Abstract
Oat (Avena sativa L.) productivity is affected by crown rust (Puccinia coronata f. sp. avenae) and stem rust (Puccinia graminis f. sp. avenae) worldwide. Control of these diseases has been through the use of host resistance genes, but frequent changes in pathogen virulence provide a continuing threat to oat production. Wild oat species have been a major source of diversity for the improvement of cultivated oat. Many rust resistance genes, as well as genes providing resistance to other major oat diseases, have been found in wild oat species, landraces as well as in cultivated species and have been utilised in plant breeding. However, the transfer of resistance from wild diploid and tetraploid species to cultivated hexaploid oat is difficult because their chromosomes do not pair readily. Nevertheless, many improved oat cultivars possess alien-derived rust resistance genes and occupy considerable acreage in the major oat-producing regions of the world. This chapter reviews the major developments and their impacts on oat breeding, specifically through alien gene transfer from wild and related species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Oat (Avena sativa L.) is an ancient cereal crop which has been cultivated at least since the time of Theophrastus (371–286 bc) and grown worldwide (Martens 1985). The genus Avena consists of diploid (2n = 2x = 14), tetraploid (2n = 4x = 28) and hexaploid (2n = 6x = 42) species, with a basic chromosome number of seven (x = 7) (Thomas 1992). The hexaploid Avena genome is comprised of three subgenomes (A, C and D), which represents modifications of a common homologous chromosome series (Rajhathy and Thomas 1974). Diploid and tetraploid species are classified into A, B, C and D genome groups, with further subdivisions based on karyotypes and chromosome pairing in interspecific hybrids. The cultivated white hexaploid oat (A. sativa L.) spread from the Near East to Europe in the late Bronze Age, while the cultivated red oat (A. byzantina C. Koch) was the main form in North Africa and in Spain. Both species were introduced into North America in the sixteenth century (Coffman 1977).
One of the major constraints to oat production worldwide is plant disease (Harder and Haber 1992). Among several diseases that attack oat, crown rust and stem rust are the most damaging, affecting both yield and quality of oat grain and forage. Up to 35 % yield loss due to severe stem rust infection has been found in Canada (Martens 1978), while up to 50 % loss due to crown rust infection has been estimated in the United States (Simons 1985). Both rust diseases have primarily been controlled through the use of host resistance genes (McCallum et al. 2007). Many accessions of wild hexaploid and diploid oat have been found to be sources of desirable rust resistance genes for incorporation into cultivated oat. The identification of wild accessions with effective resistant genes is an important step in the process of developing locally adapted cultivars with improved crown rust and stem rust resistance. The genetic characterisation of rust resistance genes in Avena species has been carried out by researchers since the 1920s.
Parker (1920) was one of the earliest workers to investigate crown rust resistance in oat. He recognised the importance of determining the inheritance of rust resistance to plant breeding, and that linkage of crown rust resistance with undesirable traits could be a hindrance to crop improvement. Rust resistance types have been categorised broadly into specific (major, vertical, seedling, etc.) or general (minor, horizontal, partial or adult plant resistance (APR)). Specific resistance, usually governed by single genes, is expressed at all growth stages, and, while relatively easy to identify and incorporate into elite germplasm, has been frequently overcome by rust races with matching virulence. Partial resistance, usually conferred polygenically, does not prevent infection completely but reduces pustule size and numbers of spores produced and extends the latency period of pustule development (Portyanko et al. 2005). Partial resistance may be more durable than specific resistance because there is less selection pressure on the pathogen and therefore slows the evolution of virulence (Simons 1972). However, partial resistance is more difficult to use in plant breeding since several genes need to be incorporated, and is evaluated at the adult plant stage. Thus, specific resistance genes are desirable in plant breeding. While most of the hexaploid oat germplasm has been exploited in the search for new rust resistance genes, this chapter reviews the utilisation of alien genes to enhance rust resistance in oat.
3.2 Crown Rust
Crown rust, caused by the fungus Puccinia coronata f. sp. avenae, is a widespread and damaging disease of oat. Oat crown rust infection is favoured by periods of high leaf moisture (rainfall or dew) and moderate temperatures (21–25 °C) during the growing season (Carson 2011). Crown rust occurs commonly on wild A. fatua in the United States and Canada (Leonard 2003; Chong et al. 2011) and on A. sterilis and other wild oat species in the Mediterranean regions of southern Europe, Northern Africa and the Middle East (Zillinsky and Murphy 1967). Losses in both yield and grain quality commonly result from epidemics of crown rust (Simons 1985), with individual oat fields suffering total crop failure. Crown rust generally attacks the leaves and interferes with transport of photosynthesised sugars from leaves to the developing grain, which causes shrivelled grain with reduced quality. Badly rusted plants develop stunted root systems and have poor drought tolerance. Control of crown rust disease can be attained using fungicides, but disease resistance is the most effective, cost-efficient and environment friendly control method.
Breeding for resistance to crown rust began in 1919 (Simons 1985), and to date over 100 alleles have been described (Table 3.1). Crown rust resistance genes have been identified from four main sources: A. byzantina, A. sativa, A. sterilis and lower ploidy material (diploid oat, mainly A. strigosa). The best sources of genes for resistance to P. coronata can be found among wild relatives of oat in the regions of its origin (Manisterski and Wahl 1995). Of the wild relatives, A. sterilis is the most important source of resistance genes (Segal et al. 1980; Simons 1985) and can be readily hybridised with cultivated oat. Although nearly 30 genes have been described in lower ploidy wild oat, relatively few have been used in oat breeding. Crown rust resistance genes (Pc genes) developed from the various sources are listed below.
3.2.1 A. byzantina
The first early successes in finding crown rust resistance genes were from A. byzantina introductions. Pc1, a dominant gene, was isolated from the cultivar Red Rustproof (Davies and Jones 1927), designated “S” by Dietz and Murphy (1930). Victoria, an introduction from Uruguay, was found to contain three genes: Pc2 (Murphy et al. 1937), Pc11 and Pc12 (Welsh et al. 1954). Bond, from Red Algerian/Gold Rain out of Australia (Welsh et al. 1953), contained two dominant complementary genes for crown rust resistance: Pc3 and Pc4 (Hayes et al. 1939). Pc5 was a gene found in Landhafer (Litzenberger 1949) and was an introduction from Germany, but it probably originated in South America (Coffman 1961). Another South American introduction was Santa Fe, which was the source of three resistance genes: Pc6 (Litzenberger 1949), Pc7 and Pc8 (Osler and Hayes 1953). Klein was an A. byzantina introduction from Argentina which came to North America from Australia (Welsh et al. 1953), and Klein 69b was found to contain Pc10 (Finkner 1954). Simons (1956) identified two genes in the variety Ascencao: one was determined to be Pc2 while the second, Pc14, was shown to be epistatic to Pc2.
