Abstract
The liver possesses a remarkable ability to restore, through compensatory hyperplasia or regeneration, its original mass following partial or massive parenchymal cell loss. However, this ability is compromised in most relevant pathological conditions of clinical interest, with liver transplantation being at present the only resolutive treatment for severe acute liver failure (ALF), chronic inborn, or acquired end-stage liver diseases.
Replacing diseased hepatocytes and stimulating endogenous and exogenous regeneration by stem cells represent the main aims of liver-oriented cell therapy. Recent developments in stem cell technology have raised the hopes of identifying new expandable sources of liver cells for use in regenerative medicine and prompted studies on the best support for their growth.
In this chapter we will offer an overview of concept and data from available current literature by focusing the attention first on liver regeneration and the role of liver progenitor cells or adult liver stem cells and to then analyze current status of the therapeutic use of extrahepatic stem cells for liver diseases in either preclinical or clinical studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hepatic adult stem cells
- Bipotent hepatic progenitor cells
- Acute liver failure
- Chronic liver diseases
- Hepatic regenerative medicine
1 The “Clinical” Liver Scenario and the Need for Hepatic Regenerative Medicine
1.1 Acute and Chronic End-Stage Liver Diseases: Epidemiological Data
As it is well known, the liver possesses a remarkable ability to restore its original mass following partial or massive parenchymal cell loss by ensuing compensatory hyperplasia or regeneration. However, in most relevant pathological conditions of clinical interest orthotopic liver transplantation (OLT) is indeed the only resolutive treatment for severe acute liver failure (ALF), chronic inborn, or acquired end-stage liver diseases [1].
Along these lines, ALF is commonly defined as a multi-organ syndrome occurring in previously healthy subjects (that is, in the absence of underlying liver disease) which is characterized by severe hepatocellular dysfunction and often rapid progression to death. Major causes of ALF are represented by acetaminophen or non-acetaminophen drug-induced toxicity, prominent in Western countries, as well as by hepatitis viruses (mainly developing countries). Although the last two decades have been characterized by a consistent overall improvement in critical care and OLT, ALF is still associated with high mortality rate (30–100 %) and the USA data indicate that ALF has an incidence of 3.5 deaths per million population, then accounting for approx. 5–6 % of all OLT [2, 3].
Epidemiological data for chronic end-stage liver diseases (CLD) are more relevant and outline a global scenario dominated by an increasing worldwide prevalence of liver cirrhosis, mostly related to chronic infection by hepatitis C or B virus, alcohol consumption and nonalcoholic fatty liver disease (NAFLD) [4–8]. At present, approx. 170 million patients worldwide are estimated to be affected by a form of CLD and 25–30 % of these patients are expected to develop with time significant fibrosis and eventually cirrhosis and related complications. The latter scenario has a relevant clinical impact since, among disease of the GI tract, liver cirrhosis now represents the most common non-neoplastic cause of death in Europe and USA, as well as the seventh most common cause of death in Western countries. Moreover, particularly in Western countries, cirrhosis also represents the main predisposing cause for hepatocellular carcinoma (HCC), accounting for 85–90 % of primary liver cancers and representing the fifth most common human cancer and the third most common cause of cancer mortality worldwide [9].
The overall liver scenario, as for current epidemiological analysis, needs to take into account a peak for advanced CLD which is predicted to occur in the next decade, resulting then in a significant increase of the numbers of patients reaching end-stage disease and potentially requiring OLT that should face a predictable shortage of donor livers. Moreover, current knowledge suggests that OLT procedures can be further complicated by immunological incompatibilities and by the fact that OLT is not always effective. Clinical evidence has outlined the existence of a subset of patients, particularly within those undergoing OLT for hepatitis C virus (HCV)-related cirrhosis, which can develop fulminant fibrotic progression to cirrhosis within a relatively short period of time (i.e., 2–3 years) [4–7]. Although OLT could be considered as an essentially successful surgical procedure, it should be emphasized that, in addition to the problem of the shortage of donor organs, OLT still suffers from operative damage. Therefore, recent attention has been focused on the ability to use cellular resources to bridge patients until transplantation or to restore liver mass and function [10].
1.2 The Need for Hepatic Regenerative Medicine: Introductory Remarks
Liver transplantation is the gold standard procedure for treating acute and chronic end-stage liver disease and the demand for treatment of end-stage liver disease will continue to rise and will drive development of alternatives [1]. Hepatocyte transplantation has been proposed to replace whole liver transplantation at least for selected cases of inherited liver disorders, but there are several limitations for the use of liver cell therapies. Studies on stem cells and on their potential sources have been intensified in recent years, given the promise of their clinical application, especially in regenerative medicine [11]. The behavior and composition of both multipotent and pluripotent stem cell populations are exquisitely controlled by a complex interplay of extracellular matrix and cell–cell interaction. An interesting review was recently published on developments of arrayed cellular environments and their contribution and potential in stem cells and regenerative medicine. Arrayed cellular environments provide a set of experimental elements with variation of one or several classes of stimuli across elements of the array with the capability to provide an understanding of the molecular and cellular events that underlie expansion and specification of stem cell and therapeutic cell populations [12].
Success for stimulating stem cells to differentiate into hepatocytes and other liver cell types has been reported; however, it appears that it is very difficult to obtain differentiated human hepatocytes from human cord blood or human cord mesenchymal stem cells. These cells only mimic the hepatocyte function and are usually called hepatocyte-like cells [13].
Replacing diseased hepatocytes and stimulating endogenous and exogenous regeneration by stem cells represent the main aims of liver-oriented cell therapy [14, 15]. Recent developments in stem cell technology have raised the hopes of identifying new expandable sources of liver cells for use in regenerative medicine [16] and prompted studies on the best support for their growth. Embryonic stem cells can be considered the best model of multipotency, but their use is limited due to ethical concerns [17] and the neoplastic risks after their in vivo use [18] have led to adult stem cells being considered a more acceptable source.
Adult stem cells have consequently been widely explored in recent years as a more acceptable source of cells, including the mesenchymal stem cells (MSCs), a population of multipotent progenitors capable of differentiating towards adipogenic, osteogenic [19], and hepatogenic lineages [20, 21] with a low immunogenicity [22].
