Abstract
Underlying mechanisms leading to pituitary plasticity by which the gland adapts the number of hormone-producing cell to the continuously changing physiological requirements are still poorly understood. Adult stem cells were shown to direct homeostatic cell maintenance, regeneration, and functional plasticity in several organs and tissues. Only recently potential stem cells were identified and phenotypically characterized in adult pituitary. Multiple possible stem/progenitor cell candidates were proposed, but different studies have been only partially reconciled. Here, we critically analyzed the reports addressing the identification of adult pituitary stem cells, trying, when possible, to reunite the results of the different studies. Nonetheless, in light of the still non-complete characterization of these cells, some discrepancies among the published studies are still apparent. Importantly, long-term in vitro self-renewal, a defining feature of stem cells, remains to be unequivocally demonstrated. Finally, the potential role of adult pituitary stem (or progenitor) cells in pituitary adenoma development will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The existence of stem-like/progenitor cell population was hypothesized since 1969 in studies in which the transplantation of undifferentiated (chromophobe) pituitary cells in hypophysectomized rats was reported, to originate differentiated (hormone-producing) pituitary cells [1].
Moreover, indirect evidence of the presence of multipotent cells within pituitary was obtained by the adaptive responsiveness of pituitary to both physiological and pathological conditions.
In vertebrates pituitary develops from the most anterior part of the anterior neural ridge [2]. During early development oral and neural ectoderms are located in close proximity: differentiating signals start from neural ectoderm to oral ectoderm that invaginates in the Rathke’s pouch. Subsequently, Rathke’s pouch forms a closed epithelial structure separating from the oral ectoderm, giving rise to anterior pituitary, which is composed of several types of specialized endocrine cells able to produce and release the different hypophyseal hormones, namely, GH (somatotrophs), prolactin (lactotrophs), TSH (thyrotrophs), POMC (corticotrophs), and LH/FSH (gonadotrophs). On the other hand, from neural ectoderm originate the infundibulum and the pituitary neural lobe containing hypothalamic neuron terminal and releasing vasopressin and oxytocin [3].
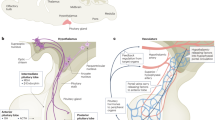
Several studies analyzing cell proliferation and differentiation within Rathke’s pouch identified two levels of regulation of stem/progenitor cell activity involving embryogenesis and postnatal days. In mouse embryo, between days 11.5 and 18.5, pituitary cells shift from mainly proliferating to differentiating populations, while proliferating cells can be observed only around the ridge of the Rathke’s pouch, named marginal zone [4]. Marginal zone (also defined as the cleft separating anterior and intermediate pituitary lobes) has been indeed identified as putative “stem cell niche” in pituitary [5]. Functional and morphological evidence supports the assumption that cells located in this region could actually represent stem cells. For example, cells in marginal zone do not express secretory granules, as differentiated pituitary cells, and are characterized by reduced endoplasmic reticulum, by abundance of free ribosomes and polysomes, and by the expression of potential stem cell markers, such as Sox2 [3]. Importantly, while during pituitary organogenesis proliferating Sox2-expressing cells are readily observed throughout the gland, after development they are mainly localized within the marginal zone.
In the adulthood, pituitary proliferating cells are highly reduced in number, and, concomitantly, the number of differentiated cells increases. While all hormone-producing cell types are already developed at birth, pituitary is not completely formed yet and, in rodents, its growth and maturation continue for a few weeks in postnatal period [6]. It was shown that terminally differentiated hormone-secreting cells during this period can reenter the cell cycle to increase a given hormone-producing population cell number, although current evidence proposes that the increase in pituitary cell number is mainly derived from a population of so-called “transient-amplifying cells” derived from differentiation of cells in the stem compartment that migrate into pituitary parenchyma where they start to actively proliferate [7].
The continuous generation of “transient-amplifying cells” from stem cells would also represent the mechanism by which, in normal conditions, the whole adult pituitary cell compartment is completely renewed every 5–8 weeks [3, 8, 9]. In fact, in “basal” conditions, in the absence of hypothalamic or other hormonal stimuli, most of these cells undergo apoptosis commitment, limiting the proliferation activity to the replacement of dying cells. Nevertheless, in several physiological conditions (growth, puberty, pregnancy, lactation), in which a general reorganization of pituitary structure and cell composition is required, the activity of these cells can largely increase [8, 9]. For example, after adrenalectomy or orchiectomy a great surge in the number of corticotroph or gonadotroph cells occurs in adult pituitary [10, 11]. In a more physiological setting, lactotroph number increases several folds during pregnancy and lactation to adapt the prolactin secretion required in those conditions [12].
Still three mechanisms can account for adult pituitary plasticity: (1) differentiated (hormone-producing) cells enter mitosis [13]; (2) transdifferentiation of differentiated cell populations [14]; and (3) recruitment of putative adult pituitary stem/progenitor cells [15].
The first two mechanisms, mitotic activation of differentiated/hormone-producing pituitary cells and transdifferentiation from a different pituitary cell type (mostly conversion of somatotrophs in lactotrophs), are believed to represent only a minor way of pituitary plasticity. In fact, hormonally null cells comprise more than 90 % of mitotic events observed during adrenalectomy, and only after few weeks differentiate corticotroph cells are generated (for review, see [16, 17]). Similarly, genetic lineage trace experiments demonstrated that new prolactin-secreting cells, developed in response to estrogens (for example, during pregnancy), rarely derive from differentiated somatotroph cells [18], but, again, mainly derive from a self-limiting wave of proliferation of the nonhormonal pool of pituitary cells [12]. Thus, most of basal pituitary cell turnover and cell lineage changes, occurring as dynamic adaptation in physiological or pathological conditions, are driven by recruitment of pituitary cell subpopulation endowed with stem/progenitor cell characteristics [15].
In the past years, a large effort was dedicated to the search of putative pituitary stem or progenitor cells, and only in recent years convincing data were produced to sustain this hypothesis, opening a completely novel and rapidly growing field of research. In fact, the definitive demonstration of pituitary stem cells may have significant clinical impact, for example, in adult-onset hypopituitarism in the context of regenerative medicine [19], as proposed for pancreatic β-cells in type I diabetes or, more importantly, for pituitary tumors, in light of the cancer stem cell theory of tumor development [20].