3.2.2 A. sativa
It would appear that A. sativa contains relatively few naturally occurring resistance genes useful against P. coronata. Ukraine (Hutica), an introduction from Russia, contained two dominant complementary genes, Pc3c and Pc4c (Weetman 1942). Finkner (1954) showed that two closely linked alleles, Pc6c and Pc9, conferred resistance in Ukraine. But a later work by Sanderson (1960) showed that Ukraine resistance was due to a single dominant gene, Pc9. Trispernia, an introduction from Romania (Welsh et al. 1953), contained three genes for resistance, one of which was Pc6d (Finkner 1954). Pc13 was found to be present in the widely grown US oat cultivar Clinton (Finkner et al. 1955). Pc22 was isolated from the Welsh variety Ceirch du Bach (McKenzie 1961). The cultivar Garry in Canada was found by Upadhyaya and Baker (1960) to possess three seedling genes (Pc24, Pc25, Pc26) and two APR genes (Pc27, Pc28). Kyto oat was found to contain the dominant gene Pc44 (Martens et al. 1968). More recently, Pc96 was identified in A. sativa (Chong and Brown 1996) and is currently effective against predominant races of P. coronata in North America (Chong et al. 2011), thus is useful for combining with other effective Pc genes.
3.2.3 A. sterilis
A. sterilis is a wild hexaploid oat species which is native in the Mediterranean regions (Wong et al. 1983). Accessions were collected in Israel and other Mediterranean countries during the 1960s and the early 1970s (Simons et al. 1978). The transfer of resistance from wild hexaploid A. sterilis to cultivated oat, A. sativa, was reported to be more successful than from other related wild species (Martens and Dyck 1989). A. sterilis has been the richest source of crown rust resistance genes of all species. To date there have been 43 genes described, many of which are linked or allelic (Table 3.1). Most Pc genes derived from A. sterilis have been defeated in North America, due to single gene deployment and rapid virulence changes in the P. coronata population. For example, Pc68 was derived from A. sterilis and was one of the most effective major resistance genes deployed globally against crown rust, but virulence to this gene is now common in North America. Most of the A. sterilis genes for crown rust resistance are dominant or incompletely dominant, but there are exceptions as Pc54 was shown to be an incompletely recessive gene (Martens et al. 1980).
At least 16 genes from A. sterilis have been deployed at various times in North America (Leonard 2003). Pc38, Pc39 and Pc68, and to a lesser extent Pc48, have been used in developing resistant oat cultivars in the eastern prairie region of Canada. Pc39 was the first gene derived from A. sterilis to be deployed in Canada, with the release of “Fidler” in 1980. This was followed by the release of a series of cultivars (such as “Dumont”, “Robert” and “Riel”) with both Pc38 and Pc39 from 1982 to 1993. Pc38 and Pc39 became ineffective in the eastern prairie region in the mid-1990s, due to a major shift in virulence to these two genes in the prairie rust population (Chong and Seaman 1997). Subsequently, a series of cultivars with the Pc38 + 39 + 68 gene combination were released, starting with “AC Assiniboia” in 1995, followed by six other cultivars with the same gene combination. Since Pc38 and Pc39 were no longer effective in the 1990s, these cultivars basically were protected only by Pc68. In 1998, “Triple Crown” was released and its resistance was based on gene Pc48. In 2001, late-planted fields of this cultivar were severely damaged by crown rust (Chong and Zegeye 2004). By 2005 cultivars with the Pc38, Pc39, Pc68 gene combination (“AC Assiniboia” and “Ronald”) were severely damaged by crown rust (Chong et al. 2008), indicating that Pc68 was no longer effective in Canada. Since then, two putative new genes from A. sterilis (Temp_Pc97 and Temp_Pc98) have been described and are resistant to most current P. coronata races in Canada (Chong et al. 2011).
3.2.4 Diploid and Tetraploid Oat
Several wild diploid species of Avena, particularly A. strigosa, possess a high degree of resistance to crown rust. A. strigosa is primarily found in Western Europe and in countries of the former USSR. This species was widely cultivated for grain-fodder (Holden 1979) and is currently grown to a limited extent in Germany, the United Kingdom (Wales) and Australia. It is also used as a fodder oat in Brazil (Leonard and Martinelli 2005). Transfer of genes for crown rust resistance from A. strigosa to hexaploid oat was initially accomplished by producing an autotetraploid of A. strigosa followed by crossing to A. sativa (Zillinsky and Derick 1960). However, the resistant hexaploid lines isolated in the later generations exhibited a high degree of sterility and cytological instability (Dyck and Zillinsky 1963). Wild diploid A. strigosa accessions have also been found to be a better source of adult plant resistance when compared to accessions of the tetraploid species, A. barbata (Cabral et al. 2011). There have been 22 genes described from A. strigosa (Table 3.1). Of these genes, only Pc23 and Pc94 have been incorporated into a stable A. sativa background (Dyck and Zillinsky 1963; Aung et al. 1996).
The lack of effective crown rust resistance genes in cultivated oat and the ability of the pathogen to produce new races have resulted in the continuous search for new sources of resistance. The useful lifetime of a resistance gene can be relatively short due to changes in virulence in the pathogen population following widespread deployment of an introduced resistance gene. Early oat breeding efforts incorporated resistance genes from cultivated hexaploid oat (Martens and Dyck 1989). When all available sources of resistance were defeated by the late 1950s, new sources of crown rust resistance were identified in A. sterilis. More than 40 resistance genes have been described, but few are useful for oat breeding. Moderate levels of virulence already existed in the P. coronata f. sp. avenae population to many of these genes, even though they had not previously been deployed in North America (Chong and Kolmer 1993). Some of the genes were tightly linked or allelic, and virulence to some of the genes was associated (Chong and Brown 1996; Leonard et al. 2005b). Few crown rust genes have been described from wild diploid and tetraploid species, even though they are a rich source of genetic diversity (Simons et al. 1959). Pc91 is a highly effective gene derived from a cross between tetraploid A. magna Murphy and Terrell and diploid A. longiglumis Durr. (Rooney et al. 1994), with A. magna being the donor. Pc91 is currently the most effective crown rust resistance gene available in North America (McCartney et al. 2011). Pc94 was derived from A. strigosa (Aung et al. 1996) and was highly effective during 2002–2006 (Chong et al. 2008). Currently, oat breeders are interested in pyramiding Pc91 and Pc94 to slow the breakdown of these resistance genes. Pyramiding these genes in conjunction with APR genes may stabilise crown rust resistance.
3.3 Stem Rust
Stem rust is another major disease that threatens oat production worldwide. The disease is caused by the fungus Puccinia graminis Pers. f. sp. avenae Eriks. and E. Henn. The most severe epidemic recorded in Canada caused an estimated 35 % yield loss in the eastern prairie region in 1977 (Martens 1978). The most recent epidemic of oat stem rust in Canada occurred in the eastern prairie region in 2002, with estimated yield losses of 6.6 % (worth $12.6 million) in Manitoba and 0.5 % (worth $1.0 million) in Saskatchewan (Fetch 2005). The disease can be effectively controlled with host resistance or with fungicides. However, fungicides can be an expensive option for producers besides having environmental impacts. Therefore, the most efficient, economical and environmentally sound method of controlling the disease is through the use of host resistance.
