Abstract
This review will focus on the impact of hyperglycemia-induced oxidative stress in the development of diabetes-induced vascular and neural dysfunction. Oxidative stress occurs when the balance between the production of oxidation products and the ability of antioxidant mechanisms to neutralize these products is tilted in the favor of the former. The production of reactive oxygen species has been shown to be increased in patients with diabetes. The possible sources for the overproduction of reactive oxygen species are widespread and include enzymatic pathways, autoxidation of glucose, and the mitochondria. Increase in oxidative stress has clearly been shown to contribute to the pathology of vascular disease not only in diabetes but also in hypertension, stroke, and ischemia. Since the etiology of diabetic neuropathy is considered to have a large vascular component, prevention of oxidative stress in diabetes is considered by many investigators to be a primary defense against the development of diabetic vascular disease. Moreover, increased oxidative stress has been promoted as a unifying hypothesis for diabetic complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1.1 Introduction
Oxidative stress is an important component of diabetes and its complications [1–15]. Studies showing that treatment with antioxidants prevents diabetes- and hyperglycemia-induced impairment of endothelium-dependent relaxation suggest that oxidative stress is a major factor in the development of diabetic vascular disease [7, 16–20]. In addition, treatment of streptozotocin-induced diabetic rats with antioxidants has demonstrated that oxidative stress and vascular dysfunction may be a major factor in the development of diabetic neuropathy [5, 6, 8, 21–23]. In this chapter I will present past studies from my laboratory that have focused on the effect of streptozotocin-diabetes-induced oxidative stress on vascular reactivity of epineurial arterioles and neural function.
1.2 Diabetes-Induced Oxidative Stress and Vascular Dysfunction in Epineurial Arterioles
My laboratory has for many years focused on the effect of diabetes on vascular and neural dysfunction. Our studies first demonstrated that vascular impairment of epineurial arterioles, blood vessels that provide circulation to the sciatic nerve, and reduced endoneurial blood flow precede neural dysfunction as determined by slowing of motor nerve conduction velocity (see Fig. 1.1 [24]). Our studies demonstrated that one week after the induction of diabetes using streptozotocin, that vasodilation in response to a low dose of acetylcholine was significantly impaired and after two weeks of diabetes, maximum impairment in acetylcholine-mediated vascular relaxation was observed [24]. During this time period endoneurial blood flow of the sciatic nerve was also reduced. Impairment in motor nerve conduction velocity was not observed until after two weeks of diabetes suggesting that vascular dysfunction may be an early development in diabetes and a major factor contributing to diabetic neuropathy.
The effect of streptozotocin-diabetes in the development of vascular and neural dysfunction. Vascular reactivity to acetylcholine by epineurial arterioles (section A ED50), endoneurial blood flow (section B), and motor nerve conduction velocity (section C) was examined in control (0) and streptozotocin-induced diabetic rats following 6–30 days of diabetes. Data is presented as the mean ± SEM. *P < 0.05 compared to control (0)
Acetylcholine-induced vasodilation of epineurial arterioles is mediated by two mechanisms involving the production of nitric oxide (NO) and endothelium-derived hyperpolarizing factor (EDHF) [25]. This is important since one mechanism by which superoxide/oxidative stress can cause vascular dysfunction is by quenching the bioactivity of NO (see below). A primary factor contributing to diabetes-/hyperglycemia-induced impairment in vascular relaxation in epineurial arterioles is increased oxidative stress [26, 27]. Oxidative stress occurs when the balance between the production of oxidation products and the ability of antioxidant mechanisms to neutralize these products is shifted in the favor of formation/accumulation of oxidative stress products.
