segments and 60 % for two segments. The reference single LNT with no SCR catalyst had only 30 % deNOx efficiency. The authors also show reduced N2O, NH3, HC, and CO emissions with the segmented systems. Various dynamics are operative, but the segmented systems tend to better-match the NO and ammonia concentrations in the SCR, and alternating SCR slices better-adsorb HCs for enhanced utility. Harold et al. [56] went a step further, and layered the SCR catalyst on top of the LNT catalyst. Relative to an LNT alone, the layered combination reduced 25 % (absolute) more NO at 200 and 250 °C with very high selectivity to nitrogen (95 %+) compared to 30–75 % for the LNT alone. Further, they added ceria to the LNT formulation in the back zone of the catalyst to achieve better low-temperature performance due mainly to improved water–gas shift reaction and additional LT NOx storage.
Xu et al. [57] reported vehicle and laboratory testing on a second-generation LNT + SCR system. The DOC + LNT + SCR + DPF system was installed on a prototype F-150 pickup truck (2,610 kg, 4.4 l V8, turbo-diesel). The aged system (64 h, 750 °C) reduced NOx by 96 % to 13.5 mg/mile, and HC emissions were 14 mg/mile (−99 %), bringing the vehicle to within the emerging California Low Emission Vehicle 3 (LEVIII) limit values (30 mg/mile HC + NOx) on the standard certification test cycle. The laboratory work focused on HC reductions from the system. The SCR component reduced HCs about 75 %, mainly by adsorption under rich conditions and oxidation under lean conditions. Cavataio et al. [58] compared this capability to that of a urea-SCR system for meeting the US EPA Tier 2 Bin 2 (or California LEVIII fleet average) standards. Although the LNT + SCR system is 18 % smaller it had met the target emissions, while the SCR system fell short. Further, the LNT + SCR system is estimated to be slightly cheaper, but has most of the cost tied up in precious metal (with its inherent price volatility). On the downside, the fuel penalty was high at 10 %, versus 2 % for the SCR system. Also, sulfur management of the LNT + SCR system was not considered.
Although the LNT + SCR system has not been commercialized since being used on the 2009 Mercedes Benz E320 in the US, it is still generating significant interest and we may see its renewed adaption as understanding and performance improves. It is quite possible that lean-gasoline applications will use a version of the technology, perhaps with a TWC instead of an LNT. NOx emissions are too high to make urea-based SCR as attractive as it is for diesel, and reports are surfacing that are attractive [59, 60].
7 Outlook
SCR is the leading lean deNOx technology today. SCR was first introduced tested on trucks in the mid-1990s. The deNOx efficiency was about 50–60 % and the swept volume ratio (SCR catalyst/engine displacement) was about 6:1. Today, we are entering the fourth generation of commercialized SCR catalysts, and NOx emissions dropped 90 % using a system half the size. An analogy might be used for the three-way catalyst in the mid-1990s. At that time it was in its third or fourth generation. Today, 15 years later, modern TWCs have 95 % lower emissions and 70 % less precious metal. There is still plenty of opportunity for improvement in the SCR system.
One significant opportunity is improving low-temperature performance. Areas for improvement are delivering ammonia at temperatures down to 100 °C, understanding and improving ammonia storage in the catalysts, and improving the low-temperature NOx reduction activity of the catalyst.
Another opportunity is in understanding and improving how SCR catalyst systems age. In HD applications the catalyst may need to operate for a million kilometers. Thermal aging and poisoning need to be better understood to allow tighter control and high deNOx efficiency throughout the useful life.
There is much emerging opportunity in size reduction through consolidation of components. Engines in all applications will go lean and need deNOx. Many of these, such as smaller cars and NR applications (tractors, small construction equipment), do not have much space for large systems. Illustrated above, consolidation of the SCR and DPF can save space and cost with little compromise in performance. TWCs today are layered and zoned, and Harold et al. [56] show the potential of extending this approach to SCR systems.
Perhaps the most significant consideration going forward will be on CO2 reductions. New fuels and combinations will be used, exhaust temperatures will drop, and the charges will be lean. NOx emissions regulations will not loosen, and are showing signs of tightening again. SCR will be a vital technology to address these future challenges.