Currently, only 17 oat stem rust resistance genes (Pg genes) and the Pg-a complex have been described (Martens 1985; Fetch and Jin 2007) and are listed in Table 3.2. Most are from A. sativa, but a few are from wild oat (Martens 1985). Oat stem rust resistance genes are rare (Martens and Dyck 1989), and only a few genes (Pg2, Pg13, Pg-a) provide protection against the current races in the North American population of P. g. f. sp. avenae (Mitchell Fetch and Fetch 2011). Harder (1994) reported that only five genes (Pg1, Pg2, Pg4, Pg9 and Pg13) had been intentionally deployed in Canadian oat cultivars, and most resistant cultivars likely possessed the Pg2 + Pg13 combination. This combination was effective against all stem rust races in North America, but new races (NA67, NA76) with virulence to these genes evolved and are common in North America (Fetch and Dunsmore 2004).
Virulence in the population of P. g. f. sp. avenae appears to be increasing (Mitchell Fetch and Fetch 2011); thus new genes are highly desirable. Only Pg6, Pg10, Pg11, Pg12, Pg16 and the Pg-a complex confer resistance to NA67 and NA76 (Fetch and Dunsmore 2004). Genes Pg10, Pg11, Pg12 and the Pg-a complex are from a hexaploid background. To date, only the Pg-a complex has been deployed in oat cultivars in Canada and the United States, but the frequency of virulent races to Pg-a is increasing in North America. The Pg10 gene has been used in Canadian oat breeding programmes, but no cultivars have been developed to date. This gene confers characteristic large necrotic halos around rust pustules and on stems of adult plants (Harder 1999), and while no virulence has been detected in North America, there is virulence to Pg10 in Ethiopia (Fetch, unpublished data). Gene Pg11 is an APR gene and is effective to all races of oat stem rust, but is associated with chlorophyll deficiency (Harder et al. 1971) and is undesirable for breeding. Gene Pg12 is effective only at the seedling stage (Martens 1985).
Since the virulence spectrum in P. g. f. sp. avenae is increasing and most known genes are either ineffective or undesirable, new genes are needed. A recent study evaluated nearly 10,000 lines from various species of Avena for resistance to race NA67. Results indicated that A. strigosa is the most promising source of resistance and that additional resistance genes in hexaploid oat are unlikely to be found (Gold Steinberg et al. 2005). Gene Pg16 has been successfully transferred from the tetraploid A. barbata into hexaploid oat by irradiation (Brown 1985); however it is a 44-chromosome addition line and appears to reduce yield by about 10 % (J. Mitchell Fetch, unpublished). Efforts to reduce the chromosome number to 42 and maintain the resistance have not been successful. Gene Pg6 derives from the diploid species A. strigosa and is resistant to most races of oat stem rust in North America except BLD (NA1) and CLD (NA70) (Fetch and Jin 2007). This gene reportedly was transferred into the American oat cultivar “Delredsa” (Rothman 1984), but multipathotype tests indicated Pg6 was not present (Fetch, unpublished data). Recent efforts have been made by Zegeye (2008) to transfer resistance identified in the Gold Steinberg et al. (2005) study from A. strigosa into A. sativa. Efforts were partially successful, but resistant lines were chromosome addition lines, and no hexaploid derivatives containing A. strigosa resistance to oat stem rust has yet been developed.
3.4 Transfer of Rust Resistance from Wild Oat Accessions to Common Oat
There are many sources of rust resistance from diploid and tetraploid wild oat species. However, while transferring resistance from lower ploidy material to hexaploid wheat has been highly successful (Knott 1987), this has not been realised in oat. There are seven different genomes identified in diploid oat species and at least three in tetraploid species (Rajhathy and Thomas 1974). These genomes have differing affinities for pairing with the A. sativa genome, thus developing meiotically stable progeny from interploidy crosses has been difficult. Interploidy transfer, particularly from diploid to hexaploid genomes, often requires special manipulations such as embryo rescue or development of a synthetic hexaploid, which can be crossed to a hexaploid cultivar (Innes and Kerber 1994). For example, Pc91 is a highly effective gene in hexaploid oat that was transferred using a synthetic hexaploid derived from a cross between tetraploid A. magna Murphy and Terrell and diploid A. longiglumis Durr. (Rooney et al. 1994). In oat, diploid to hexaploid resistance gene transfers have also been facilitated by generation of autotetraploid, derived tetraploid and amphiploid lines (Sadanaga and Simons 1960). In contrast, the transfer of rust resistance genes from wild A. sterilis is easy as this species pairs readily with A. sativa.
The process of resistance gene transfer from wild to cultivated oat is often hindered by sterility barriers (Aung et al. 1977). Thus, generation of fertile progeny may involve the rescue of hybrids or F1 embryos. The choice of the female and pistillate parent in interploidy crosses is also an important consideration, with the lower ploidy genotype being the preferred pistillate parent (Rajhathy and Thomas 1974). On the contrary, retaining the higher ploidy hexaploid as the pistillate parent yielded more vigorous F1 seeds from interspecific crosses of hexaploid oat cv. “Wintaroo” and diploid A. strigosa genotypes (Cabral et al. 2013). Additionally, instances of suppressor genes/factors of the donor parent interfering with the expression of resistance in interspecific F1 progeny have been reported. Rines et al. (2007) reported a suppressor factor in diploid line CI6954SP that contributed to susceptible F1 progeny when crossed with A. sativa. The genePc38 suppresses the expression of Pc94 (Aung et al. 1996), which has been introgressed into A. sativa from A. strigosa.
Even if generation of fertile progeny from interspecific crosses is achieved, the introgression of resistance into the genome of hexaploid oat cultivars is very difficult to achieve by means of regular backcrossing procedures. This is mainly due to the low frequency of chromosome pairing between wild species with cultivated oat, consequently reducing the chance of recombination and gene transfer. Rajhathy and Thomas (1974) reported that a genotype of A. longiglumis (CW57) suppressed the activity of the gene(s) controlling regular bivalent pairing in A. sativa and induced pairing between nonhomologous chromosomes, resulting in translocation between two homologous chromosomes as well as between unrelated chromosomes. The CW57 gene(s) induced pairing between line RL1697 (A. strigosa) and line Sun II (A. sativa), which facilitated the transfer of Pc94 crown rust into a hexaploid genetic background (Chong et al. 2011).
Though the transfer of resistance genes from wild species into hexaploid oat is difficult, several success stories and methods have been reported. Ladizinsky (1995) described the domestication of two wild tetraploid oat species not by selection of rare mutations but by transfer of genes into cultivated oat through hybridisation. He later developed a synthetic hexaploid oat (2000) by crossing the A. strigosa (2n = 14) cv. “Saia” with A. magna (2n = 28). Chromosome doubling of the resulting sterile triploid hybrid produced a synthetic hexaploid, which was intermediate between its parents in panicle shape and lemma colour. Progeny were similar to the tetraploid parent in spikelet structure and to the diploid parent in having a single, albeit partially shrivelled seed per spikelet and low protein content. Fox (1989) crossed three tetraploid accessions with Rodney O (hexaploid A. sativa) and produced highly fertile progeny with resistance to crown rust races CR13 and CR 50, but two diploid accessions crossed with Rodney O did not produce any viable seeds.