Cell transplantation is a practical procedure compared with organ transplantation. It can be performed with much less risk to the patient and much reduced cost for the healthcare system. Furthermore, given the little invasiveness of systemic administration, this method could be also applied to patients who are severely ill and would not be able to tolerate organ transplantation.
Recently, we have characterized a novel MSC population obtained from human umbilical cord (UCMSCs) and we have induced their differentiation towards hepatic lineages in vitro seeking the best cell support for this purpose. The main aim of our study was to evaluate the therapeutic potential of adult UCMSCs in a murine model of acute liver injury using carbon tetrachloride, a potent hepatotoxic chemical. Phenotypic analysis showed a profile compatible with MSCs and the simultaneous high expression of CD166, CD105, and CD73 demonstrated that our cells were a novel MSCs population. The morphological features, loss of MSC phenotype, gene expression changes, immune-cytochemical staining, albumin secretion, urea production, and glycogen storage all suggested that these cells can grow and differentiate into functional hepatocyte-like cells without any biological support [23].
However, we had previously reported that stem cell differentiation can be stimulated by growth factors and extracellular matrix components used as a cell culture support. Using a homologous acellular matrix derived from surgical specimens represents an interesting tissue engineering approach since the matrix is biocompatible, contains adhesion molecules and growth factors, and is obtained from a healthy organ [24, 25].
Interestingly enough, more recently, the field of cell microencapsulation technology has opened many new perspectives. The immobilization of cells into polymeric scaffolds releasing therapeutic factors, such as alginate microcapsules, has been widely employed as a drug delivery system for numerous diseases for many years. Stem cells represent an ideal tool for cell immobilization and so does alginate as a biomaterial of choice in the elaboration of these biomimetic scaffolds [26].
Bone marrow (BM) is considered the main source of MSCs [27], but their number decreases significantly with age [28, 29] and this has led to the evaluation of alternative sources such as adipose tissue [30] and embryo-derived tissues, e.g., placenta [31], amniotic fluid [32], umbilical cord blood [33], and umbilical cord [34].
Moreover, regenerative medicine methods and technologies are currently being developed to manufacture different segments of the entire digestive tube [35]. The conveyance of these results into clinical practice would need to be considered with caution because more information is needed on cell behavior in vivo before any clinical applications can be hypothesized.
Continued research in this area and continued industry attention focused on developing liver support and cellular therapies should accelerate because of the ever pressing demand. It is this demand that has and will continue to drive us to push the limits, test new hypotheses, and take new risks [1].
In this chapter we will offer an overview of concept and data from available current literature by focusing the attention first on liver regeneration and the role of liver progenitor cells or adult liver stem cells and to then analyze current status of the therapeutic use of extrahepatic stem cells for liver diseases in either preclinical or clinical studies.
2 Liver Regeneration and Liver Progenitor Cells/Adult Liver Stem Cells
The liver has a remarkable capacity for regeneration [36]. This capacity is known since the ancient Greek myth of Titan Prometheus and his punishment for deceiving Zeus and protecting mankind. The myth of Prometheus is known to most members of the scientific community who study hepatic diseases, mainly because Prometheus’s liver was the target of torture. The Myth of Prometheus is also known and cherished by many, because, according to one version, Prometheus created the first man. The ancient poet Hesiod (eighth century BC) [37] records that Prometheus twice tricked the gods. First, he offered mortals the best meat from a slaughtered cow and gave the fat and bones to the gods. Then, when an infuriated Zeus punished man by taking fire, Prometheus stole it back for mankind. Accordingly, Zeus punished Prometheus binding him on the mountain Caucasus. More explicitly, for students of the liver, an eagle fed from his liver each day, but the liver regenerated overnight [38, 39]. Self-renewal of hepatocytes is the main mechanism responsible for liver mass homeostasis and for liver regeneration after acute (moderate) liver injury and reduction of liver mass [40]. However, in conditions of chronic liver injury or submassive liver cell loss, such capacity for self-renewal is overwhelmed, exhausted, or impaired, leading to liver failure or insufficiency. In those conditions, hepatic progenitor cells (HPCs), which are dormant and found in periportal location in a healthy liver, actively proliferate and yield transit-amplifying cells (or oval cells). Since the 1950s, when Opie and Farber described a category of small hepatic cells that they called oval cells, emerging from the canal of Hering, where bile canaliculi connect with bile ducts, it has become a hackneyed term used to define a highly heterogeneous population of cells whose fate is classically bipotent giving rise to both hepatocytes and cholangiocytes at least in vitro and at least in rodents [41].
This reaction is known as ductular reaction in human beings or oval cell proliferation in rodents [42–44]. Although the initiating mechanisms of liver regeneration may be similar in rodents and humans, the time course of the process differs among species. Nevertheless, in rats and mice, the original liver mass is restored to approximately 100 % in 7–10 days. In humans, there is a very rapid increase in liver mass during the first 7 days after partial liver transplantation, leading to complete restoration by 3 months [45].
2.1 Cellular Mechanisms of Liver Regeneration
As already mentioned before, what is unique to the liver is that differentiated hepatocytes constitute the first line of response to injury or resection, while progenitor cells function as a reserve compartment. This differs from other tissues, such as skeletal muscle, in which differentiated myocytes do not replicate, but regeneration after injury can occur through the proliferation of precursor cells (satellite cells) [46] or the heart, in which there is little if any proliferation of differentiated myocytes or immature precursors [47].
Furthermore in highly proliferating tissues such as the skin and the gut, progenitor cells continuously produce transit-amplifying cells that differentiate and replenish short-lived mature cells [48, 49]. By contrast, the liver has very low levels of cell turnover, and it primarily relies on replication of highly differentiated parenchymal hepatocytes to regenerate in response to loss of liver mass [50–52].
Physiological turnover of liver parenchyma was originally proposed to follow the model of the “streaming liver,” an hypothesis suggesting that young hepatocytes are formed in the portal area and then migrate towards the central vein to progressively replace older cells [53]. Although this concept has received some confirmation [54] other data do not support this hypothesis [43]. Whatever the mechanism or model involved, two concepts are widely acknowledged: (1) physiological hepatocyte turnover is slow, with a reported average life span of approx. 200–300 days, and (2) liver parenchyma turnover mostly depends on proliferation of adult hepatocytes and bile duct epithelial cells (BDEC), with a still debated, presumably minor, contribution by progenitor or stem cells [43].