8 Conclusions
8.1 Regulations and Engine Technologies
Tightening mobile NOx regulations are driving lean deNOx control, especially in the HD sector, but also in the US and Japanese LD diesel market, and soon in Europe. HD engine fuel consumption can benefit significantly by running at high engine-out NOx. DeNOx efficiencies of 98+ % can have significant advantages in fuel consumption and less engine hardware (EGR). In the LD sector, this level of deNOx efficiency is needed to meet the US regulations, with the main opportunity in reducing cold start emissions. Although most European LD diesels can meet the emerging Euro 6 regulation without NOx aftertreatment, it is desired to reduce NO2 emissions and save fuel through higher NOx engine calibrations. Urea-SCR is the leading approach in all segments, and is accomplishing deNOx efficiencies of 95 % with reasonable systems and temperature ranges.
8.2 Onboard Ammonia Delivery Systems and SCR Catalyst Systems
Urea-water solutions are the only commercialized source for ammonia for SCR systems. This requires an onboard urea storage and injection system. These are highly engineered systems to provide for the freezing of urea, and monitoring and pumping it for injection. Injection temperatures are limited to about 180 °C due to deposit formation, but hydrolysis catalysts are evolving to drop this temperature. Urea-exhaust mixers are critical to get good SCR performance. Gaseous ammonia systems are in development, enabling ammonia injection down to 100 °C.
Much of the new reports on DOCs concern the interplay of precious metal formulations on cost reduction, HC oxidation, NO oxidation to NO2, and the formation of N2O. HC and CO oxidation is promoted by replacement of platinum with palladium, but NO2 formation is compromised. NO2 cannot form if HCs are present, as the HCs will reduce any NO2 back to NO. HCs are also instrumental in reducing NO to N2O, particularly at ~200–250 °C if CO is not present.
Commercial SCR catalysts fall into three types: vanadia, copper zeolite, and iron zeolite. Copper zeolite is the best performing across the temperature range and has excellent thermal durability. Vanadia catalysts are the lowest cost, and can perform well at low temperatures but need good NO2 management, as do iron zeolites. Iron zeolites have the best high-temperature performance. Much effort is being put on understanding and improving durability. Vanadia systems deteriorate at high temperature (>550 °C), but are not sensitive to sulfur poisoning. Copper zeolite needs to be periodically desulfated at >500 °C, and both copper and iron zeolites are susceptible to HC poisoning. N2O formation in the SCR system is under much investigation now. Ammonia storage and management is critically important for good low-temperature performance and much progress is being made in this regard.
Emerging systems design includes consolidation of the SCR and DPF into one unit. Impacts on deNOx efficiency are minimal, but passive DPF regeneration with NO2 is reduced. PNA are emerging. They adsorb NOx at low temperatures and then passively release the NOx, upon further heating. They can be coupled with appropriate SCR catalysts for much-improved LT performance. Some effort is being made on generating ammonia onboard using lean NOx traps (LNTs), with preferences migrating to putting the LNT and SCR in closer proximity to one another (sequential slices or layering on the same catalyst).
8.3 Outlook
The outlook is optimistic that SCR systems will achieve 98+ % deNOx efficiency. Emerging issues are related to improved low-temperature performance, enhanced durability, and reduced size and cost.
References
Johnson, T. V. (2012) Diesel Emissions in Review, SAE Int. J. Engines, May 2012 5:216–234; doi:10.4271/2012-01-0368.
Johnson, T. V. (2011) Diesel Emissions in Review, SAE Int. J. Engines, June 2011 4:143–157; doi:10.4271/2011-01-0304.
Johnson, T. V. (2010) Review of Diesel Emissions and Control, SAE Int. J. Fuels Lubr. August 2010 3:16–29; doi:10.4271/2010-01-0301.
Stanton, D. (2012) Diesel Engine Technologies to Meet Future On‐Road and Off‐Road US EPA Regulations, presentation at 4th CTI International Conference, NOx Reduction, Current and Future Solutions for On-Road and Off-Road Applications, Detroit, June 19–20, 2012.