Zillinsky and Derick (1960) first reported the transfer of genes from wild diploid A. strigosa into hexaploid oat. They created an autotetraploid line by doubling the chromosome number of diploid material using colchicine and crossed the progeny to hexaploid oat. However, these transfers were genetically unstable and autotetraploids were partially sterile (Dyck and Zillinsky 1962; Sadanaga and Simons 1960). Marshall and Myers (1961) directly crossed A. strigosa with A. sativa and had no difficulty in obtaining seeds, but were only water filled and became extremely shrivelled when dry. Much work was done with CD 3820, an A. strigosa line, where several genes for crown rust resistance were found. Dyck and Zillinsky (1963) showed the presence of two independent genes, Pc15 and Pc23. Pc15 proved to be located on a chromosome that failed to pair with any A. sativa chromosomes. In lines homozygous for Pc15, the chromosome number was 44 where Pc15 was found on the extra chromosome pair. Pc23 appeared to be completely incorporated into normal A. sativa (Dyck and Zillinsky 1963).
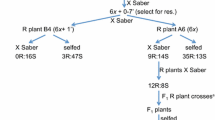
Rines et al. (2007) attempted two methods to transfer crown rust resistance from a diploid A. strigosa into a hexaploid A. sativa. The first method directly crossed the diploid line CI6954SP with a hexaploid to obtain tetraploid F1 progeny, which were subsequently treated with colchicine to generate a synthetic octaploid for subsequent backcrossing to the hexaploid parent. The second method crossed CI6954SP with a tetraploid A. murphyi line to make a synthetic hexaploid for subsequent crossing into a hexaploid background. Although the direct method requires the laborious crossing and embryo rescue to develop a fertile octaploid line, it provided faster recovery of plants with high fertility, full transmission of resistance and desired plant and seed phenotypes. Similar work was done by Zegeye (2008) to directly transfer stem rust resistance from A. strigosa into hexaploid oat. The general crossing scheme and methodology followed is shown in Fig. 3.1. Crosses and reciprocal crosses between the diploid A. strigosa accessions and hexaploid A. sativa cultivar Sun II were made, and embryo rescue and colchicine treatment produced octoploid progeny. Synthetic octaploid seeds were larger than the parental seeds (Fig. 3.2) and the seedlings were more vigorous. Progeny were selfed to the F2 and F3 generation and backcrossed to Sun II. Cytological evaluation of resistant BC1F2 progenies found 43–47 chromosomes (Fig. 3.3) and progeny tests of BC1F2 and BC1F3 with NA67 identified resistant seedlings. Rajhathy and Thomas (1974) reported that the line Cw57 can be used to induce chromosome pairing between the “As” genome of A. strigosa and the “A” genome of A. sativa. Thus, F1 octaploid seeds were produced from crossing diploid A. longiglumis (Cw57) with Sun II, which was subsequently crossed to highly resistant BC1F3 progeny lines containing 43–44 chromosomes from CN57130/Sun II//Sun II. F1 hybrid seeds were obtained, which will be repeatedly backcrossed to Sun II until the resistance from A. strigosa is stabilised in a 42-chromosome background.
The use of irradiation to facilitate the transfer of resistance genes has also been reported. Mildew resistance was transferred from tetraploid A. barbata to cultivated oat A. sativa (Aung and Thomas 1976; Aung et al. 1977) using irradiation to induce translocations in a disomic addition line (2n = 6x = 44) of an A. sativa that contained a pair of A. barbata chromosomes carrying the mildew resistance gene. Sharma and Forsberg (1977) treated a monosomic substitution line with thermal neutrons and were successful in transferring the Pc15 gene from A. strigosa into the cv. Clarian, which showed normal breeding behaviour and could be used in a breeding programme. The main limitation of the irradiation-induced gene transfers is that they are the products of reciprocal translocation and involve a deletion and duplication (Thomas 1992).
3.5 Relationship of Rust Resistance Genes and Molecular Markers
The exact position of rust resistance genes on oat chromosomes has not yet been described, because unlike the other cereal grains, there has not yet been any specific chromosome numbers allocated. Currently, there are 34 linkage groups mapped (Tanhuanpää et al. 2012), but work is underway to anchor molecular markers and develop a 21-chromosome map. Since no rust resistance genes have yet been located to a specific chromosome position, many genes could be alleles or the same gene since in most cases no full genetic studies exist. In crown rust, five linkage groups that have been identified and cultivars Santa Fe, Ukraine and Trispernia have exhibited allelism or close linkage for at least one gene (Finkner 1954). A number of crown rust resistance genes are clustered in the oat genome, including Pc46, Pc50 and Pc68 (Wong et al. 1983); Pc38, Pc62 and Pc63 (Harder et al. 1980); Pc39 and Pc55 (Kiehn et al. 1976); Pc35, Pc54 and Pc96 (Martens et al. 1980; Chong and Brown 1996); and Pc68, Pc44, Pc46, Pc50, Pc95 and PcX (Chong et al. 1994). Leonard et al. (2005a) suggested that genes Pc39, Pc55 and Pc71 may be identical or nearly identical alleles. It has also been postulated that gene Pc94 might be close to or part of PcA (Rines et al. 2007). In stem rust, genes Pg1, Pg2and Pg8 are clustered, Pg3 and Pg9 are linked, and Pg4 and Pg13 are associated (Martens 1985).
Crown rust and stem rust resistance genes have also been shown to be linked together. Martens et al. (1968) suggested that there were three alleles for crown rust resistance and two alleles for stem rust resistance at or near the Pc44 locus, and Pc44 was linked in repulsion to Pg9. Pg9 was associated with crown rust resistance in Ukraine oat (McKenzie et al. 1965). The mapping of resistance gene analogues (RGA) on the Kanota × Ogle (KO) by Sanz et al. (2012) showed that both the nucleotide binding site (NBS) and protein kinase (PK)-based markers significantly co-localised with loci conferring resistance to P. coronata, i.e. Pc39 (KO16_23), Pc54/Pc59 and Pc68 (KO4_12) and Pc58 (KO17), as well as with a locus conferring resistance to P. graminis, i.e., Pg13 (KO3 + 38).