Several cell populations in adult pituitary showing phenotypes and biological activity resembling those of stem cells (i.e., undifferentiated cells expressing stemness markers, able to self-renew, residing in specific areas of each tissue named “niches,” endowed of resistance to drugs or toxins due to high activity of DNA repair mechanisms and ABC transporters, and importantly to generate all the cell populations in a given organ or tissue [21]) were identified in studies mainly involving murine models, but a definitive consensus about the characteristics of these cells has not been reached so far.
In the following paragraphs are reported the more relevant studies aimed to define adult pituitary stem cells, trying to highlight both differences and concordances among the features of the cell populations proposed.
In Table 1 are reported the main genes involved in the putative pituitary stem cell activity.
2 Side Population
Increased activity of ABC transporters has been the first stem-like cell feature exploited to identify the presence of this cell subpopulation in mouse pituitary. The activity of ABC transporters causes the extrusion of potential toxicants from the cells, representing a defining characteristic of stem cells granting their survival and protection against genotoxic insults for all the life span of an organism. In fact, due to their prolonged survival stem cells are exposed to continuous environmental injuries that require the development of efficient defensive systems, including DNA repair mechanisms and extruding pumps [22]. This characteristic was applied to identify stem cells in different tissues as the cell population able to extrude fluorescent dyes, such as Hoechst 33342, from the cytosol. Importantly, these cells can be visualized by FACS analysis as a “side population” (SP) forming a small “streak” separated from the main population (MP) that retains the dye [23].
SP cells have been identified in adult pituitary in mouse and dog, although with some difference in the phenotype among the species [24, 25].
SP cells comprise about 1.5 % of 3–8 weeks old mouse anterior pituitary cells. These cells are characterized by the overexpression of several stem cell-associated markers (nestin, Sca1, Nanog, CD133, Oct-4) and molecular effectors of self-renewal (Notch, Wnt, Shh) [24, 26], while in a small percentage (5.8 % of total SP) a phenotype resembling folliculo-stellate (FS) cells was detected [24]. Conversely, all these markers were not (or minimally) expressed in the pituitary MP [24].
SP cells are clonogenic, growing as non-adherent spheres (named following their origin as pituispheres) [24], a feature that, when retained after serial passages, is an index of active self-renewal characterizing the growth of stem/progenitor cells of multiple tissues [27, 28]. Spherogenesis activity was restricted to a subgroup of the cells in pituitary SP, representing about 0.02 % of the seeded anterior pituitary cells. This small cell population was phenotypically characterized as the cells that express Oct-4, CD133, and components of the Notch, Wnt, and Shh pathways, while did not contain pituitary hormones. This pattern of expression represents a molecular picture largely shared by SP cells in several tissues [24].
The differences in the clonogenicity among SP cells fostered a more detailed analysis of SP cells. In these studies two cell subpopulations were selected, accordingly to the level of expression of the stem cell antigen 1 (Sca1) that, conversely, was minimally expressed in the MP cells. In particular, it was proposed that SP cells expressing Sca1 at low level (named non-Sca1high fraction) and representing about 40 % of the SP compose the putative adult pituitary progenitor population. In fact, differently from MP cells and the SP cells expressing high levels of Sca1 (Sca1high population), non-Sca1high cells grow as pituispheres (at least for a limited number of passages) and are able to differentiate in the pituitary hormone-secreting cell types. As compared to the Sca1high SP cells, non-Sca1high cells overexpress (up 25-fold more) most of the transcription factors controlling pituitary development (Hesx1, Prop1, Pax6, Lhx3, Lhx4, OTX2, PITX1, and PITX2), inhibitors of apoptosis, and components of MAP kinase, Wnt, and Notch pathways [29]. In particular, the activation of Notch pathway maintains stem cells in undifferentiated status [19], playing a relevant role in the stem cells proliferation being modulated by growth factors commonly used to select stem cells in vitro (EGF, bFGF, and LIF) [29]. Interestingly non-Sca1high cells co-express pituitary transcription factors normally identified at different stages during pituitary development [19], suggesting that this population is heterogeneous including both stem-like cells and more differentiated progenitors [3].
Sox2 and Sox9 were also detected only in non-Sca1high cells (about 50 % and 30 %, respectively) but were virtually absent in Sca1high cells, while nestin, as well as OCT4 and Bmi-1, was equally detected in the two populations [29], suggesting that these markers (representing protein involved in self-renewal of stem cells) are not specific for pituitary stem cells. The majority of the cells in pituispheres derived from non-Sca1high cells co-express Sox2 and nestin [30]. This observation suggests that self-renewal activity mainly rests in Sox2-expressing cells (representing 50 % of the non-Sca1high population). After differentiation in hormone-producing cells, Sox2 expression ceased, confirming the specificity of the expression of this transcription factor in putative pituitary stem cells. Importantly, in agreement with other studies [30], Sox2+ cells were identified in small number in proximity of the pituitary stem cell niche (the marginal zone around the cleft) [5]. However, clusters of Sox2+ cells are also scattered within the anterior pituitary parenchyma [24, 30–32], being suggestive of the existence of multiple niches [5]. On the other hand, the high level of expression of nestin in Sca1high cells is in agreement with data showing that nestin is also expressed in non-endocrine pituitary cells, including folliculo-stellate (FS) cells and pericytes that, as a consequence, were interpreted as subsets of the Sca1high cell component. Indeed, microarray analysis showed that Sca1high cells also express S100β and several angiogenesis-related genes, and thus, in consideration of the recognized role of Sca1 in endothelial development [33], it was proposed that these cells may represent a subset of FS cells and/or endothelial progenitors rather than pituitary progenitors [29].
3 SOX2+/SOX9– Cells
In agreement with the studies on pituitary SP, using different approaches, several other studies focused on Sox2 as a marker for putative pituitary stem cells. Sox2, a transcription factor of the high mobility group (HMG) family, is a reasonable candidate to identify these subpopulations since it is expressed at high levels in embryos, playing a relevant role in CNS development [34], while it is downregulated during cell differentiation. Thus, it was proposed that, in adulthood, only stem cells retain Sox2 expression. Importantly, Sox2 is also required for normal pituitary development [35].
In murine postnatal pituitary, the pattern of Sox2 expression is similar to that of developing embryonic gland, being localized in about 3 % of the postnatal anterior pituitary cells localized in the marginal zone between anterior and intermediate lobe and occasionally scattered throughout the adenohypophysis [29, 30, 36]. Sox2+ cells often co-express E-cadherin, suggesting that epithelial to mesenchymal transition might be required to Sox2-expressing cells to become rapidly dividing and differentiate [30]. In postnatal pituitary, Sox2+ cells were also reported to express PROP1, a transcription factor involved in pituitary organogenesis, but their number rapidly decreases at postnatal day 15, suggesting that a significant qualitative transition occurs during this early phase of development [36]. As far as the meaning of the Sox2+ cells scattered throughout the anterior pituitary parenchyma, it was hypothesized either a transition to a “niche-independent” state or that supporting cells, such as FS, could represent a sort of diffuse “mini-niche”.