Liver regeneration involves coordinated action of distinct cytokines and growth factors, which regulate three temporal stages of hepatocyte proliferation, namely, priming, DNA synthesis, and cell division, followed by growth termination. Tumor necrosis factor α (TNFα) and interleukin 6 (IL-6) are critical priming factors, which facilitate G0 to G1 transition of hepatocytes, rendering them competent to respond to growth factors. Mice lacking TNF receptor 1 show delayed liver regeneration, which could be reversed by administration of IL-6, whereas IL-6 deficiency induces severe apoptosis because IL-6-induced STAT3 activation is essential for liver regeneration. Following priming, growth factors provide mitogenic signals that facilitate competent hepatocytes to progress through the cell cycle [55–57]. For example, under the standard experimental conditions of 2/3 partial hepatectomy, any hepatocyte may undergo one or two rounds of cell division (sustained by HGF, IL-6, TNF, TGFα, EGF) within 24–48 h. This is followed by wave of proliferation involving other hepatic cell populations called non-parenchymal cells (NPCs).
NPCs in the liver include stellate cells/myofibroblasts, which are the main producers of collagen; macrophages, which are involved in tissue remodeling and fibrosis resolution after extensive damage; endothelial cells, which are able to form new vessels; and other leukocytes recruited by local inflammation. NPCs produce cytokines and growth factors, like transforming growth factor β, that influence oval cells/HPCs and hepatocyte proliferation, but most of the signals they exchange with the oval cell/HPC compartment and their role in regulating oval cell/LPC behavior has yet to be fully elucidated. Moreover, studies have demonstrated that in liver injury a proportion of myofibroblasts and macrophages are recruited from the BM. It has been claimed that oval cells are of BM origin; however, other studies have found that oval cells are intrinsic to the liver and not of BM origin [58–60].
2.2 Liver Progenitor Cells
Impairment of the replicative capacity of most remnant hepatocytes induces an alternate regenerative process from HPCs. These cells (also called oval cells in rodent) are located in the most peripheral branches of the biliary tree (canal of Hering).
Once activated, HPCs proliferate in the portal region and migrate into the hepatic lobule where they undergo further differentiation into hepatocytes or bile duct cells to repopulate the hepatic parenchyma (Fig. 1). This proliferative response characterized by the appearance of bile duct-like structures in humans is referred to as atypical ductular reaction [61].
Immune-histochemistry for cytokeratin 7 (CK-7, used as a marker to identify HPCs and cells derived from HPCs) in a human liver specimen obtained from a HCV-related cirrhotic patient. Positive stain is found in cholangiocytes and ductular-like structures (either typical or atypical) as well as in HPCs at the border of fibrotic septa or hepatocyte-like cells within the pseudo-lobule. Original magnification is indicated
While the term oval cell is widely used to describe liver progenitors, investigators do not agree on the phenotype and molecular signature of these cells. The terminal bile ductular system (i.e., the canal of Hering) is thought to be the main source of oval cells [62].
The oval cell compartment can probably not be attributed to a single cell type [63]. In order to avoid misunderstandings, the term oval cell activation is used to describe the heterogeneous cellular changes accompanying the appearance of progenitor cells, whereas the term oval cells refer to the progenitors themselves. Oval cells are considered bipotential transit-amplifying cells derived from normally quiescent “true stem cells” that reside in the biliary tree [62]. Proliferating oval cells constitute a heterogeneous population justifying the different names used to describe them: ductular progenitor cells, atypical ductular cells, periductular liver progenitor cell, or individual progenies [63].
We summarize the current complexity of terminology as follows:
-
(a)
Hepatic adult stem cells (HASC), normally quiescent and otherwise termed “oval” or HPC-precursor cells [43] that reside in portal areas within the canals of Hering, envisaged as the hepatic niche for progenitor cells.
-
(b)
Activation of the HASC compartment (as in ALF or chronic liver injury) leads to the appearance of oval cells, described as bipotential transient-amplifying cells or as bipotential HPC.
-
(c)
The classic view implies that during activation of the stem/progenitor compartment adult liver bipotential HPC can generate either hepatocytes or BDEC. A variant of this model hypothesizes the existence of different steps of maturation, with the most mature oval cell being bipotential and able to generate precursors of either hepatocytes or cholangiocytes, sometimes referred to as pre-hepatocytes or intermediate hepatocyte-like cells [58].
Furthermore oval cell activation can be envisaged as a process involving four distinct phases: [43] (1) activation, the phase in which the stem/precursor cell compartment is activated, leading to the emergence of oval cell or HPC, sustained and induced to proliferate by growth factors (Oncostatin M or OSM, IL-6, LIF) signaling through the JAK/STAT and other factors (TNF, TWEAK, IFNγ, SCF, COX-2); (2) oval cell population is further amplified by several polypeptide growth factors (TGFα, TGFβ, HGF, FGF-1, Sonic and Indian Hedgehog); (3) migration of progenitor cells, in response to the chemokine SDF-1 (CXCL12) or factors like uPA and tPA of the plasmin activator cascade; and (4) differentiation, the final step leading to either hepatocytes or BDEC in response again to LIF, OSM, or Dlk. This scenario has been recently implemented by an excellent in vivo study showing that in both human diseased liver and mouse models of the ductular reaction Notch and Wnt signaling are relevant in directing specification of HPCs via their interactions with activated myofibroblasts or macrophages [64]. In particular, during biliary regeneration, expression of Jagged 1 (a Notch ligand) by myofibroblasts promoted Notch signaling in HPCs and thus their biliary specification to cholangiocytes. Alternatively, during hepatocyte regeneration, macrophage engulfment of hepatocyte debris induced Wnt3a expression that resulted in canonical Wnt signaling in nearby HPCs, thus maintaining in these cells expression of the factor Numb and then promoting their specification towards hepatocyte phenotype. It is suggested that indeed these two pathways can regulate and/or promote adult parenchymal regeneration during chronic liver injury [64]. Another very recent experimental study has investigated in vivo the capacity of HPC to differentiate into hepatocytes and to contribute to liver regeneration. By performing lineage-tracing murine experiments (involving either regeneration and/or injury of liver parenchyma) in order to follow the fate of HPC and biliary cells, authors were able to show that hepatobiliary precursors do not contribute to liver mass homeostasis or to liver regeneration in the healthy liver [44]. By contrast, in conditions of chronic liver injury expanded transit-amplifying cells (HPC) were able to give rise to a small proportion of hepatocyte-like cells that were shown to be well differentiated, polarized, and respond to pro-mitogenic stimuli as normal hepatocytes. Repopulation efficiency by HPC and/or biliary cells increased when extracellular matrix and laminin deposition were reduced.