Many race-specific resistance genes have been mapped, and markers that are closely linked to crown and stem rust genes have been identified (Table 3.3). An avenin protein marker is linked to Pg3 and Pg9 (Howes et al. 1992; Chong et al. 1994), and three avenin storage protein loci as well as two RGA markers are very tightly linked to Pc68 (Satheeskumar et al. 2011). A random amplified polymorphic DNA (RAPD) marker has been linked in repulsion to Pg3 (Penner et al. 1993a). The latter RAPD primers also produced a marker which, together with three restriction fragment length polymorphism (RFLP) markers and a third RAPD marker, were shown to be linked to Pg9 (O'Donoughue et al. 1996). Gene Pg13 has been mapped in two populations to linkage groups homologous to KO3 (O'Donoughue et al. 1996) and a second stem rust resistance gene Pg4 (McKenzie et al. 1970). Pg13 is also linked to a 56.6-kDa avenin storage protein marker (Howes et al. 1992; Chong et al. 1994). Personal Communication (2013) developed SCAR, CAPS and SSR markers based on amplified fragments linked to Pg3, Pg9 and Pg13 obtained using the RAPD primers ubc195, ubc269 and ubc254. Molecular markers can be used to facilitate pyramiding of genes, a breeding strategy designed to provide more durable control of rust by combining several resistance genes in one cultivar (Pedersen and Leath 1988).
Molecular markers can also be used in counter-selection. The gene Pc38 would be an excellent choice, as it is known to suppress the action of genes Pc62 (Wilson and McMullen 1997) and Pc94 (Chong and Aung 1996). Molecular markers developed for any one particular rust resistance gene in a cluster will also be useful for the study of other disease resistance genes found within the same cluster. A KASP assay was developed from the previously reported nonredundant DArTs that co-segregated with Pc91 (Gnanesh et al. 2013). The KASP assay was used for marker-assisted selection for crown rust resistance gene Pc91 in an F2 population developed from the cross of AC Morgan × Stainless (Fig. 3.4). Sanz et al. (2012) reported the PK-RFLP and PK profiling markers co-localise with Pc71 (KO11_41 + 20) and Pc91 (KO3 + 38), whereas clusters of mixed markers based on NBS and PK domains co-localise with genes Pc38 (KO17), Pc94 (KO17) and PcX (KO4_12). Comparative mapping of disease resistance loci with reference populations such as Kanota × Ogle (KO) (Wight et al. 2003), Ogle × TAM O-301 (Portyanko et al. 2001) and Ogle × MAM17-5 (OM) (Zhu and Kaeppler 2003) increases the number of potential molecular markers available for resistance genes and furthers our understanding of their organisation in the genome. The development of anchored markers on a 21-chromosome map is the first step in identifying the location of rust resistance genes and would help immensely in the identification of new resistance genes introgressed from wild species.
3.6 Future Outlook on Alien Introgression in Oat
Wild species are an important and abundant source of new rust resistance genes. Previous studies have identified numerous rust resistance genes in diploid and tetraploid wild species, but few have been successfully transferred. Although it appears that initial transfer into chromosome addition lines is relatively simple, introgression and reduction to 42-chromosome cultivated oat have been difficult. New techniques are needed to find better methods of stabilising rust resistance from wild species, as virulence in P. coronata and P. graminis f. sp. avenae appears to be increasing worldwide.
References
Aung T, Thomas H (1976) Transfer of mildew resistance from the wild oat Avena barbata into the cultivated oat. Nature 260:603–604
Aung T, Thomas H, Jones IT (1977) The transfer of the gene for mildew resistance from Avena barbata (4x) into the cultivated oat A. sativa by an induced translocation. Euphytica 26:623–632
Aung T, Chong J, Leggett M (1996) The transfer of crown rust resistance Pc94 from a wild diploid to cultivated hexaploid oat. In: Kema GHJ, Niks RE, Daamen RA (eds) Proc. 9th Int. Eur. Mediterr. Cereal Rusts and Powdery Mildews Conf. Lunteren Netherlands. Wageningen, European and Mediterranean Cereal Rust Foundation, pp 167–171
Brown PD (1985) The transfer of oat stem rust resistance gene Pg-16 from tetraploid Avena barbata Pott. To hexaploid Avena sativa L., Univ. Wisconsin, Madison, USA. Dissertation abstracts Int B Sci and Eng 45:7, p 2036B
Browning JA, Frey KJ (1959) The inheritance of new sources of oat stem rust resistance. Plant Dis Rep 43:768–771
Bush AL, Wise RP (1996) Crown rust resistance loci on linkage groups 4 and 13 in cultivated oat. J Hered 87:427–432
Bush AL, Wise RP (1998) High-resolution mapping adjacent to the Pc71 crown-rust resistance locus in hexaploid oat. Mol Breed 4:13–21
Cabral AL, Singh D, Park RF (2011) Identification and genetic characterisation of adult plant resistance to crown rust in diploid and tetraploid accessions of Avena. Ann Appl Biol 159:220–228
Cabral AL, Karaoglu H, Park RF (2013) The use of microsatellite polymorphisms to characterise and compare genetic variability in Avena strigosa and A. barbata. Genet Resour Crop Evol 60:1153–1163
Carson ML (2011) Virulence in oat crown rust (Puccinia coronata f. sp. avenae) in the United States from 2006 through 2009. Plant Dis 95:1528–1534
CDL (2010) Cereal Disease Laboratory, St. Paul, Minnesota. http://www.ars.usda.gov/Main/docs.htm?doc id = 10342
Chang TT (1959) Analysis of genes conditioning resistance of oat varieties to races of Puccinia coronata Cda.var. avenae F. and L. Dissertation abstracts 20:1133. PhD thesis, University of Minnesota, St. Paul, Minnesota
Chen G, Chong J, Gray M, Prashar S, Procunier JD (2006) Identification of single-nucleotide polymorphisms linked to resistance gene Pc68 to crown rust in cultivated oat. Can J Plant Pathol 28:214–222
Chen G, Chong J, Prashar S, Procunier JD (2007) Discovery and genotyping of high-throughput SNP markers for crown rust resistance gene Pc94 in cultivated oat. Plant Breed 126:379–384
Chong J, Aung T (1996) Interaction of the crown rust resistance gene Pc94 with several Pc genes. In: Kema GHJ, Niks RE, Daamen RA (eds) Proc. 9th Int. Eur. mediterr. Cereal rusts and powdery mildews conf. Lunteren, Netherlands. Wageningen, European and Mediterranean Cereal Rust Foundation, pp 167–171