In the adult, Sox2-expressing cells represent less than 5 % of total anterior lobe cell population, but, differently from embryonic cells, only 1 % of the Sox2+ cells do not express Sox9, another transcription factor involved in embryo development for sex determination and later for chondrogenesis [37, 38]. Sox9 is also involved in pituitary development, but its expression in Rathke’s pouch occurs much later than Sox2. Indeed, there is a temporal modification in the expression of these transcription factors during embryogenesis, with a progression during development from a Sox2+/Sox9– phenotype to a double positivity and only a minority of the cells remain Sox2+/Sox9–. Similar, but quantitatively different, results were obtained by SP studies [29] (see above), with non-Sca1high cells also being Sox2+, and only a low percentage of these cells was reported to be also Sox9 positive [30]. Importantly, in BrdU labeling experiments, Sox2+/Sox9+ cells were showed to retain a significant proliferation rate, suggesting that this population may represent transit-amplifying cells. In fact, the proliferation wave observed in pituitary shortly after birth is mainly represented by Sox2+/Sox9+ cells. Conversely, Sox2+/Sox9– showed persistence of the staining during label-retaining experiments [30], an index of slow cell division activity, as often observed in adult stem cells. This observation suggests that, in adult pituitaries, Sox2+/Sox9– cells represent the reserve of quiescent multipotent cells for organ maintenance, evolving into Sox2+/Sox9+ cells in case of tissue loss or in response to physiological adaptive requirements [3].
This hierarchical order in pituitary stem cells was confirmed in vitro. Pituispheres generated in vitro by potential postnatal murine pituitary stem cells using selective culture conditions (medium containing growth factors without fetal calf serum, FCS) are composed by cells showing Sox2+/Sox9– phenotype, while Sox9 expression occurs only after at least 1 week in culture [30]. FACS analysis of Sox2+ cells isolated from pituitaries of Sox2-EGFP mice [39] confirmed that Sox2 expression is required for the formation of spheres that originated only from GFP-positive cells [17]. However, it was not possible to propagate these spheres for more than two passages, suggesting that these Sox2+ cells could represent multipotent progenitors rather than “real” stem cells, for which at least five in vitro passages are considered a defining requirement [15]. Alternatively, the in vitro culture conditions used might still lack some relevant factors required to retain stemness [3]. Again, in agreement with SP studies [29], Sox2+ cells within pituispheres express also E-cadherin and are completely hormone negative [30]. However, co-expression of Sca1 was also reported [30], an observation discordant with the characteristics identified in the stem/progenitor cells within SP (non-Sca1high phenotype) [29].
Prolonged (1 week) in vitro culture of Sox2+ cells induced, besides Sox9, also the expression of nestin and S100, while Sca1 is downregulated, but no spontaneous differentiation (for pituitary hormone expression) occurred. The shift to a medium containing FCS, as reported for stem cell cultures from several tissues, caused the disaggregation of spheres and the adherence of the cells to the culture substrate inducing a FS-like phenotype. To obtain endocrine differentiation this pituitary cell population has to be cultured as cellular aggregates on matrigel without growth factors [30]. In these latter experimental conditions all the pituitary hormones were detected with, sometime, the expression of multiple hormones in same sphere, confirming the multipotentiality of these cells. Altogether these observations allowed the proposal of Sox2+/E-cadherin+/Sox9–/S100– as putative pituitary stem/progenitor cells, while Sox2+/Sox9+/S100+/E-cadherin– cells could represent FS cells with transit-amplifying properties, already committed to differentiation [17].
More recently, a substantial evidence for the role of Sox2+ cells as stem cell component was demonstrated in a transgenic mouse model of pituitary regeneration [40, 41]. To conditionally destroy selective pituitary cell types (i.e., GH- or prolactin-secreting cells) a transgene was constructed to have a conditional expression of diphtheria toxin receptor driven by the promoters for GH (GHCre/iDTR mice) [40] or PRL (PRLCre/iDTR mice) [41]: the expression of the toxin receptor leads to the disruption of the cells after activation induced by administration of diphtheria toxin, thus representing a model of selective adult pituitary cell ablation. The treatment of GHCre/iDTR mice for 3 days with diphtheria toxin causes, 1 week later, the obliteration of about 90 % of the GH cells. In these conditions a rapid surge of Sox2-expressing cells, as well as of FS cells, was detected. In situ analysis showed that Sox2+ cell expansion occurred mainly in the marginal layer. Concomitantly, double labeled Sox2/GH cells appeared throughout the gland. These data clearly support the regenerative potential of adult pituitary and that Sox2+ cells represent a main component of the subpopulation endowed with this activity. Similar results were obtained in the PRL-ablation model [41]. Throughout the regeneration period (2–6 weeks), Sox2+, as well as double Sox2+/PRL+, cells continue to be more abundant than in pituitaries of control mice. Moreover, surviving or newborn lactotrophs increase their proliferative activity, and bi-hormonal PRL+/GH+ cells become detectable, suggesting somatotroph to lactotroph transdifferentiation [41].
In conclusion, these studies provided a significant evidence that adult pituitary is able to regenerate both somatotroph and lactotroph compartments after destruction, through the activation of Sox2+ adult stem cells [40, 41].
4 Nestin-Expressing Cells
Similarly to studies in other tissues, the possible existence of stem cells in pituitary relies on the identification of stem markers in subpopulations of cells in adult tissues, and nestin is one of the most commonly studied. In rat pituitary, nestin-expressing cells were identified all over the gland [42], mainly within the marginal zone lining the cleft, the possible pituitary stem cell niche [15]. These cells do not show features of hormone-secreting nor FS cells, but in vitro cultures, established from nestin-expressing cells, demonstrated characteristics of cells possessing a mesenchymal phenotype [42].
Using transgenic mice expressing nestin-GFP fluorescent cells, this cell population was detected in the Rathke’s pouch in mouse embryos, but fluorescence was also detected in high number of cells in postnatal pituitary, mainly localized in the proximity of the cleft and showing Sox2 co-expression [43].