Roberts, C. (2011) The Pursuit of High Efficiency Engines-SwRI Programs, presentation at the Emissions 2011 Conference, Ann Arbor, MI, June 2011.
Stanton, D. (2009) Technology Development for High Efficiency Clean Diesel Engines and a Pathway to 50% Thermal Efficiency”, presentation at US Department of Energy, Directions in Engine Efficiency and Emissions Research (DEER) Conference, September 2009, Detroit.
Zybell, J. G. (2011) Technology Path to Meet Future Fuel Economy Targets in the CV Segment, presentation at Integer Diesel Emissions Conference and DEF Forum, Atlanta, October 2011.
Kobayashi, M., Aoyagi, Y., Adachi, T, Murayama, T., Hashimoto, M., Goto, Y., Suzuki, H. (2011) Effective BSFC and NOx Reduction on Super Clean Diesel of Heavy Duty Diesel Engine by High Boosting and High EGR Rate, SAE Technical Paper 2011-01-0369, doi:10.4271/2011-01-0369.
de Ojeda, W. (2011) Development and Demonstration of a Fuel-Efficient HD Engine (Dept of Energy Supertruck Program), presentation at US Department of Energy, Directions in Engine Efficiency and Emissions Research (DEER) Conference, October 3–6, 2011, Detroit.
Vermeulen, R., Vonk, W., and Dekker, H. (2011) Real-World Exhaust Gas Emissions of a Prototype N3 Heavy Duty Vehicle with Euro VI Technology, TNO Technical Report TNO-RPT-2011-00076, 27 October 2011.
Pischinger, S. (2011) Efficient Powertrain Technology for the Future, keynote presentation at the Symposium on International Automotive Technology 2011, Pune, India, January 2011.
Popuri, S., Langenderfer, D., Cunningham, M., Vajapeyazula, P., Garimella, P., Joshi, A., Cecil, A, Frazier, T., and Stanton, D. (2011) Demonstration of > 10% FTP75 Cycle Efficiency Improvement for a Light-Duty Diesel Engine System at T2B5 Tailpipe Emissions Levels, presentation at SAE Light-Duty Diesel Emissions Symposium, Detroit, November 2011.
Ruth, M. (2012) Cummins’ Next Generation Tier 2, Bin 2 Light Truck Diesel Engine, presentation at the US Department of Energy Directions in Engine Efficiency and Emissions Research (DEER), Dearborn, MI, October 2012.
Balland, J., Schreurs, B., Hardam, H., Yasui, Y. (2012) A New Clean Diesel Concept for US LEV III SULEV - Advanced Air/Fuel Control for NOx Reduction and for SCR Heat Up, presentation at 4th IAV MinNOx Conference 12–13 June 2012, Berlin.
Regner, G., Herold, R. E., Wahl, M. H., Dion, E., Redon, F., Johnson, D., Callahan, B. J., and McIntyre, S. (2011) The Achates Power Opposed-Piston Two-Stroke Engine: Performance and Emissions Results in a Medium-Duty Application, SAE Technical Paper 2011-01-2221, doi:10.4271/2011-01-2221.
Casarella, M. (2011) Urea Dosing Systems and Controls for Light Duty Diesel Applications, presentation at SAE Light-Duty Diesel Emissions Symposium, Ypsilanti, MI, November 2011.
Ostertag, M. (2008) Urea Reservoir Systems for Off-Highway and Heavy Duty Market, presentation at CTI NOx Reduction Forum, December 2008, Detroit.
Needham, D., Spadafora, P., Schiffgens, H. J., Kirwan, J. E., Cabush, D. D., Kalina, A. (2012) Delphi SCR Dosing System – An Alternative Approach for Close-Coupled SCR Catalyst Systems, proceedings of 21st Aachen Colloquium Automobile and Engine Technology October 2012, Aachen, Germany.
Zhan, R., Li, W., Eakle S. T., Weber, P. A. (2010) Development of a Novel Device to Improve Urea Evaporation, Mixing and Distribution to Enhance SCR Performance, SAE Technical Paper 2010-01-1185, doi:10.4271/2010-01-1185.