Chong J, Brown PD (1996) Genetics of resistance to Puccinia coronata f. sp. avenae in two Avena sativa accessions. Can J Plant Pathol 18:286–292
Chong J, Kolmer JA (1993) Virulence dynamics and phenotypic diversity of Puccinia coronata f.sp. avenae in Canada from 1974 to 1990. Can J Bot 71:248–255
Chong J, Seaman WL (1997) Incidence and virulence of Puccinia coronata f. sp. avenae in Canada in 1995. Can J Plant Pathol 19:176–180
Chong J, Zegeye T (2004) Physiologic specialization of Puccinia coronata f. sp. avenae, the cause of oat crown rust, in Canada from 1999 to 2001. Can J Plant Pathol 26:97–108
Chong J, Howes NK, Brown PD, Harder DE (1994) Identification of the stem rust resistance gene Pg9 and its association with crown rust resistance and endosperm proteins in ‘Dumont’ oat. Genome 37(3):440–447
Chong J, Reimer E, Somers D, Aung T, Penner GA (2004) Development of sequence-characterized amplified region (SCAR) markers for resistance gene Pc94 to crown rust in oat. Can J Plant Pathol 26:89–96
Chong J, Gruenke J, Dueck R, Mayert W, Woods S (2008) Virulence of oat crown rust [Puccinia coronata f. sp. avenae] in Canada during 2002–2006. Can J Plant Pathol 30:115–123
Chong J, Gruenke J, Dueck R, Mayert W, Mitchell Fetch J, McCartney C (2011) Virulence of Puccinia coronata f. sp. avenae in the Eastern Prairie Region of Canada during 2007–2009. Can J Plant Pathol 33:77–87
Coffman FA (1961) Origin and history. In: Coffman FA (ed) Oats and oat improvement. American Society of Agronomy, Madison, WI, pp 15–40
Coffman FA (1977) Oat history, identification and classification. Technical bull. no. 1516. USDA-ARS, Washington, DC
Davies DW, Jones ET (1927) Further studies on the inheritance of resistance to crown rust (P. coronata Corda) in F3 segregates of a cross between Red Rustproof (A. sterilis) Scotch Potato oats (A. sativa). Welsh J Agric 11:232–235
Dietz SM (1928) Inheritance of resistance in oats to Puccinia graminis avenae. J Agric Res 37:1–23
Dietz SM, Murphy HC (1930) Inheritance of resistance to Puccinia coronata avenae, p.f. III. Phytopathology 20:120
Dyck PL (1966) Inheritance of stem rust resistance and other characteristics in diploid oats, Avena strigosa. Can J Genet Cytol 8:444–450
Dyck PL, Zillinsky FJ (1962) Segregation for crown and stem rust resistance in diploid and autotetraploid Avena strigosa. Can J Genet Cytol 4:469–474
Dyck PL, Zillinsky FJ (1963) Inheritance of crown rust resistance transferred from diploid to hexaploid oats. Can J Genet Cytol 5:398–407
Fetch TG Jr (2005) Races of Puccinia graminis on wheat, barley, and oat in Canada, in 2002 and 2003. Can J Plant Pathol 27:572–580
Fetch TG Jr, Dunsmore KM (2004) Physiologic specialization of Puccinia graminis on wheat, barley, and oat in Canada in 2001. Can J Plant Pathol 26:148–155
Fetch TG Jr, Jin Y (2007) Letter code system of nomenclature for Puccinia graminis f. sp. avenae. Plant Dis 91:763–766
Finkner VC (1954) Genetics factors governing resistance and susceptibility of oats to Puccinia coronata Corda var. avenae, F. and L., race 57. Iowa Agric Exp Stn Res Bull 411:1039–1063
Finkner RE, Atkins RE, Murphy HC (1955) Inheritance of resistance to two races of crown rust in oats. Iowa State Coll J Sci 30:211–228
Fleischmann G, McKenzie RIH (1968) Inheritance of crown rust resistance in Avena Sterilis L. Crop Sci 8:710–713
Fleischmann G, McKenzie RIH, Shipton WA (1971a) Inheritance of crown rust resistance in Avena sterilis L. from Israel. Crop Sci 11:451–453
Fleischmann G, McKenzie RIH, Shipton WA (1971b) Inheritance of crown rust resistance genes in Avena sterilis collections from Israel, Portugal, and Tunisia. Can J Genet Cytol 13:251–255
Fox SL (1989) Crown rust resistance in wild oats: presence, inheritance, and interspecific gene transfer to common oats. University of Manitoba, MSc thesis, Manitoba, USA
Fox SL, Brown PD, Chong J (1997) Inheritance of crown rust resistance in four accessions of Avena sterilis L. Crop Sci 37:342–345
Garber RJ (1921) A preliminary note on the inheritance of rust resistance in oats. Am Soc Agron J 13:41–43
Gnanesh BN, Mitchell Fetch J, Menzies JG, Beattie AD, Eckstein PE, McCartney CA (2013) Chromosome location and allele-specific PCR markers for marker-assisted selection of the oat crown rust resistance gene Pc91. Mol Breed:1-8. doi:10.1007/s11032-013-9900-6
Gold Steinberg J, Mitchell Fetch J, Fetch TG (2005) Evaluation of Avena spp. accessions for resistance to oat stem rust. Plant Dis 89:521–525
Harder DE (1994) Identification of new races of Puccinia grminis f.sp. avenae. Plant Dis 78(4):367–368
Harder DE (1999) Usefulness of gene Pg10 as a source of stem rust resistance in oat breeding. Phytopathology 89:1214–1217
Harder DE, Haber S (1992) Oat diseases and pathological techniques. In: Marshall HG, Sorrells ME (eds) Oat science and technology. Madison, WI, ASA
Harder DE, Martens JW, McKenzie RIH (1971) Changes in chlorophyll and carotenoid content in oats associated with the expression of adult plant resistance to stem rust conferred by gene Pg-11. Can J Bot 49:1783–1785
Harder DE, McKenzie RIH, Martens JW (1980) Inheritance of crown rust resistance in three accessions of Avena sterilis. Can J Genet Cytol 22:27–33
Harder DE, McKenzie RIH, Martens JW (1984) Inheritance of adult plant resistance to crown rust in an accession of Avena sterilis. Phytopathology 74:352–353
Harder DE, Chong J, Brown PD, Martens JW (1990) Inheritance of resistance to Puccinia coronata avenae and P. Graminis avenae in an accession of Avena sterilis from Spain. Genome 33:198–202
Harder DE, Chong J, Brown PD (1995) Stem and crown rust resistance in the Wisconsin oat selection X1588-2. Crop Sci 35:1011–1015
Hayes HK, Moore MB, Stakman EC (1939) Studies of inheritance in crosses between Bond, Avena byzantina, and varieties of A. sativa. Minn Agric Expt Sta Tech Bull 137:1–38
Hoffman DL, Chong J, Jackson EW, Obert DE (2006) Characterization and mapping of a crown rust resistance gene complex (Pc58) in TAM O-301. Crop Sci 46:2630–2635
Holden JHW (1979) Oats Avena spp. (Gramineae-Avenae). In: Simmonds NW (ed) Evolution in crop plants. Longman, London, pp 86–90
Howes NK, Chong J, Brown PD (1992) Oat endosperm proteins associated with resistance to stem rust of oats. Genome 35:120–125
Innes RL, Kerber ER (1994) Resistance to wheat leaf rust and stem rust in Triticum tauschii and inheritance in hexaploid wheat of resistance transferred from T. tauschii. Genome 37:813–822