Cell lineage studies using nestin-Cre mice, in which the progeny of nestin-expressing cells are permanently GFP positive, demonstrated that about 2 % of fluorescent cells are located in the pituitary of newborn mice, but this percentage increases up to 20 % in adults [43]. While nestin-expressing cells can originate all pituitary endocrine cells, they are heterogeneous for Pit1 expression, suggesting that only a subpopulation of nestin-expressing cells could represent pituitary progenitors. However, the discrepancy between the lower percentage of nestin-positive cells in embryos than in adult endocrine cells allows to hypothesize that adult pituitary stem cells may be formed by a different pool of cells with respect to the embryonic progenitors. In agreement with this hypothesis it was demonstrated that nestin-expressing cells originate from the differentiation of Sox2+ embryonic progenitors [30], possibly representing “transient-amplifying” population from which endocrine cells may derive.
Nestin-GFP cells were reported to be clonogenic in vitro and, when grown in “differentiation culture medium” (additioned with FCS or high concentration of cAMP induced by forskolin treatment), initially co-express nestin and Sox2 and, after time, differentiate in cells expressing all pituitary hormones [43].
A similar observation was obtained in autoptic human pituitary specimens. Nestin-expressing cells identified in the perivascular space of pituitary capillaries did not express pituitary hormones or FS and endothelial cell markers (as also demonstrated in rat and mouse pituitaries [42, 43]). Conversely, nestin was co-expressed with smooth muscle actin (SMA), suggesting a possible differentiation in pericytes. Human nestin+ pituitary cells grown on fibroblast feeder layer allowed the identification of the existence of two independent populations, of which the first was characterized as differentiated pericytes (phenotypically nestin+ and SMA+) and the second (only nestin+) was proposed as human pituitary progenitor cells accordingly to self-renewal ability (spherogenesis) and differentiative potential (induction of prolactin expression in the presence of high cAMP levels) [44]. However, since a direct derivation of colonies from nestin+ human cells was still not reported and long-term self-renewal never evaluated, further studies are required to definitively confirm these data.
Although nestin-GFP cells having a clonogenic pattern of growth in vitro do not express Sca1, nestin expression was identified in non-Sca1high pituitary SP [29]. This discrepancy may depend on the different timings of analysis (soon after explant for the SP or after few days of in vitro culture in the nestin-GFP experiments), possibly confirming that progenitors express differential markers at various development stages.
However, recent studies, raising the possibility that ectopic expression of the nestin transgene may occur in a group of cells in the Rathke’s pouch [45], cast doubts on the interpretation of these data. In fact, ectopic activity in the embryonic pituitary and, as observed with the nestin-GFP transgene in Rathke’s pouch progenitors, would cause a significant staining in postnatal anterior pituitary cells that however does not reflect the actual expression in adult pituitary. Since nestin+ cells mainly divide after birth, it was proposed that these cells are quiescent progenitors required for the initial wave of pituitary cell proliferation occurring after birth, and to maintain pituitary function in adults, but not the cells responsible of embryonic pituitary development. In particular, to define nestin-expressing cells as actual stem cells, it would be required to show exclusive co-localization of Cre and nestin [45]. In any case, from these studies it was hypothesized that nestin is expressed by different cell populations during pituitary development and in the adulthood, all labeled by nestin-GFP and including both stem/progenitor cells (Sox2+ and LHX3+), supportive (FS) cells, or vascular progenitors. In this line, it is to note that nestin is expressed in both pituitary SP groups, classified as non-Sca1high (believed to represent pituitary progenitors) and Sca1high (interpreted as vascular-endothelial progenitors) [29].
Thus, to date no definitive evidence has been provided about different studies on nestin+ putative pituitary stem/progenitor cell origin, and further studies will be required to establish their role in pituitary development.
5 GFRα2-PROP1-Stem Cells
Another potential stem-like cell population in rat adult pituitary was identified according to the expression of GFRα2, the GDNF co-receptor (altogether with the tyrosine kinase receptor c-Ret) [31].
GFRα2-expressing cells represent about 0.9 % of all pituitary cells, as expected for adult stem cells [31]. All these cells also express E-cadherin, β-catenin, and stem cell markers such as OCT4 and SSEA4, about 90 % of them express Sox2 and Sox9, and about 50 % is S100+. On the contrary, nestin was not detected in these cells, at odds with the studies described above [29, 42, 43]. Interestingly, GFRα2-expressing cells also express PROP1, a transcription factor required to induce pituitary progenitors to differentiate in Pit1-positive cells [46]. In embryos, Sox2 co-localizes with PROP1 (but not with Pit1), while about 10 % of the cells express both PROP1 and Pit1. In adults, differentiation commitment makes hormone-expressing cells retaining Pit1 but not PROP1 [47]. This complex phenotype (expression of Sox9 and S100, co-localization of GFRα2 and PROP1) allowed the hypothesis that GFRα2-PROP1-stem (GPS) cells may represent transit-amplifying cells committed to differentiation rather than multipotent stem cells [17], very similar to the Sox2+/Sox9+ described by Fauquier [30], which retain the short-term ability to form spheres. GPS cell niche was identified in the periluminal zone in both rodents and humans, in which GPS cells are organized in an oriented manner, as single cell layer bordering the cleft [31]. Thus, the most concordant evidence from all the studies looking for pituitary stem cells is that the region of the cleft could represent pituitary niche.
Neurturin, a GFRα2 ligand, is expressed in anterior pituitary but not in the niche, suggesting that the secretion of this growth factor may regulate the activity of the GPS cells and their directional migration from the niche to the anterior pituitary [31]. GPS cells, isolated by FACS sorting, generate spheroids when grown in the absence of FCS, also without the addition of EGF and bFGF, growth factors commonly used to select stem cells, while neurturin, likely acting on GFRα2, increased the sphere-forming efficiency, producing a trophic effect on the survival of these cells [31]. The pituispheres express OCT4, E-cadherin, and PROP1 and, when dissociated, can differentiate in cells expressing all pituitary hormones and/or β-III-tubulin (a neuronal marker), when grown in monolayer onto collagen IV- or poly-l-lysine-coated plates. The expression of pituitary hormones required the incubation in specific media, each one able to induce the expression of one pituitary hormone. Differentiated cells showed a downregulation of GFRα2, OCT-4, and PROP1 expression and cell growth arrest [31]. More recently, cells expressing the same phenotype as rat GPS cells were identified in human pituitary, further supporting the relevance of this subpopulation as putative adult pituitary stem cells [48].