However, it should be underlined that at present human HPCs have not been used in clinical trials. Although they can be differentiated in vitro into hepatocyte-like and cholangiocyte-like cells and effectively transplanted and engrafted into immune-compromised mice, HPCs exhibit phenotypical instability and in certain cases produce tumors in mice. Meanwhile, in vitro expansion of HPCs prior to the differentiation or transplantation into mice opens the prospect for development of liver stem cell therapy and exploitation of “humanized mouse models” [65].
3 Therapeutic Use of Extrahepatic Stem Cells in Liver Diseases: Preclinical Studies
According to the previous section, one can say that the application in clinical practice of hepatocyte transplantation as well as the use of hepatocytes in bio-artificial livers still poses considerable problems, including also the intrinsic difficulty of obtaining human hepatocytes as well as to maintain them viable and into the differentiate phenotype when cultured in vitro. These limitations have favored the alternative cell therapy approach consisting in the use of stem cells and growth factor. Indeed, a growing range of potential applications for therapeutic use of stem cells in liver diseases can be envisaged, with many pilot clinical studies already undertaken. As properly suggested in a recent editorial [66], one may identify a number of areas in which stem cell therapy could reasonably represent a future realistic aim, including the attempt: (a) to improve liver repopulation and reduce excess deposition of extracellular matrix and scarring by upregulating hepatic’s own regenerative processes; (b) to inhibit immune-mediated liver injury; and (c) to obtain hepatocyte-like cells from stem cells and to employ them either for cell transplantation (i.e., to support or replace hepatocyte function) or in extracorporeal bio-artificial liver apparatus. The first available literature data in this field already suggest that a critical issue may be represented by the choice of therapeutic cell to be employed that may be tailored to the specific type of liver disease.
3.1 Stem Cells from Bone Marrow (BM-SCs) and Other Extrahepatic Sources
The rational suggestion to use autologous transplantation of BM-SCs as a putative strategy of intervention in liver diseases was initially proposed more than a decade ago on the basis of pioneer studies [67–69]. However, positive (although relatively modest) features from these initial experimental studies, some based on transplantation of hematopoietic stem cells and indicating recruitment to the injured liver of these cells and their apparent differentiation into hepatocyte-like cells, were later shown to have resulted essentially from fusion between transplanted donor cells and resident recipient hepatocytes [70–72].
Having established this relevant point, several laboratories adopted a different strategy and reported successful “in vitro” differentiation of extrahepatic multipotent stem cells into hepatocyte-like cells (i.e., cells expressing defined hepatocellular antigens and functional properties). The list of multipotent cells employed for this purpose includes (1) so-called multipotent adult progenitor cells [73, 74], a unique population, originating from long-term culture of non-hematopoietic adherent cells (i.e., mesenchymal stem cells [MSC)]) from bone marrow, displaying the ability to differentiate into multiple lineages [75]; (2) MSC derived from either bone marrow [21, 76–78] as well as adipose tissue [79, 80], umbilical blood cord [23, 81], or even dental pulp [82]; and (3) multipotent stem cells from amniotic fluid and membranes [83, 84].
Whatever the source of multipotent stem cells, several laboratories reported successful “in vitro” differentiation of these extrahepatic cells into hepatocyte-like cells, that is, cells expressing defined hepatocellular antigens and functional properties that should include at least the following: (a) phenotypic changes leading to the acquisition of a polygonal (i.e., polarized) morphology; (b) expression of specific proteins like albumin, alpha-fetoprotein (α-FP), and cytokeratin 18 (CK-18); (c) the ability to synthetize urea as well as to synthetize and store glycogen; (d) acquisition of further antigens and/or functional activities such as expression of isoforms of cytochrome P450 and of drug metabolism-related enzymes; and (e) expression/activity of more selective proteins or enzymes, including glucose-6-phosphatase, tyrosine aminotransferase, triptophane-2,3-dioxygenase, hepatic nuclear factor 4, and canalicular antigen 9B2, to name just a few [58].
In most of these studies “in vitro” differentiation was based on a rather common scheme of experimental protocol which typically required first a so-defined differentiation step followed then by a maturation step. The protocol established for human MSCs by Lee and coworkers [21] may serve as a paradigm. In this study the differentiation step was sustained by treatment of MSCs with hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and nicotinamide; in the maturation cells were exposed to a medium containing oncostatin M (OSM), dexamethasone, insulin, transferrin, and selenium. Once hepatocyte-like cells were obtained, several laboratories transplanted in vivo these cells to test their efficiency in animal models of ALF or of CLD and most of these experimental studies have reported that transplanted cells can effectively engraft injured liver parenchyma [73, 84–90]. However, results in terms of repopulation were not numerically impressive, possibly depending on the specific protocol adopted [21, 73–79]. Best results were obtained in those experiments in which MSC-derived hepatocyte-like cells were transplanted. The overall scenario from these preclinical studies can be completed by a number of encouraging results reported in experimental protocols designed to prevent liver fibrosis [85, 86] or in studies that reported some improvement in parameters related to ALF [87–89]. Concerning prevention of liver fibrosis, however, it should be cautionary recalled that at least two studies could not document any significant anti-fibrotic effect following transplantation of either murine MSC or human MSC (in NOD-SCID mice) [77, 90]. Moreover, at least three different laboratories have provided evidence indicating that BM-SCs engrafting the liver during the course of experimental model of chronic liver injury, in particular MSC [77, 91, 92] or fibrocytes [93], have the potential to differentiate into hepatic myofibroblast-like cells (MFs). As originally proposed by Forbes and coworkers, these results envisage a potential risk for these transplanted cells to contribute to liver fibrogenesis, although several authors believe that such a contribution should be considered as minor with hepatic stellates and portal fibroblasts being by far the most relevant sources of hepatic MFs [94–96].