Irigoyen ML, Loarce Y, Fominaya A, Ferrer E (2004) Isolation and mapping of resistance gene analogs from the Avena strigosa genome. Theor Appl Genet 109:713–724
Kiehn FA, McKenzie RIH, Harder DE (1976) Inheritance of resistance to Puccinia coronata avenae and its association with seed characteristics in four accessions of Avena sterilis. Can J Genet Cytol 12:230–236
Knott DR (1987) Transferring alien genes to wheat. In: Heyne EG (ed) Wheat and wheat improvement, 2nd edn. American Society of Agronomy, Madison, WI, pp 462–471
Kremer CA, Lee M, Holland JB (2001) A restriction fragment length polymorphism based linkage map of a diploid Avena recombinant inbred line population. Genome 44:192–204
Kulcheski FR, Graichen FAS, Martinelli JA, Locatelli AB, Federizzi LC, Delatorre CA (2010) Molecular mapping of Pc68, a crown rust resistance gene in Avena sativa. Euphytica 175:423–432
Ladizinsky G (1995) Domestication via hybridization of the wild tetraploid oats Avena magna and A. murphyi. TAG Theor Appl Genet 91(4):639–646
Leonard KJ (2003) Regional frequencies of virulence in oat crown rust in the United States from 1990 through 2000. Plant Dis 87:1301–1310
Leonard KJ, Martinelli JA (2005) Virulence of oat crown rust in Brazil and Uruguay. Plant Dis 89:802–808
Leonard KJ, Anikster Y, Manisterski J (2005a) Virulence associations in oat crown rust. Phytopathology 95:53–61
Leonard KJ, Huerta-Espino J, Salmeron JJ (2005b) Virulence of oat crown rust in Mexico. Plant Dis 89:941–948
Litzenberger SC (1949) Inheritance of resistance to specific races of crown and stem rust, to Helminthosporium blight, and of certain agronomic characters of oats. Iowa Agric Expt Sta Res Bull 370:453–496
Mac Key J, Mattsson B (1972) Breeding for race specific resistance against Scandinavian oat stem rust. Sveriges Utsädesf Tidskr 82:186–203
Manisterski JA, Wahl I (1995) Studies on oat crown rust in Israel. Petria 5:50–54
Marshall HG, Myers WM (1961) A cytogenetic study of certain interspecific Avena hybrids and the inheritance of resistance in diploid and tetraploid varieties to races of crown rust. Crop Sci 1:29–34
Martens JW (1978) Stem rust of oats in Canada in 1977. Can Plant Dis Surv 58:51–52
Martens JW (1985) Stem rust of oats. In: Roelfs AP, Bushnell WR (eds) The cereal rusts: volume II: diseases, distribution, epidemiology, and control. Academic, New York, pp 103–129
Martens JW, Dyck PL (1989) Genetics of resistance to rust in cereals from a Canadian perspective. Can J Plant Pathol 11:78–85
Martens JW, McKenzie RIH, Fleischmann G (1968) The inheritance of resistance to stem and crown rust in Kyto oats. Can J Genet Cytol 10:808–812
Martens JW, Roelfs AP, McKenzie RIH, Rothman PG, Stuthman DD, Brown PD (1979) System of nomenclature for races of Puccinia graminis f. sp. avenae. Phytopathology 69:293–294
Martens JW, McKenzie RIH, Harder DE (1980) Resistance to Puccinia graminis avenae and P. coronata avenae in the wild and cultivated Avena populations of Iran, Iraq and Turkey. Can J Genet Cytol 22:641–649
Martens JW, Rothman PG, McKenzie RIH, Brown PD (1981) Evidence for complementary gene action conferring resistance to Puccinia graminis avenae in Avena sativa. Can J Genet Cytol 23:591–595
McCallum BD, Fetch T, Chong J (2007) Cereal rust control in Canada. Aust J Agric Res 58:639–647
McCartney CA, Stonehouse RG, Rossnagel BG, Eckstein PE, Scoles GJ, Zatorski T, Beattie AD, Chong J (2011) Mapping of the oat crown rust resistance gene Pc91. Theor Appl Genet 122:317–325
McKenzie RIH (1961) Inheritance in oats of reaction to race 264 of oat crown rust. Can J Genet Cytol 3:308–311
McKenzie RIH, Fleischmann G (1964) The inheritance of crown rust resistance in selections from two Israeli collections of Avena sterilis. Can J Genet Cytol 6:232–236
McKenzie RIH, Green GJ (1965) Stem rust resistance in oats. I. The inheritance of resistance to race 6AF in six varieties of oats. Can J Genet Cytol 7:268–274
McKenzie RIH, Martens JW (1968) Inheritance in the oat strain C.I. 3034 of adult plant resistance to race C 10 of stem rust. Crop Sci 8:625–627
McKenzie RIH, Fleischmann G, Green GJ (1965) A close association of stem and crown rust resistance in ‘Ukraine’ and ‘Rosen's mutant’ oats. Crop Sci 5:551–552
McKenzie RIH, Martens JW, Rajhathy T (1970) Inheritance of oat stem rust resistance in a Tunisian strain of Avena sterilis. Can J Genet Cytol 12:501–505
Mitchell Fetch J, Fetch T (2011) Inheritance of resistance to oat stem rust in the cultivars Ronald and AC Gwen. Can J Plant Sci 91:419–423
Murphy HC, Stanton TR, Harland S (1937) Breeding winter oats resistant to crown rust, smut, and cold. Agron J 29:622–637
Murphy HC, Zillinsky FJ, Simons MD, Grindeland R (1958) Inheritance of seed color and resistance to races of stem and crown rust in Avena strigosa. Agron J 50:539–541
O’Donoughue LS, Chong J, Wight CP, Fedak G, Molnar SJ (1996) Localization of stem rust resistance genes and associated molecular markers in cultivated oat. Phytopathology 86:719–727
Osler RD, Hayes HK (1953) Inheritance studies in oats with particular reference to the Santa Fe type of crown rust resistance. Agron J 45:49–53
Parker JH (1920) A preliminary study of the inheritance of rust resistance in oats. Agron J 12(1):23–38
Pavek JJ, Myers WM (1965) Inheritance of seedling reaction to Puccinia graminis Pers. f. sp. avenae race 13A in crosses of oat strains with four different reactions. Crop Sci 5:501–504
Pedersen WL, Leath S (1988) Pyramiding major genes for resistance to maintain residual effects. Ann Rev Phytopathol 26:369–378
Penner GA, Chong J, Lévesque-Lemay M, Molnar SJ, Fedak G (1993a) Identification of a RAPD marker linked to the oat stem rust gene Pg3. Theor Appl Genet 85:702–705
Penner GA, Chong J, Wight CP, Molnar SJ, Fedak G (1993b) Identification of an RAPD marker for the crown rust resistance gene Pc68 in oats. Genome 36:818–820
Portyanko VA, Hoffman DL, Lee M, Holland JB (2001) A linkage map of hexaploid oat based on grass anchor DNA clones and its relationship to other oat maps. Genome 44:249–265
Portyanko VA, Chen G, Rines HW, Phillips RL, Leonard KJ, Ochocki GE, Stuthman DD (2005) Quantitative trait loci for partial resistance to crown rust, Puccinia coronata, in cultivated oat, Avena sativa L. Theor Appl Genet 111:313–324
Rajhathy T, Thomas H (1974) Cytogenetics of oats (Avena L.). Misc Publ Genet Soc Can 2:1–90, Ottawa, ON