Alano, E., Jean, E., Perrot, Y., Brunel, J.-P., Ferrand, N., Ferhan, M., Chapel, J., and Pajot, K (2011) Compact SCR for Passenger Cars, SAE Technical Paper 2011-01-1318 doi:10.4271/2011-01-1318.
Kröcher, O., Elsener, M., Mehring, M., Bernhard, A. (2010) Highly-Developed Thermal Analysis Methods for the Characterization of Soot and Deposits in Urea-SCR Systems, in AVL 6th International Exhaust Gas and Particulate Emissions Forum, March 2010, Ludwigsburg, Germany.
Johannessen, T. Next Generation SCR System for Fuel‐Efficient NOx Reduction, presentation at SAE Light-Duty Diesel Emissions Symposium, Detroit, November 2011.
Hansen, K. F., Hägg, A., Stenfeldt, M., Lepage, M., Lafossas, F. A., Yasui, Y., Fischer, M. (2012) Solid Ammonia Storage for Third Generation SCR, presentation at the IAV MinNOx Symposium, June 2012, Berlin.
Thomas, D., and Highfield, T. (2011) Ammonium Formate/Urea Based Diesel Exhaust Fluid for Superior Low Temperature SCR Performance, presentation at SAE Light-Duty Diesel Emissions Symposium, Detroit, November 2011.
Henry, C., Currier, N., Ottinger, N., Yezerets, A., Castagnola, M., Chen, H.-Y., and Hess, H. (2011b) Decoupling the Interactions of Hydrocarbons and Oxides of Nitrogen over Diesel Oxidation Catalysts, SAE paper 2011-01-1137, doi:10.4271/2011-01-1137.
Spurk, P., Frantz, S., Schütze, F. W., Noack, H. D., Müller, W. (2010) NO2 Formation on the DOC/DPF System – A System Thought, in AVL 6th International Exhaust Gas and Particulate Emissions Forum, March 2010, Ludwigsburg, Germany.
Jen, H.-W., Girard, J. W., Cavataio, G., Jagner, M. J. (2008) Detection, Origin and Effect of Ultra-Low Platinum Contamination on Diesel-SCR Catalysts, SAE paper 2008-01-2488, doi:10.4271/2008-01-2488.
Cavataio, G., Jen, H.-W., Girard, J. W., Dobson, D., Warner, J. R., Lambert, C. K. (2009) Impact and Prevention of Ultra-Low Contamination of Platinum Group Metals on SCR Catalysts Due to DOC Design, SAE Technical Paper 2009-01-0627, doi:10.4271/2009-01-0627.
Kim, C. H., Schmid, M., Schmieg, S. J., Tan J., and Li, W. (2011) The Effect of Pt-Pd Ratio on Oxidation Catalysts Under Simulated Diesel Exhaust, SAE paper 2011-01-1134, doi:10.4271/2011-01-1134.
Wang, W., McCool, G., Kapur, N., Yuan, G., Shan, B., Nguyen, M., Graham, U. M., Davis, B. H., Jacobs, G. Cho, K., Hao, X. (2012) Mixed-Phase Oxide Catalyst Based on Mn-Mullite (Sm, Gd)Mn2O5 for NO Oxidation in Diesel Exhaust, Science 337, 832 (2012), doi:10.1126/science.122509.
Ishizaki, K., Mitsuda, N., Ohya, N., Ohno, H., Naka, T., Abe, A., Takagi, H., Sugimoto, A. (2012) A Study of PGM-Free Oxidation Catalyst YMnO3 for Diesel Exhaust Aftertreatment, SAE Technical Paper 2012-01-0365, doi:10.4271/2012-01-0365.
Glover, L., Douglas, R., McCullough, G., Keenan, M., Revereault, P., and McAtee, C. (2011) Performance Characterization of a Range of Diesel Oxidation Catalysts: Effect of Pt:Pd Ratio on Light Off Behavior and Nitrogen Species Formation, SAE Technical Paper 2011-24-0193, doi:10.4271/2011-24-0193.
Kamasamudram, K., Henry, C., Currier, N., and Yezerets, A., (2012) N2O Formation and Mitigation in Diesel Aftertreatment Systems, SAE Int. J. Engines 5: doi 10.4271/2012-01-1085.