3.2 The Use of Induced Pluripotent Stem Cells
A more recent approach for producing hepatocyte-like cells has taken advantage of induced pluripotent stem cells (iPSCs), one of the most exciting recent discoveries in the field of biology. iPSC cells by definition are somatic cells (of either murine or human origin) that are engineered (and then reprogrammed) in order to express combinations of defined transcription factors and to become pluripotent, remarkably resembling embryonic stem (ES) cells [97–100]. iPSCs are typically generated by retroviral induction of transcription factors such as Oct3/4, Sox2, KLF4, and c-Myc, in fibroblasts. Lentivirus and adenovirus induction, induction with other gene combinations and virus-free approaches such as using plasmids, small molecules, and recombinant proteins have also been reported (reviewed in [100]). In addition, it has been shown that iPSCs can be generated from a variety of cell types such as pancreatic cells, meningiocytes, keratinocytes, hematopoietic cells differentiated from ESCs, and primary human hepatocytes [101–103].
iPSCs are indeed very promising cells potentially able to overcome controversies and ethical concerns associated with the use of human embryonic stem (ES) cells and their availability has theoretically opened the way to their use for a number of perspectives and applications, including the possibility to use these cells in order to (1) design and test patient-customized (i.e., autologous) cell therapy with no need for immune suppression; (2) modeling inherited metabolic human diseases and investigate in detail pathogenic mechanisms; (3) drug discovery and testing, possibly patient customized. Unfortunately, at present the use of iPSCs for regenerative medicine is limited by two major and still unresolved concerns, the oncogenic potential of these cells and the so-called epigenetic transcriptional memory of somatic cells, that can affect the desired differentiation into the desired specific lineages [99, 100, 104, 105].
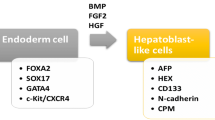
Whether the use of iPS cells in relation to liver diseases is concerned, different laboratories have used protocol similar to those designed for ES cells in order to obtain hepatocyte like cells from human [106–108] or murine [109, 110] somatic cells. This protocol usually requires from three to four steps with the following sequence: (a) endodermal differentiation step following exposure to activin A; (b) hepatic specification step as for exposure to fibroblast growth factor-2 (FGF-2) and bone morphogenetic protein-4 (BMP-4); (c) proliferation step elicited by HGF; and (d) maturation step by a specifically designed hepatocyte culture medium (HCM) containing oncostatin M (OSM) [111].
According to limitations for the use of iPS cells previously described, attempts to obtain engraftment and proliferation of hepatocytes-like cells from iPS cells led to limited and mostly disappointing results (reviewed in [111]) with just one apparent exception for a murine study [112]. In the latter study murine somatic cells were reprogrammed to iPSCs and when transplanted in vivo in the experimental model of mice carrying fumaryl-acetoacetate hydrolase deficiency (FAH −/− mice), these iPS cells apparently underwent normal ontogenic development into mature hepatocytes [111]. In particular, these murine iPSCs cells were injected into blastocysts of FAH −/− mice originating a generation of chimeric mice in which iPSC-derived hepatocytes proliferated (even responding to two/third partial hepatectomy) and repopulated the liver rescuing the chimeric mice. However, the FAH −/− mice is a rather unique and favorable model in the scenario of hepatic regenerative medicine and one should emphasize the fact that such a procedure (i.e., to inject iPS cells into blastocysts) is rather irrelevant for regenerative medicine if the final goal is to obtain safe and adult/mature hepatocyte-like cells to transplant under postnatal settings.
Literature indeed suggests that iPSC lines can be generated from patients suffering from specific diseases, providing a unique source for study and disease modeling. Along these lines, iPSC generation has been reported from individuals affected by several diseases, including neurodegenerative diseases, juvenile diabetes mellitus, muscular dystrophy, hematological diseases, Down syndrome, as well as ischemic heart failure [112]. In another recent study, human hepatocyte-like cells derived from iPSCs (obtained by reprogramming dermal fibroblasts) have been obtained from patients affected by inherited metabolic disorders like α1-antitrypsin deficiency (AAT), glycogen storage disease type 1a deficiency (GSD1), hereditary tyrosinemia type 1, Crigler–Najjar syndrome, and familial hypercholesterolemia (FH) [113]. Authors were able to generate a library of patient-specific human iPS cells to be then differentiated into hepatocyte-like cells and then characterized iPS cell-derived hepatocytes from patients affected by AAT, GSD1, and FH. Of interest, hepatocyte-like cells obtained in this way exhibited all phenotypic abnormalities of primary hepatocytes from patients carrying these diseases and, as also suggested by other researchers in a different study, potentially used in order to investigate disease pathogenesis and to test drugs in a patient-customized manner [114].
An even more interesting approach, with a potential future application in the field of ALF, is the one recently published in a study in which iPSCs as well as ESCs have been injected into hollow fiber (HF)/organoid culture in order to form organoid in the lumen of HF [115]. This study reported that the exposure of iPSCs and ESCs to agents able to promote differentiation resulted in upregulation of differentiation-related genes and a very efficient cell proliferation and organoid formation inside HFs characterized by a high cell density and promising results in terms of gain of liver-specific functions. This may represent a critical report implying the use of these cells as source for obtaining a hybrid-type artificial liver (HAL).
4 Therapeutic Use of Extrahepatic Stem Cells in Liver Diseases: Clinical Studies
There is an increasing range of potential applications of stem cells in liver diseases, with many clinical studies already undertaken. Whilst there have been advances in our understanding of the role of stem cells in liver damage and repair as well as encouraging results using stem cells as cell therapy in preclinical animal models, the precise mode of action and optimal cell usage has not been completely defined.
Cell therapy can be defined as “the use of living cells to restore, maintain, or enhance tissue and organ function” [116] and has several potential advantages when compared to OLT, since transplantable cells can be (a) expanded in vitro and cryopreserved, thus abolishing the limit of organ shortage; (b) genetically manipulated, to correct inborn errors of metabolism; c) cryopreserved for future use and infused without major surgery; and (d) obtained from the same patient, avoiding risk of rejection and need for lifelong immune-suppression [117].