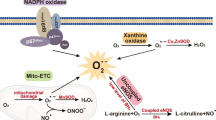
It is widely known that diabetes causes an increase in the production of reactive oxygen species [18, 28–30]. The most common forms are superoxide (O2 −), hydrogen peroxide (H2O2), hydroxyl radical (OH−), and peroxynitrite (ONOO−) [31]. There are many potential sources for production of these compounds. Superoxide can be produced by the electron transport chain of the mitochondria, NADH oxidase, NAD(P)H oxidase, xanthine oxidase, nitric oxide synthases, cyclooxygenase, lipoxygenase, and cytochrome P-450 [31]. Superoxide can spontaneously acquire an electron to form hydrogen peroxide. Hydrogen peroxide can also be formed from superoxide via superoxide dismutase (SOD), of which there are three isoforms: manganese (Mn)-SOD, which is located in the mitochondria, and two isoforms of copper and zinc (Cu, Zn)-SOD, which are located in the cytosol or extracellularly, respectively [31]. Hydrogen peroxide can be converted to water by the action of catalase or by glutathione peroxidase in the presence of reduced glutathione [31]. However, in the presence of trace metals such as iron (Fe), hydrogen peroxide can form OH− via a process known as the Fenton reaction [31]. The formation of peroxynitrite is also important pathologically and occurs by the reaction of O2 − and NO [30, 31]. We have demonstrated that superoxide and peroxynitrite, as indicated by the presence of nitrotyrosine staining, formation is increased in epineurial arterioles from diabetic rats (see Fig. 1.2 [26, 27]).
In a hallmark study Brownlee et al. [32, 33] presented a unifying hypothesis that increased production of superoxide by the mitochondrial chain is a causal link between elevated glucose and three of the main biochemical pathways (glucose-induced activation of protein kinase C, increased formation of glucose-derived advanced glycation end products, and increased glucose flux through the aldose reductase pathway) responsible for diabetes/hyperglycemia complications [32, 33]. Our studies have indicated that in epineurial arterioles from diabetic rats, the increased formation of superoxide seems to be primarily derived from the mitochondria [34]. We had previously demonstrated that reducing superoxide formation and oxidative stress in diabetic rats by treatment with several different types of antioxidants improved vasodilation by acetylcholine in epineurial arterioles of the sciatic nerve [26, 27]. In studies designed to investigate the source of superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats, we demonstrated that antioxidants were capable of preventing superoxide formation and reversing diabetes-induced vascular impairment in vitro. Dihydrolipoic acid and to a lesser extent α-lipoic acid were effective in decreasing superoxide formation and restoring acetylcholine-mediated vasodilation to arterioles from diabetic rats. α-Lipoic acid is capable of scavenging hydroxyl radicals, hypochlorous acid, and singlet oxygen, but not superoxide or peroxyl radicals [35, 36]. α-Lipoic acid is also effective at chelating transition metals. In contrast, in its reduced form as dihydrolipoic acid, it is a good scavenger of superoxide and prevents initiation of lipid peroxidation [35, 36].
In vivo α-lipoic acid can be converted into dihydrolipoic acid [35]. In addition, both α-lipoic acid and dihydrolipoic acid can regenerate other cellular antioxidants including dehydroascorbate, ubiquinol, oxidized glutathione, and, indirectly, the tocopherols [35]. The combination of these properties was likely responsible for the effectiveness of α-lipoic acid and dihydrolipoic acid in decreasing superoxide formation [34]. Tempol, a superoxide dismutase mimetic, also reversed the diabetes-induced impairment of acetylcholine-mediated vasodilation and increased superoxide formation in epineurial arterioles [37]. This is in agreement with other studies, which demonstrated that tempol or M40403, another superoxide dismutase mimetic, restores diabetes-induced endothelial dysfunction [27, 37, 38]. The decrease in superoxide formation by α-lipoic acid, dihydrolipoic acid, or tempol and the reversal of the diabetes-induced impairment in vasodilation suggest that the increased formation of superoxide and perhaps scavenging of nitric oxide is responsible in part for the reduced vascular response to acetylcholine in epineurial arterioles from diabetic rats. This is supported by our previous studies demonstrating the formation of superoxide and/or peroxynitrite by epineurial arterioles of the sciatic nerve from diabetic rats causes vascular dysfunction that is prevented with treatment by antioxidants in vivo [26, 27]. This was further supported by studies demonstrating that pretreatment with l-arginine in vitro improved acetylcholine-mediated vasodilation in epineurial arterioles from diabetic rats without decreasing the formation of superoxide by these vessels. Acute pretreatment with l-arginine of vessels from diabetic rats as well as l-arginine treatment of diabetic animal models and humans has led to the suggestion that reduced availability of nitric oxide during periods of hyperglycemia may be responsible for impaired vascular relaxation [39–43]. This may be due to a limitation in arginine as a substrate for nitric oxide synthase in diabetes or an increase in scavenging of nitric oxide by superoxide [26, 27, 44]. Our studies would support the latter conclusion. We have demonstrated increased superoxide and peroxynitrite formation in epineurial arterioles of diabetic rats and impairment in endothelium-dependent vascular relaxation that is prevented by antioxidant treatment [26, 27].