Walker, A. (2012) Current and Future Trends in Catalyst-Based Emission Control System Design”, presentation at the SAE Heavy-Duty Diesel Emission Control Symposium, September 2012, Gothenburg.
Tang, W., Huang, X., and Kumar, S. (2011) Sulfur Effect and Performance Recovery of a DOC + CSF + Cu-Zeolite SCR System, presentation at US Department of Energy, Directions in Engine Efficiency and Emissions Research (DEER) Conference, October 3–6, 2011, Detroit.
Chapman, D.M., Fu, G., Augustine S., Crouse, J., Zavalij, L., Watson, M., Perkins-Banks, D. (2010) New Titania Materials with Improved Stability and Activity for Vanadia-Based Selective Catalytic Reduction of NOx, SAE Technical Paper 2010-01-1179, doi:10.4271/2010-01-1179.
Walker, A. (2010) Optimising Future Catalyst Systems, presentation at SAE Heavy-Duty Diesel Emissions Control Symposium, Gothenburg, September 2010.
Narula, C., Yang, X., Bonnesen, P., and Hagaman, E., (2011) High Performance NH3 SCR Zeolite Catalysts for Treatment of NOx in Emissions from Off-Road Diesel Engine, SAE Technical Paper 2011-01-1330, doi:10.4271/2011-01-1330.
Yang, X., Narula, C. (2010) Simple Approach to Tuning Catalytic Activity of MFI-Zeolites for Low Temperature SCR of NOx, poster at US Department of Energy Directions in Engine Efficiency and Emissions Research (DEER) Conference, September 27–30, 2010, Detroit.
Prikhodko, V., Pihl, J., Lewis, S. and Parks, J. (2012) Hydrocarbon Fouling of SCR During PCCI Combustion, SAE Int. J. Engines 5(3):2012, doi:10.4271/2012-01-1080.
Luo, J-Y, Yezerets, A., Henry, C., Hess, H., Kamasamudram, K., Chen, H-Y, Epling, W. S. (2012), Hydrocarbon Poisoning of Cu-Zeolite SCR Catalysts, SAE Technical Paper 2012-01-1096, doi:10.4271/2012-01-1096.
Han, J., Kim, E., Lee, T., Kim, J., Ahn, N, and Han, H.-S. (2011) Urea-SCR Catalysts with Improved Low Temperature Activity, SAE Technical Paper 2011-01-1315, doi:10.4271/2011-01-1315.
Heibel, A. (2010) Advances in Substrate Technology, presentation at SAE Heavy-Duty Diesel Emissions Control Symposium, Gothenburg, September 2010.
Bartley, G. J., Chadwell, C. J., Kostek, T. W., Zhan, R. (2012) SCR Deactivation Kinetics for Model-Based Control and Accelerated Aging Applications, SAE paper 2012-01-1077, Published 04/16/2012, SAE International, doi:10.4271/2012-01-1077.
Matsui, W., Suzuki, T., Ohta, Y., Daisho, Y., Suzuki, H., and Ishii, H. (2011) A Study on the Improvement of NOx Reduction Efficiency for a Urea-SCR System (Sixth Report) - Clarifying N2O formation mechanism, JSAE Technical Paper 20115720, October 2011.
Holderbaum, B., and Kwee (2009) Integration of DPF and SCR - Interfaces and Interactions, presentation at the 5th International CTI Forum, SCR Systems, Fellbach, Germany, April 2009.
Henry, C., Langenderfer, D., Yezerets, A., Ruth, M., Chen, H.-Y., Hess, H., and Naseri, M. (2011a) Passive Catalytic Approach to Low Temperature NOx Emission Abatement, presentation at US Department of Energy, Directions in Engine Efficiency and Emissions Research (DEER) Conference, October 3–6, 2011, Detroit.
Henry, C., Gupta, A., Currier, N., Ruth, M., Hess, H., Naseri, M., Cumaranatunge, L., Chen (2012) Advanced Technology Light Duty Diesel Aftertreatment System, presentation at the US Department of Energy Directions in Engine Efficiency and Emissions Research (DEER), Dearborn, MI, October 2012.