Ideally, allogenic hepatocytes, ex vivo derived hepatocytes, or cells capable of hepatocyte differentiation could be administered directly and repopulate the failing liver. Allogenic hepatocyte transplantation has been explored as an alternative to OLT in ALF and metabolic liver diseases. However, difficulties in harvesting and storing sufficient quantities of hepatocytes and significant cell loss following transplantation have so far limited the potential of this therapy [118].
Given the right environment and stimuli, stem cells and certain progenitor cells can differentiate into hepatocytes. Stem cells are undifferentiated cells capable of proliferation, self-maintenance, and differentiation into functional progeny with flexibility or plasticity in these options [119]. Embryonic stem cells (ESCs) have pluripotency and unlimited capacity for self-renewal. In contrast, adult stem cells have a restricted differentiation capability and because of this they may be more correctly called progenitor cells. Despite the apparently limited differentiation capability of progenitor cells, given appropriate stimuli, progenitor cells can trans-differentiate into other cell lines. The ideal cell source to support hepatic regeneration must be reliably identifiable, be able to generate hepatocytes efficiently, evade the immune defenses, and behave predictably with a high safety profile.
Successful cell therapy depends on the innate clonogenicity of the administered cells or on the favorable condition in which transplanted cells have a selective growth advantage over the indigenous population. In the diseased human liver there may not be the substantial selective growth advantage for transplanted cells that pertains in many rodent models where it is possible to enrich for cells that continue to expand in the recipient liver in the absence of a major growth stimulus. Such cells might simply be fetal cells or a subpopulation of antigenically distinct adult cells [120].
Of the clinical studies published, the overwhelming data suggest that stem cell therapy is safe [121], although there are possible concerns regarding the route of delivery of cell therapy. Whilst no studies report superior outcomes when cells are directly injected into the liver (portal vein or hepatic artery), there have been complications such as hepatic artery dissection [122] and increased portal hypertensive bleeding [123] following this approach. Furthermore, the intravenous administration of autologous BM mononuclear cells resulted in hepatic homing of the injected cells suggesting this easier, safer route may be an adequate option for cell delivery [124, 125]. Assuming that delivery to the liver is important for stem cell infusions to exert their optimal effect, then developing a better understanding of the mechanisms regulating their hepatic ingress may allow for further improvements to treatment protocols. Whilst patients with a wide range of disease severity have been included in clinical trials, the priority remains to irrefutably confirm the efficacy of cell/stem cell therapy. In this regard, choosing patients in which the benefit may be most reliably determined and of greatest value is important. Patients verging on the cusp of requiring a liver transplant (e.g., with MELD score approaching/just below 15) are good candidates as even a small percentage improvement in liver function may be sufficient to significantly delay or indeed remove altogether the need for liver transplant.
Abbreviations
- AAT:
-
α1-antitrypsin deficiency
- ALF:
-
Acute liver failure
- α-FP:
-
Alpha-fetoprotein
- BDEC:
-
Bile duct epithelial cells
- BM:
-
Bone marrow
- BM-SCs:
-
Bone marrow stem cells
- CK-18:
-
Cytokeratin 18
- CLD:
-
Chronic end-stage liver diseases
- COX2:
-
Cyclooxygenase type 2
- EGF:
-
Epidermal growth factor
- ESCs:
-
Embryonic stem cells
- FGF-1:
-
Fibroblast growth factor 1
- FH:
-
Familial hypercholesterolemia
- GSD1:
-
Glycogen storage disease type 1a
- HAL:
-
Hybrid-type artificial liver
- HASC:
-
Hepatic adult stem cells
- HCC:
-
Hepatocellular carcinoma
- HCM:
-
Hepatocyte culture medium
- HCV:
-
Hepatitis C virus
- HF:
-
Hollow fiber
- HGF:
-
Hepatocyte growth factor
- HPCs:
-
Hepatic progenitor cells
- IFNγ:
-
Interferon γ
- IL-6:
-
Interleukin 6
- iPSCs:
-
Induced pluripotent stem cells
- LIF:
-
Leukemia inhibitory factor
- LPCs:
-
Liver progenitor cells
- MELD:
-
Model for end-stage liver disease
- MFs:
-
Myofibroblast-like cells
- MSCs:
-
Mesenchymal stem cells
- NAFLD:
-
Nonalcoholic fatty liver disease
- NOD-SCID mice:
-
Non-obese diabetic-severe combined immune deficiency mice
- NPCs:
-
Non-parenchymal cells
- OLT:
-
Orthotopic liver transplantation
- OSM:
-
Oncostatin M
- SCF:
-
Stem cell factor
- SDF-1:
-
Stromal cell-derived factor 1
- STAT3:
-
Signal transducer and activator of transcription 3
- TGFα:
-
Transforming growth factor α
- TGFβ:
-
Transforming growth factor β
- TNFα:
-
Tumor necrosis factor α
- UCMSCs:
-
MSCs from human umbilical cord
References
Burra P, Freeman R (2011) Trends in liver transplantation. J Hepatol 56(Suppl 1):S101–S111
Larson AM (2010) Diagnosis and management of acute liver failure. Curr Opin Gastroenterol 26:214–221
Bernal W, Auzinger G, Dhawan A, Wendon J (2010) Acute liver failure. Lancet 376:190–201
Poynard T, Bedossa P, Opolon P (1997) Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR and DOSVIRC groups. Lancet 349:825–832
Armstrong GL, Alter MJ, McQuillan GM et al (2000) The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 31:777–782
Wong JB, McQuillan GM, McHutchison JG et al (2000) Estimating future hepatitis C morbidity mortality, and costs in the United States. Am J Public Health 90:1562–1569
Pinzani M, Romanelli RG, Magli S (2001) Progression of fibrosis in chronic liver diseases – time to tally the score. J Hepatol 34:764–767
Parola M, Marra F, Pinzani M (2008) Myofibroblast-like cells and liver fibrogenesis: emerging concepts in a rapidly moving scenario. Mol Aspects Med 29:58–66
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576
Dalgetty DM, Medine CN, Iredale JP et al (2009) Progress and future challenges in stem cell-derived liver technologies. Am J Physiol Gastrointest Liver Physiol 297:G241–G248
Stocum DL (2001) Stem cells in regenerative biology and medicine. Wound Repair Regen 9:429–442
Titmarsh DM, Chen H, Wolvetang EJ, Cooper-White JJ (2012) Arrayed cellular environments for stem cells and regenerative medicine. Biotechnol J 8:822–834