Rines H, Porter HL, Carson ML, Ochocki GE (2007) Introgression of crown rust resistance from diploid oat Avena strigosa into hexaploid cultivated oat A. sativa by two methods: direct crosses and through an initial 2x.4x synthetic hexaploid. Euphytica 158:67–79
Rooney WL, Rines HW, Phillips RL (1994) Identification of RFLP markers linked to crown rust resistance genes Pc 91 and Pc 92 in oat. Crop Sci 34:940–944
Rothman PG (1984) Registration of four stem rust and crown rust resistant oat germplasm lines. Crop Sci 24:1217–1218
Sadanaga K, Simons MD (1960) Transfer of crown rust resistance of diploid and tetraploid species to hexaploid oats. Agron J 52:285–288
Sanderson KE (1960) Inheritance of reaction to several races of crown rust. Puccinia coronata avenae Erikss. in two crosses involving Ukraine Oats. Can J Plant Sci 40(2):345–352
Sanz MJ, Loarce Y, Fominaya A, Vossen JH, Ferrer E (2012) Identification of RFLP and NBS/PK profiling markers for disease resistance loci in genetic maps of oats. Theor Appl Genet 126:203–218
Satheeskumar S, Sharp PJ, Lagudah ES, McIntosh RA, Molnar SJ (2011) Genetic association of crown rust resistance gene Pc68, storage protein loci, and resistance gene analogues in oats. Genome 54:484–497
Segal A, Manisterski J, Fischbeck G, Wahl I (1980) How plant populations defend themselves in natural ecosystems. In: Horsfall JG, Cowling EB (eds) Plant disease: an advanced treatise. Acad. Press, New York, pp 75–102
Sharma DC, Forsberg RA (1977) Spontaneous and induced interspecific gene transfer for crown rust resistance in Avena. Crop Sci 17(6):855–860
Simons MD (1956) The genetic basis of the crown rust resistance of the oat variety Ascencao. Phytopathology 46:414–416
Simons MD (1965) Seedling resistance to Puccinia coronata avenae race 264 found in Avena sterilis. Phytopathology 55:700–701
Simons MD (1972) Polygenic resistance to plant disease and its use in breeding resistant cultivars. J Environ Qual 1:232–240
Simons MD (1985) Crown rust. In: Roelfs AP, Bushnell WR (eds) The cereal rusts, vol. II, disease, distribution, epidemiology and control. Academic, New York, USA, pp 131–172
Simons MD, Murphy HC (1954) Inheritance of resistance to two races of Puccinia coronata Cda.var. avenae Fraser and Led. Iowa Acad Sci 61:170–176
Simons MD, Sadanaga K, Murphy HC (1959) Inheritance of resistance of strains of diploid and tetraploid species of oats to races of the crown rust fungus. Phytopathology 49:257–259
Simons MD, Martens JW, McKenzie RIH, Nishiyama I, Sadanaga K, Sebesta J, Thomas H (1978) Oats: a standardized system of nomenclature for genes and chromosomes and catalog of genes governing characters. U.S. Dept. of Agric. Hand book. US Department of Agriculture, Madison, WI, p 509
Tanhuanpää P, Manninen O, Beattie A, Eckstein P, Scoles G, Rossnagel B, Kiviharju E (2012) An updated doubled haploid oat linkage map and QTL mapping of agronomic and grain quality traits from Canadian field trials. Genome 55:289–301
Thomas H (1992) Cytogenetics of Avena. In: Marshall HG, Sorrells ME (eds) Oat science and technology. American Society of Agronomy Inc., Madison, WI, pp 473–507
Upadhyaya YM, Baker EP (1960) Studies on the mode of inheritance of Hajira type stem rust resistance and Victoria type crown rust resistance as exhibited in crosses involving the oat variety Garry. Proc Linn Soc NSW 85:157–179
Waterhouse WL (1930) Australian rust studies III. Initial results of breeding for rust resistance. Proc Linn Soc NSW 55:596–636
Weetman LM (1942) Genetic studies in oats of resistance to two physiologic races of crown rust (Abstract). Phytopathology 32:19
Welsh JN, Johnson T (1954) Inheritance of reaction to race 7a and other races of oat stem rust, Puccinia graminis avenae. Can J Bot 32:347–357
Welsh JN, Carson RB, Cherewick WJ, Hagborg WAF, Peturson B, Wallace HAH (1953) Oat varieties—past and present. Publ. 891. Canada Department of Agriculture, Ottawa, ON
Welsh JN, Peturson B, Machaek JE (1954) Associated inheritance of reaction to races of crown rust, Puccinia coronata avenae Erikss, and to Victoria blight, Helminthosporium victoriae M. and M., in oats. Can J Bot 32:55–68
Wight CP, Tinker NA, Kianian SF, Sorrells ME, O’Donoughue LS, Hoffman DL, Groh S, Scoles GJ, Li CD, Webster FH, Phillips RL, Rines HW, Livingston SM, Armstrong KC, Fedak G, Molnar SJ (2003) A molecular marker map in 'Kanota' × 'Ogle' hexaploid oat (Avena sp.) enhanced by additional markers and a robust framework. Genome 46:28–47
Wight CP, O'Donoughue LS, Chong J, Tinker NA, Molnar SJ (2004) Discovery, localization, and sequence characterization of molecular markers for the crown rust resistance genes Pc38, Pc39, and Pc48 in cultivated oat (Avena sativa L.). Mol Breed 14:349–361
Wilson WA, McMullen MS (1997) Dosage dependent genetic suppression of oat crown rust resistance gene Pc-62. Crop Sci 37:1699–1705
Wong LSL, McKenzie RIH, Harder DE, Martens JW (1983) The inheritance of resistance to Puccinia coronata and of floret characters in Avena sterilis. Can J Genet Cytol 25:329–335
Yu GX, Wise RP (2000) An anchored AFLP- and retrotransposon-based map of diploid Avena. Genome 43:736–749
Zegeye T (2008) Stem rust resistance in Avena strigosa Schreb.: inheritance, gene transfer, and identification of an amplified fragment length polymorphism (AFLP) marker. PhD thesis, University of Manitoba
Zhu S, Kaeppler HF (2003) A genetic linkage map for hexaploid, cultivated oat (Avena sativa L.) based on an intraspecific cross 'Ogle/MAM17-5'. Theor Appl Genet 107:26–35
Zillinsky FJ, Derick RA (1960) Crown rust resistant derivatives from crosses between autotetraploid Avena strigosa and A. sativa. Can J Plant Sci 40:366–370
Zillinsky FJ, Murphy HC (1967) Wild oat species as sources of disease resistance for the improvement of cultivated oats. Plant Dis Rep 51:391–395
Acknowledgements
The first author is thankful to Prairie Oat Growers Association (POGA), Regina, Saskatchewan, Canada, for supporting his fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Gnanesh, B.N., Fetch, J.M., Zegeye, T., McCartney, C.A., Fetch, T. (2014). Oat. In: Pratap, A., Kumar, J. (eds) Alien Gene Transfer in Crop Plants, Volume 2. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9572-7_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9572-7_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9571-0
Online ISBN: 978-1-4614-9572-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)