6 Pituitary Colony-Forming Cells/Folliculo-Stellate Cells
A different approach to identify stem/progenitor cells from adult pituitary was the colony-forming assay: pituitary cells isolated from Rathke’s pouch can clonally expand in vitro as colonies, representing 0.2 % of the total pituitary cell number [49]. Not surprisingly, some of these embryonic clones are multipotent and give origin to all pituitary hormonal cell types, both in vitro and in vivo, after hypothalamic transplantation in hypophysectomized rats [50, 51]. More recently, pituitary colony-forming cells were shown to belong to the FS compartment accordingly to their ability to internalize the fluorescent dipeptide β-Ala-Lys-Nε-AMCA [52]. It was calculated that about 12 % of mouse pituitary FS cells (phenotypically characterized as S100+ and GFAP+) was clonogenic and in about 40 % of the cells also Sca1 was expressed. Within the colonies formed, few GH- or prolactin-expressing cells were detected, suggesting the differentiation from pluripotent progenitors [49].
Using a more sophisticated in vivo approach, about 3.3 % of the colony-forming FS cells was demonstrated to be able to differentiate in GH-expressing cells [53]. It was also detected that the FS cell marker S100β was expressed in 80 % of the Sox2+ pituitary cells, identified as putative pituitary stem cells (see above), and that after in vitro treatment with retinoic acid and bFGF, a small number of them can differentiate in Pit1-expressing or GH-secreting cells [54].
These studies provided evidence that FS are composed of several subpopulations, also comprising putative pituitary progenitors [53]. Conversely, other cells within this population may overlap, at least phenotypically, with the Sca1high SP (high expression of S100 and Sca1) [29]. Importantly, the clonogenic population is localized in the marginal zone of the pituitary cleft (the marginal zone between intermediate and anterior lobes), the proposed pituitary stem cell niche [55]. Thus, some postnatal pituitary FS cells exhibit stem cell-associated features such as the in vitro expansion as adherent colonies. However, given the limited differentiation capacity observed (mostly somatotrophs), these cells were interpreted as already committed progenitor cells.
7 Adult Pituitary Stem Cells and Tumor Development: The Role of Cancer Stem Cells
According to the current view of carcinogenesis, tumors possess a heterogeneous cell type patterning, including a large number of “tumor NON-initiating cells” (or “differentiated tumor cells”) constituting the tumor mass, and a small fraction of phenotypically distinct “tumor-initiating cells” (TIC), comprising the so-called “cancer stem cells” (CSCs). Similarly to what was observed in normal tissues, these tumor cell populations are biologically distinct as (1) slowly dividing stem cells (CSCs) from which all the other tumor cells are originated, (2) precursor cells (rapidly dividing) and transit-amplifying cells, and (3) differentiated cells that form the mass of the tumoral tissue [56].
In this model, CSCs are named after their ability to develop tumors (in the same way by which normal stem cells are responsible of organ development) and do not necessarily represent the malignant transformation of stem cells. In fact, different theories were provided on this issue including some evidence in support of the actual origin of CSCs from the oncogenic transformation of normal stem cells and other data demonstrating the possibility of a redifferentiation/dedifferentiation of committed progenitors or differentiated cells; likely both mechanisms can be active, in different conditions, to give origin of CSCs [57]. Independently from their origin, CSCs may arise from normal stem or progenitor cells by alterations of proto-oncogenes that, as a result of accumulative oncogenic events, may grant a deregulated self-renewal ability to transform normal cells into CSCs. On the other hand, cancer development and progression may derive from modification of the microenvironment surrounding the stem cells (for example, the niche), leading to loss of extrinsic proliferation control of normal stem cells or progenitors, and the development of CSCs. Several genes and intracellular signaling pathways, representing important regulators of normal stem cell self-renewal and proliferation, are deregulated in cancer: Sox2, Notch1, Hedgehog, Wnt, and nestin, among others [58, 59].
Many biological features of normal stem cells are retained in CSCs: long life span, including the capacity of self-renewal, the expression of common markers, the possibility to differentiate into different cell types, and the strong resistance to chemotherapeutic drugs. The high self-renewal activity renders CSCs, differently from all other tumor cells, to be capable of a potentially unlimited proliferation activity that allows the maintenance and expansion of the tumor, although they themselves are often slow growing. Conversely, differentiated tumor cells proliferate at high rate, but for a limited number of divisions. Thus, CSCs represent a reservoir of tumor cells necessary to sustain tumor development. In light of CSC theory, a reconsideration of pharmacological approaches for tumors is ongoing, since the elimination of the differentiated and rapidly dividing tumor cells, as occurring with the classical chemotherapeutic agents, could fail to obtain a successful long-term disease remission if CSCs are not eliminated. Most of the currently used cytotoxic drugs are not able to affect the survival of undifferentiated and slow proliferating CSCs that, surviving to the treatment, represent a cell reservoir able to rapidly repopulate the tumor. Drug resistance of CSCs was ascribed to the expression at high levels of DNA repair enzyme that can elude genotoxic effects of antitumoral drugs, and of ABC-family transporters that can pump chemotherapeutic agents out of the cell. For these and other cellular properties, distinct from the rest of tumor cell populations CSCs often escape the traditional cancer therapy that becomes insufficient to clear up the “tumor-initiating cells” from the organism [60].
Recent studies focused on the role of CSCs in pituitary tumor development.
Mice bearing mutations that alter pituitary β-catenin proteolysis, leading to constitutive activation of the WNT/β-catenin pathway (obtained by crossing a Hesx1-CRE knock-in strain to a β-catenin strain that produces degradation-resistant β-catenin mutant, upon recombination), spontaneously develop tumors histologically and phenotypically resembling the human adamantinomatous craniopharyngiomas [61]. Importantly, pituitary tumorigenesis in these mice was dependent on the selective expression of the β-catenin mutant in pituitary cells endowed with progenitor/stem cells features. These cells were described as Sox2+ and colony-forming cells, both characteristics proposed for adult pituitary stem cells (see above). In these trangenic mice, Sox2+ cells were increased in number and showed a higher proliferation rate than the w.t. counterpart. Importantly, when cultured in vitro these cells showed a long-lasting (at least eight passages) clonogenic activity [61], clearly indicating a powerful, likely deregulated, self-renewal activity [15]. Moreover, in this study, a significant support to the origin of CSC from oncogenic transformed normal adult stem cells was provided, since the expression of the same proteolysis-resistant β-catenin isoform in differentiated pituitary cells was not tumorigenic [61]. Accordingly, it was reported that a novel mutation in SOX2 gene did not impair transactivation or DNA binding, but failed to repress β-catenin-mediated target activation, resulting in WNT/β-catenin increased activity [62]. Thus, the β-catenin pathway, normally involved in the control of the balance between self-renewal and differentiation of stem cells, is inactivated in normal adult pituitary, and when this downregulation does not occur sustained progenitor proliferation is induced, leading, eventually, to tumor development [63]. Moreover, mice, in which a conditional deletion of the tumor suppressor retinoblastoma gene is induced, developed silent corticotroph pituitary adenomas originating from Pax7+ progenitors. In normal pituitary Pax7+ cells are located in the Rathke’s pouch cleft and derive from nestin+ progenitors, not only in mice but also in primate pituitaries and were identified in both human functioning and silent ACTH-secreting adenomas, although a direct involvement of CSC in these tumors was not studied [64].