Burra P, Bizzaro D, Ciccocioppo R et al (2011) Therapeutic application of stem cells in gastroenterology: an up-date. World J Gastroenterol 17:3870–3880
Burra P, Tomat S, Bizzaro D et al (2008) Stem cells in hepatology. Organs Tissues Cells 1:15–22
van Poll D, Parekkadan B, Cho CH et al (2008) Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 47:1634–1643
Burra P, Tomat S, Villa E et al (2008) Experimental hepatology applied to stem cells. Dig Liver Dis 40:54–61
Denker HW (2006) Potentiality of embryonic stem cells: an ethical problem even with alternative stem cell sources. J Med Ethics 32:665–671
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Kern S, Eichler H, Stoeve J et al (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Campard D, Lysy PA, Najimi M et al (2008) Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 134:833–848
Lee KD, Kuo TK, Whang-Peng J et al (2004) In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40:1275–1284
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822
Burra P, Arcidiacono D, Bizzaro D et al (2012) Systemic administration of a novel human umbilical cord mesenchymal stem cells population accelerates the resolution of acute liver injury. BMC Gastroenterol 12:88
Burra P, Tomat S, Conconi MT et al (2004) Acellular liver matrix improves the survival and functions of isolated rat hepatocytes cultured in vitro. Int J Mol Med 14:511–515
Tomat S, Burra P, Gringeri E et al (2006) Metabolic activity of rat hepatocytes cultured on homologous acellular matrix and transplanted into Gunn rats. Int J Mol Med 18:837–842
Garate A, Murua A, Orive G et al (2012) Stem cells in alginate bioscaffolds. Ther Deliv 3:761–774
Bianco P, Riminucci M, Gronthos S et al (2001) Bone marrow stromal cells: biology and potential application. Stem Cells 19:180–192
Mareschi K, Ferrero I, Rustichelli D et al (2006) Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem 97:744–754
Mendes SC, Tibbe JM, Veenhof M et al (2002) Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng 8:911–920
Rodriguez AM, Elabd C, Amri EZ et al (2005) The human adipose tissue is a source of multipotent stem cells. Biochimie 87:125–128
Miao Z, Jin J, Chen L et al (2006) Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int 30:681–687
Yu SJ, Soncini M, Kaneko Y et al (2009) Amnion: a potent graft source for cell therapy in stroke. Cell Transplant 18:111–118
Lee OK, Kuo TK, Chen WM et al (2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103:1669–1675
Wang HS, Hung SC, Peng ST et al (2004) Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 22:1330–1337
Orlando G, Garcia-Arras JE, Soker T et al (2012) Regeneration and bioengineering of the gastrointestinal tract: current status and future prospectives. Dig Liver Dis 44:714–720
Grisham J, Thorgeirsson S (1997) Liver stem cells. In: Potten CS (ed) Stem cells. Academic, London, pp 233–282
Hesiod (1999) Theogony-works and days. Oxford University Press, New York
Morfrod MPO, Lenardon RJ (2007) Classical mythology. Oxford University Press, New York
Smith W (2005) Dictionary of Greek and Roman biography and mythology. IB Tauris, London, p 1161
Fausto N, Campbell JS (2003) The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 120:117–130
Gilgenkrantz H, Collin de l’Hortet A (2011) New insights into liver regeneration. Clin Res Hepatol Gastroenterol 35:623–629
Shafritz DA, Dabeva MD (2002) Liver stem cells and model systems for liver repopulation. J Hepatol 36:552–564
Duncan AW, Dorrell C, Grompe M (2009) Stem cells and liver regeneration. Gastroenterology 137:466–481
Español-Suñer R, Carpentier R, Van Hul N et al (2012) Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143:1564–1575
Russo FP, Parola M (2012) Stem cells in liver failure. Best Pract Res Clin Gastroenterol 26:35–45
Tanaka EM (2003) Regeneration: if they can do it, why can’t we? Cell 113:559–562
Mathur A, Martin JF (2004) Stem cells and repair of the heart. Lancet 365:183–192
Slack JM (2008) Origin of stem cells in organogenesis. Science 322:1498–1501
Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10:207–217
Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276:60–66
Taub R (2004) Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5:836–847
Fausto N, Campbell JS, Riehle KJ (2006) Liver regeneration. Hepatology 43:S45–S53
Zajicek G, Oren R, Weinreb M Jr (1985) The streaming liver. Liver 5:293–300
Fellous TG, Islam S, Taudros PJ et al (2009) Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology 49:1655–1663
Yamada Y, Kirillova I, Peschon JJ et al (1997) Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A 94:1441–1446
Li W, Liang X, Kellendonk C et al (2002) STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem 277:28411–28417
Huh CG, Factor VM, Sanchez A et al (2004) Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A 101:4477–4482
Russo FP, Parola M (2011) Stem and progenitor cells in liver regeneration and repair. Cytotherapy 13:135–144
Chobert MN, Couchie D, Fourcot A (2012) Liver precursor cells increase hepatic fibrosis induced by chronic carbon tetrachloride intoxication in rats. Lab Invest 92:135–150
Lorenzini S, Bird TG, Boulter L et al (2010) Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 59:645–654
Zheng YW, Taniguchi H (2003) Diversity of hepatic stem cells in the fetal and adult liver. Semin Liver Dis 23:337–348
Shafritz DA, Oertel M, Menthena A (2006) Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology 43:S89–S98
Dollé L, Best J, Mei J et al (2010) The quest for liver progenitor cells: a practical point of view. J Hepatol 52:117–129
Boulter L, Govaere O, Bird TC et al (2012) Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18:572–579
Kisseleva T, Gigante E, Brenner DA (2010) Recent advances in liver stem cell therapy. Curr Opin Gastroenterol 26:395–402
Forbes SJ, Newsome PN (2012) New horizons for stem cell therapy in liver disease. J Hepatol 56:496–499
Petersen BE, Bowen WC, Patrene KD et al (1999) Bone marrow as a potential source of hepatic oval cells. Science 284:1168–1170
Theise ND, Nimmakayalu M, Gardner R et al (2000) Liver from bone marrow in humans. Hepatology 32:11–16
Alison MR, Poulsom R, Jeffery R et al (2000) Hepatocytes from non-hepatic adult stem cells. Nature 406:257
Terada N, Hamazaki T, Oka M et al (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416:542–545
Wang X, Willenbring H, Akkari Y et al (2003) Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422:897–901