Folić, M., Johansen, K. (2012) SCR-DPF Integrations for Diesel Exhaust Performance and Perspectives for High SCR Loadings, presentation at the US Department of Energy Directions in Engine Efficiency and Emissions Research (DEER), Dearborn, MI, October 2012.
Tan, J., Solbrig, C., and Schmieg, S. J. (2011) The Development of Advanced Two-Way SCR/DPF Systems to Meet Future Heavy-Duty Diesel Emissions, SAE Technical Paper 2011-01-1140, doi:10.4271/2011-01-1140.
Schrade, F., Brammer, M., Schaeffner, J., Langeheinecke, K., Kraemer, L. (2012) Physico-Chemical Modeling of an Integrated SCR on DPF (SCR/DPF) System, SAE Int. J. Engines 5(3):2012, doi:10.4271/2012-01-1083.
Murata, Y., Tokui, S., Watanabe, S., Daisho, Y., Suzuki, H., Ishii, H., (2008) Improvement of NOx Reduction Rate of Urea-SCR System by NH3 Adsorption Quantity Control, SAE Technical Paper 2008-01-2498, doi:10.4271/2008-01-2498.
Yasui, Y., Matsunaga, H., Hashimoto, E., Satoh, N., Hardam, H., Balland, J., Yamada, M., Takahashi, T. (2012) A New Clean Diesel Concept for US LEV-III SULEV - First Report - A New Emission Strategy and Aftertreatment Management Control, presentation at the IAV MinNOx Conference, June 2012, Berlin.
Weibel, W., Waldbüßer, N., Wunsch, R., Chatterjee, D., Bandl-Konrad, B., Krutzsch, B. (2009) A Novel Approach to Catalysis for NOx Reduction in Diesel Exhaust Gas, presentation at 8th International Catalysis for Automotive Pollution Control, Brussels, April 2009.
Theis, J. R., Dearth, M., and McCabe, R. (2011) LNT + SCR Catalyst Systems Optimized for NOx Conversion on Diesel Applications, SAE Technical Paper 2011-01-0305, doi:10.4271/2011-01-0305.
Harold, M., Luss, D., Liu, Yi (2012) Dual Layered Catalysts for Lean Reduction of NOx by In Situ Generation of Ammonia, presentation at the Catalysts for Automotive Pollution Control 9 (CAPoC9), September 2012, Brussels.
Xu, L., McCabe, R., Tennison, P., and Jen, H-W. (2011) Laboratory and Vehicle Demonstration of “2nd-Generation” LNT + in situ SCR Diesel Emission Control Systems, SAE Technical Paper 2011-01-0308, doi:10.4271/2011-01-0308.
Cavataio, G., Guo, K., Xu, L., Dobson, D., Warner, J., Dearth, M., Theis, J., Ruona, W., McCabe, R., Lambert, C., (2011) Comparing Urea SCR to In-situ LNT+SCR After Treatment Systems for Light Duty Vehicles, SAE 2011 Light Duty Diesel Emissions Control Symposium, November 2–3, 2011, Ann Arbor.
Guralp, O., Qi, Q., Li, W., and Najt, P. (2011) Experimental Study of NOx Reduction by Passive Ammonia-SCR for Stoichiometric SIDI Engines, SAE Technical paper 2011-01-0307, doi:10.4271/2011-01-0307.
Toops, T. J., Parks II, J. E., Pihl, J. A., DiGiulio, C. D., Amiridis, M. D. (2012) Lean Gasoline Emissions Control: NH3 Generation Over Commercial Three-Way Catalysts and Lean-NOx Traps, presentation at US Department of Energy, Directions in Engine Efficiency and Emissions Research (DEER) Conference, October 2012, Detroit.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Johnson, T.V. (2014). Review of Selective Catalytic Reduction (SCR) and Related Technologies for Mobile Applications. In: Nova, I., Tronconi, E. (eds) Urea-SCR Technology for deNOx After Treatment of Diesel Exhausts. Fundamental and Applied Catalysis. Springer, New York, NY. https://doi.org/10.1007/978-1-4899-8071-7_1
Download citation
DOI: https://doi.org/10.1007/978-1-4899-8071-7_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4899-8070-0
Online ISBN: 978-1-4899-8071-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)