In studies to investigate the possible sources of superoxide formation in epineurial arterioles of diabetic rats, we found that increased formation of superoxide by epineurial arterioles was attenuated by preincubation with rotenone but not m-chlorophenylhydrazone (CCCP) or thenoyltrifluoroacetone (TTFA) [34]. Rotenone is an inhibitor of complex I of the mitochondrial electron transport chain, TTFA is an inhibitor of complex II, and CCCP is an uncoupler of oxidative phosphorylation. We are unsure why CCCP was less effective than rotenone in reducing superoxide formation by epineurial arterioles of the sciatic nerve of diabetic rats. It is possible that CCCP did not penetrate the vascular wall under the incubation conditions. Nonetheless, this study implicated complex I of the mitochondrial electron transport chain in the production of superoxide by epineurial arterioles of the sciatic nerve of the diabetic rat. In our studies increased formation of superoxide by epineurial arterioles from diabetic rats was also partially decreased by diphenylene iodonium (DPI). DPI has been used for many years as a NAD(P)H oxidase inhibitor [45]. Therefore, our studies at first would suggest that NAD(P)H oxidase may also be a source for the production of superoxide by epineurial arterioles of the diabetic rat. However, Li and Trush have reported in studies with monocytes that DPI at concentrations that inhibit NAD(P)H oxidase diminished the production of superoxide by mitochondrial respiration [46]. They found that DPI was as potent as rotenone in inhibiting the production of superoxide by the mitochondria, likely by complex I. If the studies by Li and Trush are correct, we cannot unequivocally state that NAD(P)H oxidase is a source of superoxide formation by epineurial arterioles of the sciatic nerve.
1.3 Neural Dysfunction
In two separate studies we examined the effect of treating streptozotocin-diabetic rats with α-lipoic acid or M40403 on vascular dysfunction, endoneurial blood flow, and nerve activity, as determined by measuring motor nerve conduction velocity [26, 27]. These studies demonstrated that treating diabetic rats using a prevention protocol with α-lipoic acid or M40403 prevented the diabetes-induced decrease in motor nerve conduction velocity and endoneurial blood flow (Fig. 1.3) and impairment of acetylcholine-mediated vascular relaxation by epineurial arterioles (Fig. 1.4). These treatments generally improved markers of oxidative stress including serum thiobarbituric acid reactive substance and superoxide and nitrotyrosine staining of epineurial arterioles [26, 27]. These studies imply that diabetes causes the increased production of superoxide and peroxynitrite in neural microvascular tissue and this is responsible for impaired vascular function. Moreover, improving vascular function in diabetes by use of antioxidants also restores endoneurial blood flow and neural activity.
Diabetes has been shown to cause an increase flux of glucose through the aldose reductase pathway that leads to the accumulation of sorbitol by nerve and other tissues [33]. Numerous investigators have demonstrated that treating diabetic rats with an aldose reductase inhibitor improves nerve function, and we have shown that treatment with an aldose reductase inhibitor also improves vascular dysfunction in epineurial arterioles [47]. The mechanism responsible for improving diabetes impaired vascular and nerve function by aldose reductase inhibitor treatment is unclear but in part may be due to reducing oxidative stress [47]. Previously we had reported that treating streptozotocin-induced diabetic rats with 0.5 % α-lipoic acid (see above) provides maximum protection against diabetes-induced oxidative stress and the development of vascular and neural dysfunction [26]. We have also reported that sorbinil, an aldose reductase inhibitor, partially prevented the development of diabetes-induced vascular and neural defects but were not as