Another recent study showed the expression of GPS cell markers in human adamantinomatous craniopharyngiomas suggesting a common origin from these putative adult pituitary stem cells, with the only difference in the lack of expression of GFR2α that was interpreted as a way of deregulation of growth in the tumor cells [48].
Thus, although this is a still open issue, several evidence supports that, at least as far as some pituitary tumor is concerned, CSC might originate from genetic or epigenetic alterations in adult pituitary stem cells or progenitors.
Much less clear are the role of CSCs in pituitary adenoma development and the origin of these cells from normal adult pituitary stem cells. Although still debated, a growing bulk of evidence is now supporting the role of CSC as tumor-initiating cells also in benign tumors [20].
To date, molecular and cellular determinants of pituitary adenoma pathogenesis are still largely unknown [65], and although not definitively proved, the hypothesis that the formation of CSC subpopulation from stem or progenitor cells may also cause the formation of pituitary adenomas is currently under investigation.
Several indirect evidence was provided. For example, it was shown that CXCR4, a chemokine receptor identified as a marker in several stem cell populations [66] including pituitary stem/progenitors during development and in SP cells in adulthood [5], is expressed in subpopulations of human normal pituitary (about 30 % of the cells) with its expression shared by subpopulations of GH-, prolactin-, and ACTH-secreting cells, strongly suggesting a lineage derivation from common precursor cells [67]. In several CXCR4+ cells also its ligand (CXCL12) was expressed, suggesting a possible autocrine/paracrine mechanism of activation, and also a few CXCR4+/hormone negative cells, scattered throughout the anterior pituitary, were identified [67]. Although the co-expression of CXCR4/CXCL12 with stemness marker (Sox2, nestin) was not evaluated in these studies, it could be hypothesized that these hormone negative cells could be pituitary stem cells (see before). CXCR4 and CXCL12 expression was also analyzed in a large series of human secreting and nonfunctioning) pituitary adenomas, and both molecules were found highly overexpressed, with all tumoral cells positive for both CXCR4 and CXCL12. Moreover, in vitro studies showed that CXCL12 activation is a mitogen for human pituitary adenoma cells [67]. Thus, it was proposed that putative CSCs for human pituitary adenomas may derive from CXCR4+/CXCL12+ cells in normal pituitary and that, due to the proliferative advantage granted by the autocrine activation of CXCR4 via the constitutive CXCL12 secretion, these cells could be one of the cell populations that clonally expand during pituitary tumorigenesis [68].
In another study, the expression of stem cell/progenitor markers (Sox2, nestin, Lhx3), but not pituitary hormones, was identified in cells composing hyperplastic pituitary nodules developed in nestin-GFP/Rb+/– mice. Moreover, in adult mice, the tumors contained twofold more nestin/Sox2-expressing cells than normal tissues, although the real nature of these masses was not really characterized [43].
However, to date, only one study formally analyzed the possible role of CSCs in human pituitary adenomas pathogenesis [69]. In this study, putative CSCs, called “pituitary adenoma stem-like cells” (PASCs), were obtained from one GH-secreting and one clinically nonfunctioning human pituitary adenomas, culturing dispersed postsurgical specimens in stem cell permissive medium (DMEM/F12, enriched with B27 supplement, bFGF, and EGF). After 1 week in vitro, few pituitary adenoma cells generated spheroids that were able to generate secondary spheres [69]. Spheroid cells were nestin and CD133 (prominin-1) positive but did not release GH, when the single GH-secreting adenoma was analyzed. Quantitative RT-PCR analysis performed in one PASC spheroid culture showed high expression of stem cell-associated genes (CD90, OCT-4, Musashi-1, NOTCH4, JAG2, and DLL-1). Conversely, culturing the cells in differentiation medium (containing FCS, without growth factors) several neural markers were induced (β-tubulin III, GFAP). Cells from dissociated spheroids, cultured for 2 weeks in differentiation medium (additioned with FCS, but devoid of growth factors), were able to release pituitary hormones in response to hypothalamic peptides [69]. In particular, the cells derived from the GH-secreting tumor did not express GH or LH before differentiation, but these hormones were detected in significant amounts after treatment with GHRH and GnRH, respectively, in differentiation medium. On the contrary, undifferentiated cells released PRL and TSH in response to PRL-releasing peptide and TRH, while the secretory activity was reduced after differentiation. This unexpected result allowed to hypothesize that in the spheroids generated by PASCs are also present differentiated cells originated by spontaneous differentiation also in stem-permissive culture conditions [69].
However, although phenotype characterization was still not completely defined and totally convincing, the most important evidence shown in this study was that spheroid-derived cells (1 × 104 cells), but not the adherent/differentiated cells (1 × 105), were able to reproduce the tumors when implanted into the forebrain of immunodeficient mice, since in vivo tumorigenicity is still the best feature to define bona fide CSCs. After 6 months from the transplant, tumor cells were harvested and dissociated giving rise to new spheroids that again were tumorigenic when reinjected into the brain of the NOD/SCID mice. Cell masses were immunopositive to human antigens, and some of them also expressed human GH [69].
While to date this is the best evidence about the existence of CSC in pituitary adenomas, inconclusive answers and few inconsistencies are still present (see [20]), and in particular, since only two human tumors were analyzed, the reproduction of these data from more specimens is required to definitely prove the CSC existence in pituitary adenomas, as well as their derivation from adult pituitary stem cells.