Vassilopoulos G, Wang PR, Russell DW (2003) Transplanted bone marrow regenerates liver by cell fusion. Nature 422:901–904
Jiang Y, Jahagirdar BN, Reinhardt RL et al (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49
Schwartz RE, Reyes M, Koodie L et al (2002) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 109:1291–1302
Reyes M, Verfaillie CM (2001) Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci 938:231–233
Lange C, Bassler P, Lioznov MV et al (2005) Hepatocytic gene expression in cultured rat mesenchymal stem cells. Transplant Proc 37:276–279
Valfrè di Bonzo L, Ferrero I, Cravanzola C et al (2008) Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 57:223–231
Ishii K, Yoshida Y, Akechi Y et al (2008) Hepatic differentiation of human bone marrow-derived mesenchymal stem cells by tetracycline-regulated hepatocyte nuclear factor 3beta. Hepatology 48:597–606
Seo MJ, Suh SY, Bae YC et al (2005) Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun 328:258–264
Aurich H, Sgodda M, Kaltwasser P et al (2009) Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 58:570–581
Hong SH, Gang EJ, Jeong JA et al (2005) In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun 330:1153–1161
Ishkitiev N, Yaegaki K, Calenic B et al (2010) Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J Endod 36:469–474
De Coppi P, Bartsch G Jr, Siddiqui MM et al (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106
Miki T, Lehmann T, Cai H et al (2005) Stem cell characteristics of amniotic epithelial cells. Stem Cells 23:1549–1559
Oyagi S, Hirose M, Kojima M et al (2006) Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol 44:742–748
Abdel Aziz MT, Atta HM, Mahfouz S et al (2007) Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem 40:893–899
Banas A, Teratani T, Yamamoto Y et al (2008) IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 26:2705–2712
Kuo TK, Hung SP, Chuang CH et al (2008) Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology 134:2111–2121
Yan Y, Xu W, Qian H et al (2009) Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int 29:356–365
Carvalho AB, Quintanilha LF, Dias JV et al (2008) Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells 26:1307–1314
Forbes SJ, Russo FP, Rey V et al (2004) A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology 126:955–963
Russo FP, Alison MR, Bigger BW et al (2006) The bone marrow functionally contributes to liver fibrosis. Gastroenterology 130:1807–1821
Kisseleva T, Uchinami H, Feirt N et al (2006) Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 45:429–438
Lee UE, Friedman SL (2011) Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol 25:195–206
Forbes SJ, Parola M (2011) Liver fibrogenic cells. Best Pract Res Clin Gastroenterol 25:207–217
Dranoff JA, Wells RG (2010) Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 51:1438–1444
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Yu J, Vodyanik MA, Smuga-Otto K et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Yamanaka S, Blau HM (2010) Nuclear reprogramming to a pluripotent state by three approaches. Nature 465:704–712
Okita K, Yamanaka S (2010) Induction of pluripotency by defined factors. Exp Cell Res 316:2565–2570
Yamanaka S (2009) A fresh look at iPS cells. Cell 137:13–17
Liu H, Ye Z, Kim Y et al (2010) Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology 51:1810–1819
Yu J, Thomson JA (2008) Pluripotent stem cell lines. Genes Dev 22:1987–1997
Ben-David U, Benvenisty N (2011) The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 11:268–277
Si-Tayeb K, Noto FK, Nagaoka M et al (2010) Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51:297–305
Song Z, Cai J, Liu Y et al (2009) Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res 19:1233–1242
Sullivan GJ, Hay DC, Park IH et al (2010) Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology 51:329–335
Gai H, Nguyen DM, Moon YJ et al (2010) Generation of murine hepatic lineage cells from induced pluripotent stem cells. Differentiation 79:171–181
Li W, Wang D, Qin J et al (2010) Generation of functional hepatocytes from mouse induced pluripotent stem cells. J Cell Physiol 222:492–501
Behbahan IS, Duan Y, Lam A et al (2011) New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Rev 7:748–759
Espejel S, Roll GR, McLaughlin KJ et al (2010) Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest 120:3120–3126
Rashid ST, Corbineau S, Hannan N et al (2010) Modeling metabolic inherited disorders of the liver using human pluripotent stem cells. J Clin Invest 120:3127–3136
Soto-Gutierrez A, Tafaleng E, Kelly V et al (2011) Modeling and therapy of human liver diseases using pluripotent stem cells: how far we come ? Hepatology 53:708–711
Amimoto N, Mizumoto H, Nakazawa K et al (2011) Hepatic differentiation of mouse embryonic stem cells and induced pluripotent stem cells during organoid formation in hollow fibers. Tissue Eng Part A 17:2071–2078
Sipe JD (2002) Tissue engineering and reparative medicine. Ann N Y Acad Sci 961:1–9
Piscaglia AC, Campanale R, Gasbarrini G et al (2010) Stem cell-based therapies for liver diseases: state of the art and new perspectives. Stem Cells Int 2010:259461. doi:10.4061/2010/259461
Han B, Lu Y, Meng B et al (2009) Cellular loss after allogenic hepatocyte transplantation. Transplantation 87:1–5
Dan Y, Yeoh G (2008) Liver stem cells: a scientific and clinical perspective. J Gastroenterol Hepatol 23:687–698
Alison MR, Islam S, Lim S (2009) Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J Pathol 217:282–298
Houlihan DD, Newsome PN (2008) Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology 135:438–450
Couto BG, Goldenberg RC, da Fonseca LM et al (2011) Bone marrow mononuclear cell therapy for patients with cirrhosis: a Phase 1 study. Liver Int 31:391–400
Salama H, Zekri AR, Bahnassy AA et al (2010) Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol 16:5297–5305
Terai S, Ishikawa T, Omori K et al (2006) Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 24:2292–2298
Lyra AC, Soares MB, da Silva LF et al (2010) Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol 22:33–34
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Russo, F.P., Burra, P., Parola, M. (2014). Adult Liver Stem Cells. In: Turksen, K. (eds) Adult Stem Cells. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-9569-7_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9569-7_13
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-9568-0
Online ISBN: 978-1-4614-9569-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)