efficacious as antioxidant therapies [26, 47]. We next sought to determine whether combining these therapies at lower doses may be synergistic [48]. We found that the combination of 0.25 % α-lipoic acid and fidarestat (3 mg/kg B.W.), an aldose reductase inhibitor, completely prevented the diabetes-induced impairment of acetylcholine-mediated vascular relaxation in epineurial arterioles of the sciatic nerve (Fig. 1.5) and that this combination was more effective in preventing diabetes-induced vascular dysfunction than monotherapy of either compound. Our explanation for these results was that treatment of diabetic rats with fidarestat in combination with α-lipoic acid favored the formation of dihydrolipoic acid. α-Lipoic acid is a good metal chelator and is capable of scavenging hydroxyl radicals, hypochlorous acid, and singlet oxygen, but not superoxide or peroxyl radicals [35, 36, 49, 50]. However, in its reduced form, as dihydrolipoic acid, it is a good scavenger of superoxide and prevents initiation of lipid peroxidation [35, 36, 49, 50]. In vivo, the conversion of α-lipoic acid to dihydrolipoic acid requires either NADH or NADPH [49, 51]. In the mitochondria, preferentially R(+)-α-lipoic acid is converted to dihydrolipoic acid by the action of dihydrolipoamide dehydrogenase which requires NADH [50, 51]. Both stereo-isoforms of α-lipoic acid can be reduced in the cytosol by glutathione reductase or thioredoxin reductase, both require NADPH [50–52]. In neutrophils, as well as rat heart, kidney, and brain, NADH-dependent reduction of α-lipoic acid is prominent, whereas with rat liver, NADH- and NADPH-dependent pathways were about equally active [50, 52]. In erythrocytes and endothelial cells, NADPH is the primary reducing cofactor for α-lipoic acid [50, 53]. In diabetes, NADPH levels are reduced due to the increased flux of glucose through the aldose reductase pathway [40, 41]. Therefore, blocking the aldose reductase pathway with an aldose reductase inhibitor such as fidarestat likely protects cellular NADPH levels permitting the formation of dihydrolipoic acid. This explanation is supported by our studies demonstrating that serum dihydrolipoic acid levels are increased in diabetic rats treated with α-lipoic acid and fidarestat [48]. These studies suggest that in addition to protecting glutathione production, treatment of diabetic rats with an aldose reductase inhibitor may promote the formation of dihydrolipoic acid. This result may explain the antioxidant properties of aldose reductase inhibitors [54]. In these studies there was a synergistic effect on improving lens glutathione levels when treating diabetic rats with the combination of α-lipoic acid and fidarestat. Treatment of diabetic rats with fidarestat alone independently improved endoneurial blood flow and motor nerve conduction velocity, by 50 and 60 %, respectively, and reduced superoxide formation in the aorta. Furthermore, treating diabetic rats with 3 or 15 mg/kg body weight of fidarestat had a concentration-dependent effect on improving endoneurial blood flow, motor nerve conduction velocity, and acetylcholine-mediated vasodilation in epineurial arterioles. Taken together our results imply that some markers of oxidative stress and neural function are significantly improved by monotherapy using α-lipoic acid; however, the greatest beneficial effects were observed on all markers of oxidative stress and vascular function when the combination treatment consisting of α-lipoic acid and fidarestat was used.
In summary, diabetic neuropathy is a multifactorial disorder and vascular dysfunction in part due to an increase in oxidative stress is a contributing factor. Since diabetic vascular and neural disease is multifactorial, combination therapy may be the best approach for an effective treatment. The studies presented above suggest that an effective combination therapy should include an antioxidant such as α-lipoic acid and an aldose reductase inhibitor.