8 Conclusions and Future Perspectives
In this chapter we report the main studies proposing the existence of multipotent stem/progenitor cells in adult pituitary and the possibility that a deregulation of the activity of these cells may result in pituitary tumor development. Although not all the studies are completely concordant, several features, including Sox2 expression, SP nature, and a niche-like configuration, seem to characterize the phenotype of these cells in all the studies to date available. The main limit of almost all the evidence reported to characterize pituitary stem cells is that they are performed in murine models. A necessary step ahead will be the reproduction of these conclusions in human tissues.
The role of stem/progenitor cells in adult pituitary cell homeostasis, regeneration after injury, genetic endocrine deficits, and tumor pathogenesis will have significant clinical relevance, and the possibility of isolation and functional analysis of this cell population will provide important information to define these issues. Importantly, the characterization of pituitary stem/progenitor cells could also allow a better understanding of the biological basis of some pituitary pathologies including hypopituitarism and adenoma tumorigenesis also allowing the identification of potential novel pharmacological targets for pituitary tumors.
Abbreviations
- CSC:
-
Cancer stem cells
- FCS:
-
Fetal calf serum
- FS cells:
-
Folliculo-stellate cells
- GPS cells:
-
GFRα2-PROP1-STEM cells
- HMG:
-
High mobility group
- MP:
-
Main population
- PASCs:
-
Pituitary adenoma stem-like cells
- Sca1:
-
Stem cell antigen 1
- SMA:
-
Smooth muscle actin
- SP:
-
Side population
- TIC:
-
Tumor-initiating cells
References
Yoshimura F, Harumiya K, Ishikawa H, Otsuka Y (1969) Differentiation of isolated chromophobes into acidophils or basophils when transplanted into the hypophysiotrophic area of hypothalamus. Endocrinol Jpn 16:531–540
Kawamura K, Kouki T, Kawahara G, Kikuyama S (2002) Hypophyseal development in vertebrates from amphibians to mammals. Gen Comp Endocrinol 126:130–135
Castinetti F, Davis SW, Brue T, Camper SA (2011) Pituitary stem cell update and potential implications for treating hypopituitarism. Endocr Rev 32:453–471
Bilodeau S, Roussel-Gervais A, Drouin J (2009) Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol 29:1895–1908
Vankelecom H (2010) Pituitary stem/progenitor cells: embryonic players in the adult gland? Eur J Neurosci 32:2063–2081
Carbajo-Perez E, Watanabe YG (1990) Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res 261:333–338
Levy A (2008) Molecular and trophic mechanisms of tumorigenesis. Endocrinol Metab Clin North Am 37:23–50, vii
Levy A (2002) Physiological implications of pituitary trophic activity. J Endocrinol 174:147–155
Levy A (2008) Stem cells, hormones and pituitary adenomas. J Neuroendocrinol 20:139–140
Nolan LA, Kavanagh E, Lightman SL, Levy A (1998) Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. J Neuroendocrinol 10:207–215
Nolan LA, Levy A (2006) The effects of testosterone and oestrogen on gonadectomised and intact male rat anterior pituitary mitotic and apoptotic activity. J Endocrinol 188:387–396
Nolan LA, Levy A (2009) The trophic effects of oestrogen on male rat anterior pituitary lactotrophs. J Neuroendocrinol 21:457–464
Kominami R, Yasutaka S, Taniguchi Y, Shinohara H (2003) Proliferating cells in the rat anterior pituitary during the postnatal period: immunoelectron microscopic observations using monoclonal anti-bromodeoxyuridine antibody. Histochem Cell Biol 120:223–233
Frawley LS, Boockfor FR (1991) Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev 12:337–355
Vankelecom H (2007) Stem cells in the postnatal pituitary? Neuroendocrinology 85:110–130
McNicol AM, Carbajo-Perez E (1999) Aspects of anterior pituitary growth, with special reference to corticotrophs. Pituitary 1:257–268
Rizzoti K (2010) Adult pituitary progenitors/stem cells: from in vitro characterization to in vivo function. Eur J Neurosci 32:2053–2062
Castrique E, Fernandez-Fuente M, Le Tissier P, Herman A, Levy A (2010) Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J Endocrinol 205:49–60
Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT (2009) Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829
Florio T (2011) Adult pituitary stem cells: from pituitary plasticity to adenoma development. Neuroendocrinology 94:265–277
Furusawa C, Kaneko K (2012) A dynamical-systems view of stem cell biology. Science 338:215–217
Kim M, Morshead CM (2003) Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci 23:10703–10709
Challen GA, Little MH (2006) A side order of stem cells: the SP phenotype. Stem Cells 24:3–12
Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H (2005) The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146:3985–3998
van Rijn SJ, Gremeaux L, Riemers FM, Brinkhof B, Vankelecom H, Penning LC, Meij BP (2012) Identification and characterisation of side population cells in the canine pituitary gland. Vet J 192:476–482
Chen J, Crabbe A, Van Duppen V, Vankelecom H (2006) The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol 20:3293–3307
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17:1253–1270
Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D (2004) Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 22:1115–1124
Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H (2009) Pituitary progenitor cells tracked down by side population dissection. Stem Cells 27:1182–1195
Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912
Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, Alvarez CV (2009) A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One 4:e4815
Gremeaux, L, Fu QV, Van Duppen V, Van den broeck A, Wouters J, van Loon J, Bex M, and Vankelecom H (2009) Cancer stem cells in human pituitary adenoma: identification and characterization of a tumor ‘side population’. In: Abstract, Oncoforum 2009. Leuven, Belgium
Cherqui S, Kurian SM, Schussler O, Hewel JA, Yates JR 3rd, Salomon DR (2006) Isolation and angiogenesis by endothelial progenitors in the fetal liver. Stem Cells 24:44–54
Pevny LH, Nicolis SK (2010) Sox2 roles in neural stem cells. Int J Biochem Cell Biol 42:421–424
Alatzoglou KS, Kelberman D, Dattani MT (2009) The role of SOX proteins in normal pituitary development. J Endocrinol 200:245–258