References
Pieper GM, Langenstroer P, Gross GJ (1993) Hydroxyl radicals mediate injury to endothelium-dependent relaxation in diabetic rat. Mol Cell Biochem 122:139–145
Pieper GM, Siebeneich W (1997) Diabetes-induced endothelial dysfunction is prevented by long-term treatment with the modified iron chelator, hydroxyethyl starch conjugated-deferoxamine. J Cardiovasc Pharmacol 30:734–738
Pieper GM, Siebeneich W (1998) Oral administration of the antioxidant N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J Cardiovasc Pharmacol 32:101–105
Cameron NE, Cotter MA (1995) Neurovascular dysfunction in diabetic rats: potential contribution of autoxidation and free radicals examined using transition metal chelating agents. J Clin Invest 96:1159–1163
Cameron NE, Cotter MA (1999) Effects of antioxidants on nerve and vascular dysfunction in experimental diabetes. Diabetes Res Clin Pract 45:137–146
Cameron NE, Cotter MA, Horrobin DH, Tritschler HJ (1998) Effects of α-lipoic acid on neurovascular function in diabetic rats: interaction with essential fatty acids. Diabetologia 41:390–399
Pieper GM (2000) Hyperglycemia and diabetes-induced vascular dysfunction: role of oxidative stress. In: Keaney JF (ed) Oxidative stress and vascular disease. Kluwer Academic, Norwell
Keegan A, Cotter MA, Cameron NE (1999) Effects of diabetes and treatment with the antioxidant α-lipoic acid on endothelial and neurogenic responses of corpus cavernosum in rats. Diabetologia 42:343–350
Obrosova IG, Fathallah L, Greene DA (2000) Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina. Eur J Pharmacol 398:139–146
Cakatay U, Telci A, Kayali R, Sivas A, Akcay T (2000) Effect of α-lipoic acid supplementation on oxidative protein damage in the streptozotocin-diabetic rat. Res Exp Med 199:243–251
Haak E, Usadel KH, Kusterer K, Amini P, Frommeyer R, Tritschler HJ, Haak T (2000) Effects of α-lipoic acid on microcirculation in patients with peripheral diabetic neuropathy. Exp Clin Endocrinol Diabetes 108:168–174
Andrew R, Skyrme-Jones P, O’Brien RC, Berry KL, Meredith IT (2000) Vitamin E supplementation improves endothelial function in type I diabetes mellitus: a randomized placebo-controlled study. J Am Coll Cardiol 36:94–102
Gocmen C, Secilmis A, Kumcu EK, Ertug PU, Onder S, Dikmen A, Baysal F (2000) Effects of vitamin E and sodium selenate on neurogenic and endothelial relaxation of corpus cavernosum in the diabetic mouse. Eur J Pharmacol 398:93–98
Ishii H, Koya D, King GL (1998) Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med 76:21–31
Ammar RF Jr, Gutterman DD, Brooks LA, Dellsperger KC (2000) Free radicals mediate endothelial dysfunction of coronary arterioles in diabetes. Cardiovasc Res 47:595–601
Tribe RM, Poston L (1996) Oxidative stress and lipids in diabetes: a role in endothelium vasodilator dysfunction? Vasc Med 1:195–206
Giugliano D, Ceriello A, Paolisso G (1996) Oxidative stress and diabetic vascular complications. Diabetes Care 19:257–267
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9
West IC (2000) Radicals and oxidative stress in diabetes. Diabet Med 17:171–180
De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM (2000) Endothelial dysfunction in diabetes. Br J Pharmacol 130:963–974
Cameron NE, Cotter MA, Archibald V, Dines KC, Maxfield EK (1994) Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and streptozotocin-diabetic rats. Diabetologia 37:449–459
Cameron NE, Cotter MA, Maxfield EK (1993) Anti-oxidant treatment prevents the development of peripheral nerve dysfunction in streptozotocin-diabetic rats. Diabetologia 36:299–304
Karasu C, Dewhurst M, Stevens EJ, Tomlinson DR (1995) Effects of anti-oxidant treatment on sciatic nerve dysfunction in streptozotocin-diabetic rats; comparison with essential fatty acids. Diabetologia 38:129–134
Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA (2000) Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res 1:131–143
Terata K, Coppey LJ, Davidson EP, Dunlap JA, Gutterman DD, Yorek MA (1999) Acetylcholine-induced arteriolar dilation is reduced in streptozotocin induced diabetic rats with motor nerve dysfunction. Br J Pharmacol 128:837–843
Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA (2001) Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes 50:1927–1937
Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Salvemini D, Yorek MA (2001) Effect of M40403 treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Br J Pharmacol 134:21–29
Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L (2001) The role of oxidative stress in the onset and progression of diabetes and its complications. Diabetes Metab Res Rev 17:189–212
Lum H, Roebuck KA (2001) Oxidant stress and endothelial cell dysfunction. Am J Physiol 280:C719–C741
Yorek MA (2003) The role of oxidative stress in diabetic vascular and neural disease. Free Radic Res 37:471–480
Schnackenberg CG (2002) Physiological and pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 18:176–184
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek M, Beebe D, Oates PJ, Hammes HP, Giardinl I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature 404:787–790
Brownlee M (2001) Biochemistry and molecular biology of diabetic complications. Nature 414:813–820
Coppey LJ, Gellett JS, Davidson EP, Yorek MA (2003) Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res 37:33–40
Coleman MD, Eason RC, Bailey CJ (2001) The therapeutic use of lipoic acid in diabetes: a current perspective. Environ Toxicol Pharmacol 10:167–172
Packer L, Kraemer K, Rimbach G (2001) Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 17:888–895
Schnackenberg CG, Wilcox CS (2001) The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int 59:1859–1864
Nassar T, Kadery B, Lotan C, Da’as N, Kleinman Y, Haj-Yehia A (2002) Effects of the superoxide dismutase-mimetic compound tempol on endothelial dysfunction in streptozotocin-induced diabetic rats. Eur J Pharmacol 436:111–118
Pieper GM, Peltier BA (1995) Amelioration by l-arginine of a dysfunctional arginine/nitric oxide pathway in diabetic endothelium. J Cardiovasc Pharmacol 25:397–403
Pieper GA, Siebeneich W, Dondlinger LA (1996) Short-term oral administration of l-arginine reverses defective endothelium-dependent relaxation and cGMP generation in diabetes. Eur J Pharmacol 317:317–320
Giugliano D, Marfella R, Verrazzo G, Acampora R, Coppola L, Cozzolino D, D’Onofrio F (1997) The vascular effects of l-arginine in humans. J Clin Invest 99:433–438
Giugliano D, Marfella R, Coppola L, Verrazzo G, Acampora R, Giunta R, Nappo F, Lucarelli C, D’Onofrio F (1997) Vascular effects of acute hyperglycemia in humans are reversed by l-arginine. Circulation 95:1783–1790
Yildirim S, Ayan S, Sarioglu Y, Gultekin Y, Butuner C (1999) The effects of long-term oral administration of l-arginine on the erectile response of rabbits with alloxan-induced diabetes. BJU Int 83:679–685
Pieper GA, Dondlinger LA (1997) Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J Pharmacol Exp Ther 283:684–691
Kim YK, Lee MS, Son SM, Kim I, Lee WS, Rhim BY, Hong KW, Kim CD (2002) Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes 51:522–527
Li Y, Trush MA (1998) Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun 253:295–299
Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA (2002) Effect of treating streptozotocin-induced diabetic rats with sorbinil, myo-inositol or aminoguanidine on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Int J Exp Diabetes Res 3:21–36
Yorek MA, Coppey LJ, Gellett JS, Davidson EP, Lund DD (2004) Effect of Fidarestat and α-lipoic acid on diabetes-induced epineurial arteriole vascular dysfunction. Exp Diabesity Res 5:123–135
Dincer Y, Telci A, Kayah R, Yilmaz IA, Cakatay U, Akcay T (2002) Effect of α-lipoic acid on lipid peroxidation and anti-oxidant enzyme activities in diabetic rats. Clin Exp Pharmacol Physiol 29:281–284
Jones W, Li X, Zhi-Chao Q, Perriott L, Whitesell RR, May JM (2002) Uptake, recycling, and antioxidant actions of α-lipoic acid in endothelial cells. Free Radic Biol Med 33:83–93
Haramaki N, Han D, Handelman GJ, Tritschler HJ, Packer L (1997) Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid. Free Radic Biol Med 22:535–542
Arner ES, Nordberg J, Holmgren A (1996) Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase. Biochem Biophys Res Commun 225:268–274
Constantinescu A, Pick U, Handelman GJ, Haramaki N, Han D, Podda M, Tritschler HJ, Packer L (1995) Reduction and transport of lipoic acid by human erythrocytes. Biochem Pharmacol 50:253–261
Nakamura J, Hamada Y, Chaya S, Nakashima E, Naruse K, Kato K, Yasuda Y, Kamiya H, Sakakibara F, Koh N, Hotta N (2002) Transition metals and polyol pathway in the development of diabetic neuropathy in rats. Diabetes Metab Res Rev 18:395–402
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Yorek, M.A. (2014). Oxidative Stress and Diabetes-Induced Vascular Dysfunction: Role in Diabetic Neuropathy. In: Obrosova, I., Stevens, M., Yorek, M. (eds) Studies in Diabetes. Oxidative Stress in Applied Basic Research and Clinical Practice. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4899-8035-9_1
Download citation
DOI: https://doi.org/10.1007/978-1-4899-8035-9_1
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4899-8034-2
Online ISBN: 978-1-4899-8035-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)