Yoshida S, Kato T, Yako H, Susa T, Cai LY, Osuna M, Inoue K, Kato Y (2011) Significant quantitative and qualitative transition in pituitary stem / progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol 23:933–943
Kawakami Y, Rodriguez-Leon J, Izpisua Belmonte JC (2006) The role of TGFbetas and Sox9 during limb chondrogenesis. Curr Opin Cell Biol 18:723–729
Sekido R (2010) SRY: a transcriptional activator of mammalian testis determination. Int J Biochem Cell Biol 42:417–420
Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH (2006) SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev 20:1187–1202
Fu Q, Gremeaux L, Luque RM, Liekens D, Chen J, Buch T, Waisman A, Kineman R, Vankelecom H (2012) The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology 153:3224–3235
Fu Q, Vankelecom H (2012) Regenerative capacity of the adult pituitary: multiple mechanisms of lactotrope restoration after transgenic ablation. Stem Cells Dev 21(18):3245–3257
Krylyshkina O, Chen J, Mebis L, Denef C, Vankelecom H (2005) Nestin-immunoreactive cells in rat pituitary are neither hormonal nor typical folliculo-stellate cells. Endocrinology 146:2376–2387
Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G (2008) Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA 105:6332–6337
Weiss S, Siebzehnrubl FA, Kreutzer J, Blumcke I, Buslei R (2009) Evidence for a progenitor cell population in the human pituitary. Clin Neuropathol 28:309–318
Galichet C, Lovell-Badge R, Rizzoti K (2010) Nestin-Cre mice are affected by hypopituitarism, which is not due to significant activity of the transgene in the pituitary gland. PLoS One 5:e11443
Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA (2005) Role of PROP1 in pituitary gland growth. Mol Endocrinol 19:698–710
Yoshida S, Kato T, Susa T, Cai LY, Nakayama M, Kato Y (2009) PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochem Biophys Res Commun 385:11–15
Garcia-Lavandeira M, Saez C, Diaz-Rodriguez E, Perez-Romero S, Senra A, Dieguez C, Japon MA, Alvarez CV (2012) Craniopharyngiomas express embryonic stem cell markers (SOX2, OCT4, KLF4, and SOX9) as pituitary stem cells but do not coexpress RET/GFRA3 receptors. J Clin Endocrinol Metab 97:E80–E87
Lepore DA, Roeszler K, Wagner J, Ross SA, Bauer K, Thomas PQ (2005) Identification and enrichment of colony-forming cells from the adult murine pituitary. Exp Cell Res 308:166–176
Bowie EP, Ishikawa H, Shiino M, Rennels EG (1978) An immunocytochemical study of a rat pituitary multipotential clone. J Histochem Cytochem 26:94–97
Shiino M, Ishikawa H, Rennels EG (1977) In vitro and in vivo studies on cytodifferentiation of pituitary clonal cells derived from the epithelium of Rathke’s pouch. Cell Tissue Res 181:473–485
Otto C, tom Dieck S, Bauer K (1996) Dipeptide uptake by adenohypophysial folliculostellate cells. Am J Physiol 271:C210–C217
Lepore DA, Thomas GP, Knight KR, Hussey AJ, Callahan T, Wagner J, Morrison WA, Thomas PQ (2007) Survival and differentiation of pituitary colony-forming cells in vivo. Stem Cells 25:1730–1736
Osuna M, Yako H, Yoshida S, Sonobe Y, Inoue K, Kato T, Kato Y (2011) S100b-expressing folliculo-stellate cells are found in SOX2-positive population in the anterior pituitary lobe and show multiple differentiation capacities in the defined culture conditions. Endocr Rev 32:P1–P386
Lepore DA, Jokubaitis VJ, Simmons PJ, Roeszler KN, Rossi R, Bauer K, Thomas PQ (2006) A role for angiotensin-converting enzyme in the characterization, enrichment, and proliferation potential of adult murine pituitary colony-forming cells. Stem Cells 24:2382–2390
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Soltysova A, Altanerova V, Altaner C (2005) Cancer stem cells. Neoplasma 52:435–440
Cerdan C, Bhatia M (2010) Novel roles for Notch, Wnt and Hedgehog in hematopoesis derived from human pluripotent stem cells. Int J Dev Biol 54:955–963
Krupkova O Jr, Loja T, Zambo I, Veselska R (2010) Nestin expression in human tumors and tumor cell lines. Neoplasma 57:291–298
Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB (2009) Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov 8:806–823
Gaston-Massuet C, Andoniadou CL, Signore M, Jayakody SA, Charolidi N, Kyeyune R, Vernay B, Jacques TS, Taketo MM, Le Tissier P, Dattani MT, Martinez-Barbera JP (2011) Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci USA 108:11482–11487
Alatzoglou KS, Andoniadou CL, Kelberman D, Kim HG, Botse-Baidoo E, Pedersen-White JR, Layman LC, Martinez-Barbera JP, Dattani MT (2011) Clinical manifestations of a novel SOX2 mutation may result from failure to repress β-catenin-mediated target activation: suggestion for a new mechanism for the interaction between SOX2 and β-catenin. Endocr Rev 32:P3–P758
Camper SA (2011) Beta-catenin stimulates pituitary stem cells to form aggressive tumors. Proc Natl Acad Sci USA 108:11303–11304
Hosoyama T, Nishijo K, Garcia MM, Schaffer BS, Ohshima-Hosoyama S, Prajapati SI, Davis MD, Grant WF, Scheithauer BW, Marks DL, Rubin BP, Keller C (2010) A postnatal Pax7 progenitor gives rise to pituitary adenomas. Genes Cancer 1:388–402
Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7:257–266
Miller RJ, Banisadr G, Bhattacharyya BJ (2008) CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol 198:31–38
Barbieri F, Bajetto A, Stumm R, Pattarozzi A, Porcile C, Zona G, Dorcaratto A, Ravetti JL, Minuto F, Spaziante R, Schettini G, Ferone D, Florio T (2008) Overexpression of stromal cell-derived factor 1 and its receptor CXCR4 induces autocrine/paracrine cell proliferation in human pituitary adenomas. Clin Cancer Res 14:5022–5032
Barbieri F, Bajetto A, Pattarozzi A, Gatti M, Würth R, Porcile C, Thellung S, Corsaro A, Villa V, Nizzari M, Florio T (2011) The chemokine SDF1/CXCL12: a novel autocrine/paracrine factor involved in pituitary adenoma development. Open Neuroendocrinol J 4:64–76
Xu Q, Yuan X, Tunici P, Liu G, Fan X, Xu M, Hu J, Hwang JY, Farkas DL, Black KL, Yu JS (2009) Isolation of tumour stem-like cells from benign tumours. Br J Cancer 101:303–311
Acknowledgments
This work was supported by a grant from the Italian association for Cancer Research (AIRC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Florio, T. (2014). Adult Pituitary Stem Cells. In: Turksen, K. (eds) Adult Stem Cells. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-9569-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9569-7_5
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-9568-0
Online ISBN: 978-1-4614-9569-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)